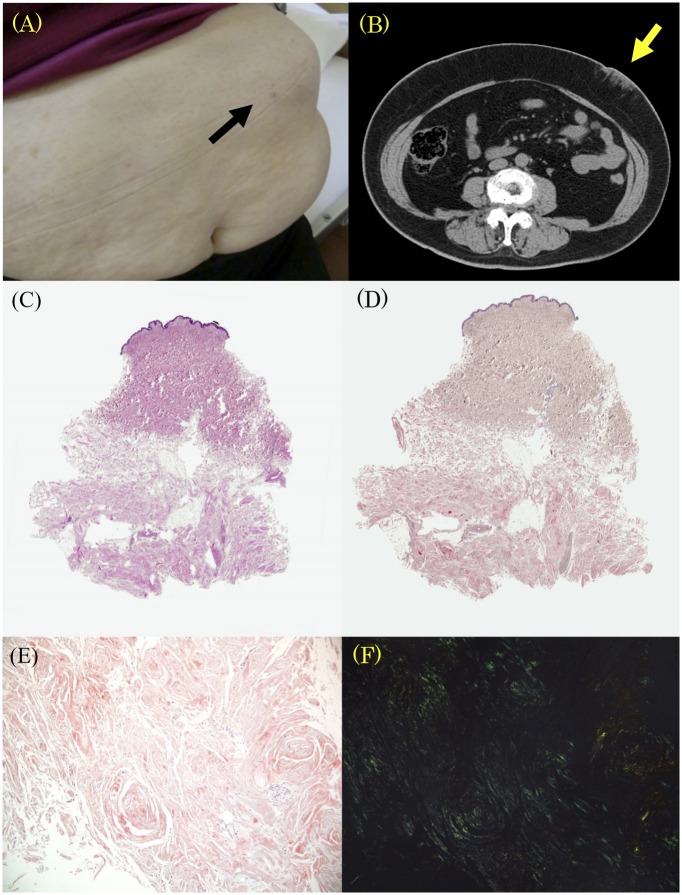
We present the case of a 69-year-old woman with type 2 diabetes mellitus who developed severe insulin resistance associated with insulin injections into a subcutaneous mass formed by repeated injections into the same site over many years. The patient (height, 150 cm; weight, 79 kg; BMI, 35.1 kg/m2) was admitted to Tenri Hospital with severe insulin resistance. She was undergoing insulin therapy since 56 years of age. Her glycemic control was fair until 63 years of age, after which it worsened and necessitated an increase in her insulin dose. This led to a 15-kg increase in body weight. On admission, her insulin regimen comprised a single injection of 33 units of neutral protamine Hagedorn insulin at bedtime and three daily injections of 33 units of premixed human insulin (70/30) before each meal. She repeatedly administered all insulin injections at the same site in her abdominal wall. Physical examination revealed a 12 × 8-cm soft, nontender, nonerythematous subcutaneous mass at the insulin injection site (Fig. 1A).
Figure 1.
(A) A subcutaneous mass in a 69-year-old patient with type 2 diabetes. (B) Computed tomography of the subcutaneous mass. (C) Light microscopic findings of the abdominal mass showing widespread deposition of eosinophilic amorphous material in the subcutaneous tissue (loupe). Congo red staining highlighting the amyloid deposit (loupe). (D) Confirmation of an amyloid deposit by Congo red staining and (E) green birefringence via polarized microscopy [(F) magnification ×200)].
Laboratory evaluation revealed the following: plasma glucose, 248 mg/dL; serum C-peptide, 1.8 ng/mL; urinary C-peptide, 20.6 μg/day; and HbA1c, 7.6%. Abdominal computed tomography revealed a subcutaneous mass with a nonhomogeneous shadow (Fig. 1B). We performed biopsy, and hematoxylin–eosin-stained sections revealed that most of the hypodermal tissue space was occupied by eosinophilic homogeneous material (Fig. 1C) that stained positively with Congo red staining (Fig. 1D and E) and was visualized by polarized microscopy (Fig. 1F).
Our patient was instructed to inject insulin external to the subcutaneous mass. Within 14 days after changing the injection site, her insulin regimen was changed to 12 units of glargine at bedtime and premixed insulin (lispro 50/50; mid mixture) before meals: 16 units before breakfast, 4 units before lunch, and 10 units before dinner. This resulted in an average four-point blood glucose reading of 176 mg/dL, resolved insulin resistance, and dramatic improvement in insulin sensitivity.
Local amyloid deposition at the site of repeated insulin injection causing subcutaneous insulin resistance is rarely reported. Insulin-degrading enzymes in inflammatory cells at the injection site reportedly cause insulin insensitivity (1). Localized amyloidosis associated with repeated insulin injections is usually misdiagnosed as lipohypertrophy, which is a common side effect of subcutaneous insulin therapy that occurs in up to 50% of type 1 diabetes patients and causes impairment of insulin absorption (2,3). Lipohypertrophy is usually diagnosed by palpation and appears as an amphibolic and lobular growth, whereas amyloid tumors are more solid and firm. Nevertheless, a physical examination alone is insufficient to distinguish them. Lipohypertrophy generally regresses soon after cessation of insulin injection, whereas localized amyloidosis does not. If a subcutaneous mass does not regress after cessation of insulin injection, we recommend histological examination for an accurate diagnosis. In any case, patients with diabetes should be advised to routinely change insulin injection sites, while health care providers should consider that an insulin ball may cause poor glycemic control that will necessitate an increased insulin dose.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.O. wrote the manuscript and researched data. Y.H. contributed to discussion and reviewed/edited the manuscript. S.K.-E. researched data. S.T. contributed to discussion and reviewed/edited the manuscript. S.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Albert SG, Obadiah J, Parseghian SA, Yadira Hurley M, Mooradian AD. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract 2007;75:374–376 [DOI] [PubMed] [Google Scholar]
- 2.Nagase T, Katsura Y, Iwaki Y, et al. The insulin ball. Lancet 2009;373:184. [DOI] [PubMed] [Google Scholar]
- 3.Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care 2005;28:2025–2027 [DOI] [PubMed] [Google Scholar]