Abstract
OBJECTIVE
To study expression of the recently identified adipokine dipeptidyl peptidase-4 (DPP4) in subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) of patients with various BMIs and insulin sensitivities, as well as to assess circulating DPP4 in relation to obesity and insulin sensitivity.
RESEARCH DESIGN AND METHODS
DPP4 expression was measured in SAT and VAT from 196 subjects with a wide range of BMIs and insulin sensitivities. DPP4 release was measured ex vivo in paired biopsies from SAT and VAT as well as in vivo from SAT of lean and obese patients. Circulating DPP4 was measured in insulin-sensitive and insulin-resistant BMI-matched obese patients.
RESULTS
DPP4 expression was positively correlated with BMI in both SAT and VAT, with VAT consistently displaying higher expression than SAT. Ex vivo release of DPP4 from adipose tissue explants was higher in VAT than in SAT in both lean and obese patients, with obese patients displaying higher DPP4 release than lean controls. Net release of DPP4 from adipose tissue was also demonstrated in vivo with greater release in obese subjects than in lean subjects and in women than in men. Insulin-sensitive obese patients had significantly lower circulating DPP4 than did obesity-matched insulin-resistant patients. In this experiment, DPP4 positively correlated with the amount of VAT, adipocyte size, and adipose tissue inflammation.
CONCLUSIONS
DPP4, a novel adipokine, has a higher release from VAT that is particularly pronounced in obese and insulin-resistant patients. Our data suggest that DPP4 may be a marker for visceral obesity, insulin resistance, and the metabolic syndrome.
Obesity is an increasing health issue worldwide and an economical burden, and as the hallmark of the metabolic syndrome the obese state is frequently associated with the development of chronic diseases, including type 2 diabetes (1,2). The association between the epidemics of obesity and diabetes has promoted research on the endocrine link between lipid and glucose homeostasis, demonstrating that adipose tissue is an endocrine organ releasing various adipokines. A complex interorgan crosstalk scenario between adipose tissue and other central and peripheral organs underlies the progression of obesity-related metabolic disorders, with adipose tissue being a key player in this scenario (3). The current view of the role of expanded adipose tissue in obesity identifies adipokines as a potential link between obesity and insulin resistance (4). This link has stimulated a further characterization of the adipocyte secretome by means of diverse proteomic profiling approaches, leading to the discovery of such novel adipokines as dipeptidyl peptidase-4 (DPP4) (5).
DPP4 is a transmembrane glycoprotein and exoprotease that cleaves N-terminal dipeptides from various substrates (6). Most importantly, DPP4 also cleaves and inactivates the incretins glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide. In this context, DPP4-inhibitors are in clinical use as antidiabetic drugs to improve glycemic control by stimulating pancreatic insulin secretion and suppressing glucagon production (7). We recently demonstrated that adipocytes release DPP4 in a differentiation-dependent manner (5). Circulating DPP4 concentrations are increased in obese subjects and correlate with fasting plasma insulin, leptin, and adipocyte size in subcutaneous adipose tissue (SAT); however, the tissue source of circulating DPP4 is not known. This study aimed to assess DPP4 expression and release in paired biopsies of SAT and visceral adipose tissue (VAT) of lean and obese patients and of patients with or without impaired glucose tolerance, as well as DPP4 release from adipose tissue in vivo. Because circulating DPP4 is increased in obese patients with the metabolic syndrome (5), we hypothesized that DPP4 expression and release in VAT are more prominent than in SAT and that VAT DPP4 could be a marker for insulin sensitivity.
RESEARCH DESIGN AND METHODS
Patients
For all studies, protocols were approved by local ethics committees. All participants gave written, informed consent.
DPP4 expression in paired biopsies from SAT and VAT.
Paired samples of VAT and SAT were obtained from 196 Caucasians (97 men and 99 women) undergoing open abdominal surgery for various reasons, including gastric banding, cholecystectomy, appendectomy, and weight-reduction surgery (8). Patients with severe conditions, including generalized inflammation or end-stage malignant disease, were not included. Age ranged from 24 to 86 years, and BMI ranged from 20.8 to 54.1 kg/m2. Sixty-seven patients had impaired glucose tolerance or type 2 diabetes. Adipose tissue specimens were frozen in liquid nitrogen immediately after excision. Clinical parameters were assessed as described previously (9). Insulin sensitivity was assessed with the euglycemic-hyperinsulinemic clamp method (10) as previously described (11). In brief, after an overnight fast and supine resting for 30 min, intravenous catheters were inserted into antecubital veins in both arms. One was used for the infusion of insulin and glucose; the other was used for the frequent sampling. After a priming dose of 1.2 nmol/m2 insulin, the infusion with insulin (Actrapid 100 U/mL; Novo Nordisk, Bagsvaerd, Denmark) was started with a constant infusion rate of 0.28 nmol/m2 body surface per minute and continued for at least 120 min. After 3 min, a variable 20% glucose infusion rate (GIR) was added. The GIR was adjusted during the clamp to maintain the blood glucose at 5.0 mmol/L. Bedside blood glucose measurements were performed every 5 min by means of the glucose dehydrogenase technique with Hemocue B (Hemocue, Angelholm, Sweden).
In vitro release of DPP4 from paired biopsies from SAT and VAT.
SAT and VAT biopsies were obtained during planned abdominal surgery (surgery for hernia, gall bladder, and other noninflammatory and nonmalignant causes) from 12 lean and 11 obese patients (BMI 22 ± 2 and 38 ± 3 kg/m2, respectively; age 62 ± 6 and 58 ± 6 years, respectively). Adipose tissue specimens were immediately transferred to medium, and explants were generated as described earlier (12). Briefly, fat explants were cultured in serum-free medium. After 24 h, the conditioned medium was collected and stored in aliquots at −80°C until further use.
Serum DPP4 and adipose DPP4 expressions in insulin-sensitive and insulin-resistant obese subjects.
Sixty morbidly obese men and women (BMI 45 ± 1.3 kg/m2) scheduled for elective cholecystectomy, explorative laparotomy, or gastric sleeve resection were selected and allocated to two experimental groups of insulin-sensitive and insulin-resistant obesity with 30 subjects each, as described previously (13). On the basis of the GIR in a euglycemic hyperinsulinemic clamp, patients were defined as either insulin sensitive (GIR >70 µmol/kg · min) or insulin resistant (GIR 60 < µmol/kg · min). Both groups were matched for sex, age, and BMI. Clinical parameters were assessed as described previously (13).
In vivo release of DPP4 from SAT.
Twenty-seven healthy volunteers (15 women, 12 men; BMI 21–41.5 kg/m2; age 32–56 years) were recruited from the greater Oxford community by advertisement or from the Oxford BioBank (14). None of the subjects were taking medication known to affect lipid metabolism, and all were normoglycemic. Arteriovenous differences were measured across abdominal SAT in the fasting state. A superficial epigastric vein draining abdominal SAT (15) and an arterialized dorsal hand vein (with the hand kept in a warming box at 60°C) were cannulated. The cannulas were kept patent with an intravenous infusion of 0.9% saline solution. Adipose tissue blood flow was measured in abdominal SAT as described previously (16) and calculated from the washout of 133Xe, assuming a partition coefficient of 10 mL/g (17). After the subjects had rested for 45 min, blood samples were taken simultaneously from the two sites. Samples were stored at −80°C until analysis. Arteriovenous differences were calculated as arterialized plasma DPP4 concentration − adipose venous plasma DPP4 concentration. Net release was calculated as arteriovenous difference × (−1) × (1-hematocrit) × blood flow, where there is a factor (−1) that converts to net release and a factor (1 − hematocrit) that converts to whole blood concentrations (assuming no DPP4 in erythrocytes). Positive values for net release indicate a net release of DPP4 from adipose tissue in vivo; negative values indicate a net uptake.
Measurement of DPP4 expression
Human DPP4 mRNA expression was measured by quantitative real-time RT-PCR in a fluorescent temperature cycler with the TaqMan assay, and fluorescence was detected on an ABI PRISM 7000 sequence detector (Applied Biosystems, Darmstadt, Germany). Total RNA was isolated with TRIzol (Life Technologies, Grand Island, NY), and 1 µg RNA was reverse transcribed with standard reagents (Life Technologies). From each RT-PCR, a 2-µL sample was amplified in a 26-µL PCR with the Brilliant SYBR green QPCR Core reagent kit from Stratagene (La Jolla, CA) according to the manufacturer’s instructions. Samples were incubated in the ABI PRISM 7000 sequence detector for an initial denaturation at 95°C for 10 min, followed by 40 PCR cycles, each cycle consisting of 95°C for 15 s, 60°C for 1 min, and 72C for 1 min. Human DPP4 mRNA expression was calculated relative to the mRNA expression of 18s rRNA, all determined by premixed assays on demand for DPP4 and 18s rRNA (Applied Biosystems). Amplification of specific transcripts was confirmed by melting curve profiles (cooling the sample to 68°C and heating slowly to 95°C with measurement of fluorescence) at the end of each PCR. The specificity of the PCR was further verified by subjecting the amplification products to agarose gel electrophoresis.
Measurement of DPP4 in serum and conditioned medium
DPP4 release by adipose tissue explants and plasma or serum concentration were determined by ELISA (R&D Systems, Wiesbaden, Germany). The assay was performed in duplicates according to the manufacturer’s instructions. The intra- and interassay variations were 5.4 and 8.1%, respectively.
Statistical analysis
Data are expressed as mean ± SEM. The Shapiro-Wilcoxon test was used to test the Gaussian distribution of biological parameters. The Student t test was used for comparison between two groups, and ANOVA followed by P for linear trend posttest was used for comparison between multiple groups. The Mann-Whitney test was used for variables that were not normally distributed. Correlations were assessed with the Pearson correlation coefficient or as indicated in the graphs. All statistical analyses were done with JMP statistics software (SAS Institute Inc., Cary, NC) or Prism (GraphPad Software, Inc., La Jolla, CA), considering a P value < 0.05 as statistically significant.
RESULTS
DPP4 expression correlates with BMI, is higher in VAT than SAT, and is increased in VAT of lean patients with impaired glucose tolerance
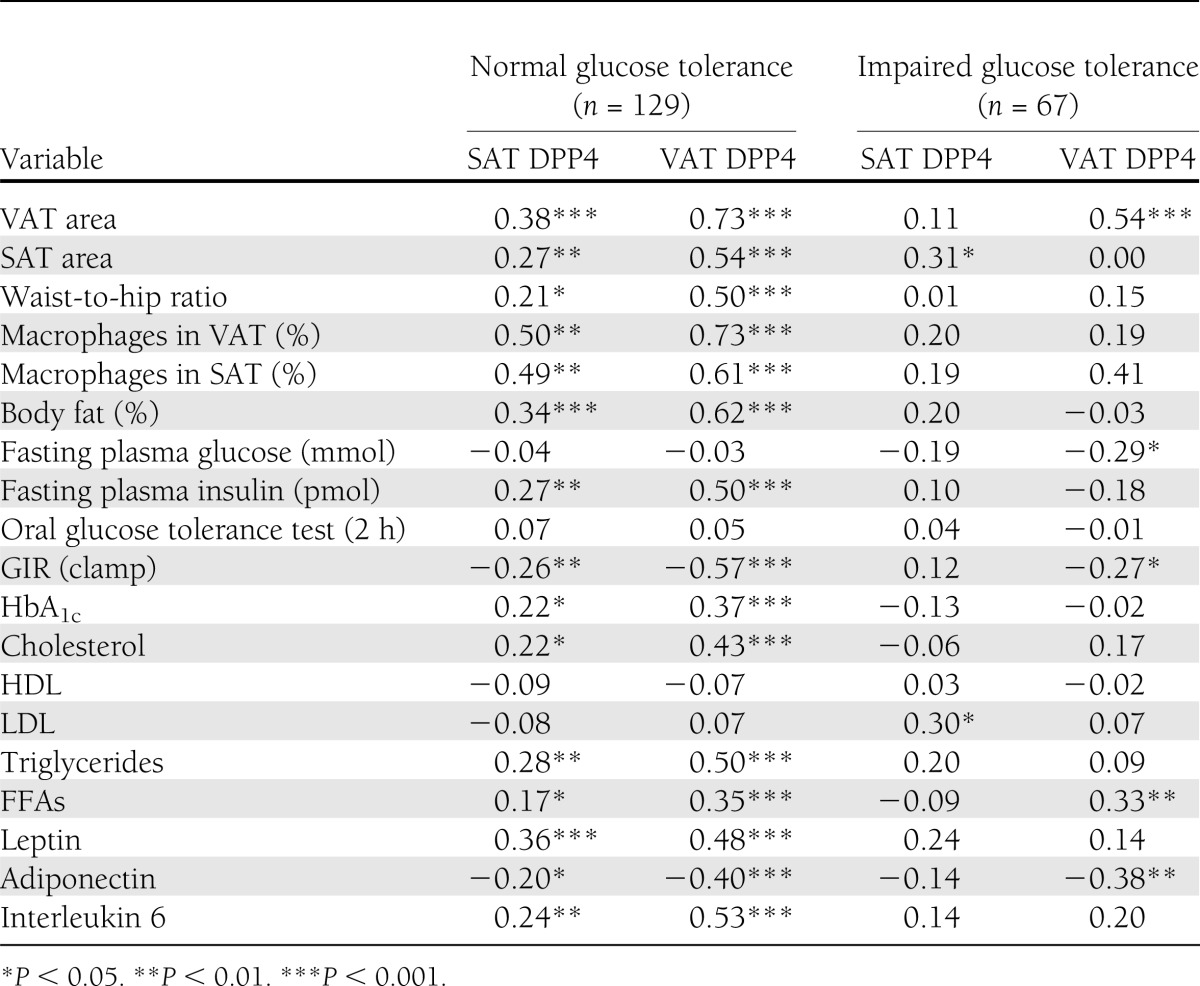
DPP4 expression in SAT and VAT was measured in 196 individuals of various BMIs that were characterized by impaired or normal glucose tolerance, as assessed by oral glucose tolerance test. DPP4 expression in both depots correlated positively with BMI (Fig. 1A and B) and correlated with each other (r = 0.28, P < 0.0001). DPP4 expression was significantly higher in VAT than in SAT. Furthermore, DPP4 expression significantly increased in both depots with increasing BMI from lean to obese subgroups (Fig. 1C). In lean individuals with impaired glucose tolerance, DPP4 was significantly increased in the VAT but not in the SAT (Fig. 1D). This difference was not related to BMI or sex in either group. In overweight and obese subjects, there was no difference in DPP4 expression in either depot according to glucose tolerance (Fig. 1E). DPP4 mRNA in both depots correlated with various clinical parameters and measures of adipose tissue inflammation (Table 1). DPP4 expression in SAT and VAT of normoglycemic patients was positively associated with several measures of obesity, including body fat, VAT and SAT areas, circulating leptin and interleukin 6, and the amount of macrophages in adipose tissue; it was negatively associated with circulating adiponectin. With respect to the metabolic state of normoglycemic patients, DPP4 expression in adipose tissue was positively correlated with fasting plasma insulin and HbA1c and negatively correlated with GIR obtained during the euglycemic-hyperinsulinemic clamp. In addition, DPP4 expression was associated with total cholesterol, free fatty acids (FFAs), and triglycerides. In both normoglycemic patients and patients with impaired glucose tolerance, DPP4 expression in VAT positively correlated with VAT area and FFAs, whereas a negative correlation was found for GIR and circulating adiponectin.
Figure 1.
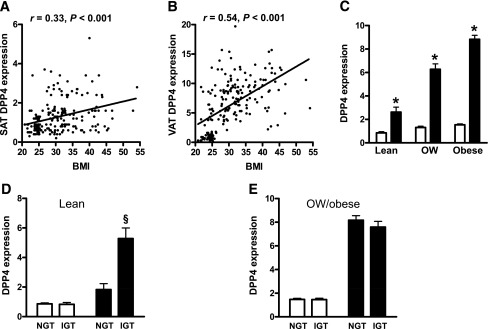
DPP4 expressions in paired SAT (white bars) and VAT (black bars) of lean, overweight (OW), and obese patients in relation to obesity and glucose tolerance. DPP4 expression in adipose tissue was measured in 197 individuals. A and B: Linear regression analysis of DPP4 expression in SAT and VAT with respect to BMI. Statistical evaluation is indicated in each graph. C–E: Comparison of SAT and VAT DPP4 expressions in lean, overweight, and obese subjects (C); in lean subjects with (IGT) and without (NGT) impaired glucose tolerance (D); and in overweight or obese subjects with and without impaired glucose tolerance (E). *P < 0.05 compared with SAT; §P < 0.05 compared with VAT of subjects without impaired glucose tolerance.
Table 1.
Pearson correlation coefficients of SAT and VAT DPP4 expressions with clinical parameters and adipose tissue measures
In vitro DPP4 release is most prominent in VAT from obese patients
Paired biopsies of SAT and VAT from lean and obese subjects were used to study DPP4 release in vitro. DPP4 release was significantly higher from VAT in both lean and obese subjects (Fig. 2A). The highest DPP4 release was measured from VAT of obese subjects. Dividing the group of obese subjects in those with type 2 diabetes and those without, we observed a significant increase of DPP4 release from VAT in those obese patients with type 2 diabetes (BMI and age not different between obese subgroups) (Fig. 2B). DPP4 release from SAT only tended to be higher in obese patients irrespective of their diabetes state (data not shown).
Figure 2.
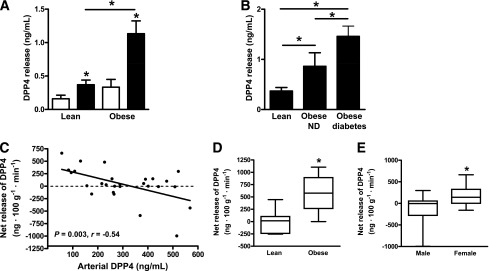
In vitro DPP4 release from paired SAT (white bars) and VAT (black bars) samples from lean and obese patients and in vivo DPP4 release from abdominal SAT samples from lean and obese men and women. A and B: DPP4 release was measured in paired adipose tissue specimens from 12 lean and 11 obese (5 with type 2 diabetes) patients by ELISA. *P < 0.05 compared with respective SAT sample or between groups as indicated. C–E: DPP4 was measured in arterialized blood and adipose tissue venous blood in lean and obese patients, and the net release of DPP4 was calculated (n = 27). C: Correlation of arterial DPP4 with DPP4 net release from adipose tissue, taking blood flow into account. Linear regression was performed with Spearman correlation. D: Net release of DPP4 from adipose tissue in lean and obese subjects with low arterial DPP4 (< 288 ng/mL, n = 14). *P < 0.05 by Mann-Whitney test. E: Net release of DPP4 from adipose tissue in men and women. *P < 0.05 by Mann-Whitney test. ND, nondiabetic.
In vivo release of DPP4 is higher in obese patients and in females
Arteriovenous differences of DPP4 were measured across abdominal SAT in lean and obese subjects as described before (15). In 16 out of all 27 subjects studied there was net release of DPP4 from adipose tissue (Fig. 2C). This was related to the arterial DPP4 concentration, with the net release of DPP4 was negatively correlated with the arterial level such that the greatest net release of DPP4 was associated with the lowest arterial concentration (Fig. 2C). In patients with lower arterial DPP4 (<288 ng/mL, n = 14), obese subjects were characterized by a significantly higher net release of DPP4 than were lean patients (Fig. 2D), and there was a correlation between DPP4 release and BMI (Spearman rank correlation 0.72, P = 0.01, data not shown). Women (n = 16) showed net release (P = 0.014 for difference from no net release, as assessed by Wilcoxon signed rank test) and showed significantly more release than men (Fig. 2E). Among the women, DPP4 release was significantly related to BMI (r = 0.59, P = 0.021, data not shown).
Circulating DPP4 is lower in BMI-matched healthy obese patients and correlates with insulin resistance and adipose tissue inflammation
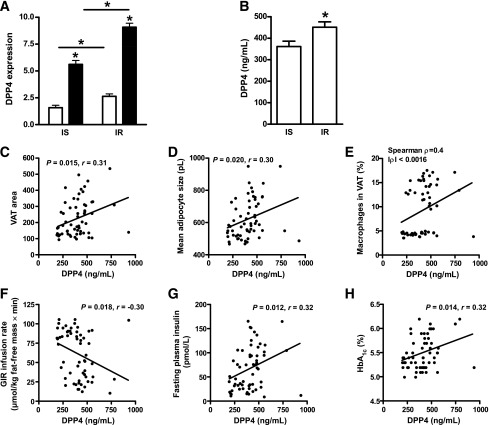
DPP4 expression and circulating levels were also measured in healthy, insulin-sensitive obese patients and compared with BMI-, age-, and sex-matched insulin-resistant obese patients. DPP4 expression was significantly elevated in both SAT and VAT of insulin-resistant patients relative to insulin-sensitive subjects (Fig. 3A). DPP4 circulating concentrations were significantly elevated in insulin-resistant versus insulin-sensitive subjects (Fig. 3B). There was no difference in circulating DPP4 in females compared with males (data not shown). Serum DPP4 correlated with the amount of VAT, as did adipocyte size and adipose tissue inflammation expressed as the percentage of macrophages in VAT (Fig. 3C–E). Furthermore, DPP4 correlated with insulin resistance because it was negatively related to GIR and positively associated with fasting insulin and HbA1c (Fig. 3F–H).
Figure 3.
DPP4 expression in adipose tissue and circulating DPP4 in insulin-sensitive (IS) and insulin-resistant (IR) morbidly obese subjects. A: DPP4 expression in adipose tissue was measured in insulin-sensitive (n = 30) and insulin resistant (n = 30) obese subjects. *P < 0.05 compared with respective SAT (white bars) or between groups as indicated. Black bars, VAT. B: Circulating DPP4 concentrations in insulin-sensitive and insulin-resistant groups. *P < 0.05 compared with insulin-sensitive group. C–H: Linear regression analysis of DPP4 serum concentration and measures of adipose tissue morphology and inflammation or insulin sensitivity. Statistical evaluation is indicated in each graph.
CONCLUSIONS
We recently identified DPP4 as a new adipokine that may be a missing link between increased adipose tissue mass in obesity and obesity-associated metabolic diseases (5). Although much attention has focused on the role of DPP4 in the degradation of GLP-1, our earlier data suggest that DPP4 also exerts direct effects, as it is able to induce insulin resistance in adipocytes and skeletal muscle cells in concentrations that can be found in the circulation of overweight and obese subjects (5). DPP4 thus may also have local effects within adipose tissue and systemic effects via the blood circulation. For a better understanding of the regulation of DPP4 in humans with different degrees of obesity and insulin sensitivity, in this study we measured DPP4 mRNA expression in adipose tissue and correlated it with clinical parameters and adipose tissue measures. DPP4 expression is systematically lower in SAT irrespective of the body fat level, suggesting that there is a depot-specific control of DPP4 expression. The fact that circulating DPP4 and DPP4 expression in adipose tissue both correlate with adipocyte size and adipose tissue inflammation also suggests that proinflammatory adipokines released from enlarged adipocytes could regulate DPP4 release.
The findings with DPP4 expression in adipose tissue in relation to BMI have been divergent, with a first report on this subject demonstrating higher DPP4 expression in adipose tissue from obese patients than in that from lean controls (18) and data from a second study describing higher DPP4 expression in lean subjects than in obese ones (19). Together with our previous publication describing DPP4 as a novel adipokine (5), we now show in different groups of patients that both DPP4 mRNA expression and DPP4 protein levels are increased in both SAT and VAT from obese subjects. By using different groups of patients, such as consecutive patients with a continuous spectrum of BMIs, as well as carefully characterized insulin-sensitive and insulin-resistant morbidly obese patients, we can furthermore demonstrate that DPP4 expression, especially in VAT, is negatively associated with insulin sensitivity in both lean and obese subjects.
To extend our understanding of how DPP4 is not only expressed in adipose tissue but also released from the tissue, we also studied DPP4 release from adipose tissue explants ex vivo. SAT biopsies from obese patients were characterized by higher DPP4 release than seen in those from lean controls. This set of data corroborates our earlier study showing that enlarged subcutaneous adipocytes from obese patients release higher amounts of DPP4 than do adipocytes from lean controls (5). Additionally, we have now shown that adipose tissue explants from VAT release more DPP4 than do SAT explants, pointing to a possible higher relative contribution by VAT to circulating DPP4 levels. DPP4 release from VAT from obese patients is again higher than that in VAT from lean controls. Although DPP4 release is significantly increased from VAT, SAT constitutes the major adipose tissue in humans and therefore may be the primary source of adipose-derived circulating DPP4, whereas VAT may contribute to increased circulating levels in morbidly obese patients with insulin resistance and more pronounced visceral obesity. It is not yet known whether higher release of DPP4 from VAT in obesity simply reflects higher DPP4 expression and therefore higher presence at the plasma membrane or enzymes involved in DPP4 shedding from the membrane are also regulated in obesity. The mechanisms of DPP4 release from the cell membrane are unknown and involve enzymes not yet identified. It could be speculated that metalloproteinases and ADAMs (a disintegrin and metalloproteinase domains) could be shedding partners for DPP4. In fact, the first so-called sheddase, ADAM17, is involved in tumor necrosis factor-α processing at the cell surface, thereby leading to tumor necrosis factor-α release into the circulation (20). Deregulation of metalloproteinases and ADAMs in association with fibrosis in expanding adipose tissue in obesity may contribute to increased DPP4 release and its depot-specific differences (21,22).
DPP4 expression and release are higher in obese patients with the metabolic syndrome and type 2 diabetes. Animal data support the notion that DPP4 expression in adipose tissue increases with developing type 2 diabetes, because rats with streptozotocin-induced diabetes display significantly increased DPP4 activity in epididymal fat (23). Furthermore, recent animal studies with DPP4 inhibitors support the notion that DPP4 may play a functional role within adipose tissue, because DPP4 inhibition has been seen to prevent adipose tissue inflammation and development of glucose intolerance in high fat diet–induced obesity (24,25). How DPP4 is regulated in this context is not known. We previously demonstrated higher DPP4 release and higher circulating DPP4 levels in obese patients with the metabolic syndrome relative to obese controls (5). In this study, we further refined these findings in a well-characterized cohort of insulin-sensitive morbidly obese patients compared with insulin-resistant BMI-matched obese subjects, showing that circulating DPP4 levels and DPP4 expression in VAT are higher in insulin-resistant patients despite similar adiposity. Some studies have already attempted to explain why DPP4 is overexpressed in adipose tissue of patients with the metabolic syndrome. A first study analyzing single nucleotide polymorphisms revealed that DPP4 polymorphisms are probably not modulating DPP4 expression and the association of DPP4 expression with cardiovascular risk (26). Conversely, DPP4 expression seems to be mediated by epigenetic effects. Methylation levels of the DPP4 promoter were found to be negatively associated with DPP4 mRNA expression in VAT in obese women with and without the metabolic syndrome and also to be associated with HDL cholesterol (27). Furthermore, three DPP4 polymorphisms were found to be significantly associated with methylation levels. Interestingly, we observed here a significant increased DPP4 expression in VAT of lean patients with impaired glucose tolerance, suggesting that mechanisms explaining higher DPP4 expression in obese subjects with the metabolic syndrome could also be translated to lean patients who have not yet fully developed insulin resistance. It should be noted that this phenomenon was restricted to VAT, which again illustrates the major differences between the SAT and VAT depots in conferring an increased metabolic risk.
Data on in vivo DPP4 release measuring arteriovenous differences suggest that abdominal SAT is not the only source of circulating DPP4, although there is net release in most of the subjects. In fact, net release is seen when the arterial concentrations of soluble DPP4 are relatively low. When concentrations are high, this fat depot can extract DPP4 from the circulation. The data are similar to those seen for steroid hormones: for both estradiol and testosterone, uptake by abdominal SAT is seen when arterial concentrations are high, but release when arterial concentrations are low (28). The data show a strong effect of obesity on net release. Interestingly, albeit unexplained, DPP4 net release is higher in women, although circulating DPP4 and adipose DPP4 expression are not different between males and females. In animals, DPP4 expression and activity in rats with streptozotocin-induced diabetes are significantly increased not only in epididymal fat but also in the liver (23). In fact, other tissues in addition to adipose tissue have been discussed as sources of circulating DPP4 including the liver, vascular cells, and immune cells (29,30). Data from humans also indicate that the liver may be a primary source of DPP4 in addition to adipose tissue, because as patients with nonalcoholic fatty-liver disease (NAFLD) have higher circulating DPP4 activity than do controls (31). It should be noted, however, that those NAFLD patients had significantly higher BMI, and DPP4 was not adjusted for BMI in that study. In addition, the authors reported that patients without NAFLD but with type 2 diabetes had similar circulating DPP4 activity to that in controls, which would not agree with our data. Patients with type 2 diabetes were about 30 years older on average than controls, however, and we know from our previous study that circulating DPP4 levels decline with age (5). Additional studies are needed to clarify whether DPP4 concentrations in the circulation are predictive of NAFLD independently of age and BMI.
Several studies indicate that the incretin system is altered in the obese state. The incretin effect is reduced in obese patients (32), and GLP-1 release and gastric inhibitory polypeptide metabolism are altered after a mixed meal test in obese subjects (33). In this context, obesity and circulating DPP4 levels may also affect efficacy of treatment with DPP4 inhibitors. Higher circulating DPP4 levels are associated with worse response to sitagliptin in patients with type 2 diabetes inadequately controlled by metformin or sulfonylurea (34). In addition, higher BMI may be predictive of less HbA1c lowering by sitagliptin treatment (35). It is not clear, however, whether increased DPP4 release from expanded adipose tissue is contributing to disturbances in the incretin system and how in detail treatment with DPP4 inhibitors is attenuated.
In conclusion, we have demonstrated that DPP4 is an adipokine with significantly higher expression in and release from VAT, with both parameters further increased in obese subjects. Circulating DPP4 is higher in insulin-resistant than insulin-sensitive obese subjects, indicating that it may be a marker for insulin resistance independently of obesity. Finally, DPP4 overexpression in VAT may be a marker of adipose tissue inflammation, which is known to be associated with insulin resistance and the metabolic syndrome. Further work is needed to elucidate the functional role of DPP4 within adipose tissue and to define whether higher DPP4 expression and serum concentration may contribute to higher efficacy of DPP4 inhibitors in patients with a high proportion of VAT.
Acknowledgments
This work was supported by the Ministerium für Wissenschaft und Forschung des Landes Nordrhein-Westfalen (Ministry of Science and Research of the State of North Rhine-Westphalia), the Bundesministerium für Gesundheit (Federal Ministry of Health), the German Research Council (SE 1922/2-1), the Commission of the European Communities (Collaborative Project ADAPT, Contract No. HEALTH-F2-2008-201100; Integrated Project HEPADIP, Contract LSHM-CT-2005-018734), and European Union COST Action BM0602. This work was further supported by the Kompetenznetz Adipositas (Competence Network for Obesity) funded by the Federal Ministry of Education and Research (German Obesity Biomaterial Bank; FKZ 01GI1128) and a grant from Deutsche Forschungsgemeinschaft the Clinical Research group Atherobesity KFO 152 (project BL 833/1-1), both to M.B. The study was also supported by grants from Swedish Research Council, EASD/Lilly, and the Novo Nordisk Foundation (P.A.).
No potential conflicts of interest relevant to this article were reported.
H.S. wrote the manuscript, researched data, and contributed to discussion. M.B. researched date and contributed to discussion. N.K., R.S., M.W., and B.A.F. researched data. F.R., W.T.K., and A.D. contributed to study design. P.A., K.N.F., and J.E. contributed to discussion. All authors reviewed and edited the manuscript. H.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl. 7):S120–S126 [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitling R. Robust signaling networks of the adipose secretome. Trends Endocrinol Metab 2009;20:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Sell H, Dietze-Schroeder D, Eckel J. The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol Metab 2006;17:416–422 [DOI] [PubMed] [Google Scholar]
- 5.Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 2011;60:1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009;30:600–607 [DOI] [PubMed] [Google Scholar]
- 7.Deacon CF, Carr RD, Holst JJ. DPP-4 inhibitor therapy: new directions in the treatment of type 2 diabetes. Front Biosci 2008;13:1780–1794 [DOI] [PubMed] [Google Scholar]
- 8.Blüher M, Bashan N, Shai I, et al. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body Insulin sensitivity. J Clin Endocrinol Metab 2009;94:2507–2515 [DOI] [PubMed] [Google Scholar]
- 9.Berndt J, Klöting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot–specific mRNA expression in humans. Diabetes 2005;54:2911–2916 [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 11.Blüher M, Unger R, Rassoul F, Richter V, Paschke R. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or Type II diabetes. Diabetologia 2002;45:210–216 [DOI] [PubMed] [Google Scholar]
- 12.Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. J Cell Mol Med 2011;15:1177–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 14.Tan GD, Neville MJ, Liverani E, et al. The in vivo effects of the Pro12Ala PPARgamma2 polymorphism on adipose tissue NEFA metabolism: the first use of the Oxford Biobank. Diabetologia 2006;49:158–168 [DOI] [PubMed] [Google Scholar]
- 15.Frayn KN, Coppack SW, Humphreys SM, Whyte PL. Metabolic characteristics of human adipose tissue in vivo. Clin Sci (Lond) 1989;76:509–516 [DOI] [PubMed] [Google Scholar]
- 16.Samra JS, Frayn KN, Giddings JA, Clark ML, Macdonald IA. Modification and validation of a commercially available portable detector for measurement of adipose tissue blood flow. Clin Physiol 1995;15:241–248 [DOI] [PubMed] [Google Scholar]
- 17.Jansson PA, Lönnroth P. Comparison of two methods to assess the tissue/blood partition coefficient for xenon in subcutaneous adipose tissue in man. Clin Physiol 1995;15:47–55 [DOI] [PubMed] [Google Scholar]
- 18.Bouchard L, Tchernof A, Deshaies Y, et al. ZFP36: a promising candidate gene for obesity-related metabolic complications identified by converging genomics. Obes Surg 2007;17:372–382 [DOI] [PubMed] [Google Scholar]
- 19.Kos K, Baker AR, Jernas M, et al. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab 2009;11:285–292 [DOI] [PubMed] [Google Scholar]
- 20.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997;385:729–733 [DOI] [PubMed] [Google Scholar]
- 21.Chavey C, Mari B, Monthouel MN, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 2003;278:11888–11896 [DOI] [PubMed] [Google Scholar]
- 22.Divoux A, Tordjman J, Lacasa D, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010;59:2817–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K, Nakahori Y. Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin-induced model using wild-type and DPP4-deficient rats. J Endocrinol 2009;200:53–61 [DOI] [PubMed] [Google Scholar]
- 24.Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab 2011;300:E410–E421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirakawa J, Fujii H, Ohnuma K, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes 2011;60:1246–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouchard L, Faucher G, Tchernof A, et al. Comprehensive genetic analysis of the dipeptidyl peptidase-4 gene and cardiovascular disease risk factors in obese individuals. Acta Diabetol 2009;46:13–21 [DOI] [PubMed] [Google Scholar]
- 27.Turcot V, Bouchard L, Faucher G, et al. DPP4 gene DNA methylation in the omentum is associated with its gene expression and plasma lipid profile in severe obesity. Obesity (Silver Spring) 2011;19:388–395 [DOI] [PubMed] [Google Scholar]
- 28.Boulton KL, Hudson DU, Coppack SW, Frayn KN. Steroid hormone interconversions in human adipose tissue in vivo. Metabolism 1992;41:556–559 [DOI] [PubMed] [Google Scholar]
- 29.Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother 2009;58:1723–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012;33:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firneisz G, Varga T, Lengyel G, et al. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: a novel liver disease biomarker. PLoS ONE 2010;5:e12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008;57:1340–1348 [DOI] [PubMed] [Google Scholar]
- 33.Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 2010;95:872–878 [DOI] [PubMed] [Google Scholar]
- 34.Aso Y, Inukai T, Kasai K. Serum level of soluble CD26/DPP-4 predicts the response to sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, in patients with type 2 diabetes inadequately controlled by metformin and/or sulfonylurea (Abstract). Diabetes 2012;61(Suppl. 1):A546–A546 [DOI] [PubMed] [Google Scholar]
- 35.Bando Y, Kanehara H, , Aoki H, Hisada K, Toya A, Tanaka D. Obesity may attenuate the HbA1c-lowering effect of sitagliptin in Japanese type 2 diabetic patients. J Diabetes Investig 2012;3:170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]