Abstract
OBJECTIVE
To investigate the impact of iron status on survival in patients with type 2 diabetes and coronary artery disease (CAD).
RESEARCH DESIGN AND METHODS
Serum ferritin, transferrin saturation (Tsat), and soluble transferrin receptor (sTfR) were measured in 287 patients with type 2 diabetes and stable CAD (65 ± 9 years of age, 78% men).
RESULTS
During a mean follow-up of 45 ± 19 months, there were 59 (21%) deaths and 60 (21%) cardiovascular hospitalizations. Both serum ferritin and sTfR strongly predicted 5-year all-cause mortality rates, independently of other variables (including hemoglobin, measures of renal function, inflammation, and neurohormonal activation). There was an exponential relationship between sTfR and mortality (adjusted hazard ratio [HR] per 1 log mg/L: 4.24 [95% CI 1.43–12.58], P = 0.01), whereas the relationship between ferritin and mortality was U-shaped (for the lowest and the highest quintiles vs. the middle quintile [reference group], respectively: adjusted HR 7.18 [95% CI 2.03–25.46], P = 0.002, and adjusted HR 5.12 [1.48–17.73], P = 0.01). Similar patterns were observed for the composite outcome of all-cause mortality or cardiovascular hospitalization, and in these multivariable models, low Tsat was related to unfavorable outcome.
CONCLUSIONS
Both low and high serum ferritin (possibly reflecting depleted and excessive iron stores, respectively) along with high serum sTfR (reflecting reduced metabolically available iron) identify patients with type 2 diabetes and CAD who have a poor prognosis.
The clinical significance of deficiency and disordered metabolism of iron in patients with chronic diseases associated with aging (1), including obesity (2), metabolic syndrome (3), coronary artery disease (CAD) (4), and heart failure (HF) (5–7), has received much attention. Iron overload and associated oxidative stress have been reported to accelerate the development of atherosclerosis (8) and cause endocrine organ dysfunction (9). However, iron deficiency (ID) is the most common dietary deficiency and the predominant cause of anemia worldwide (10).
The physiological significance of ID may be much broader than its role in erythropoiesis (1,7,11,12). Iron is also critical for cellular energy generation by mitochondria (13–15). ID will impair the function of cells with high energy demand whether due to mechanical work, high metabolic rate, or high rates of proliferation (11,12).
Diabetes may cause profound derangements of energy metabolism due to insulin resistance and mitochondrial dysfunction (16,17), contributing to a high risk of fatal and nonfatal cardiovascular events, and acting as an adjuvant substrate for other factors interfering with energy metabolism, such as iron excess or depletion. Attention has focused, until now, on the unfavorable effects of iron overload in diabetes, including oxidative stress and hemochromatosis (9), with little attention being paid to ID other than in pregnancy (18).
Accordingly, we investigated the impact of iron status assessed using circulating biomarkers on survival in patients with type 2 diabetes and CAD.
RESEARCH DESIGN AND METHODS
Study cohort
Patients with type 2 diabetes and CAD were recruited from outpatient clinics or elective admissions at the Centre for Heart Diseases, Military Hospital (Wroclaw, Poland). Inclusion criteria were as follows: 1) type 2 diabetes according to the criteria pointed out in The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) (19) of ≥6 months duration; 2) a documented history of CAD based on the ESC recommendations (19) of ≥6 months duration, i.e., the presence of significant coronary artery lesions in coronary angiography along with the symptoms of stable angina, or a confirmed episode of myocardial infarction; and 3) stable therapy for both type 2 diabetes and CAD for ≥1 month. Exclusion criteria included the following: 1) acute coronary syndrome and/or coronary revascularization within the previous 3 months; 2) unplanned hospitalization due to any other cardiovascular reason within the previous month; 3) any acute or chronic illness that might influence iron metabolism (including known malignancy, infection, severe chronic kidney disease requiring dialysis, and hematological diseases); 4) any treatment for anemia or ID in the previous 12 months; and 5) lack of informed written consent.
When subjects were screened for this study, they were asked in detail about blood transfusions, erythropoietin therapy, intravenous iron infusions, and any nutritional supplements potentially containing iron. None of subjects included in the study received such a therapy. Additionally, all anemic subjects included into the study underwent a routine clinical evaluation in order to detect any potential secondary causes of anemia. Patients with evidence of active bleeding were not included in the study. However, routine endoscopy was not required.
The study protocol was approved by the local ethics committees, and all subjects gave written informed consent. The study was conducted in accordance with the Helsinki Declaration.
Indices of iron status and other laboratory measurements assessed in peripheral blood
Venous blood samples were taken in the morning after an overnight fast and after a supine rest of at least 15 min. Hematological measurements were assessed from fresh venous blood anticoagulated with EDTA. Hemoglobin (g/dL) was measured using the ADVIA 120 automated system (Siemens Healthcare Diagnostics, Deerfield, IL). Anemia was defined as hemoglobin <12 g/dL in women and <13 g/dL in men (20).
After centrifuging of heparinized and clotted blood, supernatant plasma and serum were stored frozen at –70°C until analyses for 1) serum ferritin (μg/L) measured using electrochemiluminescence with the Elecsys 2010 System (Roche Diagnostics GmbH, Mannheim, Germany); 2) transferrin saturation (Tsat) calculated as a ratio serum iron (μg/dL) and total iron binding capacity (μg/dL) (multiplied by 100 and expressed in percentage), both assessed using a substrate method with Feren S (Thermo Fisher Scientific, Waltham, MA); and 3) serum soluble transferrin receptor (sTfR, mg/L) measured using immunonephelometry (Siemens Healthcare Diagnostics, Marburg, Germany).
Plasma N-terminal protype B natriuretic peptide (NT-proBNP, pg/mL) was measured using immunoassay based on electrochemiluminescence on the Elecsys 1010/2010 System. Serum high-sensitivity C-reactive protein (hsCRP, mg/L) was assessed using kinetic nephelometry (Dade Behring; Siemens Healthcare Diagnostics, Deerfield, IL). Renal function was assessed using the estimated glomerular filtration rate (eGFR, mL/min/1.73 m2), calculated from the Modification of Diet in Renal Disease equation (21).
HbA1c (%) was measured using an immunochemical assay (AxSYM; Abbot Laboratories, Abbot Park, IL). HbA1c was expressed also in mmol/mol, after being converted from percentage using the NGSP’s HbA1c converter (http://www.ngsp.org/convert1.asp). Insulin resistance was estimated using the homeostatic model assessment of insulin resistance (HOMA-IR) from basal (fasting) plasma glucose and insulin concentrations (22) (calculated only for patients not treated with insulin). Plasma glucose (mg/dL) was assessed using a colorimetric assay with glucose oxidase (Konelab Prime 60i/30i; Thermo Fisher Scientific, Waltham, MA). Plasma insulin (μIU/mL) was measured using an immunochemiluminescence assay (Liason Insulin; DiaSorin S.p.a., Saluggia, Italy).
Clinical follow-up
Patients were regularly seen by the study investigators in outpatient clinics. Further information regarding survival was obtained directly from patients or their relatives and the hospital system.
There were two predefined end points: 1) all-cause mortality and 2) the first event of either death or cardiovascular hospitalization (myocardial infarction, stroke, transient ischemic attack [TIA], HF hospitalization, and coronary revascularization).
There is no doubt that all-cause death is commonly accepted as the most relevant outcome end point. However, in recent years, most outcome analyses performed in patients at high risk of cardiovascular events also consider unplanned cardiovascular hospitalizations as clinically relevant end points. There is compelling evidence that cardiovascular hospitalization worsens the patient’s quality of life, may be a trigger for disease progression, and, in some cases, is also associated with higher morbidity and mortality during a longer follow-up. In order to focus on those that are the most relevant for diabetic patients with CAD (considered as the population at the highest cardiovascular risk), we prospectively decided to focus on the events pathophysiologically related to either atherothrombotic complications (myocardial infarction, a need for coronary revascularization, and stroke/TIA) or clinical evidence of HF decompensation requiring hospital admission.
The length of follow-up of patients who did not experience any aforementioned event or who experienced any event after 5 years was censored at 1,825 days (5 years).
Statistical analyses
Most continuous variables had a normal distribution and were expressed as a mean (x) (with an SD). The continuous variables with a skewed distribution (the duration of diabetes, plasma NT-proBNP, serum hsCRP, eGFR, HOMA-IR, serum ferritin, and serum sTfR) were expressed as a median (with lower and upper quartiles). Variables were log transformed as necessary in order to normalize their distribution, and log-transformed values were used for further statistical analyses. The intergroup differences in continuous variables were tested using the Student t test, whereas the relationships between continuous variables were assessed using the Pearson linear correlatory coefficients. Categorical variables were expressed as the number of patients in given categories (with a percentage). The intergroup differences in categorical variables were tested using the χ2 test.
The associations between iron status (assessed using serum ferritin, Tsat, and sTfR) and clinical variables and event-free survival during the 5-year follow-up in patients with type 2 diabetes and CAD were established using Cox proportional hazard regression analyses (both univariable and multivariable models). In the univariable analyses, the following variables were included: age, sex, BMI, systolic blood pressure (BP), the duration of diabetes (log), left ventricular ejection fraction (LVEF), plasma NT-proBNP (log), serum hsCRP (log), HOMA-IR (log), HbA1c, eGFR (log), hemoglobin, and iron status biomarkers (serum ferritin [log], Tsat, and sTfR [log]). All variables, which appeared to be significant in univariable Cox regression models, were included in the multivariable Cox regression models. All analyses were performed twice, separately for two predefined end points (as described above). For both univariable and multivariable models, hazard ratios (HRs) (with 95% CIs) with corresponding χ2 and P values were estimated for all variables incorporated into the models. The assumption of the proportional hazard was tested for each derived model.
For serum ferritin, Tsat, and sTfR, nonlinear associations with event-free survival rates were expected. Tsat appeared to be linearly related to analyzed event rates, but not serum ferritin or sTfR. In order to assess the shape of association between the later two iron status biomarkers and event-free survival rates, univariable and multivariable models were constructed, where these variables were included as 1) log transformed and 2) transformed using natural cubic splines with n knots (23). The number of knots was chosen based on Akaike Information Criterion for univariable models (24). Serum ferritin was spline transformed with three knots (demonstrating U-shaped associations with event-free survival rates), whereas sTfR with two knots (demonstrating exponential associations with event-free survival rates). The fitness of the established models was expressed using the χ2 test (with P values). Natural cubic splines are well suited, because they allow estimation of nonlinear relationships (23), which were expected to exist in our study population. As serum ferritin appeared to have a U-shaped association with event rates, it was categorized into quintiles, with quintile 3 chosen as a reference group.
All statistical analyses were performed using Statistica 9.1 and R version 2.15.2 (www.r-project.org) (25). The P value <0.05 was considered statistically significant.
RESULTS
Baseline clinical characteristics of patients with type 2 diabetes and CAD
The baseline clinical characteristics of 287 patients with type 2 diabetes and CAD are shown in Table 1, overall and by quintile of serum ferritin (quintile 1, <60.9 μg/L; quintile 2, 60.9–118.9 μg/L; quintile 3, 119.0–202.1 μg/L [reference group]; quintile 4, 202.2–304.8 μg/L; quintile 5, ≥304.9 μg/L). Among all investigated diabetic patients with CAD, 260 (91% of all) underwent coronary angiography, and in these patients, the one-, two- and three-vessel disease was found in 97 (37%), 75 (29%), and 88 (34%) subjects, respectively (percentage of those with performed coronary angiography). Additionally, 143 (55%) of those who underwent coronary angiography also experienced myocardial infarction in the past. All remaining patients who did not undergo coronary angiography (27, 9% of all) had a confirmed episode of myocardial infarction in the past.
Table 1.
Clinical characteristics of diabetic patients with CAD, also grouped according to quintiles of serum ferritin
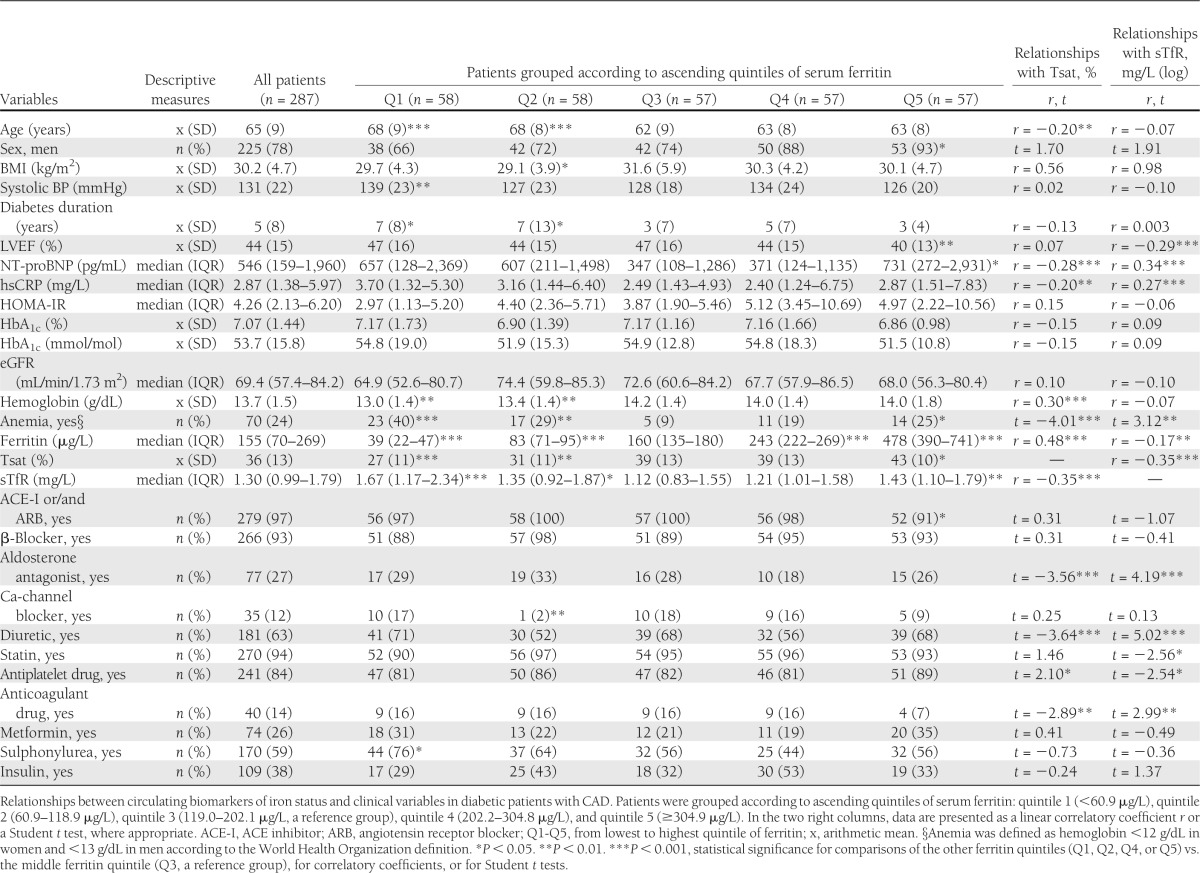
Iron status and clinical characteristics of patients with type 2 diabetes and CAD
Patients in the lowest compared with the middle quintile of serum ferritin were older; had higher systolic BP, longer duration of diabetes, lower hemoglobin, and more anemia; and were more frequently treated with sulphonylureas (all P < 0.05) (Table 1). Patients in the highest compared with the middle quintile of serum ferritin had lower LVEF and higher plasma NT-proBNP, were more likely to be men and have anemia, and were less frequently treated with ACE inhibitors or/and angiotensin receptor blockers (all P < 0.05) (Table 1).
Low Tsat was associated with older age, high plasma NT-proBNP, high serum hsCRP, and low hemoglobin (linear/log-linear relationships with P < 0.05). Low Tsat was also found in anemic patients; those treated with aldosterone antagonists, diuretics, and anticoagulant drugs; and those not receiving any antiplatelet drug (all P < 0.05) (Table 1). In a multivariable stepwise regression model, only serum hsCRP, plasma NT-proBNP, hemoglobin, and treatment with aldosterone antagonists were associated with Tsat (all P < 0.05).
High serum sTfR was associated with low LVEF, high plasma NT-proBNP, and high serum hsCRP (log-linear relationships with P < 0.05). High serum sTfR was also found in anemic patients and was associated with several treatments (all P < 0.05) (Table 1). In a multivariable stepwise regression model, only plasma NT-proBNP, serum hsCRP, the presence of anemia, therapy with aldosterone antagonists, and lack of therapy with a statin were associated with sTfR (all P < 0.05).
Mutual relationships between indices of iron status in patients with type 2 diabetes and CAD
Tsat was inversely related to serum sTfR (r = −0.35, P < 0.001) and positively to serum ferritin (r = 0.48, P < 0.001) (both log-linear relationships), and there was a weak inverse relationship between serum ferritin and sTfR (r = −0.17, P < 0.01). Patients in the lowest and highest quintiles of serum ferritin were characterized by high serum sTfR compared with patients in the middle ferritin quintile (both P < 0.01).
Iron status and risk of death in patients with type 2 diabetes and CAD
The mean follow-up in a whole cohort was 1,356 ± 578 days (median 1,692 days, range 1–1,825 days), and the mean follow-up for survivors was 1,546 ± 438 days (median 1,825 days, range 365–1,825 days). By 5 years, 59 patients (21%) had died, and the mean time to death was 621 ± 455 days (median 577 days, range 1–1,587 days).
The proportionality assumption and the assumption of a log-linear relationship between the continuous variables and the hazard function were fulfilled, except for serum ferritin.
Univariable models.
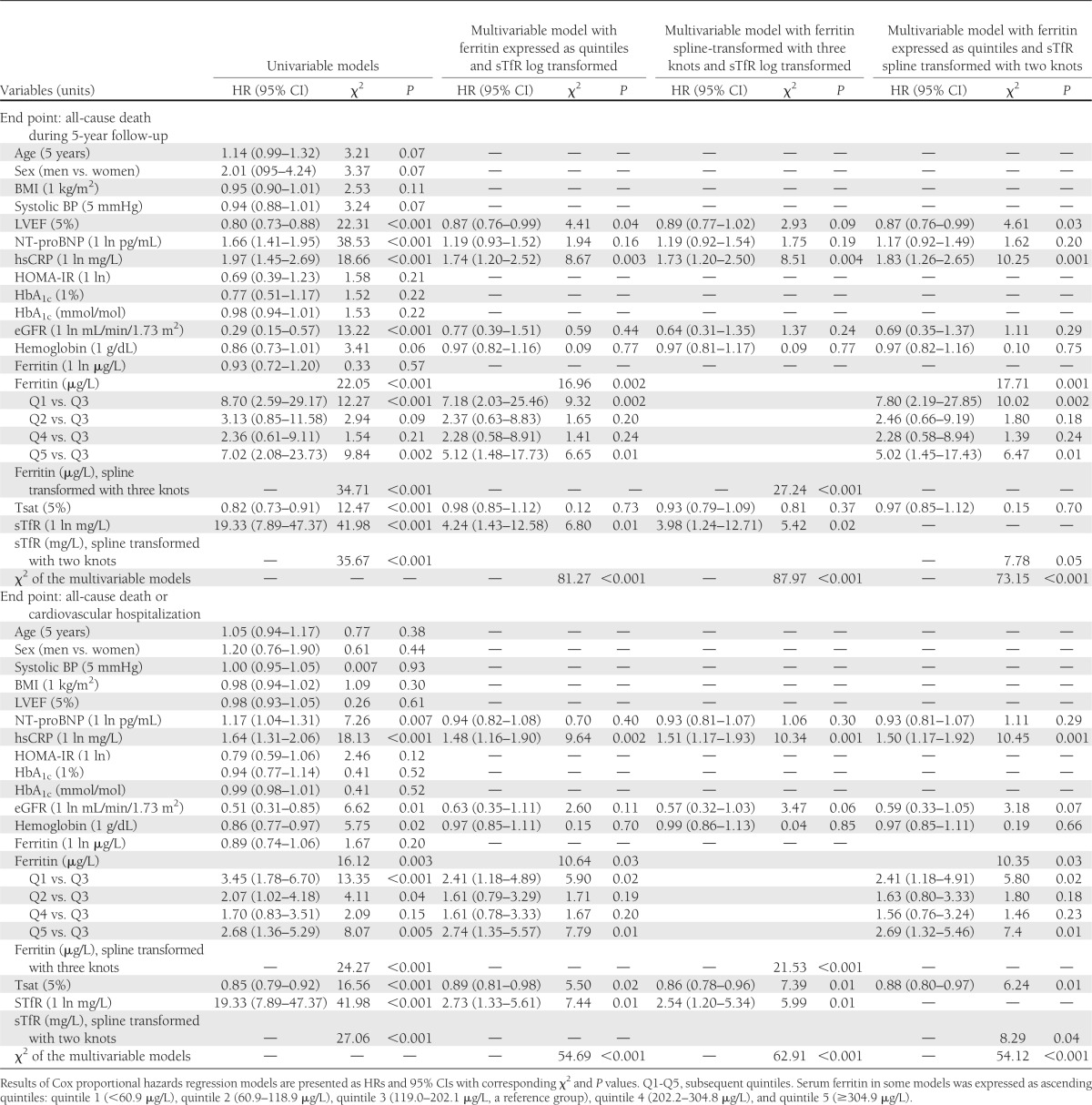
In univariable Cox proportional hazard regression models, the following variables predicted an increased 5-year mortality: low LVEF, high plasma NT-proBNP (log), high serum hsCRP (log), reduced eGFR (log) (all P < 0.05), and borderline reduced hemoglobin (P = 0.06) (Table 2). Low Tsat and high serum sTfR (log) were also associated with an increased 5-year mortality, and the Cox proportional hazard regression models of the spline-transformed serum ferritin with three knots and the spline-transformed serum sTfR with two knots were also statistically significant (all P < 0.001) (Table 2). When serum ferritin was expressed in quintiles, patients in the middle quintile had the most favorable outcome compared with both the lowest and highest quintiles (both P < 0.01) (Table 2).
Table 2.
Prognosticators of 5-year all-cause mortality and 5-year all-cause death and cardiovascular hospitalization rates in diabetic patients with CAD (univariable and multivariable Cox proportional hazards regression models)
Multivariable models.
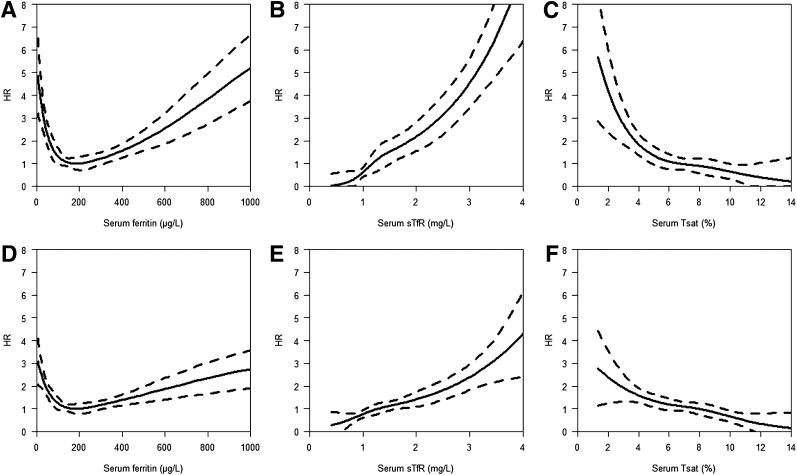
In multivariable models, serum sTfR (both when log and spline transformed) and serum ferritin (both when expressed in quintiles and when spline transformed), but not Tsat, predicted all-cause mortality (all P ≤ 0.05) (Table 2). The relationship between serum ferritin and mortality remained U-shaped (Fig. 1A), whereas the relationship between serum sTfR and mortality was exponential (Fig. 1B).
Figure 1.
Serum ferritin, serum sTfR, and Tsat by relative hazard of events (all-cause deaths [A–C]; all-cause deaths and cardiovascular hospitalizations [D–F]) using cubic splines during the 5-year follow-up in diabetic patients with CAD.
Iron status and risk of death or cardiovascular hospitalization in patients with type 2 diabetes and CAD
The mean follow-up free of cardiovascular hospitalization or death in a whole cohort was 1,162 ± 636 days (median 1,278 days, range 1–1,825 days), and the mean follow-up for those who did not experience any predefined event (death or cardiovascular hospitalization) was 1,538 ± 433 days (median 1,825 days, range 365–1,825 days). By 5 years, 112 patients (39%) had experienced cardiovascular hospitalization (including 21 HF hospitalizations, 18 coronary revascularizations, 15 myocardial infarctions, and 6 stroke/TIA) or had died, and the mean time to the first predefined event was 576 ± 423 days (median 524 days, range 1–1,659 days).
Univariable models.
In univariable Cox proportional hazard regression models, the following variables predicted an increased risk of death or cardiovascular hospitalization: high plasma NT-proBNP (log), high serum hsCRP (log), reduced eGFR (log), and reduced hemoglobin (all P < 0.05). Low Tsat and high serum sTfR (log) were associated with increased rates of death or cardiovascular hospitalization, and the Cox proportional hazard regression models of the spline-transformed serum ferritin with three knots and the spline-transformed serum sTfR with two knots were also statistically significant (all P < 0.001) (Table 2). When serum ferritin was expressed in quintiles, patients with serum ferritin in the middle quintile had the most favorable outcome compared with those in the lowest and highest quintiles (both P < 0.01) (Table 2).
Multivariable models.
In multivariable models, serum sTfR (both when log and spline transformed), serum ferritin (both when expressed in quintiles and when spline transformed), and Tsat were associated with death or cardiovascular hospitalization (all P < 0.05) (Table 2). The relationship between serum ferritin and outcome was again U-shaped (Fig. 1D), but those for serum sTfR and Tsat were exponential (Fig. 1E) and linear (Fig. 1F), respectively.
CONCLUSIONS
We have demonstrated that measures of iron status predict long-term outcome in patients with type 2 diabetes and clinically overt CAD, even after adjusting for conventional (e.g., age, sex, and renal function) and some more advanced (e.g., hsCRP and NT-proBNP) prognostic markers.
We deliberately assessed three different markers of iron status. Ferritin is the major intracellular protein storing iron (26), and serum concentrations <30 μg/L are specific for absolute ID (27). However, serum ferritin is commonly elevated when inflammatory pathways are activated, including atherosclerosis and renal dysfunction (27,28). A normal serum ferritin may be misleading. Extremely high serum ferritin (>1,000 μg/L) indicates a high probability of hemochromatosis (29). Importantly, high circulating ferritin provides no insight about the amount of intracellular iron available for metabolic (including energetic) needs (26). Tsat is a direct measure of the circulating iron pool, but it is unclear if this reflects the amount in peripheral tissues (27,28). Transferrin receptor is the major entrance pathway for iron to most hematopoietic and extrahematopoietic cells (26). When cells become deficient in iron for metabolic processes (mainly in mitochondria), this particle is overexpressed on the cellular membrane and shed into the circulation, and thus, high circulating sTfR reflects intracellular iron depletion (30,31). We found only moderate (at the most) associations between Tsat and serum ferritin and sTfR. Importantly, patients with both low and high serum ferritin had increased circulating sTfR. There are two possible explanations for cellular ID in the presence of high serum ferritin. It is possible that in patients with more advanced disease, an inflammatory state exists, causing serum ferritin to rise despite cellular ID. Alternatively, it is possible that tissue stores of iron are high but not metabolically available for hematopoesis or other cell functions, or even that iron overload causes an inflammatory response.
We found no major associations between iron indices and measures of glucose control or the severity of insulin resistance in patients with type 2 diabetes and CAD. Relationships between low serum ferritin and high HbA1c have been reported by some authors in young adults (32) and a small group of pregnant women with type 2 diabetes (18). Such differences may more reflect the presence of advanced and complex cardiovascular disease (CVD) in our patients (59% with previous myocardial infarction and 26 and 30% with two- and three-vessel disease, respectively), the larger population studied, or the greater array of measurements included in our multivariable models. In contrast, we have demonstrated associations between markers of iron status and measures of the severity of CVD (i.e., plasma NT-proBNP and LVEF) and of inflammation (serum hsCRP) most likely related to the burden of atherosclerotic disease.
For the first time, we have shown in patients with type 2 diabetes and clinically overt CAD that ID, expressed as both depleted iron stores and as diminished iron available for metabolic needs, critically impairs homeostasis in general, and markedly worsens prognosis. Circulating biomarkers of iron status allow the prediction of the long-term follow-up (5 years), and the discriminative value of these parameters has also been observed as early as after 3–6 months of clinical follow-up (data not shown). The relationships with outcomes have been demonstrated to be linear for Tsat, U-shaped for serum ferritin, and exponential for sTfR (Fig. 1).
Available evidence linking iron status and cardiovascular morbidity and mortality is equivocal. Regarding cardiovascular morbidity, the analysis of 2,874 subjects from the Monitoring of Trends and Determinants in Cardiovascular Disease (DAN-MONICA) I study revealed no links between serum ferritin and the risk of either CVD or CAD during the 10-year follow-up (33). However, in two studies performed in elderly subjects ≥65 (34) and ≥71 years of age (35), low circulating iron was independently associated with the higher occurrence of CVD and CAD during the long-term follow-up. Also, in an unselected cohort of 906 women (mean age 61 ± 17 years), low nonheme (but not heme) iron intake assessed based on a questionnaire was related to the increased rates of nonfatal cerebrovascular and coronary events during the 10-year follow-up (36). Finally, among >5,000 subjects 45–74 years of age free of stroke at baseline from the first National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Followup Study, there was a U-shaped relationship between Tsat (high and low) and the risk of incident stroke in women (but not in men) during the 12-year follow-up (37).
The results of studies that investigated the associations between iron status and survival are even more heterogeneous. There is evidence established in general populations on the associations between increased all cause-mortality and low circulating iron (34,35) and low Tsat (38,39), between high cardiovascular mortality and low Tsat (38,39), and between high mortality due to CAD and low Tsat (40). However, the other authors have not confirmed the links between mortality rates and different indices of iron status, including Tsat and serum ferritin (38,41–43). Finally, among 6,102 women 45–64 years of age without known heart disease at baseline, there was the U-shaped relationship between Tsat and mortality due to CAD during the 12-year follow-up (44). Among an unselected population of 906 women (mean age 61 ± 17 years), low nonheme (but not heme) iron intake assessed based on a questionnaire increased the risk of fatal cerebrovascular and coronary events during the 10-year follow-up (36). On the other hand, among >25,000 healthy men 40–79 years of age, free of stroke, CAD, and cancer at baseline, dietary iron intake assessed based on a questionnaire was positively associated with the higher mortality from both stroke and CAD (45). There is also an observation from the NHANES II that individuals with high Tsat (>55%) and high LDL cholesterol (>160 mg/dL) had markedly increased both CVD mortality and all-cause mortality (46). In a recent study, high circulating catalytic iron (not bound to either transferrin or ferritin) in 1,701 patients with acute coronary syndromes has been shown to predict increased all-cause mortality during the median 10-month follow-up, but without any associations with further cardiovascular nonfatal events, such us recurrent ischemia, myocardial infarction, and HF (47). Finally, it has been shown that in patients with HF, ID is strongly and independently associated with high mortality during the long-term follow-up, in those with and without anemia, with and without CAD, and with and without systolic dysfunction (5,48,49).
We acknowledge the observational character of our study, which was not designed to investigate either the cause of abnormal iron status or the mechanisms linking iron disorder to poor outcomes. The observed associations between serum measures of ID other than ferritin and high hsCRP suggest that inflammation could interfere with iron metabolism, as it has been shown in chronic kidney disease (28,50) but not in HF (48). Another potential explanation linking abnormal iron status and outcomes in these patients could be the fact that iron is a micronutrient crucial for the cellular metabolism and survival involved in numerous biochemical reactions, including oxygen storage and transport, oxidative metabolism and mitochondrial functioning, and turnover of lipids, proteins, and ribonucleic acids, and there are premises that both ID and iron excess may negatively affect the efficacy of these reactions (7,11–15). These theories deserve further studies. Ultimately, studies of iron supplementation, e.g., in patients with HF (51,52), ideally showing only those with ID benefit, and of iron depletion in those with possible iron overload are required to determine the real functional significance of perceived abnormalities. A major impediment to research in this area is defining the precise biochemical criteria by which to define ID and iron overload. Existing standard laboratory ranges may only be an approximate guide.
In conclusion, both low and high serum ferritin along with high serum sTfR identify patients with type 2 diabetes and clinically overt CAD who have a poor prognosis. Additionally, patients with low Tsat have an increased risk of death or cardiovascular hospitalization during long-term follow-up. If the primary intention of assessing iron status is to detect ID, then sTfR alone may be preferred. However, measurement of serum ferritin appears to add prognostic information. These hypotheses require verification in prospective studies.
Acknowledgments
This research was funded by the statutory grant of the Department of Physiology, Wroclaw Medical University (ST-660) (costs of laboratory assessments).
S.v.H. has received consulting honoraria, speaker's fees, and travel support from Vifor Pharma and Thermo Fisher Scientific and consulting honoraria from Pfizer, Solartium Dietetics, and Respicardia. W.D. has received consulting honoraria, speaker's fees, and research support from Bristol-Myers Squibb, Nutricia, Sanofi, Solartium Dietetics, and Vifor Pharma. S.D.A. has received consulting fees from Alere, Amgen, Thermo Fisher Scientific, Abbott Laboratories, Takeda, NOXXON, and Vifor Pharma; honoraria from Alere, Amgen, BRAHMS GmbH, and Vifor Pharma; and research support from Thermo Fisher Scientific and Vifor Pharma. J.G.F.C. has received research funding from Amgen and Vifor Pharma. E.A.J. has received honoraria for lectures and participation in advisory boards from Vifor Pharma, related travel/accommodation expense support from Vifor Pharma, and research support from Vifor Pharma. Wroclaw Medical University received an unrestricted grant from Vifor Pharma. No other potential conflicts of interest relevant to this article were reported.
B.Po. conceived and designed the study, obtained funding, analyzed and interpreted data, and drafted the manuscript. T.S. performed statistical analysis, analyzed and interpreted data, and critically revised the manuscript for important intellectual content. B.Pa., M.O., and S.P. acquired data and critically revised the manuscript for important intellectual content. L.B.-N. obtained funding, analyzed and interpreted data, and critically revised the manuscript for important intellectual content. K.R., S.v.H., W.D., S.D.A., and J.G.F.C. analyzed and interpreted data and critically revised the manuscript for important intellectual content. E.A.J. conceived and designed the study, performed statistical analysis, analyzed and interpreted data, drafted the manuscript, and supervised the study. B.Po. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics 2009;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet 2012;112:391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datz C, Felder TK, Niederseer D, Aigner E. Iron homeostasis in the metabolic syndrome. Eur J Clin Invest 2013;43:215–224 [DOI] [PubMed] [Google Scholar]
- 4.Varma A, Appleton DL, Nusca A, et al. Iron deficiency anemia and cardiac mortality in patients with left ventricular systolic dysfunction undergoing coronary stenting. Minerva Cardioangiol 2010;58:1–10 [PubMed] [Google Scholar]
- 5.Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872–1880 [DOI] [PubMed] [Google Scholar]
- 6.Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011;17:899–906 [DOI] [PubMed] [Google Scholar]
- 7.Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013;34:816–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan JL. Iron in arterial plaque: modifiable risk factor for atherosclerosis. Biochim Biophys Acta 2009;1790:718–723 [DOI] [PubMed]
- 9.Utzschneider KM, Kowdley KV. Hereditary hemochromatosis and diabetes mellitus: implications for clinical practice. Nat Rev Endocrinol 2010;6:26–33 [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370:511–520 [DOI] [PubMed] [Google Scholar]
- 11.Haas JD, Brownlie T., 4th Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001;131(2S-2):676S–688S; discussion 688S–690S [DOI] [PubMed] [Google Scholar]
- 12.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001;131(Suppl. 2):568S–579S; discussion 580S [DOI] [PubMed] [Google Scholar]
- 13.Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol 2005;6:345–351 [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Paw BH. Cellular and mitochondrial iron homeostasis in vertebrates. Biochim Biophys Acta 2012;1823:1459–1467 [DOI] [PMC free article] [PubMed]
- 15.Walter PB, Knutson MD, Paler-Martinez A, et al. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc Natl Acad Sci USA 2002;99:2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:92–103 [DOI] [PubMed] [Google Scholar]
- 17.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab 2012;23:477–487 [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K, Osugi T, Noguchi S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care 2010;33:509–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox K, Garcia MA, Ardissino D, et al. Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. ESC Committee for Practice Guidelines (CPG) Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341–1381 [DOI] [PubMed] [Google Scholar]
- 20.Blanc B, Finch CA, Hallberg L. Nutritional anemias. Report of a WHO Scientific Group Geneva, World Health Org. (Tech. Rep. Ser., 1968;405:1–40) [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 22.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 23.Therneau TM, Grambsch PM. Modelling Survival Data: Extending the Cox Model. New York, NY, Springer, 2001 [Google Scholar]
- 24.Anderson DR. Model Based Inference in the Life Sciences. New York, NY, Springer, 2008 [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing, 2010. Vienna, Austria, R Foundation for Statistical Computing. Available at http://www.R-project.org Accessed 15 February 2013
- 26.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell 2010;142:24–38 [DOI] [PubMed] [Google Scholar]
- 27.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010;116:4754–4761 [DOI] [PubMed] [Google Scholar]
- 28.Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006;1(Suppl. 1):S4–S8 [DOI] [PubMed] [Google Scholar]
- 29.Waalen J, Felitti VJ, Gelbart T, Beutler E. Screening for hemochromatosis by measuring ferritin levels: a more effective approach. Blood 2008;111:3373–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis 2009;18:345–352 [PubMed] [Google Scholar]
- 31.Skikne BS. Serum transferrin receptor. Am J Hematol 2008;83:872–875 [DOI] [PubMed] [Google Scholar]
- 32.Hardikar PS, Joshi SM, Bhat DS, et al. Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young Indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care 2012;35:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrich N, Milman N, Völzke H, Linneberg A, Jørgensen T. Is serum ferritin within the reference range a risk predictor of cardiovascular disease? A population-based, long-term study comprising 2874 subjects. Br J Nutr 2009;102:594–600 [DOI] [PubMed] [Google Scholar]
- 34.Hsu HS, Li CI, Liu CS, et al. Iron deficiency is associated with increased risk for cardiovascular disease and all-cause mortality in the elderly living in long-term care facilities. Nutrition 2013;29:737–743 [DOI] [PubMed] [Google Scholar]
- 35.Corti MC, Guralnik JM, Salive ME, et al. Serum iron level, coronary artery disease, and all-cause mortality in older men and women. Am J Cardiol 1997;79:120–127 [DOI] [PubMed] [Google Scholar]
- 36.Casiglia E, Tikhonoff V, Bascelli A, et al. Dietary iron intake and cardiovascular outcome in Italian women: 10-year follow-up. J Womens Health (Larchmt) 2011;20:1565–1571 [DOI] [PubMed] [Google Scholar]
- 37.Gillum RF, Sempos CT, Makuc DM, Looker AC, Chien CY, Ingram DD. Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I Epidemiologic Followup Study. National Health and Nutrition Examination Survey. Am J Epidemiol 1996;144:59–68 [DOI] [PubMed] [Google Scholar]
- 38.Kim KS, Son HG, Hong NS, Lee DH. Associations of serum ferritin and transferrin % saturation with all-cause, cancer, and cardiovascular disease mortality: Third National Health and Nutrition Examination Survey follow-up study. J Prev Med Pub Health 2012;45:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sempos CT, Looker AC, Gillum RF, Makuc DM. Body iron stores and the risk of coronary heart disease. N Engl J Med 1994;330:1119–1124 [DOI] [PubMed] [Google Scholar]
- 40.Mørkedal B, Laugsand LE, Romundstad PR, Vatten LJ. Mortality from ischaemic heart disease: sex-specific effects of transferrin saturation, serum iron, and total iron binding capacity. The HUNT study. Eur J Cardiovasc Prev Rehabil 2011;18:687–694 [DOI] [PubMed] [Google Scholar]
- 41.Sempos CT, Looker AC, Gillum RE, McGee DL, Vuong CV, Johnson CL. Serum ferritin and death from all causes and cardiovascular disease: the NHANES II Mortality Study. National Health and Nutrition Examination Study. Ann Epidemiol 2000;10:441–448 [DOI] [PubMed] [Google Scholar]
- 42.Menke A, Muntner P, Fernández-Real JM, Guallar E. The association of biomarkers of iron status with mortality in US adults. Nutr Metab Cardiovasc Dis 2012;22:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Asperen IA, Feskens EJ, Bowles CH, Kromhout D. Body iron stores and mortality due to cancer and ischaemic heart disease: a 17-year follow-up study of elderly men and women. Int J Epidemiol 1995;24:665–670 [DOI] [PubMed] [Google Scholar]
- 44.Reunanen A, Takkunen H, Knekt P, Seppänen R, Aromaa A. Body iron stores, dietary iron intake and coronary heart disease mortality. J Intern Med 1995;238:223–230 [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Iso H, Ohira T, et al. Associations of dietary iron intake with mortality from cardiovascular disease: the JACC study. J Epidemiol 2012;22:484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells BJ, Mainous AG, 3rd, King DE, Gill JM, Carek PJ, Geesey ME. The combined effect of transferrin saturation and low density lipoprotein on mortality. Fam Med 2004;36:324–329 [PubMed] [Google Scholar]
- 47.Steen DL, Cannon CP, Lele SS, et al. Prognostic evaluation of catalytic iron in patients with acute coronary syndromes. Clin Cardiol 2013;36:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013;34:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575–582, e3 [DOI] [PubMed] [Google Scholar]
- 50.De Lima GA, Mazzali M, Gentil AF, Plotegher L, Grotto HZ. Anemia in chronic renal disease: evaluation of inflammatory activity on erythropoiesis and iron metabolism in patients not submitted to dialysis treatment. Clin Lab 2012;58:695–704 [PubMed] [Google Scholar]
- 51.Anker SD, Comin Colet J, Filippatos G, et al. FAIR-HF Trial Investigators Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448 [DOI] [PubMed] [Google Scholar]
- 52.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008;51:103–112 [DOI] [PubMed] [Google Scholar]