Abstract
OBJECTIVE
Impaired awareness of hypoglycemia (IAH) and defective counterregulation significantly increase severe hypoglycemia risk in type 1 diabetes (T1D). We evaluated restoration of IAH/defective counterregulation by a treatment strategy targeted at hypoglycemia avoidance in adults with T1D with IAH (Gold score ≥4) participating in the U.K.-based multicenter HypoCOMPaSS randomized controlled trial.
RESEARCH DESIGN AND METHODS
Eighteen subjects with T1D and IAH (mean ± SD age 50 ± 9 years, T1D duration 35 ± 10 years, HbA1c 8.1 ± 1.0% [65 ± 10.9 mmol/mol]) underwent stepped hyperinsulinemic-hypoglycemic clamp studies before and after a 6-month intervention. The intervention comprised the HypoCOMPaSS education tool in all and randomized allocation, in a 2 × 2 factorial study design, to multiple daily insulin analog injections or continuous subcutaneous insulin infusion therapy and conventional glucose monitoring or real-time continuous glucose monitoring. Symptoms, cognitive function, and counterregulatory hormones were measured at each glucose plateau (5.0, 3.8, 3.4, 2.8, and 2.4 mmol/L), with each step lasting 40 min with subjects kept blinded to their actual glucose value throughout clamp studies.
RESULTS
After intervention, glucose concentrations at which subjects first felt hypoglycemic increased (mean ± SE from 2.6 ± 0.1 to 3.1 ± 0.2 mmol/L, P = 0.02), and symptom and plasma metanephrine responses to hypoglycemia were higher (median area under curve for symptoms, 580 [interquartile range {IQR} 420–780] vs. 710 [460–1,260], P = 0.02; metanephrine, 2,412 [−3,026 to 7,279] vs. 5,180 [−771 to 11,513], P = 0.01). Glycemic threshold for deterioration of cognitive function measured by four-choice reaction time was unchanged, while the color-word Stroop test showed a degree of adaptation.
CONCLUSIONS
Even in long-standing T1D, IAH and defective counterregulation may be improved by a clinical strategy aimed at hypoglycemia avoidance.
Hypoglycemia remains an important barrier to achievement of near-normal glucose control in people with insulin-treated diabetes (1–3). Severe hypoglycemia (SH), (defined as an event requiring assistance of another person to administer carbohydrate, glucagon, or other resuscitative measures) affects 30–50% of individuals with established type 1 diabetes (T1D) each year and remains one of the most feared complications, as it can result in loss of consciousness, seizures or even sudden death (4,5).
In people without diabetes, hypoglycemia is prevented by the existence of a well-coordinated hierarchy of responses including an early cessation of insulin secretion. If blood glucose is lowered sufficiently (usually under experimental conditions), glucagon secretion increases and a brisk sympathodrenal response leads to a rise in epinephrine, symptom generation, and self-awareness of hypoglycemia. In established T1D, the first and second physiological defenses against hypoglycemia (decrease in insulin and increase in glucagon release) are lost, and in many individuals, epinephrine and other counterregulatory and symptomatic responses are also diminished, leading to the syndromes of “defective glucose counterregulation” and “impaired awareness of hypoglycemia” (IAH). IAH affects ~25% of people with T1D, and it is associated with a sixfold increased risk of SH (6).
Although the pathogenesis is not entirely clear, it is generally accepted that hypoglycemia per se is responsible in part for the syndromes of defective glucose regulation and IAH, leading to a vicious cycle of hypoglycemia (7). In a seminal study by Heller and Cryer, a single episode of antecedent hypoglycemia caused a generalized reduction of the neuroendocrine and symptomatic responses to subsequent hypoglycemia (8). This finding has been subsequently reproduced in people without diabetes (9,10) and those with T1D (11) or type 2 diabetes (12). Further support for this concept derives from recovery of IAH and defective counterregulation after eliminating recurrent hypoglycemia in people with insulinoma (13,14) and in people with T1D after pancreas (15,16) and islet cell transplantation (17), as well as through adherence to treatment protocols aimed at meticulous prevention of hypoglycemia (18–21).
Use of continuous subcutaneous insulin infusion (CSII) is reported to reduce SH (22), but relatively few studies have compared CSII with modern long-acting analog insulins, such as insulin glargine as the basal insulin (23,24). Studies involving use of real-time continuous glucose monitoring (RT-CGM) have also shown reduced rates of biochemical hypoglycemia (25,26). In all such studies, improvement of HbA1c has been the primary outcome, with prevention of SH a secondary (and sometimes underpowered) end point. No study to date has evaluated the efficacy of RT-CGM in combination with CSII or optimized analog-based multiple daily injections (MDIs) with the primary objective of reversing IAH. A recent study in adolescents with relatively short duration of T1D (5.2 ± 1.4 years) showed improved epinephrine responses after 4-week use of RT-CGM with preset low alarms at 6 mmol/L and advice to institute standard hypoglycemia treatment for blood glucose levels <6 mmol/L (27).
The HypoCOMPaSS (Comparison of Optimised MDI versus Pumps with or without Sensors in Severe Hypoglycemia) trial (28) is a U.K.-based, prospective, multicenter, randomized controlled trial (RCT) with the primary aim of comparing the ability to avoid hypoglycemia and reversal of IAH using either optimized subcutaneous insulin analog regimen (MDI) or insulin pump therapy (CSII) with or without adjunctive RT-CGM in a 2 × 2 factorial design. The primary outcome of the main HypoCOMPaSS study was the difference in hypoglycemia awareness determined by Gold score (6) between study interventions at 24 weeks. All eligible trial participants were invited to participate in optional hyperinsulinemic-hypoglycemic clamp substudies before and after the trial intervention, with the aim of objectively demonstrating reversibility of IAH and counterregulatory responses during controlled hypoglycemic challenge. The primary outcome for the clamp substudy was the glucose concentration at which participants felt hypoglycemic during progressive hypoglycemia. Here, we describe the results from those who participated in paired clamp studies before and 24 weeks after the study intervention.
RESEARCH DESIGN AND METHODS
The full HypoCOMPaSS trial protocol (including details of the hyperinsulinemic-hypoglycemic clamp substudy) has previously been published (28), but key details are summarized here. The study protocol, participant information sheets, and consent forms were approved by an independent research ethics committee, and the study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant for the main trial, and separate written consent was obtained for the optional clamp study described here.
Between July 2010 and June 2011, 96 adults were recruited to the main HypoCOMPaSS trial from five U.K. tertiary referral and academic hypoglycemia/CSII centers. Four of the five centers participated in the optional clamp study: Addenbrooke’s Hospital, Cambridge; Newcastle Diabetes Centre, Newcastle upon Tyne; and Derriford Hospital, Plymouth, and Sheffield Teaching Hospitals, Sheffield.
Key inclusion criteria for the main HypoCOMPaSS trial participation were as follows: 1) age 18–74 years inclusive, 2) T1D according to World Health Organization criteria, 3) serum C-peptide <50 pmol/L with simultaneous exclusion of biochemical hypoglycemia (glucose <4.0 mmol/L), and 4) IAH confirmed by a Gold score (6) of ≥4 with or without history of SH in the preceding 12 months (as defined by the American Diabetes Association [29]). Key exclusion criteria comprised any condition precluding informed consent, unwillingness to undertake intensive insulin therapy and use study devices, and history of intolerance to insulin glargine. Additional exclusion criteria were applied to the optional stepped hyperinsulinemic-hypoglycemic clamp studies as follows: age >60 years, history of epilepsy (seizures not primarily induced by hypoglycemia), and known ischemic heart disease or other significant disease that in the judgment of the investigators would increase the risks associated with taking part in the substudy.
HypoCOMPaSS study intervention
After a 4-week run-in period, participants were randomized to one of four treatment arms: MDI with conventional self-monitoring of blood glucose (SMBG), MDI with SMBG and RT-CGM, CSII with SMBG, and CSII with SMBG and RT-CGM. The primary goal of insulin dose titration throughout the 24-week RCT period was the absolute avoidance of all glucose levels <4 mmol/L as determined by CGM and SMBG. Participants were advised to treat all glucose levels <4 mmol/L with 15 g glucose with repeat SMBG every 15 min until glucose >4 mmol/L and to consider prospective insulin dose reduction. All participants received a focused individualized education session (“My HypoCOMPaSS” tool) at the start of the 24-week RCT period aimed at avoidance and early detection of all blood glucose levels <4 mmol/L with the goal of preventing progression to significant hypoglycemia while maintaining overall glycemic control. Further details about the educational tool and follow-up of participants during the 6 months intervention are provided in the Supplementary Data. Importantly, there was no difference in the clinical contact time or follow-up between treatment groups (28).
Clamp study procedures
Clamp studies were conducted in a dedicated clinical research facility within respective institutions before and after the 24-week HypoCOMPaSS trial intervention. Participants were fitted with a retrospective CGM sensor (Medtronic iPro; Minimed) to be worn for 5–7 days preceding the study day. This was downloaded on the morning of the clamp study to determine whether any antecedent biochemical hypoglycemia occurred over the 24-h period prior to the clamp. Studies were rescheduled after repeat CGM profile if biochemical hypoglycemia (≤3 mmol/L) was detected over the preceding 24 h. All participants were advised to fast from 10:00 p.m. the night before and to avoid caffeine for 24 h before the study.
Participants were admitted to the clinical research facility at 7:00 a.m. on the day of their clamp studies. On arrival, an intravenous cannula was inserted in the antecubital vein of the nondominant arm and blood glucose was stabilized using sliding-scale Actrapid insulin (Novo Nordisk, Baegsvard, Denmark) insulin infusion aiming initially for blood glucose 6.0–7.0 mmol/L and then 5.0–6.0 mmol/L between 10:30 a.m. and 11:00 a.m. for clamp initiation.
A second retrograde cannula was inserted into a vein on the dorsum of the nondominant hand, which was heated to 50–60°C to arterialize venous blood. During this period of stabilization, participants practiced cognitive function tests demonstrated to be sensitive to hypoglycemia: four-choice reaction time (19,30,31) and Stroop (32,33) tests. At the start of the clamp, a primed infusion of 60 mU/m2/min soluble human Actrapid insulin in a 4% solution of autologous blood in 0.9% sodium chloride was started via the nondominant antecubital vein catheter. In parallel, the rate of infusion of 20% dextrose was adjusted as needed, aiming to stabilize plasma glucose at 5.0 mmol/L at 40 min followed by sequential lowering to 3.8 mmol/L, 3.4 mmol/L, 2.8 mmol/L, and 2.4 mmol/L in 40-min steps. Samples for plasma glucose were obtained every 5 min and analyzed in real time. Participants remained blinded to their real-time glucose levels throughout the study.
At the end of each clamp stage, participants completed a previously validated questionnaire (Edinburgh Hypoglycemia Score) (34) consisting of 11 items requiring rating of four autonomic symptoms (pounding heart, shaking/tremor, hunger, and sweating) and five neuroglycopenic symptoms (drowsiness, difficulty speaking, clumsiness/incoordination, odd behavior, and confusion). We omitted the two nonspecific symptoms (nausea and headache) from our analysis. Each item was scored from 1 (absent) to 7 (maximal) and for ease of interpretation converted to a scale of 0–6 with a minimum-maximum possible score range of 0–54. The symptom questionnaire was followed by the four-choice reaction-time test and the Stroop test.
Additional blood samples were taken at regular intervals for the later measurement of insulin, metanephrine, growth hormone, glucagon, and cortisol. Heart rate and blood pressure were recorded every 20 min.
Analytical methods
Arterialized plasma glucose was analyzed in real time using Yellow Springs analyzer (YSI STAT Plus, Farnborough, U.K.) (intra-assay coefficient of variation [CV] 1.5% and interassay CV 2.8%) Plasma insulin was measured by ELISA (Dako, Glostrup, Denmark) (intra-assay CV 1.8% and interassay CV 7.8%). Glucagon was measured by ELISA (Alpco Diagnostics) (intra-assay CV 1.6% and interassay CV 2.4%). Cortisol levels were measured using a two-step sandwich immunoassay (Roche Modular E-170 platform, Elecsys cortisol reagents) (intra-assay CV 1.4% [based on a mean value of 593 nmol/L] and interassay CV 4.7% [based on a mean value of 535 nmol/L]). Plasma metanephrine (separate from normetanephrine) was measured using ELISA (Alpco Diagnostics) (intra-assay CV 12% [based on a mean value of 652 pmol/L] and interassay CV 12.2% [based on a mean value of 350 pmol/L]). Growth hormone was measured using ELISA (Alpco Diagnostics) (intra-assay CV 1.4% and interassay CV 4.5%). All samples were measured in a single central laboratory in the same batch.
Data and statistical analysis
Glucose thresholds for the onset of symptoms, counterregulatory hormone responses, and impairment of cognitive function were determined according to previously published protocols (19,30,35,36). Briefly, glucose thresholds for onset of hormone responses were defined as the time of onset of a sustained (≥2 successive time points) increase in hormone concentrations ≥2 SDs above the mean of the five baseline measurements for that hormone. Thresholds for an increase in total, autonomic, and neuroglycopenic symptoms were determined as the time at which the symptoms score increased ≥2 over baseline on ≥2 successive time points. Where no defined changed occurred, the lowest measured glucose level during the respective clamp was used as the threshold for that individual in keeping with published literature. Incremental areas under the curve for symptoms were calculated using incremental symptom scores (subtracting the symptom score at the end of stage 1 of the clamp from the scores obtained in stages 2–5). Incremental areas under the curve for hormones were calculated by subtracting the mean hormone levels achieved during euglycemia (first 40 min of the clamp) from subsequent hormone levels during the rest of the clamp. For hormones, single missing values were replaced with linear interpolation and the trapezoidal rule was used for calculation of the area under curve. For the four-choice reaction time, glucose thresholds were determined as the plasma glucose level when the reaction time first exceeded twice the CV of the stable baseline measurements. For Stroop tests, glucose thresholds were determined as the plasma glucose level where performance first deteriorated ≥2 SDs below the mean baseline performance.
Data are presented as mean (SE) or median (interquartile range) unless stated otherwise. Normally distributed data/parameters were compared using paired-samples t tests, while non–normally distributed data/parameters were compared using the Wilcoxon signed rank test. Statistical analyses were conducted using SPSS, version 19 (IBM Software, Hampshire, U.K.), and P < 0.05 was considered statistically significant.
RESULTS
Study participants
In total, 30 participants consented to the baseline clamp study, and 27 participants consented to the post-RCT clamp study. Stepped hypoglycemic clamp studies were successfully completed in 25 participants at baseline and 22 participants post-RCT. The most common reason for premature clamp termination was difficulty obtaining arterialized blood from the retrograde cannula. One clamp study was prematurely terminated owing to transient hypotension. Results presented in this report refer to the 18 participants (mean ± SD age 50 ± 9 years, T1D duration 35 ± 10 years, and HbA1c 8.1 ± 1.0% [65 ± 10.9 mmol/mol]) who completed paired baseline and post-RCT clamp studies. Detailed demographic and other baseline parameters for the paired clamp cohort as well as nonclamp HypoCOMPaSS trial participants are summarized in Supplementary Table 1. The participants undergoing paired clamp studies had significantly longer duration of diabetes, earlier onset of disease, and lower BMI compared with other HypoCOMPaSS participants. Baseline HbA1c and Gold score were comparable between two groups.
Treatment allocations and effects on glycemic control and hypoglycemia
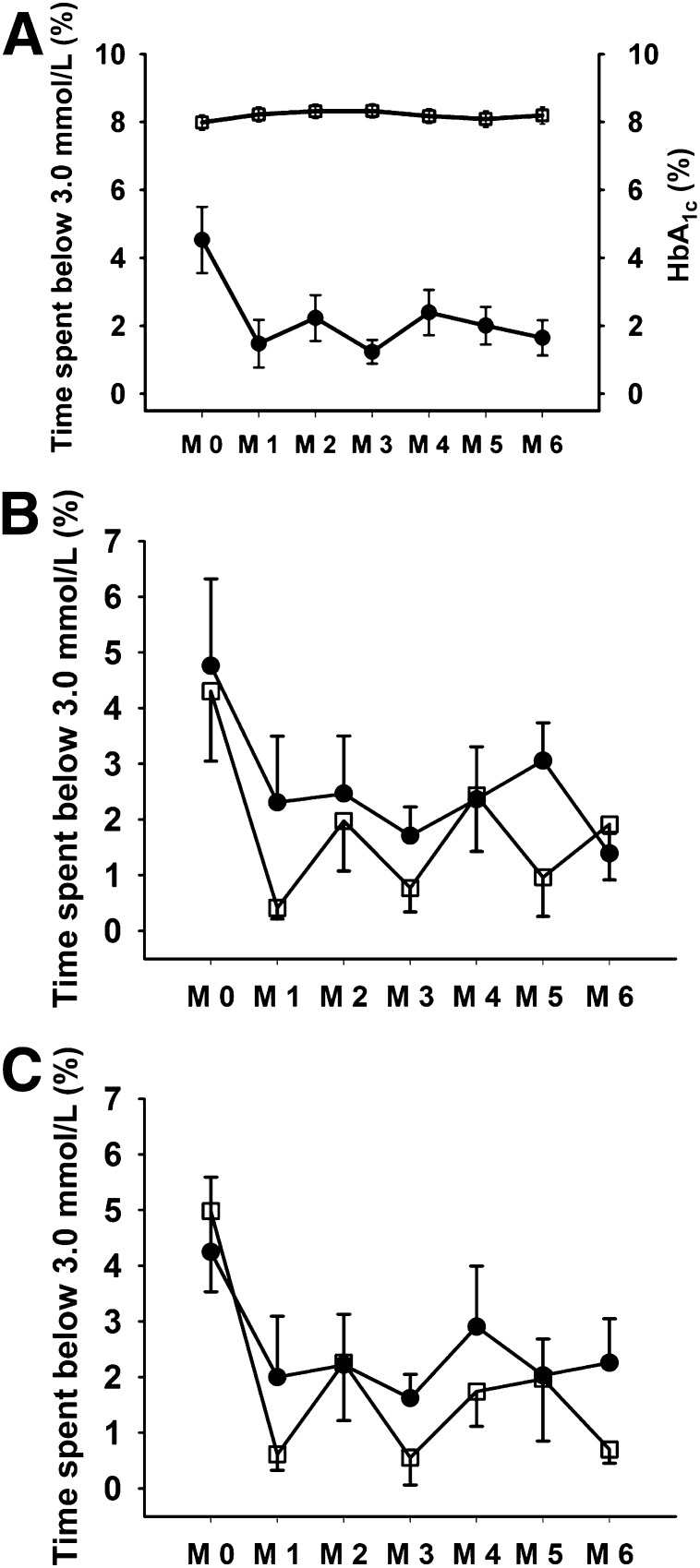
Of the 18 participants who completed paired clamp studies, 9 were randomized to CSII and 9 to MDI, with 11 participants randomized to conventional monitoring and 7 subjects to RT-CGM [2 × 2 study design]). Changes in monthly HbA1c and percentage of time spent below 3.0 mmol/L during monthly blinded CGM for participants undergoing paired clamp studies are shown in Fig. 1A. Compared with baseline, the percentage of time spent in biochemical hypoglycemia (blood glucose <3.0 mmol/L [54 mg/dL]) was reduced by ~65% from baseline to study end (4.5 ± 0.9% vs. 1.6 ± 0.5%, P = 0.015). Further, the percentage of time spent with blood glucose <3.9 mmol/L (70 mg/dL) was reduced by ~50% from baseline to study end (11.3 ± 2.1% vs. 5.6 ± 1.0%, P = 0.025). Importantly, this was achieved without an overall deterioration in glycemic control as measured by HbA1c (baseline vs. 24 weeks: 8.1 ± 0.2% vs. 8.2 ± 0.2% or 65 mmol/mol vs. 66 mmol/mol, P = 0.66). Compared with the previous 6 months, annualized SH rates were significantly lower during the 6-month study intervention: 4 (interquartile range 0–7) vs. 0 (0–0), P = 0.001. Compared with baseline, post-RCT Gold scores were significantly lower (baseline vs. 24 weeks: 5.2 ± 0.2 vs. 4.3 ± 0.4, P = 0.009), with 7 of 18 participants showing reversal (Gold score <4 at 24 weeks) and a further 5 of 18 showing an improvement in IAH.
Figure 1.
A: Time spent <3.0 mmol/L (%) (●) during monthly blinded continuous glucose monitoring and HbA1c (%) (□) during the study intervention for the whole clamp cohort (N = 18). Data shown are mean ± SEM. (M 0, baseline, M 1 to M 6, end of each study month.) B: Time spent <3.0 mmol/L (%) during monthly blinded continuous glucose monitoring for MDI (N = 9) (●) and CSII (N = 9) (□) groups. C: Time spent <3.0 mmol/L (%) during monthly blinded continuous glucose monitoring for non–RT-CGM (N = 11) (●) and RT-CGM (N = 7) (□) groups.
As shown in Fig. 1B and C, overall time spent in hypoglycemia (blood glucose <3.0 mmol/L [54 mg/dL]) was not statistically different with CSII and RTCGM compared with MDI and SMBG (mean ± SE area under the curve, CSII vs. MDI 640 ± 191 vs.852 ± 219, P = 0.47, and RTCGM vs. SMBG 658 ± 223 vs. 797 ± 193, P = 0.64).
Results from clamp studies
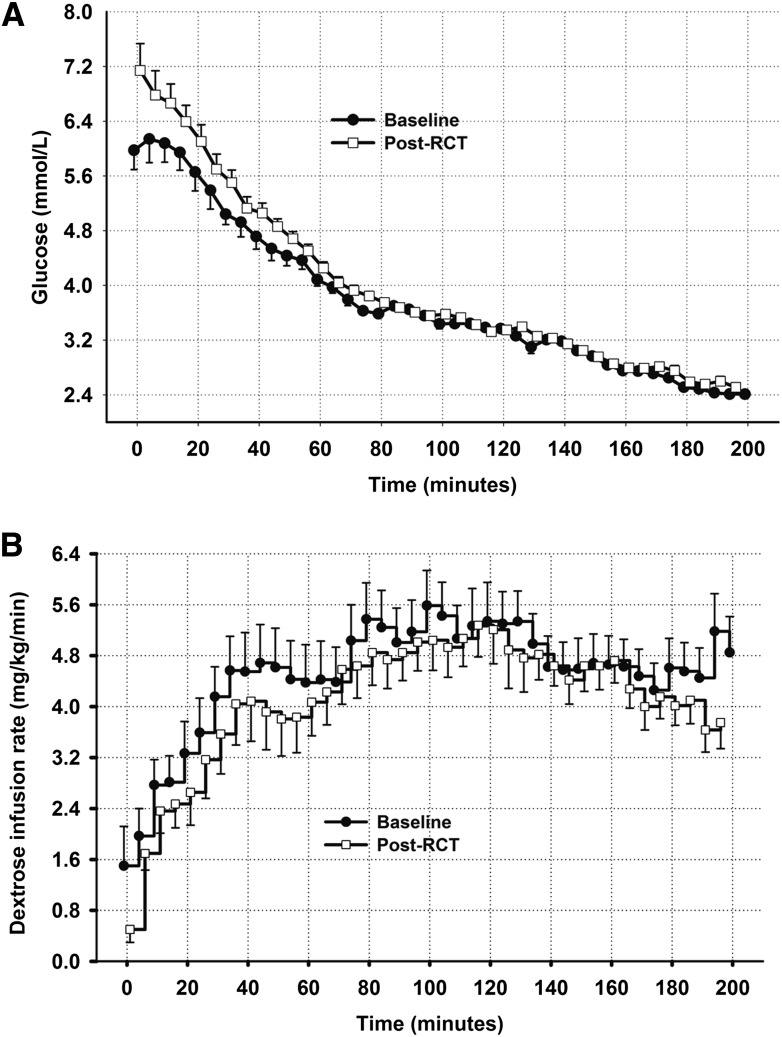
Plasma glucose levels and dextrose infusion rates during baseline and post-RCT clamp studies are shown in Fig. 2A and B. Mean starting glucose level of the post-RCT clamps was slightly higher than in the baseline clamps (6.0 ± 0.3 vs. 7.0 ± 0.4 mmol/L), but by the end of euglycemic phase (>40 min) glucose levels were comparable (4.7 ± 0.2 vs. 5.0 ± 0.1 mmol/L). Participants achieved similar glucose levels during the hypoglycemic phase of the clamp studies (>80 to >200 min). There was a tendency for the amount of dextrose required to maintain plasma glucose at the desired level to be lower during the final stage of the postintervention clamp (Supplementary Fig. 1B) (>160 to >200 min). During the final 20 min of studies, mean dextrose infusion rates were 4.8 ± 1.9 vs. 4.0 ± 1.4 mg/kg/min, P = 0.058, baseline vs. post-RCT. Importantly, steady state plasma insulin levels were comparable between baseline and post-RCT clamps (data not shown).
Figure 2.
A: Plasma glucose in baseline and post-RCT clamp studies for the whole clamp cohort (N = 18). B: Dextrose infusion rates in baseline and post-RCT studies for the whole clamp cohort (N = 18).
Self-awareness of hypoglycemia
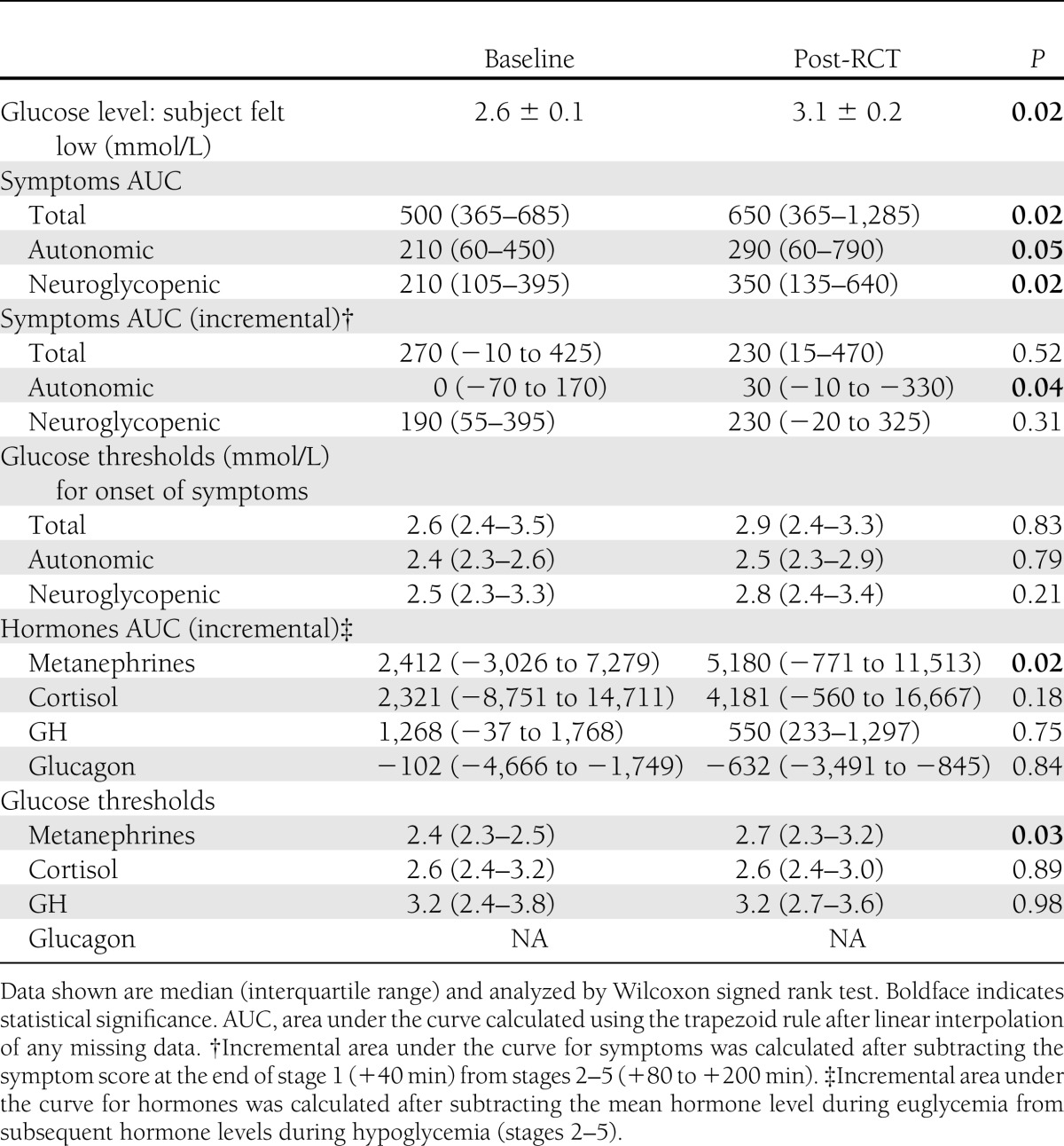
The plasma glucose concentration at which subjects first felt hypoglycemic (answer to the question, “Do you feel hypoglycemic?”) increased from 2.6 ± 0.1 mmol/L at baseline to 3.1 ± 0.2 mmol/L post-RCT (P = 0.017).
Symptom scores
As expected from the study population, questionnaire symptom scores were low during baseline clamp studies (Supplementary Fig. 1). During post-RCT clamp studies, symptom scores were higher throughout, with a greater increment during the final stage (160–200 min [Supplementary Fig. 1A and B for autonomic and neuroglycopenic symptoms]). Although thresholds for increase in symptom scores were similar, the total area under the curve values for total, autonomic, and neuroglycopenic symptoms were higher during the postintervention clamp studies (Table 1), but the incremental area under the curve was only increased significantly for autonomic symptoms.
Table 1.
Symptom and hormonal responses to clamped hypoglycemia in clamp substudy participants (N = 18)
Hormones
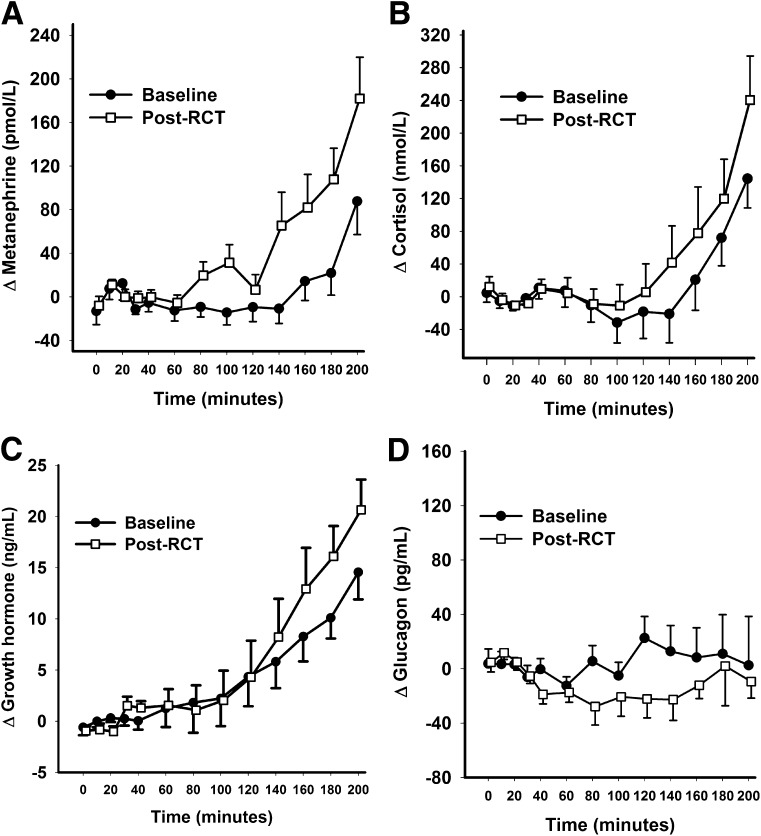
Plasma metanephrine, cortisol, and growth hormone levels rose with progressive hypoglycemia, with higher mean levels during post-RCT studies (Supplementary Tables 2 and 3), but statistical significance was only achieved for plasma metanephrine response (Table 1). As expected in this group with long-standing T1D, glucagon showed no response to hypoglycemia. Incremental plasma hormone levels during baseline and post-RCT clamp studies are shown in Fig. 3. Area under the curve values for incremental hormone levels and glucose thresholds for initiation of hormone responses are shown in Table 1.
Figure 3.
Incremental plasma hormone levels during baseline and post-RCT clamp studies for the whole clamp cohort (N = 18). Data shown are mean ± SE.
Baseline and post-RCT response by RCT intervention
Symptom and hormonal responses to hypoglycemia stratified according to the 2 × 2 study design are shown in Supplementary Tables 4 and 5. Compared with baseline, participants in the CSII arm achieved higher symptom and hormonal responses after the trial intervention, reaching statistical significance in several key parameters including the threshold for metanephrine secretion. In contrast, participants using MDI showed smaller responses, which were non–statistically significant compared with baseline. Similarly, those who were allocated to RT-CGM showed similar trends, but statistically significant improvements were only seen in total symptoms and the threshold for secretion of metanephrine, while no statistical significance was seen in the Non–RT-CGM group.
Direct head-to-head comparison of change in subjective awareness, symptoms, and hormonal responses from baseline to post-RCT are shown in Supplementary Table 6. There was a tendency for the CSII group to show more pronounced improvements compared with the MDI group, but no statistical significance was achieved.
Cognitive function tests
We did not observe any statistically significant differences in cognitive function test performance at the start of the baseline and post-RCT clamp studies. Performance in both four-choice reaction-time Stroop tests deteriorated significantly with progressive hypoglycemia (Supplementary Tables 7–10). Thresholds for deterioration of cognitive function were similar in baseline and post-RCT clamps as measured by four-choice reaction time (2.7 ± 0.1 vs. 2.7 ± 0.1 mmol/L, P = 0.90), Stroop black and white reading (2.8 ± 0.1 vs. 2.9 ± 0.2 mmol/L, P = 0.47), and color “X” components (2.6 ± 0.1 vs. 2.8 ± 0.1 mmol/L, P = 0.30). In contrast, the plasma glucose threshold for deterioration of cognitive function based on the color word interference component of the Stroop test changed from 2.6 ± 0.1 mmol/L at baseline to 3.0 ± 0.2 mmol/L in the post-RCT clamp (P = 0.045).
CONCLUSIONS
We used insulin clamp studies to create controlled reproducible hypoglycemic challenges to measure objectively responses to hypoglycemia in a subset of participants within the HypoCOMPaSS trial. Our results show that IAH may be improved, even in adults with long-standing T1D using a treatment strategy avoiding hypoglycemia without worsening overall glycemic control. During post-RCT clamp studies, most participants had higher symptom scores despite an equal hypoglycemic stimulus to baseline and first felt hypoglycemic at a higher blood glucose value. Both the concentration and the threshold for secretion of plasma metanephrine (metabolite of the key counterregulatory hormone epinephrine) were significantly improved during post-RCT clamps.
The degree of reversibility of IAH (symptom scores and glucose threshold for onset of symptoms) demonstrated in our study was less than in previous studies documenting the reversal of IAH where researchers were able to achieve near-complete avoidance of hypoglycemia (18–20,37). To achieve this, previous reports in small single-center experimental medicine studies without an RCT setting necessitated a highly focused approach with significant time and resource investment by the study team. For example, in the study by Cranston et al. (19) there was an absolute requirement for participants to consume snacks between meals and at bedtime together with reduction in insulin doses. They were contacted weekly for insulin dose adjustment, and the second clamp was only performed after 3 weeks of absolute avoidance of hypoglycemia. Similarly, in the study by Fanelli et al. (20) participants were telephoned up to four times daily. In the study by Fritsche et al. (37), preprandial target glucose was raised from 5.6 to 8.3 mmol/L and target bedtime glucose was increased from 5.6 to 10 mmol/L. In contrast, we aimed to maintain tight glycemic targets throughout the study, did not require participants to make any significant changes to lifestyle, and did not ask them to take regular snacks between meals or at bedtime. Within the current HypoCOMPaSS multicenter trial, we were not able to eliminate completely hypoglycemia in this group with long-standing T1D and IAH. Based on blinded CGM data, significant biochemical hypoglycemia (<3.0 mmol/L) was reduced by >60% over the first 4 weeks and maintained throughout the study period in the whole cohort. Although significantly lower compared with baseline, this ongoing biochemical hypoglycemia (compared with absolute avoidance) may explain the lesser degree of reversibility in our study. In addition, it is important to note that our study participants had long duration of diabetes (mean duration of ~35 years). This is considerably longer than the previous clamp studies, which demonstrated reversibility of IAH (18–20)
In this study, we did not demonstrate a definite advantage for one treatment modality over another. The trend for more marked improvements in awareness and responses to hypoglycemia in the CSII group compared with MDI (also for RT-CGM for some parameters) failed to reach statistical significance. It is also worth noting that participants in the CSII arm had lower symptom area under the curve and lower incremental metanephrine area under the curve than did the MDI group during baseline clamp. Our data suggest that even in long-standing T1D, defenses against hypoglycemia may be improved by clinical strategies aimed at minimizing ongoing biochemical hypoglycemia (including close professional support, education, and the judicious use of CSII or RT-CGM where appropriate).
We found no difference in the threshold for cognitive deterioration based on four-choice reaction time. This observation is consistent with previous studies (19,30) and supports the notion that there is no adaptation of the glucose level at which cognitive dysfunction occurs (30). However, the most difficult component of the Stroop test (color-word interference) did show change in the threshold for impairment from 2.6 ± 0.1 mmol/L at baseline to 3.0 ± 0.2 mmol/L in the post-RCT clamp (P = 0.045). Several previous studies, which have used a battery of cognitive function tests, have also reported such changes in glycemic thresholds (13,18,20) (downward shift in participants with IAH and subsequent upward shift during reversal of unawareness). It is possible that different cognitive function tests relate to different areas in the brain and some tests (and brain areas) are more sensitive to hypoglycemia than others and may also have different levels of adaptation to recurrent hypoglycemia. Alternatively, it is possible that performance of some cognitive function tests is affected by the distraction caused by recovery of symptoms during post-RCT clamps.
Our study has a number of strengths. It is the first RCT clamp study series to our knowledge to examine modern treatment strategies including the use of contemporary CSII and RT-CGM in a group of subjects with long-standing T1D and IAH. Further, clinically meaningful improvements have been confirmed by insulin clamp studies for the first time within a multicenter RCT involving interventions that might be incorporated into routine clinical practice. Limitations of our substudy include the lack of a control group, which did not undergo any intervention; the relative lack of power to establish the superiority of a single treatment; and the potential for contamination in a single center and measurement of plasma metanephrine rather than epinephrine. We collected samples for measurement of plasma epinephrine, but technical failures with assays prevented this. Since >90% of circulating metanephrine is produced within the adrenal medulla by the conversion of epinephrine to metanephrine by catechol-O-methyl transferase (38), plasma metanephrine level may act as a surrogate marker of epinephrine production. However, since the conversion of epinephrine to metanephrine takes some time, use of metanephrine may have underestimated the onset and magnitude of catecholamine response. It is also worth noting that experimental hypoglycemia induced during clamp studies may not be totally representative of hypoglycemia under real-world conditions, but the objective of this substudy was to compare responses before and after a treatment strategy using a reproducible hypoglycemic challenge.
In conclusion, results from the clamp substudy of the HypoCOMPaSS trial show that even in adults with long-standing diabetes, meaningful recovery of subjective awareness and counterregulatory responses to hypoglycemia can be achieved using a management strategy aimed at avoidance of hypoglycemia without relaxation of overall glycemic control. Further work is underway to identify the baseline and other characteristics associated with recovery and nonrecovery of hypoglycemia awareness and counterregulatory responses in this cohort with long-standing T1D.
Acknowledgments
This study was funded by a peer-reviewed grant from Diabetes UK and supported by the Cambridge National Institute for Health Research Biomedical Research Centre.
No pharmaceutical company or medical device manufacturer has had any role in the design or funding of this trial. J.S. is a member of the Accu-Check Advisory Board (Roche Diagnostics Australia). Her research group has received unrestricted educational grants from Medtronic and Sanofi Diabetes; has received sponsorship to host or attend educational meetings from Lilly, Medtronic, MSD, Novo Nordisk, Roche Diagnostics Australia, and Sanofi Diabetes; and has received consultancy income from Roche Diagnostics Australia and Sanofi Diabetes. M.L.E. has received travel support from Roche and Medtronic; has received support for studies from Roche, Medtronic, and Abbott Diabetes Care; sits on advisory boards for Medtronic, Roche, and CellNovo; and has received speakers fees from Animas. S.R.H. has carried out consultancy work for pump/meter and insulin companies, LifeScan, Sanofi, Novo Nordisk, and Lilly and has received research support from Medtronic and speaker fees from Novo Nordisk, Eli Lilly, and LifeScan. D.F. has received speaker fees from Animas and Novo Nordisk. J.A.M.S. has in the past taken part in Medical Advisory Boards for Novo Nordisk, Sanofi, Johnson & Johnson, and Medtronic and is receiving travel support for conference attendance and grant funding from Medtronic, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
L.L. conducted clamp studies, designed the statistical analysis plan, wrote the manuscript, and read and approved the final manuscript. S.A.L., E.W., H.K.T., A.L.-S., and K.K. conducted clamp studies, critiqued the manuscript, and read and approved the final manuscript. A.P.L. was responsible for biochemical assays, critiqued the manuscript, and read and approved the final manuscript. T.C. gave advice regarding the statistical analysis plan, critiqued the manuscript, and read and approved the final manuscript. S.M.M. was responsible for biochemical assays, critiqued the manuscript, and read and approved the final manuscript. J.S. participated in the design of the clamp studies, critiqued the manuscript, and read and approved the final manuscript. D.F. participated in the design of the clamp studies, supervised the clamp studies, critiqued the manuscript, and read and approved the final manuscript. S.R.H. participated in the design of the clamp studies, supervised the clamp studies, gave advice regarding the statistical analysis plan, critiqued the manuscript, and read and approved the final manuscript. J.A.M.S. participated in the design of the clamp studies, supervised the clamp studies, designed the statistical analysis plan, critiqued the manuscript, and read and approved the final manuscript. M.L.E. participated in the design of the clamp studies, supervised the clamp studies, designed the statistical analysis plan, wrote the manuscript, critiqued the manuscript, and read and approved the final manuscript. M.L.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the Diabetes UK Annual Professional Conference, 13–15 March 2013, Manchester, U.K.
The authors thank study participants and staff at the clinical research facilities at participating centers. The authors also thank Prof. Brian Frier, University of Edinburgh, Edinburgh, U.K., and Prof. Stephanie Amiel and Dr. Pratik Choudhary, Kings College London, London, U.K., for their advice during planning of this study.
Footnotes
Clinical trial reg. nos. ISRCTN52164803, www.isrctn.org, and EudraCT2009-015396-27, http://eudract.ema.europa.eu/.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1004/-/DC1.
References
- 1.Cryer PE. Elimination of hypoglycemia from the lives of people affected by diabetes. Diabetes 2011;60:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes 2010;59:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiel SA. Hypoglycemia: from the laboratory to the clinic. Diabetes Care 2009;32:1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Characterizing sudden death and dead-in-bed syndrome in Type 1 diabetes: analysis from two childhood-onset Type 1 diabetes registries. Diabet Med 2011;28:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–1147 [DOI] [PubMed] [Google Scholar]
- 6.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE. Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. A vicious cycle. Diabetes 1992;41:255–260 [DOI] [PubMed] [Google Scholar]
- 8.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991;40:223–226 [DOI] [PubMed] [Google Scholar]
- 9.Davis MR, Shamoon H. Counterregulatory adaptation to recurrent hypoglycemia in normal humans. J Clin Endocrinol Metab 1991;73:995–1001 [DOI] [PubMed] [Google Scholar]
- 10.Widom B, Simonson DC. Intermittent hypoglycemia impairs glucose counterregulation. Diabetes 1992;41:1597–1602 [DOI] [PubMed] [Google Scholar]
- 11.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993;91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SN, Mann S, Briscoe VJ, Ertl AC, Tate DB. Effects of intensive therapy and antecedent hypoglycemia on counterregulatory responses to hypoglycemia in type 2 diabetes. Diabetes 2009;58:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitrakou A, Fanelli C, Veneman T, et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. N Engl J Med 1993;329:834–839 [DOI] [PubMed] [Google Scholar]
- 14.Maran A, Taylor J, Macdonald IA, Amiel SA. Evidence for reversibility of defective counterregulation in a patient with insulinoma. Diabet Med 1992;9:765–768 [DOI] [PubMed] [Google Scholar]
- 15.Kendall DM, Rooney DP, Smets YF, Salazar Bolding L, Robertson RP. Pancreas transplantation restores epinephrine response and symptom recognition during hypoglycemia in patients with long-standing type I diabetes and autonomic neuropathy. Diabetes 1997;46:249–257 [DOI] [PubMed] [Google Scholar]
- 16.Paty BW, Lanz K, Kendall DM, Sutherland DE, Robertson RP. Restored hypoglycemic counterregulation is stable in successful pancreas transplant recipients for up to 19 years after transplantation. Transplantation 2001;72:1103–1107 [DOI] [PubMed] [Google Scholar]
- 17.Leitão CB, Tharavanij T, Cure P, et al. Restoration of hypoglycemia awareness after islet transplantation. Diabetes Care 2008;31:2113–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 1993;42:1683–1689 [DOI] [PubMed] [Google Scholar]
- 19.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994;344:283–287 [DOI] [PubMed] [Google Scholar]
- 20.Fanelli C, Pampanelli S, Epifano L, et al. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia 1994;37:1265–1276 [DOI] [PubMed] [Google Scholar]
- 21.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994;43:1426–1434 [DOI] [PubMed] [Google Scholar]
- 22.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 23.Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004;27:1554–1558 [DOI] [PubMed] [Google Scholar]
- 24.Hirsch IB, Bode BW, Garg S, et al. Insulin Aspart CSII/MDI Comparison Study Group Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28:533–538 [DOI] [PubMed] [Google Scholar]
- 25.Beck RW, Hirsch IB, Laffel L, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ly TT, Hewitt J, Davey RJ, Lim EM, Davis EA, Jones TW. Improving epinephrine responses in hypoglycemia unawareness with real-time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care 2011;34:50–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little S, Chadwick T, Choudhary P, et al. Comparison of Optimised MDI versus Pumps with or without Sensors in Severe Hypoglycaemia (the Hypo COMPaSS trial). BMC Endocr Disord 2012;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 30.Maran A, Lomas J, Macdonald IA, Amiel SA. Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia 1995;38:1412–1418 [DOI] [PubMed] [Google Scholar]
- 31.Heller SR, Macdonald IA. The measurement of cognitive function during acute hypoglycaemia: experimental limitations and their effect on the study of hypoglycaemia unawareness. Diabet Med 1996;13:607–615 [DOI] [PubMed] [Google Scholar]
- 32.Lezak MD. Neuropsychological Assessment. Oxford, U.K., Oxford University Press, 1995 [Google Scholar]
- 33.Bench CJ, Frith CD, Grasby PM, et al. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 1993;31:907–922 [DOI] [PubMed] [Google Scholar]
- 34.Deary IJ, Hepburn DA, MacLeod KM, Frier BM. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia 1993;36:771–777 [DOI] [PubMed] [Google Scholar]
- 35.Choudhary P, Lonnen K, Emery CJ, et al. Comparing hormonal and symptomatic responses to experimental hypoglycaemia in insulin- and sulphonylurea-treated Type 2 diabetes. Diabet Med 2009;26:665–672 [DOI] [PubMed] [Google Scholar]
- 36.Bingham E, Hopkins D, Pernet A, Reid H, Macdonald IA, Amiel SA. The effects of KATP channel modulators on counterregulatory responses and cognitive function during acute controlled hypoglycaemia in healthy men: a pilot study. Diabet Med 2003;20:231–237 [DOI] [PubMed] [Google Scholar]
- 37.Fritsche A, Stefan N, Häring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta-adrenergic sensitivity in type 1 diabetes. Ann Intern Med 2001;134:729–736 [DOI] [PubMed] [Google Scholar]
- 38.Eisenhofer G, Rundquist B, Aneman A, et al. Regional release and removal of catecholamines and extraneuronal metabolism to metanephrines. J Clin Endocrinol Metab 1995;80:3009–3017 [DOI] [PubMed] [Google Scholar]