Abstract
Dysregulated complement is thought to play a central role in AMD pathogenesis, but the specific mechanisms have yet to be determined. In maculas of AMD specimens, we found that the complement regulatory protein, CD59, was increased in regions of uninvolved retinal pigmented epithelium (RPE) of early AMD, but decreased in the RPE overlying drusen and in geographic atrophy, an advanced form of AMD. While CD46 immunostaining was basolaterally distributed in the RPE of unaffected controls, it was decreased in diseased areas of early AMD samples. Since oxidized low density lipoproteins (oxLDL) collect in drusen of AMD and are a known complement trigger, we treated ARPE-19 cells with oxLDL and found that cellular CD46 and CD59 proteins were decreased by 2.9-fold and 9-fold (p<0.01), respectively. OxLDLs increased complement factor B mRNA and Bb protein, but not factor D, I, or H. OxLDLs increased C3b, but not C3a, C5 or C5b-9. C5b-9 was increased by 27% (p<0.01) when medium was supplemented with human serum, which was sufficient to induce poly (ADP-ribose) polymerase cleavage, a marker of apoptosis. The decreased levels of CD46 and CD59 were in part, explained by their release in exosomal and apoptotic membranous particles. In addition, CD59 was partially degraded through activation of IRE1α. Collectively, these results suggest that a combination of impaired complement regulators results in inadequately controlled complement by the RPE in AMD that induces RPE damage.
Keywords: Aging, Age-related macular degeneration, apoptosis, complement, exosome, oxidative stress
Introduction
Determining the events that distinguish chronological aging from early age-related disease would provide the foundation for developing novel targeted early therapy. Age-related macular degeneration (AMD), a prototypical complex aging related disease, is the most common cause of low vision among the elderly in the United States. In early AMD, retinal pigmented epithelial (RPE) cell apoptosis and the formation of drusen deposits within Bruch’s membrane are cardinal changes[1-3]. Drusen contain a heterogeneous accumulation of lipids, proteins, and cellular debris, many of which are oxidized[4]. Apolipoprotein B100 (apoB100) lipoproteins have captured our interest because they accumulate and become oxidized at the same location within Bruch’s membrane prior to drusen[5-9]. Geographic atrophy is an advanced, late form of AMD, and is characterized by continued atrophy or loss of the RPE and degeneration of the overlying photoreceptors[10].
The discovery that polymorphisms in complement factor H (CFH), a circulating regulator of the alternative complement pathway, are strongly associated with AMD risk, has solidified complement as a key element in AMD pathophysiology[11-14]. In general, cells protect themselves from excessive complement activation through fluid-phase regulators such as CFH, or by cell membrane molecules such as CD46 and CD55, which regulate the C3 and C5 convertases, and CD59, which prevents the formation of C5b-9 complexes. In late-onset diseases such as AMD, local regulation of the alternative pathway may be overwhelmed by cellular injury or the accumulation of debris[15,16]. Despite intensive study, the exact mechanism of how complement contributes to AMD remains unresolved[12,17,18]. Herein, we addressed the hypothesis that a combination of complement regulators, and not any single complement factor, is required to protect the RPE from a complement trigger.
Materials & Methods
Immunohistochemistry
Autopsy eyes (n=18) were obtained from the Wilmer Eye Institute Pathology Division after approval from the Human Subjects Committee at Johns Hopkins University. “Unaffected” eyes (n=9) had no AMD history or microscopic evidence of drusen, basal deposits, or loss of RPE cuboidal epithelial morphology. Early AMD donors (n=9) had an AMD history and macular drusen, but no late stage disease. Eyes were fixed in 4% formaldehyde, paraffin embedded, sectioned to 4μm thickness, and deparaffinized with xylene and an ethanol gradient. Geographic atrophy eyes (GA; n=7) were provided by the UAB Age-related Maculopathy Laboratory[19]. These eyes were fixed in 4% paraformaldehyde, embedded in sucrose OCT, cryopreserved, and sectioned at 10μM.
Antigens were retrieved with the Target Retrieval System (Dako, Inc., Carpinteria, CA). Sections were incubated with blocking serum for 1 h; with mouse anti-human CD59 monoclonal antibody (5μg/ml for early AMD or 20μg/ml for GA; clone MEM-43; Abcam, Inc., Cambridge, MA) or rabbit anti-human CD46 monoclonal (1/200 dilution; clone EPR4014; Abcam, Inc.), and equivalent concentratinos of mouse IgG2α isotype control (eBioscience, Inc., San Diego, CA) for CD59 and rabbit IgG isotype control (Abcam, Inc.; Cambridge, MA) for CD46 overnight at 4°C; with biotinylated anti-mouse (CD59) or anti-rabbit (CD46) IgG for 60 min, and then with ABC-AP (Vector labs, Burlingame, CA) for 30 min. The chromagen was developed with blue substrate working solution (Vector labs) supplemented with levamasole. Sections were viewed with a light microscope equipped with the Cri-Nuance system (Caliper Life Sciences, Inc. Hopkinton, MA) to subtract out the melanin. Immunolabeling was assessed for tissue distribution within each section, and differences between macular and peripheral sections. Relative immunolabeling abundance was assessed by the extent of the labeling area (i.e. thickness of staining bands). Staining intensity was a secondary criterion, but it was utilized only within the same tissue sections, or between macular and peripheral sections of the same donor to exclude unintentional confounding influences across donors. For CD59, labeling intensity was ranked relative to choroidal endothelial cell labeling because strong, consistent labeling was observed across all specimens.
Cell Culture
ARPE-19 cells were grown in DMEM/Ham’s F12 (Invitrogen-Gibco BRL; Gaithersberg, MD) plus 10% FBS (Invitrogen-Gibco BRL). For experiments, cells were grown until they were visually confluent, then an additional 10 days until they lost spindle-shaped morphology and developed a cobblestone appearance, and subsequently serum-deprived in DMEM/F12 for 24 h. LDLs, oxidized LDLs (oxLDLs) (both from Intracel, Inc.; Frederick, MD), or vehicle (0.15M NaCl-0.01% EDTA, pH 7.2) were added to the medium for 24 h. In some experiments, cells were treated with 10-25% normal human serum (Quidel, Inc.; San Diego, CA), or heat inactivated serum (52°C for 1 h) for 2 or 4 h, respectively.
Cell Viability
Cell viability was evaluated with the LIVE/DEAD® Assay (Invitrogen). After a 24 h exposure to 0-400μg/ml oxLDL, cells were treated for 30 min with calcein AM, which measures cell viability and ethidium homodimer (EthD-1), which measures cell death. Fluorescence was detected for calcein AM at ex/em 495nm/515nm and EthD-1 at ex/em 495nm/635nm, and the number of live and dead cells was counted using the Cellomics ArrayScan VTI HCS Reader (Thermo Fisher Scientific, Waltham, MA). Hoechst staining was used to identify the total number of the cells. The percentage of the live cells in each group was calculated relative to controls.
Cell Transfection
Cells grown to 90% confluence were transfected with a mixture of the RNAi duplex-Lipofectamine™ RNAiMAX complexes (Invitrogen-Gibco BRL) that contained a final concentration of 20nM RNAi duplex, IRE1siRNA, or Silencer® Select Negative Control #2 siRNA (Applied Biosystems; Foster City, CA) and 5μl Lipofectamine™ RNAiMAX that was added to cells overnight.
RNA Extraction and Quantitative Real-time PCR
Total RNA was extracted from ARPE-19 cells using the RNeasy ®Mini Plus Kit (Qiagen Inc.; Valencia, CA). RNA (2μg) was reverse-transcribed using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). Analyses of selected genes were performed with TaqMan Gene Expression Assays (Applied Biosystems) using the StepOnePlus TaqMan System Fast Mode (Applied Biosystems). Data were analyzed by using the comparative CT method.
Western Blot Analysis
Western blot analysis was performed using our standard technique[9]. Cellular protein was extracted using RIPA buffer containing a protease inhibitor (Sigma-Aldrich, Inc.; St. Louis, MO). Supernatant was centrifuged at 16000Xg for 5 min and lyophilized. The primary antibodies used were mouse monoclonal anti-human CD59 (1μg/ml), CD55 (2μg/ml), CD46 (0.5μg/ml), C3 (recognizes C3a and C3b; 0.4μg/ml), CFI (2μg/ml), CFH (2μg/ml), and CD63 (2μg/ml), all from Abcam; rabbit anti-human PARP 1/1000 dilution (Cell signaling Inc.; Danvers, MA), rabbit monoclonal anti-human GAPDH (0.1μg/ml; Abcam) and β-actin-HRP (0.2μg/ml; Santa Cruz Inc., Santa Cruz, CA). According to the company, the anti-CD46 and anti-CD59 antibodies detect two and three bands on immunoblots, respectively, as previously reported for CD46 using the same antibody [20]. Membranes were then incubated with the appropriate 2° antibody conjugated with horseradish peroxidase (0.1-0.5μg/ml; Abcam). Signal was detected either by chemiluminescence or Supersignal West Femto maximum sensitivity substrate (Thermoscientific Pierce, Rockford, IL). Band intensities were quantified with image J (v 1.42q; NIH, Bethesda, MD), and reported as arbitrary densitometric units.
ELISA measurements of C5a, sC5b-9, Bb, CFD, and C5b-9
Human C5a, Bb plus factor and SC5b-9 level in the medium was determined with a commercial ELISA kit (Quidel), or Human Factor D (R&D Systems; Minneapolis, MN), according to the manufacturer’s instructions.
Double Labeled Immunofluorescence Cytochemistry
The non-centrifuged, unfiltered conditioned medium was fixed and permeabilized with ice-cold acetone, blocked with 10% normal serum from the species of the 2° antibody, and incubated with the first primary antibody of interest. Slides were then incubated with the first secondary antibody labeled with Alexa Fluor® 488, with the second primary antibody, and finally with secondary antibody labeled with Alexa Fluor® 594 (Table S1). DAPI or wheat germ agglutinin (WGA) conjugated to Alexa FluorR 350 was used to stain nuclei or plasma membranes, respectively. Isotype mouse IgG2α, mouse IgM, rabbit polyclonal IgG, and mouse monoclonal IgG1 at equivalent concentrations were used as controls. Slides were imaged with the Axioplan2 imaging system with Axiovision4 software (Carl Zeiss Microimaging, Inc.; Thornwood, NY). Cellular particles for each stain were counted in forty 40X high power fields. Particles from smears were selected if they were in the plane of focus, labeled with either WGA or DAPI to identify cellular elements, and immunostained. The appropriate IgG was used to eliminate nonspecifically labeled particles. Particles were excluded if they appeared as pigment granules, thread shaped, or granular reagent debris with brightfield microscopy.
Statistics
Non-repeated analysis of variance (ANOVA) or unpaired Student’s t-test were used to determine differences between experimental and control mean values using Y-stat software (SAS Institute, Cary, NC). All experiments were performed at least three times (n=3).
Results
CD46 and CD59 Immunolabeling is Altered in the RPE of AMD Samples
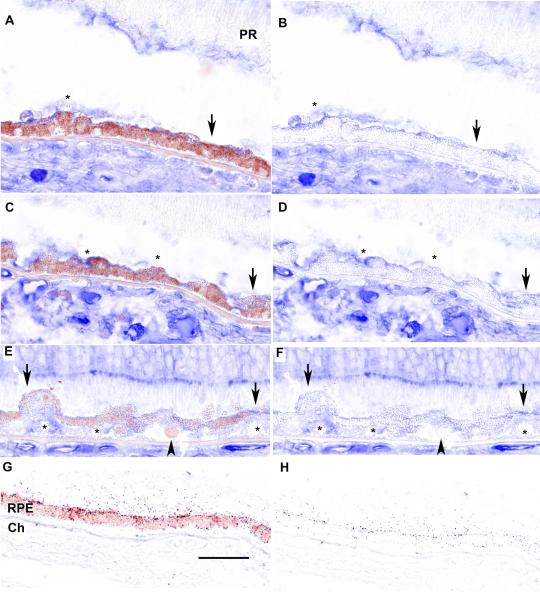
We used unaffected control donors with ages between 19-89 years (mean 68 years), early AMD donors between 60-96 years (mean 77 years), and GA donors between 81-93 years (mean 87 years). In normal human macular and peripheral RPE, distinct CD46 labeling was found at the lateral and basal surfaces of the RPE (Fig. 1A, B). In early AMD, peripheral CD46 labeling remained intact while in the macula, CD46 labeling was reduced at the lateral surface of dysmorphic RPE located adjacent to drusen. The basal line of CD46 persisted in RPE cells overlying small drusen, but it was thinner than that seen in normal areas (Fig. 1C, D). In more advanced, but early disease, such as overlying large drusen, basal CD46 labeling was lost along with the appearance of diffuse, extracellular labeling throughout the drusen (Fig. 1E, F). Table 1 summarizes the CD46 labeling pattern in our cohort of eyes. This reduced labeling pattern correlates well with the reported decreased CD46 in geographic atrophy[21].
Figure 1.
Distribution of CD46 in the RPE of early AMD eyes. A. Peripheral RPE/choroid from a 94 yr old Caucasian female with early AMD. CD46 labeling appears as a prominent line at the basal (arrowhead) and lateral (arrows) surfaces of morphologically normal RPE cells. C. Dysmorphic macular RPE cells, from the same patient, adjacent to small druse with reduced (thinner line) or undetectable CD46 labeling at their lateral surface (arrows). The basal line of CD46 labeling (arrowhead) is preserved, but thinned over the small druse. E. 96 year old Caucasian male with early AMD and a large druse (>500 μm in length). RPE cells overlying the large druse are dysmorphic and the uniform line of lateral (arrows) and basal (arrowhead) CD46 labeling is lost. CD46 labeling is diffusely distributed throughout the druse (*).G. IgG control. B, D, F, H. Same images after RPE melanin pigment is subtracted by Nuance software. Ch, choroid; Dr, druse; RPE, retinal pigmented epithelium. Bar=50μm.
Table 1.
CD46 Immunolabeling of the RPE in early AMD.
Basolateral immunolabeling of the RPE overlying unthickened Bruch’s membrane (BrM) or when overlying drusen (drusen).
| Basolateral CD46 Immunolabeling | ||||||||
|---|---|---|---|---|---|---|---|---|
| Donor | Age (Yrs) | Gender | Race | D-E (Hrs) | Macula |
Periphery |
||
| BrM | Drusen | BrM | Drusen | |||||
|
| ||||||||
|
Unaffected
| ||||||||
| 1 | 19 | M | B | 18 | Yes | NP | No | NP |
| 2 | 54 | F | B | 51 | Yes | NP | Yes | NP |
| 3 | 60 | M | W | 10 | Yes | NP | Yes | NP |
| 4 | 61 | M | W | 30 | Yes | NP | Yes | NP |
| 5 | 77 | F | W | 93 | Yes | NP | Yes | NP |
| 6 | 83 | F | W | 12 | Yes | NP | No | NP |
| 7 | 84 | M | B | 44 | Yes | NP | Yes | NP |
| 8 | 84 | F | W | 75 | Yes | NP | Yes | NP |
| 9 | 89 | M | W | 21 | Yes | NP | No | NP |
|
| ||||||||
|
Early AMD
| ||||||||
| 1 | 60 | M | B | 28 | Yes | No | Yes | NP |
| 2 | 61 | M | W | 20 | Yes | No | Yes | NP |
| 3 | 68 | F | W | 20 | Yes | No | Yes | NP |
| 4 | 69 | F | W | 19 | Yes | No | Yes | No |
| 5 | 87 | M | W | 24 | Yes | No | Yes | NP |
| 6 | 87 | F | W | 70 | Yes | No | No | NP |
| 7 | 91 | F | W | 22 | Yes | No | Yes | No |
| 8 | 94 | F | W | 8 | Yes | No | Yes | NP |
| 9 | 96 | M | W | 5 | Yes | No | No | NP |
D-E, death to enucleation time; NP, Drusen are not present; M, male; F, female; W, white; B: Black.
Macular and peripheral RPE of unaffected eyes, and peripheral RPE of early AMD eyes had minimal CD59 staining. By comparison, staining in the choroidal vascular endothelium was strong among all specimens (Fig. 2A, B). A distinct CD59 RPE labeling pattern was seen in the maculas of AMD eyes that inversely correlated with disease (Table 2). In early AMD, strong CD59 labeling, defined by a wide region of staining, was observed at the apex of morphologically normal RPE and within debris in the adjacent subretinal space (Fig. 2B-D), whereas CD59 labeling was weaker, defined as a thin region of staining, in flattened RPE overlying drusen (Fig. 2C). Some drusen exhibited patchy CD59 labeling. Decreased CD59 was also observed in areas of geographic atrophy, a late stage AMD, when compared to morphologically unaffected areas within the same samples (Fig. 3, Table 3). Reduced CD59 was seen in morphologically preserved RPE cells at the leading edge of geographic atrophy, as well as in dysmorphic RPE cells (Fig. 3).
Figure 2.
Distribution of CD59 in unaffected and early AMD eyes. A. Unaffected eye from a 62 year old African American woman. Macular (left above) and peripheral (right above) RPE do not have CD59 labeling. B. 61-year-old Caucasian woman with early AMD. Macular (left) RPE overlying normal Bruch’s membrane have strong CD59 labeling at the apex of cells. In contrast, peripheral (right) RPE have minimal staining with few CD59 labeled cells. C. When overlying drusen, dysmorphic RPE cells displayed weaker CD59 labeling (red arrows), especially apically, compared to uninvolved adjacent areas (red arrowheads). D. Different area of the same macula with a druse and material in the subretinal space that has CD59 labeling (red arrows). E. IgG control. The lower images show the RPE after melanin pigmented is subtracted with the Nuance software. BrM, Bruch’s membrane; Ch, Choroid; Dr, Drusen; RPE, Retinal pigmented epithelium; Bar=50μm.
Table 2.
CD59 immunolabeling at the apical RPE of early AMD.
Immunolabeling was scored by staining thickness (none, thin, or thick) and intensity (0=no staining, 1=staining less than CD59 labeling of the choriocapillaris endothelium; 2=staining equal or greater than CD59 labeling of the choriocapillaris endothelium). CD59 immunolabeling of subretinal deposits was rated as present (+) or absent (−).
| Donor | Age (Yrs) | Gender | Race | D-E (Hrs) | CD59 Immunolabeling |
|||
|---|---|---|---|---|---|---|---|---|
| Macula | Periphery | |||||||
|
| ||||||||
| Stain thickness |
Stain intensity |
Subretinal staining |
Stain intensity |
|||||
|
| ||||||||
|
Unaffected
| ||||||||
| 1 | 19 | M | B | 18 | None | 0 | − | 0 |
| 2 | 50 | F | W | 3 | None | 0 | − | 0 |
| 3 | 55 | M | B | 4 | None | 0 | − | 0 |
| 4 | 62 | F | B | 18 | None | 0 | − | 0 |
| 5 | 64 | M | B | 65 | None | 0 | − | 0 |
| 6 | 77 | F | W | 93 | Thick | 1 | + | 0 |
| 7 | 83 | F | W | 12 | None | 0 | − | 0 |
| 8 | 84 | M | W | 44 | None | 0 | − | 0 |
| 9 | 87 | F | W | 70 | None | 0 | − | 0 |
|
| ||||||||
|
Early AMD
| ||||||||
| 1 | 60 | M | B | 28 | Thick | 1 | 0 | |
| 2 | 61 | M | W | 20 | Thick | 2 | + | 1 |
| 3 | 68 | F | W | 20 | Thick | 2 | + | 0 |
| 4 | 69 | M | W | 46 | Thin | 2 | + | 1 |
| 5 | 69 | F | W | 19 | Thin | 2 | + | 0 |
| 6 | 87 | M | W | 24 | Thick | 2 | + | 0 |
| 7 | 91 | F | W | 22 | Thick | 1 | + | 0 |
| 8 | 94 | F | W | 8 | Thin | 1 | + | 0 |
| 9 | 96 | M | W | 5 | Thick | 2 | + | 0 |
D-E, Death to enucleation time; M, male; F, female; W, white; B: Black.
Figure 3.
Distribution of CD59 in geographic atrophy in a 91 year old Caucasian woman. A. RPE cells have uniform morphology, but with a thin line of apical CD59 labeling (arrows) compared to early AMD. Subretinal debris labels for CD59(*). Photoreceptors (PR) are truncated. C. RPE cells are dysmorphic with a thin line of apical CD59 labeling (arrows) and CD59 labeling of subretinal debris (*). E. Markedly dysmorphic RPE with thin line of CD59 labeling (arrows). Mild CD59 labeling is now seen in Bruch’s membrane (*). RPE cell that has migrated into the subRPE space (arrowhead) does not stain for CD59. G. IgG control. B, D, F, H. Same images after RPE melanin pigment is subtracted by Nuance software. Ch, choroid; RPE, retinal pigmented epithelium; Bar=50μm.
Table 3.
CD59 Immunolabeling of the RPE in Geographic Atrophy.
Immunolabeling was scored by staining thickness (none, thin, or thick) and intensity (0=no staining, 1=staining less than CD59 labeling of the choriocapillaris endothelium; 2=staining equal or greater than CD59 labeling of the choriocapillaris endothelium). CD59 immunolabeling of subretinal deposits was rated as present (+) or absent (−).
| Geographic Atrophy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Age (Yrs) |
Gender | Race | D-E (Hrs) |
CD59 Immunolabeling |
|||||
| Non-Atrophic zone | Atrophic zone | |||||||||
|
| ||||||||||
| Stain thickness |
Stain intensity |
Subretinal staining |
Stain thickness |
Stain intensity |
Subretinal staining |
|||||
|
| ||||||||||
| 1 | 81 | F | W | <6 | Thin | 1 | + | None | 0 | + |
| 2 | 83 | F | W | <6 | Thick | 1 | + | None | 0 | + |
| 3 | 88 | F | W | <6 | Thin | 1 | + | None | 0 | − |
| 4 | 89 | M | W | <6 | None | 0 | − | None | 0 | − |
| 5 | 90 | F | W | <6 | Thin | 0 | + | None | 0 | − |
| 6 | 91 | F | W | <6 | Thin | 1 | + | None | 0 | + |
| 7 | 93 | M | W | <6 | Thin | 0 | − | None | 0 | − |
|
| ||||||||||
|
Geographic Atrophy
*
| ||||||||||
| Donor |
Age
(Yrs) |
Gender | Race |
D-E
(Hrs) |
CD59 Immunolabeling
|
|||||
| Non-Atrophic zone | Atrophic zone | |||||||||
|
| ||||||||||
|
Stain
thickness |
Stain
intensity |
Subretinal
staining |
Stain
thickness |
Stain
intensity |
Subretinal
staining |
|||||
|
| ||||||||||
| 1 | 81 | F | W | <6 | Thin | 1 | + | None | 0 | + |
| 2 | 83 | F | W | <6 | Thick | 1 | + | None | 0 | + |
| 3 | 88 | F | W | <6 | Thin | 1 | + | None | 0 | − |
| 4 | 89 | M | W | <6 | None | 0 | − | None | 0 | − |
| 5 | 90 | F | W | <6 | Thin | 0 | + | None | 0 | − |
| 6 | 91 | F | W | <6 | Thin | 1 | + | None | 0 | + |
| 7 | 93 | M | W | <6 | Thin | 0 | − | None | 0 | − |
D-E, Death to enucleation time; M, male; F, female; W, White; B, Black.
Courtesy of R. Read, C. Curcio, UAB.
CD59 and CD46 are reduced by OxLDL
To investigate how these regulators are decreased, we used oxLDLs as a relevant complement trigger because they accumulate in Bruch’s membrane prior to drusen formation[9]. OxLDL is often studied at 50-400μg/ml[22-24], which is similar to plasma levels in atherosclerosis[25]. While the concentration of oxLDL in Bruch’s membrane is unknown, oxLDLs in atherosclerotic plaques are 70 times higher than in plasma[26]. Therefore, we tested doses within the range of both plasma and tissue levels. ARPE-19 cells remained viable after exposure up to 400μg/ml oxLDL, where viability was decreased by 55% (p<0.01; Fig. 4). We then explored the effect of oxLDLs on complement expression using 100μg/ml oxLDL, a non-toxic dose. OxLDL treatment did not change CD46 mRNA and decreased CD59 mRNA by 0.6 fold (p<0.05 Fig. S1). CD46 protein was decreased 2.9 fold and CD59 protein by 9 fold with 100μg/ml oxLDL compared to controls (p<0.01; Fig. 5). CD46 and CD59 protein was also decreased by a range of oxLDL doses (50-200μg/m; p<0.01; Fig. 6). We next explored another cell membrane regulator, CD55, and found that while 100μg/ml oxLDL increased its mRNA by 1.7 fold (p<0.01), the protein level did not change. Likewise, CFH mRNA or protein was not influenced by oxLDL treatment (data not shown). LDL treatment did not affect CD46, CD55, CFH, or CD59 mRNA or protein abundance.
Figure 4.

Viability of ARPE-19 cells after being treated with oxLDL. Cells were treated for 24 h with 0-400μg/ml oxLDL. Viability was assessed by the Live-Dead assay. The graph shows the results of 3 independent experiments. **p<0.001.
Figure 5.
CD59 and CD46 are reduced by 100μg/ml oxLDL. A. Representative Western blot shows reduced CD46 in cell lysates after oxLDL treatment. B. Graph shows 2.9 fold decreased CD46 intensity after oxLDL treatment, as quantified using Image J software (n=3 independent experiments). C. Representative Western blot shows reduced CD59 in cell lysates after oxLDL treatment. D. Graph shows a 9 fold decrease in CD59 after oxLDL treatment (n=3 independent experiments). **p<0.01.
Figure 6.
Decreased CD46 and CD59 after 0-200μg/ml oxLDL. A. Representative western blot showing decreased CD46 after 50-200μg/ml oxLDL. B. Graph of decreased CD46 after oxLDL treatment, compared to control (n=3 independent experiments). C. Representative western blot showing decreased CD59 after 50-200μg/ml oxLDL. D. Graph of decreased CD46 after oxLDL treatment, compared to control (n=3 independent experiments). **p<0.01.
Decreased CD59 is Mediated in Part, by Decreased Transcripts
OxLDLs induce ER stress, which can influence protein processing[27]. During the unfolded protein response, IRE1α, an ER-localized ribonuclease and kinase, cleaves mRNAs to reduce the overall protein load[28]. Since CD59 mRNA was decreased by oxLDLs, we tested if IRE1α was induced by oxLDLs. Indeed, 100μg/ml oxLDL increased IRE1α mRNA expression by 1.9 fold (p<0.05) compared to controls (Fig. 7A). We next found that an siRNA to IRE1α, which reduced IRE1α in cells treated with either vehicle or 100μg/ml oxLDL (p<0.01; Fig. 7B), increased CD59 protein 3.2 fold compared to control siRNA (p<0.01; Fig. 7C).
Figure 7.
Attenuation of CD59 is partially mediated by IRE1α. A. Quantitative RT-PCR shows 1.9 fold increased IRE1α expression after treatment with 100μg/ml oxLDL for 24 h (*p<0.05). B. Representative Western blot and graph show effective knockdown of IRE1α after siRNA treatment both with vehicle and 100μg/ml oxLDL exposure, compared to scrambled siRNA control (siRNA CTRL). (n=3 independent experiments). C. Representative Western blot and graph shows a 3.2 fold increase in CD59 after knockdown of IRE1α with siRNA compared to scrambled siRNA control (n=3 independent experiments). **p<0.01.
CD59 and CD46 are Released in Membranous Vesicles after OxLDL Treatment
The incomplete role of IRE1α prompted us to identify an additional explanation for how CD59 is reduced by oxLDLs. We reasoned that it could be released from the cell, and found increased CD59 in the supernatant after 100μg/ml oxLDL compared to controls (Fig. 8A) along with increased CD63, an exosomal marker[29] (Fig. 8B). Contamination of CD59 or CD63 from dead cells was excluded since cells remained viable by the Live Dead assay, and only centrifuged medium was used. Using sequential double immunostaining for CD59 and CD63, we found that 36% of CD59 labeled collections of particles also labeled for CD63 (Fig. 8C).
Figure 8.
Released CD59 are in membranous vesicles. The supernatant of ARPE-19 cells treated with 100μg/ml oxLDL for 24 h was analyzed by Western blot analysis using non-filtered, centrifuged, lyophilized supernatant. A, B. Representative Western blot and graph from 3 independent experiments show increased CD59 (**p<0.01) and the exosomal marker CD63 after oxLDL treatment (**p<0.01). C. Immunofluorescence microscopy of clustered membranous released particles and RPE cell fragments in the supernatant after ARPE-19 cells were treated with oxLDLs. The upper panels show a 10μm diameter cellular fragment that stained with WGA (blue, upper left), CD59 (red; upper middle left panel), exosomal marker CD63 (green; upper middle right panel), and the merged picture indicating colocalization of CD63 and CD59 binding (yellow; upper right). The lower panels show released particle clusters that stain for CD59 (red), CD63 (green), and the merged image of colocalized CD59 and CD63 (yellow) on the cell surface. Bar=5μm.
Membranous blebs can be released from cells during sublethal apoptosis. When cells undergo apoptosis, they express oxidation-specific epitopes on their cell surface, including the oxidized phospholipid, phosphorylcholine (oxPl), which is specifically recognized by the EO6 antibody[30]. Using sequential double immunostaining of the smears prepared from noncentrifuged, unfiltered supernatant, we used this oxPl to identify apoptotic particles and found that 55% of CD59 labeled membranous particles also labeled with the EO6 antibody.
Since CD46 mRNA was unchanged following oxLDL exposure, we focused on the release of membranous particles to explain its decrease. We found that 100μg/ml oxLDL also increased CD46 in the supernatant compared to controls (Fig. 9A). With sequential double immunostaining of supernatant smears, we found that 62% of the CD46 labeled membranous particles colabeled with EO6 (Fig. 9B). We also identified 40% of the CD46 labeled collections of particles labeled for CD63. These data suggest that the decreased cellular CD46 is released in apoptotic blebs and exosomes.
Figure 9.
CD46 is shed into the supernatant after oxLDL treatment. A. Representative Western blot and graph from 3 independent experiments show increased CD46 in the supernatant after 100μg/ml oxLDL (**p<0.01). B. Immunofluorescence microscopy of a 5μm diameter membranous particle that stained for WGA (blue, upper left panel), CD46 (green; upper left middle), oxPl (red; upper right middle), and the merged image of colocalized CD46 and oxPl (yellow; upper right). The lower panels show collections of membranous released particle clusters in the supernatant that were stained for CD46 (green; lower left panel), apoptotic marker oxidized phosphorylcholine (oxPl) which was identified using anti-EO6 IgM antibody (red; lower middle), and the merged image showing colocalization of CD46 and oxPl (yellow; lower right). Bar=5μm.
Impact of Reduced Membrane Regulators by OxLDLs
We tested the extent of alternative pathway activation by oxLDLs. Of factors B, D, H, and I, only factor B was altered by 100μg/ml oxLDL, with a 2 fold mRNA increase (p<0.01) and a 1.7 fold increase in the activation fragment Bb (p<0.01) after oxLDL treatment. We next determined if oxLDLs generated the anaphylatoxins C3a or C5a, which can induce a local pro-inflammatory environment, or the final common pathway leading to C5b-9. OxLDL (100μg/ml) induced C3 mRNA by 1.7 fold (p<0.05) and C3b in cell lysates by 1.5 fold (p<0.05; Fig. 10), but not C3a in the supernatant by ELISA (data not shown, n=3 independent experiments). OxLDLs did not change C5 mRNA or C5a. We next measured C5b-9, but did not detect any in the supernatant or cells after 100μg/ml oxLDL. However, the addition of normal human serum (NHS) with 100μg/ml oxLDL increased C5b-9 formation 27% when compared to vehicle plus NHS (p<0.01). In two independent experiments, a dose-related increase in C5b-9 was seen with 400μg/ml (34%) and 600μg/ml (211%) while heat inactivated serum with 600μg/ml oxLDL reduced C5b-9 complexes to 56%.
Figure 10.
C3b is increased by oxLDLs. A. Representative Western blot from 3 independent experiments shows increased C3b (187 KD) in cell lysates after 100μg/ml oxLDL treatment. B. Graph shows a 1.5 fold increase in C3b after oxLDL treatment. *p<0.05.
To determine if C5b-9 formation promoted apoptosis, we evaluated the cleavage of Poly (ADP-ribose) polymerase (PARP), a 116kDa protein that is cleaved by activated caspase 3[31-33]. Cells treated with 100-400μg/ml oxLDL did not induce PARP cleavage unless supplemented with NHS. Mild PARP cleavage was seen after cells were treated with NHS and 100μg/ml oxLDL (not shown). PARP cleavage increased in a dose dependent fashion with 200μg/ml (2.0 fold), 400μg/ml (2.1fold), and 600μg/ml (3.4 fold), while 600μg/ml with heat inactivated serum attenuated the abundance of the 89 kDa PARP fragment by 30% when compared to 600μg/ml oxLDL with NHS (Fig. 11).
Figure 11.
OxLDLs sensitize RPE cells to PARP cleavage upon exposure to serum. Representative Western blot from 3 independent experiments shows 200-600μg/ml oxLDL with the addition of 25% NHS increased PARP cleavage. Heat inactivated (HI) NHS attenuated the abundance of the 89 KD fragment.
Discussion
Herein, we show altered distribution of complement regulators CD46 and CD59 in the RPE of early and advanced AMD eyes. The CD59 labeling pattern suggests both a protective and impaired response that inversely corresponds with classic AMD changes. In early AMD, CD59 labeling was strong at the apex of morphologically normal macular RPE and in the adjacent subretinal space, compared to the RPE in the periphery and in normal maculas, which suggests a protective CD59 response to a complement stimulus at the RPE-photoreceptor junction. In the same samples, CD59 labeling was reduced in dysmorphic RPE overlying drusen, and in geographic atrophy. Together with reduced CD46 labeling in affected RPE of early AMD or in geographic atrophy[21], and the recent observation that CFH is also decreased in early AMD and geographic atrophy [34], we suggest that the impairment of multiple regulators, and not any single complement factor, is associated with features of AMD.
We used oxLDLs, which accumulate in drusen[9], as a relevant complement trigger, and found marked reduction in CD46 and CD59, but no change in CFH by RPE cells. This result suggests that if CFH is an important regulatory element for the RPE, it likely originates from either the choroid[17] or the circulation[35], and must transit through Bruch’s membrane to reach the RPE. In Bruch’s membrane, CFH mediated CFI cleavage of C3b is regulated in part, by heparan sulfate proteoglycans in the RPE basement membrane[36,37]. It is logical then, that the RPE has developed a formidable set of local regulators rather than relying solely upon factors that must pass through Bruch’s membrane, especially since Bruch’s membrane undergoes a change in the heparan sulfate proteoglycan content [38] and accumulates lipoproteins which impair diffusion with aging and AMD[39].
Oxidative stress can decrease complement regulators[40,41]. Thurman et al. found that H2O2 reduced surface expression of CD55 and CD59, but a mechanism for this decrease was not investigated[41]. Our experiments indicate several possibilities for decreasing membrane regulators after a complement trigger. We found that CD46 and CD59 were both shed as a combination of exosomes and sublethal apoptotic blebs. Exosomes are distinct particles from apoptotic blebs, and have a physiological role that depends upon their molecular content. In early AMD, we propose that following an oxidized lipid stimulus, CD59 at the apical RPE is released on exosomes into the adjacent subretinal space to regulate the complement response or serve as an avenue for cell-cell communication[16]. It is also possible that if the RPE has entered apoptosis, CD59 is released in sublethal blebs. Likewise, it is plausible that the RPE releases CD46 in exosomes or if the cell has entered apoptosis, on sublethal blebs into Bruch’s membrane. This mechanism might explain why CD46 and CD59 was decreased on the RPE, along with the appearance of CD59 in the subretinal space or CD46 within drusen in AMD[18]. Alternatively, transmembrane proteins like CD46 can be removed from the cell membrane by endocytosis or cleaved by MMPs 3, 8, and 9 as well as ADAM10[20,42,43]. The GPI-anchored proteins can also be cleaved and released by phospholipases C and D from the cell surface[44].
CD59 was also degraded by an IRE1α mechanism. Since oxLDLs can induce the unfolded protein response, and since IRE1α has ribonuclease activity, we tested and found that oxLDLs increased IRE1α, and that IRE1α knockdown partially restored CD59 levels. CD59 contains a consensus sequence (CUGCAG) that can be cleaved by IRE1α under cell free conditions, independent of ER stress[45]. Alternatively, IRE1α can process RNA by a regulated IRE1-dependent decay, but only under ER stress conditions[46]. Future studies will be needed to define whether CD59 mRNA is cleaved in RPE cells during ER stress.
CD59 is the major regulator of C5b-9 formation[47]. It follows that the oxLDL mediated reduction in CD59, in concert with decreased CD46, would promote final pathway activation with C5b-9 formation and eventually RPE cell death. RPE cells produced enough C5b-9 complexes to initiate PARP cleavage by activated caspase 3 [31-33], only when human serum was added. This result suggests that the RPE alone is unable to synthesize all of the components in sufficient quantities to activate the final pathway following a trigger such as oxLDLs, and would need additional factors from another source. In support of combined local and systemic complement contribution, Scholl et al. found increased complement factors in the serum of patients with AMD[48]. Importantly, since it is one of the most abundant proteins in plasma[35], CFH would be expected to limit complement activation after the addition of serum to RPE cells. The progression of C5b-9 with apoptosis highlights the important regulatory role of CD46 and CD59 on complement activation despite abundant CFH in the serum.
We note that the exact mechanism of cell death induced by oxLDL treatment was not fully characterized. While complement activation has traditionally been thought to kill cells by membrane destabilization or necrosis, C5b-9 complexes can induce apoptotic events such as a massive Ca2+ increase and a rapid loss of inner mitochondrial membrane potentials[49], morphological changes that are typical of apoptosis[50], and DNA fragmentation[51]. This research suggests that characterizing complement mediated cell death as purely apoptosis or necrosis is too simplistic, as reviewed in[52]. Our results indicate that apoptosis is partly involved in cell death because PARP is cleaved by activated caspase 3[31-33].
Further studies are needed to delineate the exact mechanism of how oxLDLs promote the release of CD46 and CD59 particles, and to test the effect of reduced CD46 or CD59 in vivo. Eventually, we want to determine the exact impact of decreased CD46 and/or CD59 on the development of AMD changes, such as drusen formation or RPE cell death. Even with intensive study by a number of laboratories around the world, a complete understanding of how the genetic variant of CFH causes AMD pathology has remained elusive. Our results indicate that membrane regulators, such as CD46 and CD59, are necessary for appropriate complement control after a trigger such as oxLDLs. From these results, we have developed the hypothesis that AMD pathology caused by the CFH variant requires other complement regulator impairment, such as CD46 and CD59 from a stressor such as oxLDLs, to induce pathologic complement inflammation and render the RPE cell vulnerable to cell death, a documented event in AMD.
Finally, we find translational relevance to our findings. With increased CD59 in early AMD, CD59 could represent a new complement activation biomarker for early disease. With reduced CD46 and CD59 in later stage AMD, new treatment might be targeted toward restoring these regulators. Given the possibility that impairment of multiple complement regulators are necessary to generate pathologic complement activation during AMD, a suitable alternative treatment strategy would reduce the formation or accumulation of oxidatively damaged molecules, such as oxLDLs, before complement regulation becomes impaired.
Supplementary Material
Figure S1. Complement regulator gene expression after 100μg/ml OxLDL treatment. Graph of CD46, CD59, and CD55 gene expression, as measured by Taq man RT-qPCR analysis, shows increased CD59 and CD55, but not CD46. N=3 independent experiments. *p<0.05, **p<0.01.
Acknowledgements
We thank Christian Gutierrez, David O’Brien, Laura Asnaghi, Lei Wang, and Sony Dike for technical support, Cathy Bowes Rickman for valuable advice, and Russell Read, MD and Christine Curcio, PhD, University of Alabama, Birmingham, for providing the geographic atrophy samples. Funding: NIH EY14005 (JTH), EY019904 (JTH), Thome Foundation (JTH), AHAF (JTH), Research to Prevent Blindness Senior Scientist Award (JTH), Unrestricted grant from Research to Prevent Blindness to the Wilmer Eye Institute, NIH P30EY001765 core grant, Robert Bond Welch Professorship, and a gift from the Merlau family and Aleda Wright.
Footnotes
Conflict of interest: Dr. Handa has recently given a scientific talk to Genentech and received an honoraria.
The other authors report no financial interest related to this manuscript.
Statement of author contributions: KBE contributed in study design, data collection, data analysis, data interpretation, literature search, generation of figures and writing of manuscript. NF contributed in data collection and interpretation. MC contributed in data collection and interpretation, and study design. JTH conceived and contributed in study design, data analysis, data interpretation, literature search, generation of figures and writing of manuscript.
List of online supporting information: Supplemental Table S1. Antibodies used for immunofluorescence cytochemistry
References
- 1.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44:1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 2.Dunaief JL, Dentchev T, Ying GS, et al. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 3.Del Priore LV, Kuo YH, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci. 2002;43:3312–3318. [PubMed] [Google Scholar]
- 4.Spaide RF, Ho-Spaide WC, Browne RW, et al. Characterization of peroxidized lipids in Bruch’s membrane. Retina. 1999;19:141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92:615–627. [PubMed] [Google Scholar]
- 6.Malek G, Li CM, Guidry C, et al. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CM, Chung BH, Presley JB, et al. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 8.Curcio CA, Presley JB, Malek G, et al. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Tian J, Yang Y, et al. Oxidized Low Density Lipoproteins Induce a Pathologic Response by Retinal Pigmented Epithelial Cells. J Neurochem. 2008;105:1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- 10.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 11.Edwards AO, Ritter R, Iii, Abel KJ, et al. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 12.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haines JL, Hauser MA, Schmidt S, et al. Complement Factor H Variant Increases the Risk of Age-Related Macular Degeneration. Science. 2005 doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 14.Klein RJ, Zeiss C, Chew EY, et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science. 2005 doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson JP, Goodship TH. Complement factor H and the hemolytic uremic syndrome. J Exp Med. 2007;204:1245–1248. doi: 10.1084/jem.20070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elward K, Griffiths M, Mizuno M, et al. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280:36342–36354. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 19.Vogt SD, Barnum SR, Curcio CA, et al. Distribution of complement anaphylatoxin receptors and membrane-bound regulators in normal human retina. Exp Eye Res. 2006;83:834–840. doi: 10.1016/j.exer.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Cole DS, Hughes TR, Gasque P, et al. Complement regulator loss on apoptotic neuronal cells causes increased complement activation and promotes both phagocytosis and cell lysis. Mol Immunol. 2006;43:1953–1964. doi: 10.1016/j.molimm.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Vogt SD, Curcio CA, Wang L, et al. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res. 2011 doi: 10.1016/j.exer.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seibold S, Schurle D, Heinloth A, et al. Oxidized LDL induces proliferation and hypertrophy in human umbilical vein endothelial cells via regulation of p27Kip1 expression: role of RhoA. J Am Soc Nephrol. 2004;15:3026–3034. doi: 10.1097/01.ASN.0000146425.58046.6A. [DOI] [PubMed] [Google Scholar]
- 23.Satirapoj B, Bruhn KW, Nast CC, et al. Oxidized low-density lipoprotein antigen transport induces autoimmunity in the renal tubulointerstitium. Am J Nephrol. 2012;35:520–530. doi: 10.1159/000338484. [DOI] [PubMed] [Google Scholar]
- 24.Hirose K, Iwabuchi K, Shimada K, et al. Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis. 2011;10:1. doi: 10.1186/1476-511X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holvoet P, Mertens A, Verhamme P, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21:844–848. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 26.Nishi K, Itabe H, Uno M, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 27.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 29.van Niel G, Porto-Carreiro I, Simoes S, et al. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 30.Chang MK, Bergmark C, Laurila A, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 32.Tewari M, Quan LT, O’Rourke K, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 33.Oliver FJ, de la Rubia G, Rolli V, et al. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 34.Bhutto IA, Baba T, Merges C, et al. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esparza-Gordillo J, Soria JM, Buil A, et al. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics. 2004;56:77–82. doi: 10.1007/s00251-004-0660-7. [DOI] [PubMed] [Google Scholar]
- 36.Call TW, Hollyfield JG. Sulfated proteoglycans in Bruch’s membrane of the human eye: localization and characterization using cupromeronic blue. Exp Eye Res. 1990;51:451–462. doi: 10.1016/0014-4835(90)90158-q. [DOI] [PubMed] [Google Scholar]
- 37.Kelly U, Yu L, Kumar P, et al. Heparan sulfate, including that in Bruch’s membrane, inhibits the complement alternative pathway: implications for age-related macular degeneration. J Immunol. 2010;185:5486–5494. doi: 10.4049/jimmunol.0903596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadanaka S, Kitagawa H. Heparan sulphate biosynthesis and disease. J Biochem. 2008;144:7–14. doi: 10.1093/jb/mvn040. [DOI] [PubMed] [Google Scholar]
- 39.Hussain AA, Starita C, Hodgetts A, et al. Macromolecular diffusion characteristics of ageing human Bruch’s membrane: implications for age-related macular degeneration (AMD) Exp Eye Res. 90:703–710. doi: 10.1016/j.exer.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Lauer TW, Sick A, et al. Oxidative Stress Modulates Complement Factor H Expression in Retinal Pigmented Epithelial Cells by Acetylation of FOXO3. J Biol Chem. 2007;282:22414–22425. doi: 10.1074/jbc.M702321200. [DOI] [PubMed] [Google Scholar]
- 41.Thurman JM, Renner B, Kunchithapautham K, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284:16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakulinen J, Junnikkala S, Sorsa T, et al. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol. 2004;34:2620–2629. doi: 10.1002/eji.200424969. [DOI] [PubMed] [Google Scholar]
- 43.Van Den Berg CW, De Andrade RM, Magnoli FC, et al. Loxosceles spider venom induces metalloproteinase mediated cleavage of MCP/CD46 and MHCI and induces protection against C-mediated lysis. Immunology. 2002;107:102–110. doi: 10.1046/j.1365-2567.2002.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehto MT, Sharom FJ. PI-specific phospholipase C cleavage of a reconstituted GPI-anchored protein: modulation by the lipid bilayer. Biochemistry. 2002;41:1398–1408. doi: 10.1021/bi011579w. [DOI] [PubMed] [Google Scholar]
- 45.Oikawa D, Tokuda M, Hosoda A, et al. Identification of a consensus element recognized and cleaved by IRE1 alpha. Nucleic Acids Res. 2010;38:6265–6273. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollien J, Lin JH, Li H, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Qiao F, Abagyan R, et al. Defining the CD59-C9 binding interaction. J Biol Chem. 2006;281:27398–27404. doi: 10.1074/jbc.M603690200. [DOI] [PubMed] [Google Scholar]
- 48.Scholl HP, Charbel Issa P, Walier M, et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadimitriou JC, Ramm LE, Drachenberg CB, et al. Quantitative analysis of adenine nucleotides during the prelytic phase of cell death mediated by C5b-9. J Immunol. 1991;147:212–217. [PubMed] [Google Scholar]
- 50.Papadimitriou JC, Drachenberg CB, Shin ML, et al. Ultrastructural studies of complement mediated cell death: a biological reaction model to plasma membrane injury. Virchows Archiv : an international journal of pathology. 1994;424:677–685. doi: 10.1007/BF00195784. [DOI] [PubMed] [Google Scholar]
- 51.Cragg MS, Howatt WJ, Bloodworth L, et al. Complement mediated cell death is associated with DNA fragmentation. Cell Death Differ. 2000;7:48–58. doi: 10.1038/sj.cdd.4400627. [DOI] [PubMed] [Google Scholar]
- 52.Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunologic research. 2011;51:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Complement regulator gene expression after 100μg/ml OxLDL treatment. Graph of CD46, CD59, and CD55 gene expression, as measured by Taq man RT-qPCR analysis, shows increased CD59 and CD55, but not CD46. N=3 independent experiments. *p<0.05, **p<0.01.