More than 30 years ago in Diabetes Care, Schmidt et al. (1) defined “dawn phenomenon,” the night-to-morning elevation of blood glucose (BG) before and, to a larger extent, after breakfast in subjects with type 1 diabetes (T1D). Shortly after, a similar observation was made in type 2 diabetes (T2D) (2), and the physiology of glucose homeostasis at night was studied in normal, nondiabetic subjects (3–5). Ever since the first description, the dawn phenomenon has been studied extensively with at least 187 articles published as of today (6). In this issue, Monnier et al. (7) report an additional observation on the dawn phenomenon in a large group of T2D subjects and quantify its role on overall BG control. Given this information and the extensive data to date, an assessment of our knowledge in this area should be determined. Specifically, what have we learned from the last 30 years of research on the dawn phenomenon? What is the appropriate definition, the identified mechanism(s), the importance (if any), and the treatment of the dawn phenomenon in T1D and T2D?
Physiology of glucose homeostasis in normal, nondiabetic subjects indicates that BG and plasma insulin concentrations remain remarkably flat and constant overnight, with a modest, transient increase in insulin secretion just before dawn (3,4) to restrain hepatic glucose production (4) and prevent hyperglycemia. Thus, normal subjects do not exhibit the dawn phenomenon sensu strictiori because they secrete insulin to prevent it.
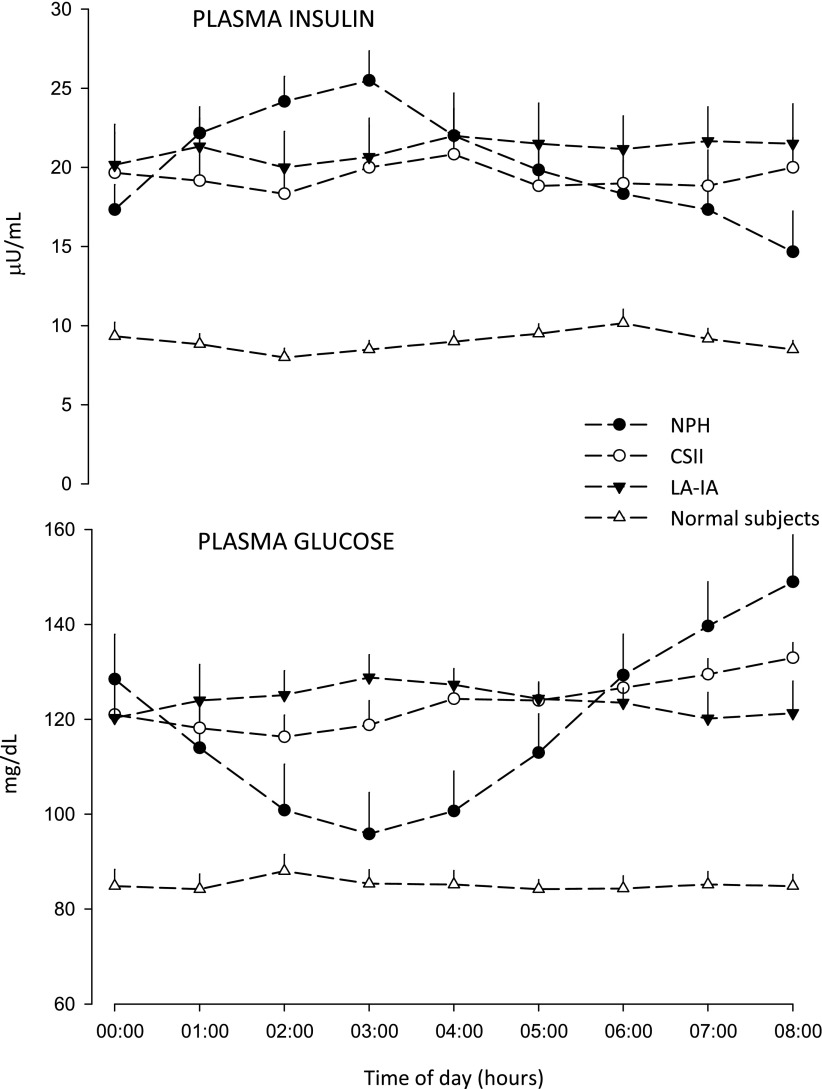
In T1D, the magnitude of BG elevation at dawn first reported was impressive and largely secondary to the decrease of plasma insulin concentration overnight (1), commonly observed with evening administration of NPH or lente insulins (8) (Fig. 1). Even in early studies with intravenous insulin by the “artificial pancreas” (Biostator) (2), plasma insulin decreased overnight because of progressive inactivation of insulin in the pump (9). This artifact exaggerated the dawn phenomenon, now defined as need for insulin to limit fasting hyperglycemia (2). When the overnight waning of insulin was prevented by continuous subcutaneous insulin infusion (CSII), even at single rate (10); intravenous infusion of albumin-added insulin by pump (11,12); or by the long-acting insulin analogs (LA-IAs) (8), it was possible to quantify the real magnitude of the dawn phenomenon—15–25 mg/dL BG elevation from nocturnal nadir to before breakfast (Fig. 1). Nocturnal spikes of growth hormone secretion are the most likely mechanism of the dawn phenomenon in T1D (13,14). The observation from early pioneering studies in T1D (10–12) that insulin sensitivity is higher after midnight until 3 a.m. as compared to the period 4–8 a.m., soon translated into use of more physiological replacement of basal insulin (CSII and the nearly peakless LA-IA [8] as compared with NPH) to reduce risk of nocturnal hypoglycemia while targeting fasting near-normoglycemia (Fig. 1).
Figure 1.
Overnight plasma insulin and glucose concentrations in normal, nondiabetic subjects (data from ref. 4) and in three groups of intensively treated subjects with T1D that had basal insulin replaced as either NPH (n = 6, NPH three times/day, at breakfast, lunch and bedtime; dose at bedtime 0.22 ± 0.03 units/kg/day), CSII (n = 6, infusion of basal insulin as rapid-acting analog at single rate 0.7 ± 0.1 U/h), or LA-IA (n = 6, dinnertime injection of glargine 0.25 ± 0.02 units/kg/day). Despite the fact that the total daily dose of NPH was fractionated into three times a day, bedtime NPH resulted in early plasma insulin peak and later insulin waning. This increases the risk of hypoglycemia after midnight and results in hyperglycemia before breakfast (dawn phenomenon 55 mg/dL). CSII at single rate prevents early peak and later overnight waning of insulin (dawn phenomenon 17 mg/dL). With LA-IA glargine, there is no dawn phenomenon since BG decreases in the second part of night as a result of continuing subcutaneous insulin absorption at dawn (7–8 h postinjection), which tends to slightly increase plasma insulin bioavailability at this time of day (G.B.B., unpublished observations).
In T2D, identification of diurnal changes in BG goes back decades, but only quite recently fasting hyperglycemia has been attributed to a transient increase in hepatic glucose production (both glycogenolysis and gluconeogenesis) at dawn in the absence of compensatory insulin secretion (15–17). Monnier et al. (7) report on the overnight (interstitial) glucose concentration (IG), as measured by continuous ambulatory IG monitoring, in three groups of 248 subjects with T2D (on diet only, on insulin sensitizers alone, or on secretagogues alone or in combination with insulin sensitizers). They observed an increase in IG from nocturnal nadir to prebreakfast values similar in the three groups (13–20 mg/dL). The prebreakfast increase in IG extended to the postbreakfast period with the highest value of day (mean values 191–208 mg/dL). Importantly, the dawn phenomenon had an impact on mean daily IG and A1C (mean increase of 0.39% [4.3 mmol/mol]), which was independent of treatment.
Two messages from the data of Monnier et al. (7) are important. First, the dawn phenomenon is confirmed as a frequent event across the heterogeneous population of T2D independent of (oral) treatment and studied in everyday life conditions, not only in the setting of specialized clinical research units. Second, the article reaffirms that the primary target of treatment in T2D is to reestablish near-normoglycemia before and after breakfast (i.e., to treat the dawn phenomenon) to lower mean daily BG and A1C (8).
The absolute overnight increase in fasting IG observed (7) is in the range of that reported previously for BG at dawn (10–12,17), and the postbreakfast IG is higher as compared with the postlunch and dinner values (7). Thus, the dawn phenomenon induces hyperglycemia not only before, but, to a larger extent, after breakfast as well (7,18). Over the years, fasting (and postbreakfast) hyperglycemia in T2D worsens as result of progressively impaired pancreatic B-cell function on the background of continued insulin resistance primarily at dawn (8,15–18) and independently of age (19). Because it is an early metabolic abnormality leading over time to the vicious circle of “hyperglycemia begets hyperglycemia” by glucotoxicity and lipotoxicity, the dawn phenomenon in T2D should be treated early and appropriately before A1C continues to increase (20).
Oral medications do not adequately control the dawn phenomenon, even when given in combination (7,18). Sulphonylureas are less than ideal due to risk of hypoglycemia in the afternoon or evening, when the dose is increased to counteract the hyperglycemia of dawn phenomenon. Incretins are designed to elegantly improve the postprandial, not the fasting, periods (20). The evening replacement of basal insulin, which abolishes the dawn phenomenon by restraining hepatic glucose production and lipolysis (21), is an effective treatment as it mimics the physiology of glucose homeostasis in normal, nondiabetic subjects (4).
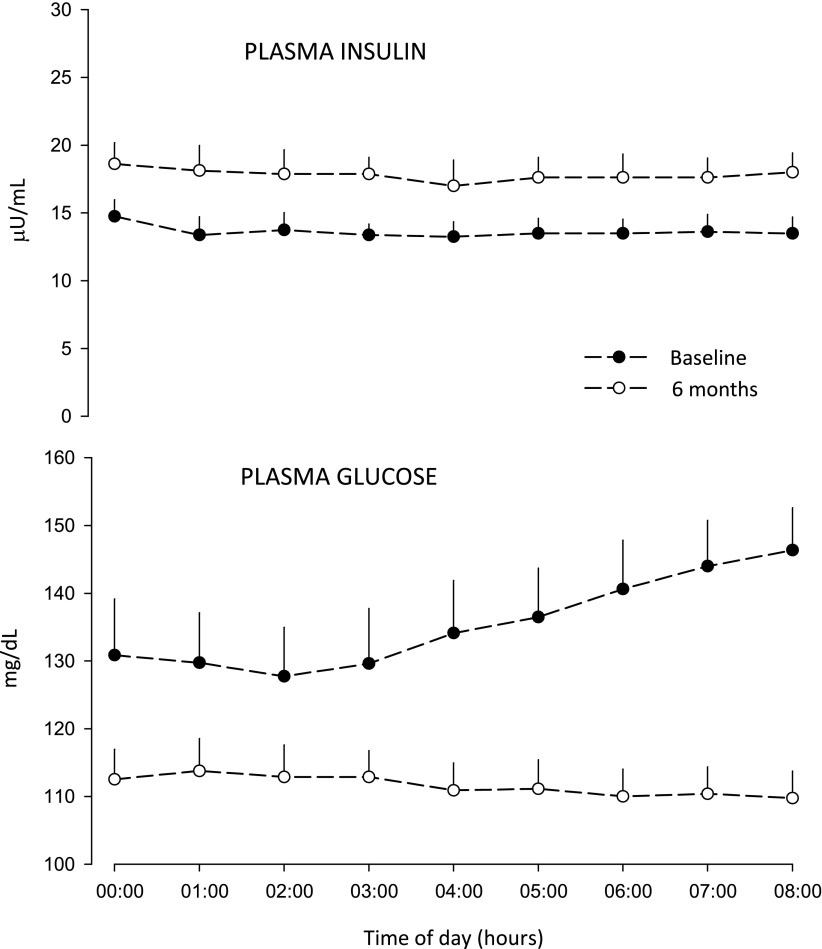
Early use of basal insulin in T2D is an add-on option treatment after failure of metformin to control A1C <7.0% (20). However, to fight the clinical inertia, it would be wise to consider initiation of basal insulin (effective, easy, safe, and “natural”) before—not after—A1C has increased well beyond 7.0%, as usually it is done in practice currently. Basal insulin may be initiated to prevent the increase of A1C >7.0% (22) by reducing nocturnal hyperglycemia and the dawn phenomenon (Fig. 2).
Figure 2.
Overnight plasma glucose and insulin concentrations in a group of T2D subjects (n = 8, age 53 ± 4 years, diabetes duration 3 ± 1 years, A1C 6.89 ± 0.05% [52 ± 0.5 mmol/mol], all on metformin only) before and after 6-month treatment with evening dose of insulin glargine (0.20 ± 0.02 U/Kg/day) as add-on to metformin. Basal insulin near-normalized the fasting BG by two mechanisms: partly by reducing the midnight BG and partly by totally abolishing the BG increase of the dawn phenomenon of the baseline study (18 mg/dL), as result of overnight sustained increase in plasma insulin concentration by ∼4 μU/mL. At the end of observation, the removal of the dawn phenomenon resulted in a decrease of A1C of 6.5 ± 0.1% (48 ± 0.7 mmol/mol). This validates the estimated contribution of the dawn phenomenon to the increased A1C calculated by Monnier et al. (7) of 0.39% (4.3 mmol/mol) (G.B.B., unpublished data).
In summary, despite the extensive number of publications today, the dawn phenomenon remains an area of research interest and, importantly, a target for clinical modulation that should be taken into account whenever choosing treatments.
Acknowledgments
F.P. has received honoraria for speaker fees and/or travel grants from Sanofi, Eli Lilly & Co., Bristol-Myers Squibb, and Merck. P.L. received travel grants for scientific meetings from Sanofi and Menarini Group. G.B.B. received honoraria from Sanofi, MannKind, and Eli Lilly & Co. for scientific advising and consulting. C.G.F. has served on scientific advisory panels for Sanofi and received honoraria for speaker fees and/or travel grants from Bristol-Myers Squibb, Merck, and Menarini Group. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See Monnier et al., p. 4057
References
- 1.Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S, Kowarski A. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care 1981;4:579–585 [DOI] [PubMed] [Google Scholar]
- 2.Bolli GB, Gerich JE. The “dawn phenomenon”—a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med 1984;310:746–750 [DOI] [PubMed] [Google Scholar]
- 3.Schmidt MI, Lin QX, Gwynne JT, Jacobs S. Fasting early morning rise in peripheral insulin: evidence of the dawn phenomenon in nondiabetes. Diabetes Care 1984;7:32–35 [DOI] [PubMed] [Google Scholar]
- 4.Bolli GB, De Feo P, De Cosmo S, et al. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes 1984;33:1150–1153 [DOI] [PubMed] [Google Scholar]
- 5.Kruszynska YT, Home PD. Night-time metabolic changes in normal subjects in the absence of the dawn phenomenon. Diabete Metab 1988;14:437–442 [PubMed] [Google Scholar]
- 6.PubMed.gov. Search results for “dawn phenomenon.” Available at www.ncbi.nlm.nih.gov/pubmed/?term=dawn+phenomemon Accessed 28 August 2013
- 7.Monnier L, Colette C, Dejager S, Owens D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care 2013;36:4057–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens DR, Bolli GB. Beyond the era of NPH insulin—long-acting insulin analogs: chemistry, comparative pharmacology, and clinical application. Diabetes Technol Ther 2008;10:333–349 [DOI] [PubMed] [Google Scholar]
- 9.Brennan JR, Gebhart SS, Blackard WG. Pump-induced insulin aggregation. A problem with the Biostator. Diabetes 1985;34:353–359 [DOI] [PubMed] [Google Scholar]
- 10.Bending JJ, Pickup JC, Collins AC, Keen H. Rarity of a marked “dawn phenomenon” in diabetic subjects treated by continuous subcutaneous insulin infusion. Diabetes Care 1985;8:28–33 [DOI] [PubMed] [Google Scholar]
- 11.De Feo P, Perriello G, Ventura MM, et al. Studies on overnight insulin requirements and metabolic clearance rate of insulin in normal and diabetic man: relevance to the pathogenesis of the dawn phenomenon. Diabetologia 1986;29:475–480 [DOI] [PubMed] [Google Scholar]
- 12.Perriello G, De Feo P, Torlone E, et al. The dawn phenomenon in type 1 (insulin-dependent) diabetes mellitus: magnitude, frequency, variability, and dependency on glucose counterregulation and insulin sensitivity. Diabetologia 1991;34:21–28 [DOI] [PubMed] [Google Scholar]
- 13.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med 1985;312:1473–1479 [DOI] [PubMed] [Google Scholar]
- 14.Perriello G, De Feo P, Torlone E, et al. Nocturnal spikes of growth hormone secretion cause the dawn phenomenon in type 1 (insulin-dependent) diabetes mellitus by decreasing hepatic (and extrahepatic) sensitivity to insulin in the absence of insulin waning. Diabetologia 1990;33:52–59 [DOI] [PubMed] [Google Scholar]
- 15.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996;45:1044–1050 [DOI] [PubMed] [Google Scholar]
- 16.Perriello G, Pampanelli S, Del Sindaco P, et al. Evidence of increased systemic glucose production and gluconeogenesis in an early stage of NIDDM. Diabetes 1997;46:1010–1016 [DOI] [PubMed] [Google Scholar]
- 17.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia 2006;49:1619–1628 [DOI] [PubMed] [Google Scholar]
- 18.Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007;30:263–269 [DOI] [PubMed] [Google Scholar]
- 19.Monnier L, Colette C, Sardinoux M, Baptista G, Regnier-Zerbib A, Owens D. Frequency and severity of the dawn phenomenon in type 2 diabetes: relationship to age. Diabetes Care 2012;35:2597–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porcellati F, Lucidi P, Candeloro P, et al. Pharmacokinetics and pharmacodynamics of insulin glargine: changes in glucose and lipid metabolism after either evening or morning injection in T2DM. Diabetes 2013;62(Suppl.1):A242 [Google Scholar]
- 22.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]