Abstract
OBJECTIVE
Type 1 diabetes is associated with a markedly increased risk of stroke, but only a few studies on the incidence of stroke in type 1 diabetes exist. Therefore, we assessed the incidence of stroke in patients with type 1 diabetes and studied the impact of diabetic nephropathy (DN) and severe diabetic retinopathy (SDR) on this risk.
RESEARCH DESIGN AND METHODS
We studied 4,083 patients with type 1 diabetes from the Finnish Diabetic Nephropathy Study. Mean age was 37.4 ± 11.8 years, duration of diabetes was 21.6 ± 12.1 years, and 52% were men. Strokes were identified from medical records, death certificates, and the National Hospital Discharge Register and classified based on medical files and brain images.
RESULTS
During 36,680 person-years of follow-up, 149 (4%) patients suffered an incident stroke (105 infarctions and 44 hemorrhages). Of the infarctions, 58 (55%) were lacunar. The incidence of stroke, cerebral infarction, and cerebral hemorrhage was 406 (95% CI 344–477), 286 (234–347), and 120 (87–161) per 100,000 person-years, respectively. In an adjusted analysis, microalbuminuria increased the risk of stroke with a hazard ratio (HR) of 3.2 (1.9–5.6), macroalbuminuria 4.9 (2.9–8.2), and end-stage renal disease 7.5 (4.2–13.3), and SDR increased the risk with an HR of 3.0 (1.9–4.5). The risk of cerebral infarction, cerebral hemorrhage, and lacunar infarction increased in a similar manner. The proportion of lacunar versus nonlacunar infarction did not change across DN groups.
CONCLUSIONS
The presence of SDR and DN, independently, increases the risk of stroke, cerebral infarction, and cerebral hemorrhage in patients with type 1 diabetes.
Type 1 diabetes accelerates the atherosclerotic process in blood vessels, leading to micro- and macrovascular complications, stroke being one of these. These complications account for the markedly increased risk for early mortality associated with type 1 diabetes (1).
Although stroke is a severely disabling complication, scarce data exist on cerebrovascular disease in type 1 diabetes. In patients with type 1 diabetes, stroke occurs fivefold more often than in nondiabetic subjects (2), and up to 20-fold more often in patients under the age of 50 years (3). The incidence of stroke and its subtypes in Finnish patients with type 1 diabetes is not known. The two major stroke subtypes are ischemic and hemorrhagic. Based on the phenotypic characteristics of the clinical stroke syndrome and the radiological features, ischemic stroke can be dichotomized as nonlacunar (i.e., large-vessel stroke mostly due to either atherosclerosis of the carotid artery or cardioembolism) or lacunar (i.e., occlusion of a single small perforating artery) (4).
Stroke is generally considered a macrovascular complication of diabetes. In nondiabetic subjects, however, every fourth ischemic stroke is lacunar (5) and, thus, of microvascular origin (6). In young patients with type 1 diabetes, even more than half of the strokes are microvascular according to the TOAST (Trial of Org 10127 in Acute Stroke Treatment) classification system (7). Type 2 diabetes has previously been thought to be associated with lacunar infarctions; however, this association was not confirmed in a meta-analysis (8), and the method used to classify strokes as microvascular influenced the association with diabetes. Although the role of diabetes in lacunar infarctions remains unclear, there seems to be a link between cerebrovascular small-vessel events and small-vessel disease in other organs such as the retina (9) and the kidney (10) in nondiabetic subjects. This could in turn suggest a link between the diabetic microvascular complications diabetic nephropathy (DN) and diabetic retinopathy and microvascular stroke.
DN and diabetic retinopathy increase the risk for stroke in patients with type 2 diabetes (11,12). In patients with type 1 diabetes, registry-based studies present similar results, especially for DN (3,13). However, how these diabetes complications independently affect the risk for cerebral infarctions, hemorrhages, and especially microvascular stroke in type 1 diabetes is unknown.
We, therefore, aimed to assess the incidence of stroke and its subtypes in a well-characterized cohort of patients with type 1 diabetes. We further aimed to study the impact of DN and severe diabetic retinopathy (SDR) on this risk.
RESEARCH DESIGN AND METHODS
All patients were part of the Finnish Diabetic Nephropathy (FinnDiane) study, a nationwide multicenter study with the aim to uncover genetic, clinical, and environmental risk factors for micro- and macrovascular complications of type 1 diabetes. The study population consists of patients with type 1 diabetes from all over Finland. All adult patients with type 1 diabetes attending the 77 participating study centers’ (Supplementary Data) diabetes and/or renal outpatient clinics were consecutively asked to participate in the study. The baseline visits began in 1998 and are still ongoing, and follow-up visits have been conducted since 2004. The patient’s attending physician completed a questionnaire where information on the patient’s medical condition appeared. For this study, we included all the 4,083 patients with type 1 diabetes in the FinnDiane database without a history of stroke at baseline and complete information on SDR as well as stroke during follow-up available. Due to unclear information on stroke at baseline or during follow-up, 15 patients were excluded. In addition, two patients were excluded due to subdural hemorrhages, one due to traumatic hemorrhage, one due to perinatal cerebral hemorrhage, and one due to hypertensive encephalopathy. The local ethics committee of each center approved the study protocol, and the study was performed in accordance with the Declaration of Helsinki. Each participating patient signed a written consent.
Diabetes and diabetes complications
Type 1 diabetes was defined as diabetes diagnosis before 40 years of age and insulin medication commencement within 1 year after diagnosis. At baseline, the mean age was 37.4 ± 11.8 years, the mean duration of diabetes was 21.6 ± 12.1 years, and 52% of the patients were men. Each patient collected timed urine samples for the measurement of the urinary albumin excretion rate (UAER). Kidney status was defined based on the UAER measured from two out of three overnight or 24-h urine collections. A normal UAER was defined as <20 µg/min or <30 mg/24 h (n = 2,482, 61%), microalbuminuria as a UAER ≥20 and <200 µg/min or ≥30 and <300 mg/24 h (n = 510, 12%), and macroalbuminuria as a UAER ≥200 µg/min or ≥300 mg/24 h (n = 549, 13%). End-stage renal disease (ESRD) was defined as ongoing dialysis treatment or kidney transplantation (n = 289, 7%). In 253 (6%) patients, the renal status remained unclassified. DN was defined as having either macroalbuminuria or ESRD. SDR was defined as laser treatment of the retina (n = 1,375, 34%), a surrogate marker for proliferative retinopathy. Of the patients with classified renal status, 2,414 (63%) had neither DN nor SDR, 578 (15%) had only SDR, 118 (3%) had only DN, and 720 (19%) had both diabetes complications.
Blood pressure was measured twice in the sitting position with a 10-min rest before the first measurement, and the mean values of these two measurements were calculated for both systolic blood pressure (SBP) and diastolic blood pressure (DBP). BMI was calculated as weight divided by square meters of length (kg/m2). Serum samples were analyzed for lipids and lipoproteins. History of smoking was defined as current smoking of at least one cigarette per day or, if the patient had quit, earlier history of smoking.
Stroke
Patients that suffered a stroke during follow-up were identified from three different sources: the FinnDiane questionnaires (either from the baseline visit or the follow-up visit), death certificates retrieved from Statistics Finland by March 2010, and the National Hospital Discharge Register based on the ICD-10 (codes I60-I64) by December 2009. Medical records, computed tomography (CT), and magnetic resonance (MR) images of the patients with an identified stroke were then ordered from the hospital where the patients had been treated.
Based on the medical files, brain images, and autopsy findings, two stroke neurologists (J.P. and R.L.) classified all strokes that presented with clinical symptoms as either cerebral hemorrhage or cerebral infarction. An experienced neuroradiologist was consulted where necessary. Cerebral infarctions were further classified as nonlacunar or lacunar. We defined lacunar infarction as one of the known lacunar syndromes with a brain imaging lesion corresponding with the occlusion of a single perforating artery. When no such lesion was visible on imaging, classification relied on established criteria of unilateral motor or sensory signs, or both, involving at least two out of three body parts (face, arm, and leg) without disturbance of cortical functions or consciousness (14). Hemorrhagic transformation of a cerebral infarction was classified as cerebral infarction. Cerebral hemorrhages were classified as deep spontaneous, lobar spontaneous, or hemorrhage into the subarachnoid space. Stroke was defined as fatal if the patient died within 30 days from onset of the symptoms. Follow-up time was calculated from the baseline visit until the last date the patients were known to be free of stroke, or until the date of the first stroke for those who suffered a stroke.
Statistical analyses
All variables were tested for normal distribution. Parametric continuous variables were analyzed with the Student t test; results are presented as means with SDs. Nonparametric continuous variables were analyzed with the Mann-Whitney U test; results are presented as medians with interquartile range. The difference in categorical variables between groups was tested with the χ2 test. Person-years of follow-up were calculated as the sum of the follow-up time in years of each patient. The age-adjusted incidence of stroke, cerebral infarction, and cerebral hemorrhage is presented per 100,000 person-years. Cox proportional hazards analysis adjusted for age, sex, SBP, DBP, BMI, LDL cholesterol, HDL cholesterol, triglycerides, and history of smoking was performed to study how DN and SDR affected the risk of stroke, cerebral infarction, cerebral hemorrhage, and lacunar infarction. The results are presented as hazard ratio (HR) with 95% CI. The proportion of lacunar infarction of the total infarctions across DN groups and SDR was calculated to assess the association of these diabetes complications and lacunar infarction. P < 0.05 was considered statistically significant. All analyses were performed with SPSS statistical software 19.0 (IBM Corporation, Armonk, NY).
RESULTS
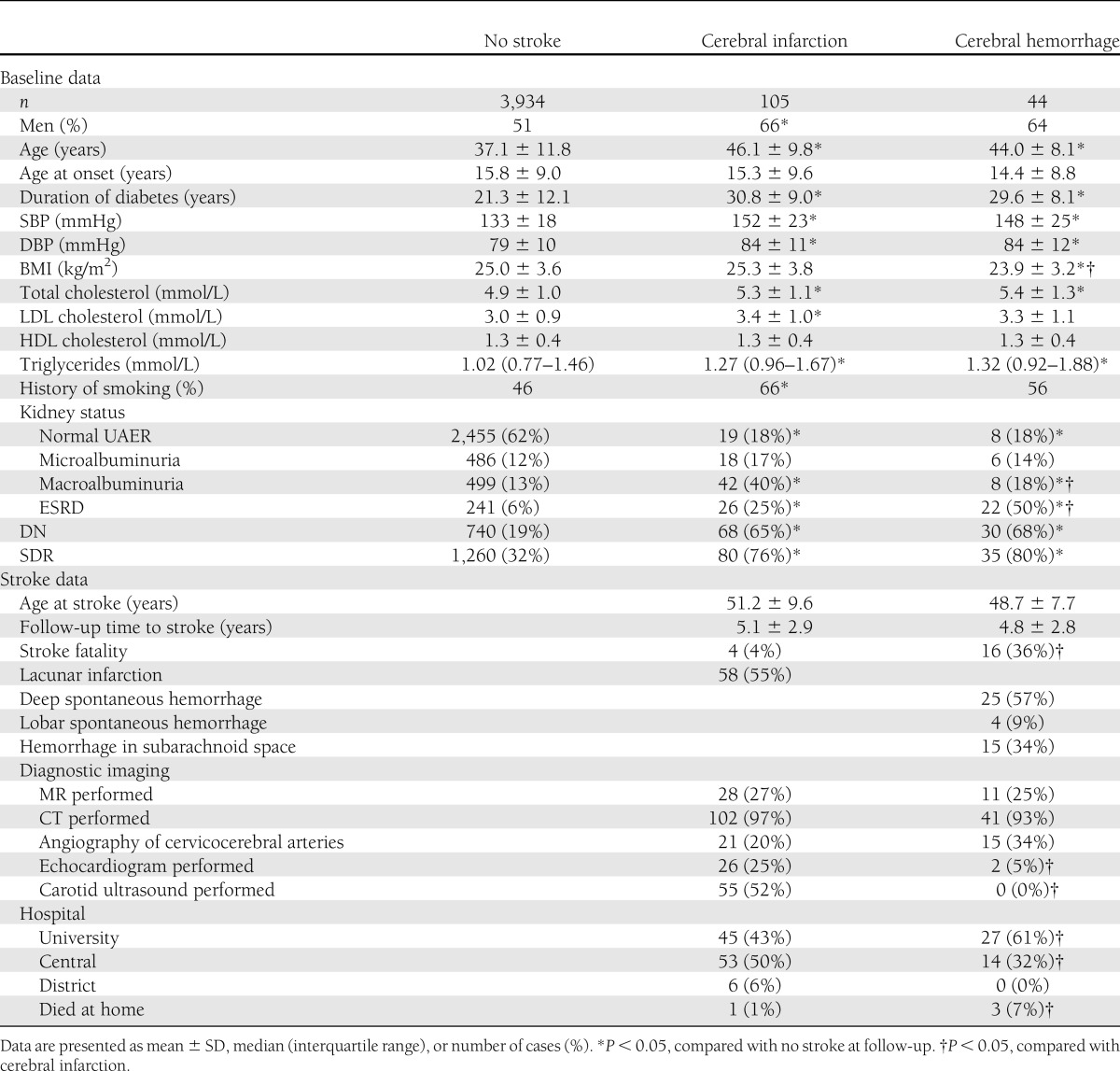
Of the 4,083 patients included in the analyses, 149 (4%) patients suffered an incident stroke between the years 1998 and 2010. Of the strokes, 105 (70%) were cerebral infarctions and 44 (30%) were cerebral hemorrhages. Table 1 shows the baseline characteristics of the patients with no stroke, cerebral infarction, and cerebral hemorrhage during follow-up. Those with a stroke were older, had a longer duration of diabetes, and had more DN and SDR at baseline. Patients who suffered a cerebral infarction compared with patients who suffered a cerebral hemorrhage were of the same age, had the same duration of diabetes, were the same proportion of men, and had the same proportion of DN and SDR at baseline. Macroalbuminuria was more common in patients with cerebral infarction, whereas ESRD was more common in those with cerebral hemorrhage.
Table 1.
Characteristics of patients with no stroke, cerebral infarction, and cerebral hemorrhage during follow-up
Cerebral infarction was more common among men, whereas no sex difference was found for cerebral hemorrhage, P = 0.101 (Table 1). After adjustment for DN in a Cox proportional hazards analysis, male sex did not affect the risk of stroke (HR 1.3 [95% CI 0.9–1.9]), cerebral infarction (1.4 [0.9–2.1]), or cerebral hemorrhage (1.3 [0.7–2.4]).
Of all the incident strokes, 4 (4%) infarctions and 16 (36%) hemorrhages were fatal, P < 0.001. Of the cerebral infarctions, 58 (55%) were lacunar infarctions. Of the cerebral hemorrhages, 25 (57%) were deep spontaneous, 4 (9%) were lobar, and 15 (34%) were hemorrhages in the subarachnoid space. Brain and blood vessel imaging was performed equally commonly on the two groups with stroke, whereas echocardiogram was performed more commonly on those with cerebral infarction, P = 0.004. The majority of the patients in both groups with stroke were treated at university (tertiary referral hospital) or central (secondary referral hospital) hospitals (Table 1).
Incidence
The mean follow-up time was 9.0 ± 2.7 years, and the total person-years of follow-up was 36,680. The incidence of stroke was 406 (95% CI 344–477) per 100,000 person-years. The incidence of cerebral infarction was 286 (234–347) per 100,000 person-years, and that of cerebral hemorrhage was 120 (87–161) per 100,000 person-years. The incidence of stroke according to kidney status is shown in Table 2. The incidence doubled with each stage of DN. A similar finding was seen in the adjusted Cox proportional hazards analysis with normal UAER as the reference group. The risk of stroke increased with each stage of DN, and if the patient had ESRD, the risk was 7.5-fold compared with normal UAER. For cerebral infarction, the risk was equally high in patients with macroalbuminuria and ESRD, whereas the risk of cerebral hemorrhage increased markedly in patients with ESRD (Table 2).
Table 2.
Incidence and adjusted HRs for stroke, cerebral infarction, and cerebral hemorrhage according to presence of DN and SDR

The incidence of stroke was markedly increased also in subjects with SDR, as shown in Table 2. In the adjusted Cox proportional hazards analysis, the risk for stroke was 3.0-fold, for cerebral infarction 2.7-fold, and for cerebral hemorrhage 3.9-fold if the patient had SDR, compared with those without SDR.
To further clarify how DN and SDR affected the risk of stroke, we combined these two complications. Both diabetes complications alone independently increased the risk for total stroke, cerebral infarction, and cerebral hemorrhage. If the patient had both complications, the risk for all subtypes of stroke was increased, that is 6.1-fold for stroke, 5.7-fold for cerebral infarction, and 7.4-fold for cerebral hemorrhage (Table 2), compared with patients with neither of the complications.
Lacunar infarction and diabetes complications
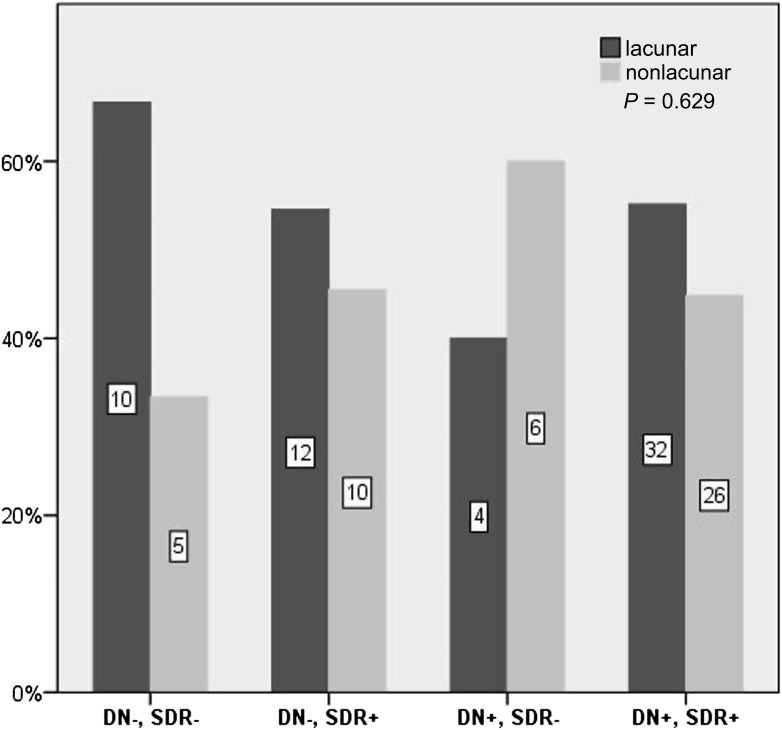
The incidence of lacunar infarction was 158 (95% CI 120–204) per 100,000 person-years. The incidence of lacunar infarction according to kidney status, i.e., normal UAER, microalbuminuria, macroalbuminuria, or ESRD, was 45 (22–83), 250 (129–437), 445 (285–663), and 490 (253–855) per 100,000 person-years, respectively. The corresponding adjusted HRs for lacunar infarction were 4.3 (95% CI 1.8–10.0), 5.7 (2.5–12.9), and 5.1 (1.9–14.1), compared with normal UAER. If the patients had SDR, the incidence was 348 (253–467), compared with 58 (32–98) per 100,000 person-years if patients did not have SDR. The adjusted HR was 2.8 (1.5–5.5), compared with no SDR. To further study the association of lacunar infarction with DN and SDR, the proportion of lacunar infarctions of the total infarctions across DN groups and SDR was assessed. Of those with cerebral infarction and normal UAER, microalbuminuria, macroalbuminuria, or ESRD, the proportion of lacunar infarction was 53, 67, 57, and 46%, respectively, P = 0.587. In patients with SDR, the proportion of lacunar infarction was 56% compared with 55% if the patient was free of SDR, P = 0.930. The proportion of lacunar infarction in patients with no DN or SDR, only SDR, only DN, and both DN and SDR is shown in Fig. 1.
Figure 1.
Proportion of lacunar and nonlacunar infarction of the total infarctions in patients with neither complication, only SDR, only DN, or both complications. The number in the bars represents the number of patients in that group.
CONCLUSIONS
In this large observational follow-up study of 4,083 patients with type 1 diabetes and 36,680 person-years of follow-up, we were able to show that the incidence of both cerebral infarction and cerebral hemorrhage increased with the presence of both SDR and advancing DN. Furthermore, both DN and SDR alone independently increased the risk for all subtypes of stroke. Cerebral hemorrhage was more fatal than cerebral infarction. To our surprise, we could not observe any association of lacunar infarctions with DN and SDR.
We found that two-thirds of the incident strokes were ischemic and one-third was hemorrhagic. This is consistent with the recent findings from the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study of patients with type 1 diabetes; of 31 incident strokes, 21 (68%) were ischemic and 8 (26%) were hemorrhagic (15). A similar ratio of ischemic versus hemorrhagic stroke has also been observed in the general population (16) and in patients with type 2 diabetes (17).
In our study, the incidence rates for stroke, cerebral infarction, and cerebral hemorrhage were 406, 286, and 120 per 100,000 person-years, respectively. These incidence rates are higher than in the Finnish general population, for whom the incidence of stroke varies between 135 and 236 per 100,000 person-years in subjects 25–74 years of age (18). To date, data on the incidence of stroke and its subtypes in patients with type 1 diabetes are scarce and are derived from studies with rather few cases with stroke. The incidence rates in the Nurses’ Health Study, including only women with type 1 diabetes and with a higher mean age than in our study, were 475 for stroke, 288 for ischemic stroke, and 43 for hemorrhagic stroke (2). In the Pittsburgh EDC Study with younger patients with childhood onset of type 1 diabetes, the incidence for stroke was 310 per 100,000 person-years (15). Although these incidence rates largely correspond with our findings, discrepancies in the incidence rates exist, especially for cerebral hemorrhage. These discrepancies may be attributed to the obvious age differences of the study populations, the methodology used for stroke classification, and the different number of strokes included in the studies.
In univariate analyses, proteinuria and DN have been shown to be associated with a 3–10-fold increased risk of stroke in type 1 diabetes (3,13,19). In these studies, however, the stroke cases have been rather few or the DN status has been retrieved from registries. Recently, the Pittsburgh EDC Study showed that overt DN was strongly predictive of stroke and increased the risk more than fourfold, independent from SBP and non-HDL cholesterol levels (15). In the current study, we confirmed that the risk of stroke is markedly increased in patients with DN. In addition, we were able to show that the risk of stroke is increased already at the stage of microalbuminuria. The incidence of stroke further increased, moving from one stage of DN to the next, and if the patient had ESRD, the risk was as high as eightfold. In contrast, in patients free of DN, the incidence of stroke was of the same magnitude as observed in the general population in Finland (18).
To date, only one study has elucidated the impact of DN on the subtypes of stroke in type 1 diabetes. In the Pittsburgh EDC Study, overt DN increased the risk of ischemic stroke 4.4-fold, but not the risk for hemorrhagic stroke, possibly due to the low number of cases (15). In contrast, in our study, we showed that DN increased the risk not only for cerebral infarction but also for cerebral hemorrhage, and the highest risk was observed in patients with ESRD. ESRD increases the risk of stroke, particularly that of ischemic stroke, in nondiabetic subjects (20). It is noteworthy that in contrast to the data in nondiabetic subjects, we found the risk for cerebral hemorrhage to be considerably higher than for cerebral infarction. This finding is not surprising, since impaired renal function is known to be associated with increased risk of gastrointestinal bleeding (21), as well as cerebral hemorrhage (22). Both are linked to dysfunction of the platelets and the endothelium, the use of antiplatelet and anticoagulative medication, as well as anemia associated with chronic kidney disease and uremia (23).
A novel finding of the current study was that SDR, independently from DN, associated with an increased risk of both incident cerebral infarction and cerebral hemorrhage. The risk of these cerebral end points was the highest in those with both DN and SDR. Diabetic retinopathy has been shown to increase the risk for ischemic stroke in type 2 diabetes (24); in that study, DN was not, however, taken into account. In type 2 diabetes, an independent association of proliferative retinopathy and nephropathy on the risk of stroke has only been shown in studies where stroke has been pooled to a common cardiovascular end point and no subanalyses for stroke have been performed (25,26). Our finding of SDR and DN as independent risk factors for stroke is consistent with earlier findings that the vasculature of the retina (27) and the kidney (28) show partly similar pathophysiological features and mechanisms as the vasculature in the brain. Retinopathy and nephropathy have, therefore, been suggested to be early surrogate markers for cerebrovascular changes before any such changes are visibly manifested on CT or MR images of the brain.
Although the incidence of lacunar infarctions increased with worsening DN and presence of SDR, surprisingly, we did not find a higher proportion of lacunar infarctions in patients with DN and/or SDR. One possible explanation for this could be the fact that asymptomatic microvascular lesions are common also in the patients with nonlacunar infarctions. Our methodology did not, however, allow us to study this in further detail, since the brain imaging technique performed varied between the patients, and the best method available, MR imaging, was performed only in 27% of the patients with cerebral infarction. Asymptomatic cerebral small vessel disease, diagnosed based on MR imaging, has been associated with chronic kidney disease, irrespective of the presence of diabetes (10). In addition, abnormalities in the retinal vasculature have been shown to be more common in patients with lacunar stroke than in any other stroke subtype (9). This association was observed especially for changes in the vasculature, such as focal arteriolar narrowing and arteriovenous nicking, whereas retinopathy signs were not associated with lacunar stroke in an analysis adjusted for the presence of diabetes. This highlights the importance of a thorough retinal examination in studying the association of SDR with lacunar infarctions. In the current study, SDR was, however, defined based solely on the presence of retinal laser treatment, and therefore milder forms of diabetic retinopathy and specific retinal vascular changes could not be considered.
The current study has some other limitations as well. Cerebral infarctions can be asymptomatic (silent or covert infarctions); therefore, some of the patients in the control group may have suffered an asymptomatic stroke without our knowledge. Nevertheless, we are convinced that all the included patients with an incident stroke indeed suffered a symptomatic stroke during follow-up, since all strokes were ascertained and thoroughly classified by experts in the field, based on medical records, brain images, or autopsy reports. Moreover, all of the patients have been closely examined by their attending physicians, and their DN status has been classified based on standard methods. It is noteworthy that the number of incident stroke cases in this study is the largest existing to this date, which allowed us to further characterize the stroke subtypes in patients with type 1 diabetes and to elucidate the impact of DN and SDR on these subtypes.
In conclusion, we showed that in patients with type 1 diabetes, DN is strongly associated with a higher incidence of stroke and its major subtypes cerebral infarction and cerebral hemorrhage. A novel finding was that SDR, independently from DN, increased the risk of both stroke subtypes. Early detection and treatment, as well as specific interventions, targeting the pathophysiological changes in the kidney and the retina could possibly lead to a decrease in the incidence of stroke in type 1 diabetes.
Acknowledgments
This study was supported by grants from the Folkhälsan Research Foundation, the Wilhelm and Else Stockmann Foundation, the Finnish Medical Society (Finska Läkaresällskapet), the Finnish Eye Foundation, the Academy of Finland (134379), the European Commission, Medicinska Understödsföreningen Liv och Hälsa, the Signe and Arne Gyllenberg Foundation, the Waldemar von Frenckell Foundation, and an EVO governmental grant (TYH 3263). J.P. was supported by the Diabetes Research Foundation.
T.T. has served on scientific advisory boards for Boehringer Ingelheim and Mitsubishi Pharma and has received speaker honorariums from the Professio Finland, the University of Helsinki, the Finnish Medical Association, the University of Donau (Austria), the Genzyme Oy, and the Finnish Neurological Association. P.-H.G. has received speaker honorariums from Boehringer Ingelheim, Cebix, Eli Lilly and Company, Genzyme Oy, Medscape, MSD, Novartis, and Novo Nordisk and is an advisory board member of Boehringer Ingelheim, Cebix, Medscape, and Novartis. No other potential conflicts of interest relevant to this article were reported.
S.H. had the main responsibility for analyzing the patient data and writing the manuscript. L.M.T. and J.P. contributed to study design, acquisition of data, data analysis, and critical revision of the manuscript. R.L. and V.H. contributed to data analysis and critical revision of the manuscript. C.M.F. contributed to study design, acquisition of data, and critical revision of the manuscript. D.G. and T.T. contributed to study design and critical revision of the manuscript. P.-H.G. contributed to study design, acquisition of data, critical revision of the manuscript, and coordination of the study. P.-H.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented orally at the 25th Annual Conference of the European Diabetic Nephropathy Study Group, Dublin, Ireland, 17–19 May 2012.
The authors acknowledge all the physicians and nurses at each center participating in the collection of patient data. The authors are indebted to Oili Salonen (Helsinki University Central Hospital) for help with neuroradiological evaluation.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0669/-/DC1.
A complete list of the FinnDiane study centers can be found in the Supplementary Data online.
References
- 1.Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janghorbani M, Hu FB, Willett WC, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses’ Health Study. Diabetes Care 2007;30:1730–1735 [DOI] [PubMed] [Google Scholar]
- 3.Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15-49: a nationwide study from Sweden. Diabet Med 2006;23:1261–1267 [DOI] [PubMed] [Google Scholar]
- 4.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701 [DOI] [PubMed] [Google Scholar]
- 5.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521–1526 [DOI] [PubMed] [Google Scholar]
- 6.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke 2009;40:e322–e330 [DOI] [PubMed] [Google Scholar]
- 7.Putaala J, Liebkind R, Gordin D, et al. Diabetes mellitus and ischemic stroke in the young: clinical features and long-term prognosis. Neurology 2011;76:1831–1837 [DOI] [PubMed] [Google Scholar]
- 8.Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke 2005;36:891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR, ARIC Study Investigators Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke 2010;41:1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada M, Nagasawa H, Iseki C, et al. Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008;272:36–42 [DOI] [PubMed] [Google Scholar]
- 11.Davis TM, Millns H, Stratton IM, Holman RR, Turner RC. Risk factors for stroke in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS) 29. Arch Intern Med 1999;159:1097–1103 [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis 2011;218:13–18 [DOI] [PubMed] [Google Scholar]
- 13.Tuomilehto J, Borch-Johnsen K, Molarius A, et al. Incidence of cardiovascular disease in type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia 1998;41:784–790 [DOI] [PubMed] [Google Scholar]
- 14.Bamford J, Sandercock P, Jones L, Warlow C. The natural history of lacunar infarction: the Oxfordshire Community Stroke Project. Stroke 1987;18:545–551 [DOI] [PubMed] [Google Scholar]
- 15.Secrest AM, Prince CT, Costacou T, Miller RG, Orchard TJ. Predictors of and survival after incident stroke in type 1 diabetes. Diab Vasc Dis Res 2013;10:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne stroke incidence study (NEMESIS). Stroke 2001;32:1732–1738 [DOI] [PubMed] [Google Scholar]
- 17.Hankey GJ, Anderson NE, Ting RD, et al. Rates and predictors of risk of stroke and its subtypes in diabetes: a prospective observational study. J Neurol Neurosurg Psychiatry 2013;84:281–287 [DOI] [PubMed] [Google Scholar]
- 18.Immonen-Räihä P, Sarti C, Tuomilehto J, et al. Eleven-year trends of stroke in Turku, Finland. Neuroepidemiology 2003;22:196–203 [DOI] [PubMed] [Google Scholar]
- 19.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl. 2):S54–S64 [DOI] [PubMed] [Google Scholar]
- 20.Seliger SL, Gillen DL, Longstreth WT, Jr, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int 2003;64:603–609 [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman GR, Cornette GL, Clouse RE, Harter HR. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med 1985;102:588–592 [DOI] [PubMed] [Google Scholar]
- 22.Iseki K, Kinjo K, Kimura Y, Osawa A, Fukiyama K. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int 1993;44:1086–1090 [DOI] [PubMed] [Google Scholar]
- 23.Sohal AS, Gangji AS, Crowther MA, Treleaven D. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res 2006;118:417–422 [DOI] [PubMed] [Google Scholar]
- 24.Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007;38:398–401 [DOI] [PubMed] [Google Scholar]
- 25.Tong PC, Kong AP, So WY, et al. Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal end points in Chinese patients with type 2 diabetes mellitus. Diabet Med 2007;24:741–746 [DOI] [PubMed] [Google Scholar]
- 26.Targher G, Bertolini L, Zenari L, et al. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabet Med 2008;25:45–50 [DOI] [PubMed] [Google Scholar]
- 27.Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol 2001;46:59–80 [DOI] [PubMed] [Google Scholar]
- 28.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–204 [DOI] [PubMed] [Google Scholar]