Hypoxia in obese adipose tissue (AT) plays an important role in the development of whole-body insulin resistance by inducing local inflammation and the release of proinflammatory cytokines (1). Yet, living at high altitude is associated with a lower prevalence of impaired fasting glucose and type 2 diabetes compared with living at low altitude (2). Furthermore, exposure to hypoxic environments increases whole-body glucose fluxes in healthy males and glucose uptake in human and murine skeletal muscle (3). In addition, exercising under hypoxic conditions improves glucose tolerance more than exercising under normoxia (4), strongly suggesting an insulin-sensitizing effect of hypoxia. Therefore, we hypothesized that exposing obese men to 10 consecutive nights of moderate hypoxia (15 ± 0.5% O2, ∼2,400 m elevation) would improve insulin sensitivity.
Eight healthy obese men (4 Caucasians, 3 African Americans, and 1 Hispanic of mean ± SEM age 28 ± 1 years, weight 96.5 ± 5.3 kg, and BMI 32.7 ± 1.3 kg/m2) without evidence of chronic disease or sleep apnea and taking no medication participated in this study. The protocol was approved by the institutional review board at Pennington Biomedical Research Center (Baton Rouge, LA). Subjects slept for 10 consecutive nights (∼10 h/night, ≥100 h in total) in a hypoxic tent (Hypoxico Inc., New York, NY) maintained at ∼15% O2 (range 14.5–15.5% O2, ∼2,400 m above sea level) using nitrogen dilution. Biopsies of abdominal subcutaneous AT and skeletal muscle were obtained at baseline and on day 11 under normoxic and hypoxic (AT only) conditions. The oxygen tension in subcutaneous AT was also measured in normoxia at baseline and under hypoxia and normoxia on day 11 using dual temperature-oxygen tension probes (Licox, Integra LifeSciences, Plainsboro, NJ) as previously described (5).
In vivo insulin sensitivity was measured by a two-step hyperinsulinemic-euglycemic clamp (low insulin, 20 mU/m2/min for 180 min; high insulin, 80 mU/m2/min for 120 min), and the glucose disposal rate (GDR) was calculated. Substrate oxidation rates and energy expenditure were assessed by indirect calorimetry (Deltatrac II; Datex-Ohmeda) at the end (30 min) of each stage of the clamp. In vitro, myotubes obtained from biopsied muscle were cultured and differentiated for 5 days and then incubated at 37°C under normoxic or hypoxic conditions (15% O2) for 4 h with measures of glucose uptake (6). Protein and gene expression were measured in skeletal muscle using Western immunoblotting and real-time PCR and adjusted to glyceraldehyde-3-phosphate dehydrogenase or Ponceau S stain and to cyclophilin A expression, respectively.
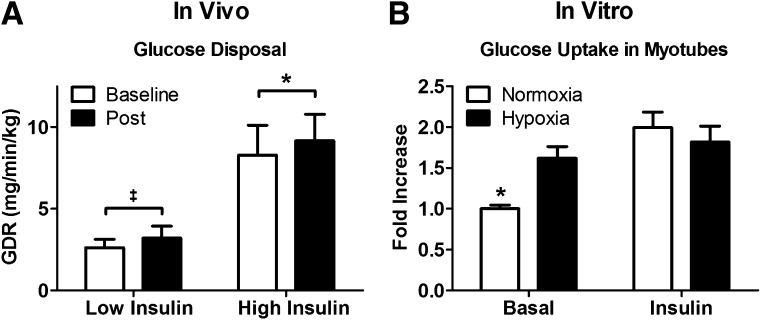
In response to the 10-night hypoxia treatment, subjects lost an average of 1.2 ± 0.3 kg (P = 0.003), and AT pO2 tended to decrease from 51.1 ± 5.7 to 40.9 ± 2.1 mmHg (P = 0.07). This was accompanied by a decrease in fasting glucose from 94.8 ± 3.3 mg/dL at baseline to 91.8 ± 2.7 mg/dL on day 12 (P = 0.04) but unchanged fasting insulin (11.1 ± 2.9 vs. 10.3 ± 2.2 mU/L; P = 0.28). At high insulin infusion, GDR increased from 8.3 ± 1.8 to 9.2 ± 1.6 mg/kg/min (P = 0.02), indicating improved whole-body insulin sensitivity (Fig. 1A). The relative change in GDR at high insulin was 20 ± 8% and was inversely correlated with baseline GDR (r = −0.71, P = 0.05) but did not correlate with weight loss (P = 0.22). GDR was somewhat increased (23 ± 17%) at low insulin infusion from 2.6 ± 0.5 to 3.2 ± 0.7 mg/kg/min (P = 0.09). Impressively, half of the subjects experienced at least a 38% improvement in GDR at either low or high insulin.
Figure 1.
A: 10 nights under moderate hypoxia improves insulin sensitivity, as measured by GDR during a hyperinsulinemic-euglycemic clamp at low (20 mU/m2/min) and high (80 mU/m2/min) insulin infusion. B: 4 h of hypoxia improves basal but not insulin-stimulated glucose uptake in cultured human myotubes. *P ≤ 0.05, ‡P ≤ 0.10.
These in vivo improvements in insulin sensitivity were corroborated by in vitro experiments showing a 62 ± 5% increase (P = 0.0006) in insulin-independent glucose uptake in primary myotubes exposed to hypoxia (15% O2) for 4 h and no change in insulin-dependent uptake (Fig. 1B). In AT, hypoxia-inducible factor 1α expression tended to be higher under hypoxia than normoxia either at baseline or at postintervention (P = 0.09). Interestingly, muscle expression of the insulin signaling proteins Akt and IRS1 and of the mitochondrial complexes I-V and peroxisome proliferator–activated receptor γ coactivator 1α were unchanged after the hypoxia treatment. However, gene expression of peroxisome proliferator–activated receptor γ coactivator 1α and COL6A3 decreased by 56 ± 7% (P = 0.02) and 48 ± 16% (P = 0.05), respectively.
In line with studies performed in rodents and isolated human skeletal muscle, this study reports for the first time a reduced fasting glucose level and improved whole-body (skeletal muscle) and hepatic insulin sensitivity after nightly exposure to moderate hypoxia. Indeed, controlled studies at altitude and after acute exposure to hypoxia during exercise report enhancements in carbohydrate metabolism, including increased glucose oxidation, reduced fasting insulin and glucose, and higher fluxes of glucose (4). The improvements in GDR at low and high doses of insulin were on average >20%, with half of the subjects experiencing a 38% or greater improvement in GDR at one of the two doses. This was corroborated by an increase in non–insulin-dependent glucose uptake in primary myotubes. Skeletal muscle protein expression and in vitro experiment data show that insulin signaling remained unaffected, suggesting enhanced insulin-independent translocation of glucose transporters by hypoxia (7), such as is encountered during exercise. Since these remarkable improvements in whole-body insulin sensitivity were greatest in those with the worst baseline insulin sensitivity, our results suggest that nightly exposure to moderate hypoxia may provide a new therapeutic avenue to improve carbohydrate metabolism in prediabetes and type 2 diabetes.
Acknowledgments
E.R. received funding for this study from an institutional grant generously donated by an anonymous philanthropist and by Nutrition Obesity Research Center grant NIH P30 DK072476. V.L. was supported by a grant from the Swiss National Science Foundation (PBLAP3_136942). The study also used the Genomics Core Facility at Pennington Biomedical Research Center, which is supported in part by the Centers of Biomedical Research Excellence (NIH P20 RR021945).
No potential conflicts of interest relevant to this article were reported.
V.L. designed the study, performed the tests and laboratory analysis, drafted the manuscript, recruited participants, and revised the manuscript. C.M.P. recruited participants, performed the tests and laboratory analysis, performed the statistical analysis, drafted the manuscript, and revised the manuscript. J.D.C. and P.J.E. performed the tests and laboratory analysis and revised the manuscript. E.A.F. performed the tests and laboratory analysis, recruited participants, and revised the manuscript. J.-M.S. designed the study, performed the tests and laboratory analysis, and revised the manuscript. E.R. designed the study, drafted the manuscript, and revised the manuscript. E.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank the clinical staff of the Pennington Biomedical Research Center for their outstanding assistance and all of the volunteers for their participation.
Footnotes
Clinical trial reg. no. NCT01872533, clinicaltrials.gov.
V.L. and C.M.P. contributed equally to this study.
References
- 1.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 2013;93:1–21 [DOI] [PubMed] [Google Scholar]
- 2.Santos JL, Pérez-Bravo F, Carrasco E, Calvillán M, Albala C. Low prevalence of type 2 diabetes despite a high average body mass index in the Aymara natives from Chile. Nutrition 2001;17:305–309 [DOI] [PubMed] [Google Scholar]
- 3.Azevedo JL, Jr, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes 1995;44:695–698 [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab 2012;97:E546–E555 [DOI] [PubMed] [Google Scholar]
- 5.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry RR, Abrams L, Nikoulina S, Ciaraldi TP. Insulin action and glucose metabolism in nondiabetic control and NIDDM subjects. Comparison using human skeletal muscle cell cultures. Diabetes 1995;44:936–946 [DOI] [PubMed] [Google Scholar]
- 7.Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol 1991;70:1593–1600 [DOI] [PubMed] [Google Scholar]