Abstract
OBJECTIVE
To test whether inhibiting inflammation with salsalate improves endothelial function in patients with type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
We conducted an ancillary study to the National Institutes of Health–sponsored, multicenter, randomized, double-masked, placebo-controlled trial evaluating the safety and efficacy of salsalate in targeting inflammation to improve glycemia in patients with T2D. Flow-mediated, endothelium-dependent dilation (FMD) and endothelium-independent, nitroglycerin-mediated dilation (NMD) of the brachial artery were assessed at baseline and 3 and 6 months following randomization to either salsalate 3.5 g/day or placebo. The primary end point was change in FMD at 6 months.
RESULTS
A total of 88 participants were enrolled in the study, and data after randomization were available for 75. Patients in the treatment and control groups had similar ages (56 years), BMI (33 kg/m2), sex (64% male), ethnicity, current treatment, and baseline HbA1c (7.7% [61 mmol/mol]). In patients treated with salsalate versus placebo, HbA1c was reduced by 0.46% (5.0 mmol/mol; P < 0.001), fasting glucose by 16.1 mg/dL (P < 0.001), and white blood cell count by 430 cells/µL (P < 0.02). There was no difference in the mean change in either FMD (0.70% [95% CI −0.86 to 2.25%]; P = 0.38) or NMD (−0.59% [95% CI −2.70 to 1.51%]; P = 0.57) between the groups treated with salsalate and placebo at 6 months. Total and LDL cholesterol were 11 and 16 mg/dL higher, respectively, and urinary albumin was 2.0 µg/mg creatinine higher in the patients treated with salsalate compared with those treated with placebo (all P < 0.009).
CONCLUSIONS
Salsalate does not change FMD in peripheral conduit arteries in patients with T2D despite lowering HbA1c. This finding suggests that salsalate does not have an effect on vascular inflammation, inflammation does not cause endothelial dysfunction in T2D, or confounding effects of salsalate mitigate favorable effects on endothelial function.
Atherosclerosis is the principal cause of death and disability among patients with type 2 diabetes (T2D), in whom it typically occurs earlier, with greater severity, and with more diffuse distribution (1). Chronic subacute inflammation is associated with obesity, insulin resistance and T2D, and atherosclerosis (reviewed by Shoelson et al. [2] and Libby [3]). Anti-inflammatory salicylates, which inhibit the nuclear factor (NF)-κB signaling pathway, are being studied as a potential new class of drugs for the treatment of these disorders, and recent studies have shown that targeting inflammation with salsalate can improve glycemia in patients with obesity and T2D (4–7).
Diabetes exerts its proatherogenic actions in part by disturbing endothelial homeostasis. Endothelium-dependent vasodilation, a marker of atherosclerosis, is impaired in patients with atherosclerotic risk factors, including diabetes (8–11). Endothelial function in peripheral conduit arteries reflects function in the human coronary circulation (12), and impaired endothelium-dependent vasodilation predicts cardiovascular events (13,14). Flow-mediated, endothelium-dependent dilation (FMD) can be quantitated as an index of vasomotor function, and the noninvasive nature of the technique permits repeated measurements over time to evaluate the effectiveness of interventions that may affect vascular health. Thus, we sought to test the hypothesis that targeting inflammation will improve endothelial function. We evaluated FMD as an informative surrogate marker of cardiovascular health following a therapeutic trial of salsalate, a novel agent being evaluated to treat T2D.
RESEARCH DESIGN AND METHODS
Trial design
The Targeting Inflammation Using Salsalate in Type 2 Diabetes (TINSAL-T2D) stage 2 trial was conducted as a single-mask lead-in, randomized, double-masked, placebo-controlled, multicenter clinical trial. In brief, the protocol, approved by human subject institutional review boards at each institution, included 1 week of screening; a 4-week, single-masked placebo run-in; a baseline evaluation before treatment; and a 48-week treatment period, with computer-generated (1:1) randomized allocation to salsalate (3.5 g/day divided) or a placebo that appeared identical, as described in detail (15). Inclusion criteria included T2D, age 18 to 75 years, and HbA1c levels of 7–9.5% (53–80 mmol/mol) at screening. Concomitant oral diabetes medication had to be at stable dosages for at least the previous 8 weeks. Patients who received low-dose aspirin (81–325 mg/day) also were eligible for the trial. Key exclusion criteria included use of thiazolidinediones, insulin, or glucagon-like peptide-1 receptor agonists; chronic use of nonsteroidal anti-inflammatory drugs, anticoagulants, or uricosuric agents; and impaired renal function with an estimated glomerular filtration rate <60 mL/min and/or urinary albumin >300 µg/mg creatinine. The Effect of Targeting Inflammation Using Salsalate on Flow-Mediated Dilation (TINSAL-FMD) was conducted as an ancillary study to the parent trial. Seven academic sites within the United States participated in the ancillary study. The protocol was approved by the human subject institutional review boards at each institution.
Vascular function studies were performed at baseline (visit 3, week 0) before randomization; at 3 months (visit 7, week 12); and at 6 months (visit 9, week 24). All nitric oxide potentiating drugs (e.g., phosphodiesterase type 5 inhibitors) were suspended for 1 week, and all antihypertensive and vasoactive drugs, alcohol, and caffeine were held for at least 12 h before vascular function measurements.
Central training and FMD methods
Endothelial function was determined in accordance with published guidelines (16). All sites received central training and underwent certification procedures to establish a uniform methodology. Each of the sites submitted to the core imaging laboratory sample images acquired from at least three volunteers for review to demonstrate the site’s ability to acquire technically adequate images for interpretation before any research subject was enrolled at the site. All sites had ultrasound equipment capable of simultaneous B-mode and electrocardiographic monitoring, with an electrocardiographic trigger, DICOM file storage capacity, a Hokanson SC5 (or equivalent 6- × 83-cm) blood pressure cuff with a manual cuff inflator, and an automatic blood pressure monitor. Subjects were studied in a quiet, temperature-controlled, dimly lit room after resting supine for a minimum of 5 min. High-resolution B-mode ultrasonography of the brachial artery was performed using a 7.5-MHz linear array probe. The brachial artery was imaged longitudinally just proximal to the antecubital fossa with the transducer position adjusted to obtain optimal images of the near and far wall of the intima. The R wave on the electrocardiogram served as a trigger to acquire digitized image frames at end diastole. After acquiring a baseline image, a sphygmomanometric cuff placed on the upper portion of the arm was inflated to suprasystolic pressure (200 mmHg) for 5 min. When the cuff is released, reactive hyperemia causes flow to increase through the brachial artery subserving the forearm. The velocity time integral was assessed by pulse Doppler within 15 s of cuff release to assess the peak velocity of hyperemic blood flow. FMD of the brachial artery was determined with images acquired 1 min after cuff deflation. FMD at this time point is largely endothelium-dependent and mediated by nitric oxide, and it can be inhibited by administration of the nitric oxide synthase antagonist NG-monomethyl-l-arginine (17). Ten minutes after release of the cuff, the brachial artery was re-imaged to ensure a return to basal conditions. Then, to determine nitroglycerin-mediated endothelium-independent vasodilation (NMD), subjects received sublingually 0.4 mg of nitroglycerin. The brachial artery was imaged 3 min later. Nitroglycerin was not administered if the systolic blood pressure was <100 mmHg. All images were analyzed at a central core laboratory by a single reader masked to treatment assignment. In the core laboratory, the intraobserver (operator) variability is 0 ± 0.15%. Arterial diameter during each experimental condition was measured using edge detection software (Brachial Analyzer for Research, version 5.8.9 SP-2; Medical Imaging Applications LLC, Coralville, IA).
Primary and secondary outcomes
The primary outcome of this study was the change in FMD from baseline to 6-month follow-up in the group treated with salsalate compared with the group treated with placebo. Change in NMD was an important secondary outcome.
Laboratory measures
Unless otherwise noted, laboratory measurements were performed at Quest Diagnostics (Chantilly, VA). Commercial immunoassays were used according to instructions for insulin and C-peptide (Mercodia, Upsala, Sweden), adiponectin, cystatin C, high-sensitivity C-reactive protein (CRP), tumor necrosis factor (TNF) and TNF receptors (ELISA kits from R & D Systems, Minneapolis, MN), and free fatty acids (reagents from VWR International, Radnor, PA).
Statistical analyses
We analyzed the data following the intention-to-treat principle. All patients with data at the baseline FMD measurement and up to the point of dropout (if dropout occurred) were included in the analyses.
Differences in baseline characteristics were compared between treatment groups using Student t test for normally distributed continuous traits and χ2 or Fisher exact test for categorical traits. For normally distributed continuous outcomes we estimated mean differences between treatment groups using linear regression mixed models, including FMD measurements adjusted for baseline levels over the 26-week study period. We assumed an autoregressive moving average covariance structure. For continuous outcomes with nonparametric distributions we used the Wilcoxon test to compare change from baseline between the placebo and salsalate groups at week 24. All statistical tests report two-sided P values; P < 0.05 was considered significant. The Holm procedure was used to correct for multiple comparisons (18).
We calculated the sample size based on mean and standard deviations of change in flow-mediated vasodilation for patients with diabetes following placebo (10). Because vascular measurements would be made at several centers we anticipated that the variation in measurements of the mean change from baseline would be 2.5-fold higher than the reported variation of 1.2%. Accordingly, based on an anticipated improvement of 25% in flow-mediated endothelium-dependent vasodilation in the group treated with salsalate compared with the group treated with placebo at 6 months and an estimated standard deviation of the mean change from baseline of 3.0%, we estimated that a total of 72 completed participants would be necessary for this study.
RESULTS
Baseline characteristics
Baseline characteristics of TINSAL-T2D subjects participating in the TINSAL-FMD ancillary study were balanced between the salsalate and placebo groups (Table 1). We enrolled 91 participants in the ancillary study. Of these, 88 participants completed the baseline assessment and 3 were unable to schedule baseline evaluations; 68 participants had visits at baseline, 3 months, and 6 months, 7 had visits at only baseline and 3 months, and 13 had only a baseline visit (Fig. 1). We excluded patients with only a baseline visit, leaving 75 patients for whom data after randomization were available for analysis. There was no statistical difference in the sex of the participants between the treatment groups.
Table 1.
Baseline characteristics of participants in the parent TINSAL-T2D study compared with the TINSAL-FMD ancillary study and characteristics by treatment group

Figure 1.
TINSAL-FMD study enrollment, randomization, and retention of the study participants.
Participants in the TINSAL-FMD ancillary study had lower systolic blood pressure and total cholesterol and were more likely to be treated with an insulin secretagogue, antihypertensive medication (especially an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker), and lipid-lowering therapy (especially hydroxymethylglutaryl-CoA reductase inhibitors [statins]) compared with the parent trial population (Table 1).
Effect of salsalate on endothelial function
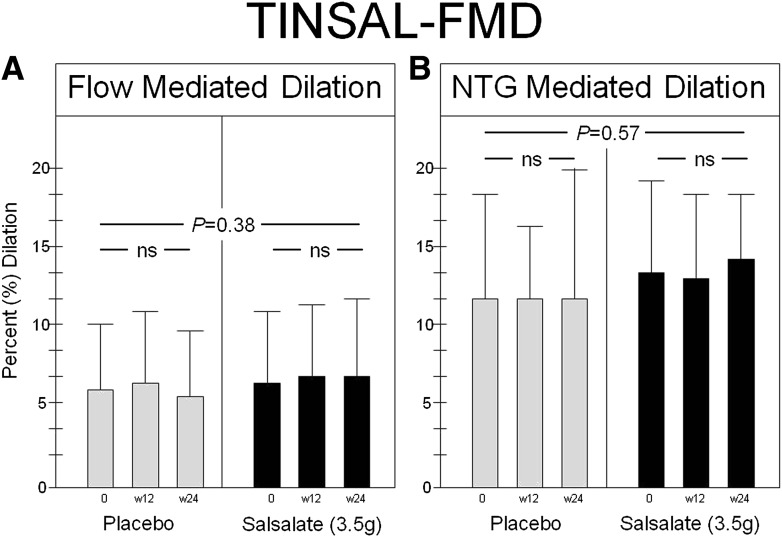
The baseline brachial artery diameter was 3.91 ± 0.62 mm in the group treated with salsalate versus 4.11 ± 0.66 mm in the group treated with placebo (P = 0.19). The baseline peak hyperemic blood flow, reported as the maximal hyperemic velocity time integral (the ratio of time averaged systolic velocity [centimeters/second] divided by the time of the cardiac cycle [seconds]), measured after cuff deflation, was 62.8 cm (interquartile range 42.0) in the group treated with salsalate and 69.6 cm (interquartile range 54.0) in the group treated with placebo (P = 0.37 between groups). There was no significant change after treatment in either brachial artery diameter or peak hyperemic velocity integral in either the salsalate or placebo treatment groups. Baseline FMD was 6.43 ± 4.55% in the salsalate group and 5.92 ± 4.02% in the placebo group. Baseline NMD was 13.14 ± 6.09% in the salsalate group and 11.78 ± 6.57% in the placebo group. Compared with placebo, salsalate had no effect on the change in FMD at either 12 or 24 weeks of treatment (P = 0.38) (Fig. 2A). Likewise, salsalate had no effect on change in NMD (P = 0.57) (Fig. 2B).
Figure 2.
FMD (A) and nitroglycerin (NTG)-mediated dilation (B) are shown for participants treated with placebo and salsalate. There was no difference in the change in FMD (the primary outcome of this study) or NTG-mediated vasodilation from baseline to the 6-month follow-up in the group treated with salsalate compared with the group treated with placebo. w12, week 12; w24, week 24.
Effect of salsalate on metabolic and cardiorenal risk factors
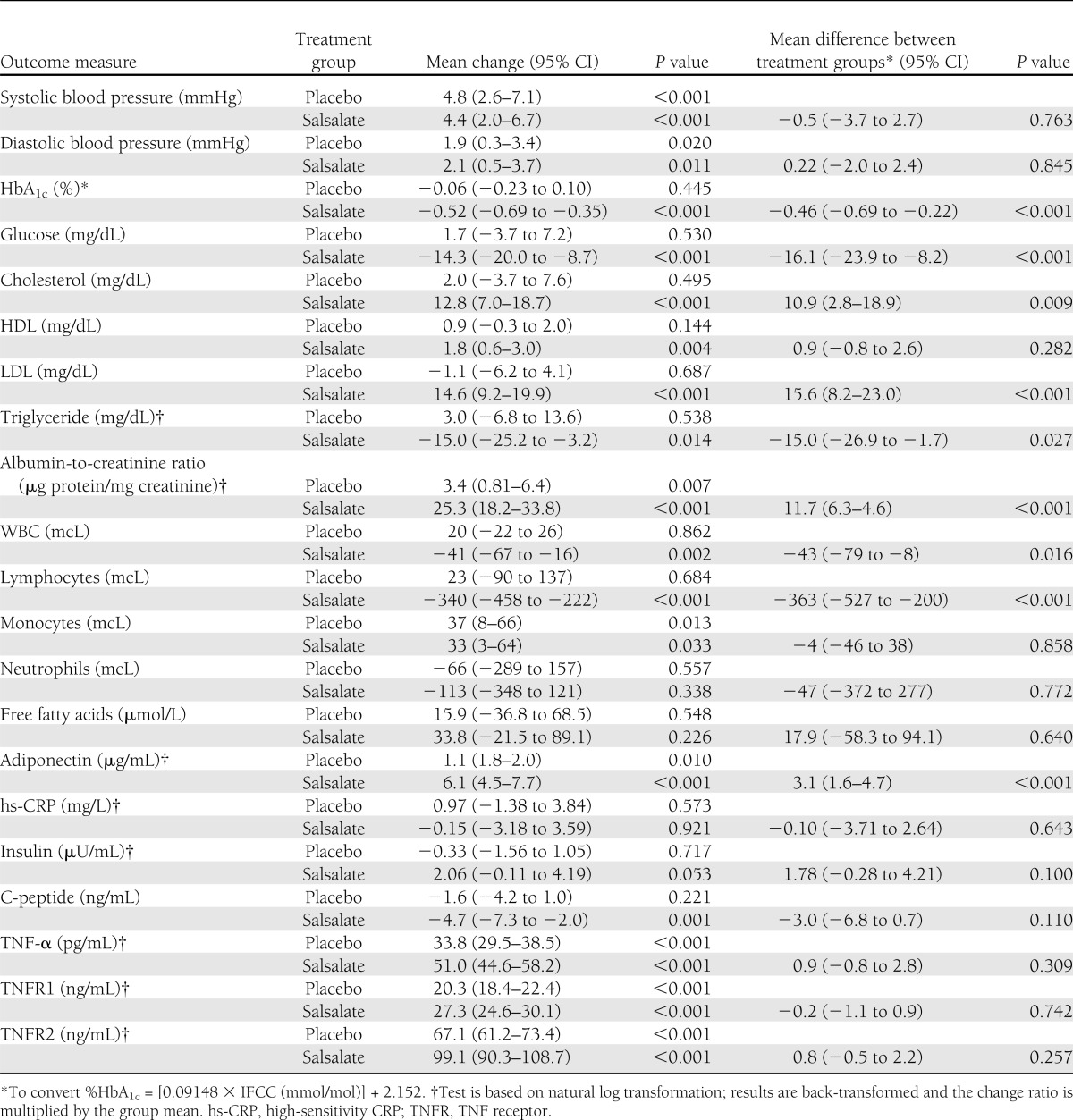
The effects of salsalate on metabolic and cardiorenal outcomes for the entire TINSAL-T2D cohort are reported by Goldfine et al. (15). The effects were similar in magnitude for glycemic, blood pressure, lipid, and inflammatory parameters in the TINSAL-FMD cohorts. Both HbA1c and fasting glucose were lowered in the group treated with salsalate compared with placebo (Table 2). There was no difference between treatment groups for systolic and diastolic blood pressures. LDL and urinary albumin, however, increased in the group treated with salsalate compared with placebo. The anti-inflammatory properties of salsalate were evident by reductions in circulating total white blood cell (WBC) and lymphocyte counts and an increase in adiponectin. There was no difference between treatment groups in the change in high-sensitivity CRP, TNF-α, or the TNF receptors 1 or 2.
Table 2.
Effect of salsalate on selected clinical and laboratory values
CONCLUSIONS
The primary finding of this study is that salsalate had no effect on FMD of the brachial artery in patients with T2D. In this study we evaluated salsalate administered over 6 months at a dose that is tolerated by the majority of patients and is under evaluation for therapeutic use in the treatment of T2D. Both HbA1c and fasting glucose were lowered in the group treated with salsalate compared with the group treated with placebo, demonstrating the positive effects of salsalate on glycemia. The anti-inflammatory effects of salsalate also were evident in the lower circulating total WBC and lymphocyte counts in the group treated with salsalate, even though there was no difference in the change in CRP between groups. Potentially adverse effects on lipids and renal function were seen. Salsalate treatment increased total and LDL cholesterol but did not affect HDL; it also increased urinary albumin, albeit without changing estimates of the glomerular filtration rate.
There are several potential explanations for our findings that neither support nor refute the findings that NF-κB expression and/or activity are increased in the endothelium of people who are resistant to insulin, with differential effects in patients with and without diabetes (19,20). First, salicylate levels achieved at the tolerable doses of salsalate used in this study may have minimal or no effect on endothelial function in people with established T2D. In a report by Pierce and colleagues (21), salsalate administered to overweight patients at higher doses (4.5 g/day) over 4 days increased the inhibition of NF-κB expression, reduced nuclear expression of NF-κB in endothelial cells, and improved endothelial function. However, these higher doses of salsalate are poorly tolerated and would not be feasible for extended administration (5,21). In addition, the positive effect of salsalate on endothelial function that was demonstrated following short-term exposure (21) may not be sustained.
Second, the participants in the TINSAL-FMD ancillary study had a higher disease index compared with the participants in the parent TINSAL-T2D study; this was manifest in the greater use of dual therapy and sulfonylureas at baseline to manage diabetes and statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers to manage lipids and hypertension, respectively. It is possible that targeting inflammation with salsalate has few additional beneficial effects on endothelial function in advanced disease or when added to lipid-lowering and antihypertensive therapies (22–24). Indeed, we recently found that salsalate did not change endothelial function, as assessed by peripheral arterial tonography, in patients with prediabetes, many of whom also were taking therapies to lower lipids and blood pressure (25). Third, confounding effects of salsalate on lipids and renal function may mitigate any improvements in endothelial function derived from salsalate such that the net effect is neutral. The mechanisms of increased LDL cholesterol and urinary albumin remain unknown. It is interesting that increased LDL concentrations have been observed with several classes of anti-inflammatory agents, including inhibition of TNF-α and interleukin (IL)-6 (26,27). High circulating WBC counts occur in patients with obesity and the metabolic syndrome (28) and predict incident T2D (29,30) and cardiovascular disease as well as poor outcomes in the latter (31–33), suggesting that reductions in the WBC count may benefit individuals with cardiometabolic risk. WBC counts are lowered by salsalate but, interestingly, not by statins. Salsalate lowered WBC counts in this study, even though the vast majority of study patients were taking statins. In contrast, salsalate had little effect on CRP levels in either the overall TINSAL-T2D trial or this FMD ancillary study, although CRP is lowered by statins. These findings are consistent with the notion that the anti-inflammatory effects of salsalate are independent from those of statins. Likewise, nonsteroidal anti-inflammatory drugs are not known to lower WBC counts, thus further distinguishing these anti-inflammatory drug classes and potential mechanisms.
Diets rich in calories, animal fats, and sugars have been shown to induce a state of chronic subacute inflammation and promote dyslipidemia, fatty liver, insulin resistance, T2D, and cardiovascular disease. At the molecular level, activation of the transcription factor NF-κB leads to the production of multiple mediators of inflammation. Stimuli that can activate NF-κB in obesity can be separated into extracellular ligands, such as the proinflammatory cytokines TNF-α, IL-1β, and IL-6 or fatty acids binding to Toll-like receptors, and intracellular stimuli, such as endoplasmic reticulum or oxidative stress and ceramides (2). These processes are associated with both T2D and increased atherosclerotic risk and thus could provide novel therapeutic targets to treat or prevent either or both processes. However, the overlap in inflammatory mechanisms driving diabetes and cardiovascular disease remains incompletely understood. The effect of NF-κB on the vasculature may occur through regulation of adipokines and cytokines that lead to vascular injury or by reducing insulin sensitivity within the endothelium, which normally signals the phosphatidylinositol-3 kinase and Akt/protein kinase B pathway, leading to activation of endothelial nitric oxide synthase.
Multiple studies demonstrate that high-dose salicylates inhibit activity of NF-κB (34–36), which regulates transcription of numerous inflammatory mediators. Compared with aspirin, salsalate, a nonacetylated dimer of salicylate, is an equipotent inhibitor of NF-κB but a much weaker inhibitor of the cyclooxygenase enzymes; therefore it is not associated with an increased risk of bleeding and is clinically safer. While salicylates inhibit NF-κB signaling, they may have additional cellular and molecular mechanisms of action. These include inhibition of mitochondrial dehydrogenases; transcription factors other than NF-κB (e.g., cAMP-responsive element–binding protein, NF of activated T cells, heat shock transcription factor-1); and cellular kinases (inhibitor of κB kinase-β, S6-kinase, p38 mitogen-activate protein kinase, ribosomal protein S6 kinase 2, Jun NH2-terminal kinase) (37–41). Salicylate also may inhibit 11-β-hydroxysteroid dehydrogenase type 1 in adipose tissue (42) and activate AMP-activated protein kinase (43). It is difficult to distinguish the relative in vivo contributions of these potential mechanisms.
Finally, it is difficult to determine whether salsalate should be further developed as a diabetes therapy given the mixed effects on metabolic markers of vascular health—including glycemic improvement, increased adiponectin, and lowered WBC, triglyceride, and uric acid levels—in the setting of increased LDL cholesterol and urinary albumin. Additional studies are underway to target inflammation using salsalate in patients with diverse diseases, including schizophrenia, anemia, myelodysplastic syndromes, spinal cord injury, polycystic ovarian syndrome, and chronic obstructive pulmonary disease. Understanding the cardiovascular impact of medications that would be intended for chronic use is of high clinical importance, but the neutral effect on endothelium-dependent dilation is especially important given the many potential diseases that might improve with targeted inflammatory response.
In conclusion, in this study we targeted inflammation in patients with T2D using a clinically tolerable dose of salsalate over 6 months to evaluate the effect on atherosclerotic risk as assessed by a measure of endothelial function. We did not find a change, either improvement or worsening, in FMD of the brachial artery, a surrogate measure of nitric oxide bioactivity, despite lowering HbA1c and markers of inflammation. These observations suggest that either inflammation does not cause endothelial dysfunction in T2D, that salsalate does not adequately inhibit inflammation of the vasculature, or that confounding effects of salsalate mitigate favorable effects on endothelial function. Yet while there was no measurable net effect on endothelial function, anti-inflammatory interventions potentially have other favorable effects on cardiovascular outcomes by interfering with the atherosclerotic process elsewhere. At this time, however, the net effects of salsalate on vascular health, as assessed by FMD, seem to be neutral, and further study is warranted before widespread use.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 HL091750, U01 DK74556, and P50 HL83813; NIH General Clinical Research Center and Clinical and Translational Science Award grants at multiple sites; and the Tullis-Tulane (V.F.) and Helen and Morton Adler Chairs (S.E.S.).
Caraco Pharmaceutical (Detroit, MI) supplied the drug and placebo; LifeScan (Miltipas, CA, a division of Johnson & Johnson) supplied home glucose-monitoring kits; and Mercodia (Uppsala, Sweden) supplied the insulin and C-peptide assay kits. No other potential conflicts of interest relevant to this article were reported.
A.B.G. and M.A.C. designed and wrote the trial protocol and wrote the manuscript. J.S.B. assisted in the development of trial materials and site training to ensure similar methods across sites and in image reading. C.D., V.F., S.E.S., and K.A.J. contributed to the manuscript. Y.-D.I.C. managed the special assay core. K.A.J. analyzed the trial data. All authors approved the final manuscript for submission. M.A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data from this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Dr. Myrlene Staten of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), for her scientific discussions.
APPENDIX
The participating investigators of the TINSAL-FMD Study are: Vanita Aroda, MD, MedStar Research Institute, Endocrinology, Diabetes, and Metabolism, Washington, DC; Robert Brook, MD, University of Michigan, Ann Arbor, MI; Mark A. Creager, MD, Principal Investigator, Brigham and Women's Hospital, Boston, MA; Cyrus Desouza, MD, University of Nebraska Medical Center, Omaha, NE; Vivian Fonseca, MD, Tulane University Health Sciences Center, New Orleans, LA; Allison B. Goldfine, MD, Joslin Diabetes Center, Boston, MA; Kathleen A. Jablonski, PhD, Biostatistics Center, Rockville, MD; Kieren Mather, MD, Indiana University, Division of Endocrinology & Metabolism, Indianapolis, IN; Catherine J. McNeal, MD, Scott & White, Temple, TX; Steven E. Shoelson, MD, PhD, Joslin Diabetes Center, Boston, MA; and Guillermo Umpierrez, MD, Emory University School of Medicine, Atlanta, GA.
Footnotes
Clinical trial reg. no. NCT00799643, clinicaltrials.gov.
J.S.B. is currently affiliated with Emory University School of Medicine, Emory University Hospital, Atlanta, Georgia.
Y.-D.I.C. is currently affiliated with the Los Angeles BioMed Institute, Los Angeles, California.
A list of the participating investigators of the TINSAL-FMD Study can be found in the Appendix.
References
- 1.Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 2013;34:2444–2452 [DOI] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874 [DOI] [PubMed] [Google Scholar]
- 4.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE, TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med 2010;152:346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfine AB, Silver R, Aldhahi W, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci 2008;1:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 2008;31:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol 2013;50:537–543 [DOI] [PubMed]
- 8.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990;323:22–27 [DOI] [PubMed] [Google Scholar]
- 9.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest 1990;86:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Keaney JF, Jr, Creager MA. Oral antioxidant therapy improves endothelial function in Type 1 but not Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 2003;285:H2392–H2398 [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 2004;109:613–619 [DOI] [PubMed] [Google Scholar]
- 12.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995;26:1235–1241 [DOI] [PubMed] [Google Scholar]
- 13.Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003;41:1769–1775 [DOI] [PubMed] [Google Scholar]
- 14.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 2009;120:502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfine AB, Fonseca V, Jablonski KA, et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med 2013;159:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265 [DOI] [PubMed] [Google Scholar]
- 17.Lieberman EH, Gerhard MD, Uehata A, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol 1996;78:1210–1214 [DOI] [PubMed] [Google Scholar]
- 18.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70 [Google Scholar]
- 19.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 2007;100:1659–1666 [DOI] [PubMed] [Google Scholar]
- 20.Tabit CE, Shenouda SM, Holbrook M, et al. Protein kinase C-β contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation 2013;127:86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-kappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 2009;119:1284–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005;111:2356–2363 [DOI] [PubMed] [Google Scholar]
- 23.Koh KK, Han SH, Chung WJ, et al. Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol 2004;93:1432–1435, A10 [DOI] [PubMed] [Google Scholar]
- 24.Sugiura T, Kondo T, Kureishi-Bando Y, et al. Nifedipine improves endothelial function: role of endothelial progenitor cells. Hypertension 2008;52:491–498 [DOI] [PubMed] [Google Scholar]
- 25.Goldfine AB, Conlin PR, Halperin F, et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia 2013;56:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vis M, Nurmohamed MT, Wolbink G, et al. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J Rheumatol 2005;32:252–255 [PubMed] [Google Scholar]
- 27.Kawashiri SY, Kawakami A, Yamasaki S, et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int 2011;31:451–456 [DOI] [PubMed] [Google Scholar]
- 28.Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30). Am J Cardiol 2002;89:1441–1443 [DOI] [PubMed] [Google Scholar]
- 29.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 2002;51:455–461 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 1999;353:1649–1652 [DOI] [PubMed] [Google Scholar]
- 31.Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: the NHANES-I epidemiologic follow-up study. Ann Epidemiol 2005;15:266–271 [DOI] [PubMed] [Google Scholar]
- 32.Grimm RH, Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA 1985;254:1932–1937 [PubMed] [Google Scholar]
- 33.Rana JS, Boekholdt SM, Ridker PM, et al. Differential leucocyte count and the risk of future coronary artery disease in healthy men and women: the EPIC-Norfolk Prospective Population Study. J Intern Med 2007;262:678–689 [DOI] [PubMed] [Google Scholar]
- 34.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001;293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 35.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994;265:956–959 [DOI] [PubMed] [Google Scholar]
- 36.Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol 1996;156:3961–3969 [PubMed] [Google Scholar]
- 37.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem 2005;280:33097–33100 [DOI] [PubMed] [Google Scholar]
- 38.Aceves M, Dueñas A, Gómez C, San Vicente E, Crespo MS, García-Rodríguez C. A new pharmacological effect of salicylates: inhibition of NFAT-dependent transcription. J Immunol 2004;173:5721–5729 [DOI] [PubMed] [Google Scholar]
- 39.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem 2003;278:24944–24950 [DOI] [PubMed] [Google Scholar]
- 40.Stevenson MA, Zhao MJ, Asea A, Coleman CN, Calderwood SK. Salicylic acid and aspirin inhibit the activity of RSK2 kinase and repress RSK2-dependent transcription of cyclic AMP response element binding protein- and NF-kappa B-responsive genes. J Immunol 1999;163:5608–5616 [PubMed] [Google Scholar]
- 41.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA 1997;94:2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nixon M, Wake DJ, Livingstone DE, et al. Salicylate downregulates 11beta-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization. Diabetes 2012;61:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 2012;336:918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]