Abstract
The purpose of this study was to investigate morphological changes of a released carpal tunnel in response to variations of carpal tunnel pressure. Pressure within the carpal tunnel is known to be elevated in patients with carpal tunnel syndrome and dependent on wrist posture. Previously, increased carpal tunnel pressure was shown to affect the morphology of the carpal tunnel with an intact transverse carpal ligament. However, the pressure-morphology relationship of the carpal tunnel after release of the transverse carpal ligament has not been investigated. Carpal tunnel release (CTR) was performed endoscopically on cadaveric hands and the carpal tunnel pressure was dynamically increased from 10 to 120 mmHg. Simultaneously, carpal tunnel cross-sectional images were captured by an ultrasound system and pressure measurements were recorded by a pressure transducer. It was found that carpal tunnel pressure significantly affected carpal arch area (p<0.001), with an increase >62 mm2 at 120 mmHg. Carpal arch height, length, and width were also found to significantly change with carpal tunnel pressure (p<0.05). As carpal tunnel pressure increased, carpal arch height and length increased, but the carpal arch width decreased. Analyses of the pressure-morphology relationship for a released carpal tunnel revealed a nine times greater compliance than that previously reported for a carpal tunnel with an intact transverse carpal ligament. This change of structural properties as a result of transecting the transverse carpal ligament helps explain the reduction of carpal tunnel pressure and relief of symptoms for patients after CTR surgery.
Keywords: carpal tunnel, pressure, morphology, release
1 Introduction
The carpal tunnel is a fibro-osseous structure composed of the carpal bones and numerous inter-carpal ligaments. The transverse carpal ligament (TCL) forms the volar aspect of the carpal tunnel and serves as the pulley for the flexor tendons1. Also, it provides stability to the bony arch of the carpal tunnel2 and acts as an anchor for the thenar and hypothenar muscles.3–4 As a biomechanical constraint of the carpal tunnel, the TCL may potentially compress the median nerve, leading to carpal tunnel syndrome. Carpal tunnel release (CTR) is routinely performed to transect the TCL as a treatment of carpal tunnel syndrome.
The morphology of the carpal tunnel has been found to be affected by transection of the TCL. MRI studies of the carpal tunnel have shown an increase in carpal tunnel volume and cross-sectional circularity after CTR.5–7 Notably, the volar carpal cross-sectional area of the carpal tunnel has been found to increase by 3.6 times after TCL transection.8 In addition, some studies have reported carpal arch width widening after CTR,2, 9 but this morphological change remains controversial because subsequent studies have found no significant change in carpal arch width during postoperative follow-up.5–6, 10
Carpal tunnel pressure in patients with carpal tunnel syndrome is higher than in healthy individuals.11–12 Despite the association between elevated pressure and carpal tunnel syndrome, the exact cause of the pressure elevation is unclear. A factor to consider is the structural mechanics of the carpal tunnel because a relatively rigid carpal tunnel is needed for pressure elevation to occur.13 Previous studies have examined the compliance of carpal tunnel between males and females14 or among humans and various animals determining that the human carpal tunnel was the least compliant.13 Another compliance study examined the influence of carpal tunnel pressure on carpal tunnel morphology and found that as carpal tunnel pressure increased, the carpal tunnel area increased and the cross-sectional shape of the carpal tunnel became rounder.15 These previous studies have investigated the compliance of the carpal tunnel with an intact transverse carpal ligament. However, the compliance of the carpal tunnel after CTR has not been investigated, despite knowledge that the carpal tunnel structure is compromised after CTR.
Even though CTR is a commonly performed surgical procedure to relieve the symptoms of carpal tunnel syndrome, its biomechanical mechanism and effectiveness remain unclear. Therefore, the purpose of this study was to investigate the morphological changes of a released carpal tunnel in response to increasing carpal tunnel pressure. We hypothesized that the carpal arch height and area would increase as carpal tunnel pressure increased. We further hypothesized that as the carpal tunnel pressure increased, the carpal arch width would decrease and that the edges of the transected TCL would further separate, therefore increasing the carpal arch height and area.
2 Methods
Preparation of Cadaveric Specimens
Nine fresh frozen cadaveric specimens (male; right hand; 56.4 ± 6.7 years old), with no history of hand and wrist injury or degeneration, were used in this study. Each specimen was thawed at room temperature prior to dissection and experimentation. An incision was made at the distal palmar crease to transect the flexor tendons and the median nerve. Another incision was made 5 cm proximal to the distal wrist crease and the carpal tunnel contents were removed through this incision. Then, all incisions were sutured (Ethicon 2-0, Ethicon, Somerville, NJ) and glued (The Original Super Glue®, Superglue Corp., Rancho Cucamonga, CA) to ensure a complete closure of the skin and superficial soft tissue.
Endoscopic carpal tunnel release was performed using a single-portal technique (SmartRelease ECTR System, MicroAire, Charlottesville, VA) with the aid of an endoscopic video camera system (Stryker Endoscopy, Kalamazoo, MI). An incision of 1 cm was made at the proximal wrist crease between the flexor carpi radialis and the flexor carpi ulnaris. The blade of the endoscopic carpal tunnel release system was placed into the tunnel, aimed toward the ring finger, and divided the TCL in the distal to the proximal direction.
Experimental Procedures
Following endoscopic carpal tunnel release, each specimen was splinted in a supine, neutral position and secured to a metal plate using Velcro® straps. The distal ends of a pressure transducer (SPC 450, Millar Instruments Inc., Houston, TX) and catheter tubing were inserted into the lumen of the carpal tunnel, free from surrounding soft tissue, via the endoscopic carpal tunnel release incision. Then, this incision was sutured and glued to seal the skin and superficial soft tissue, as well as secure the placement of the pressure transducer and the catheter tubing.
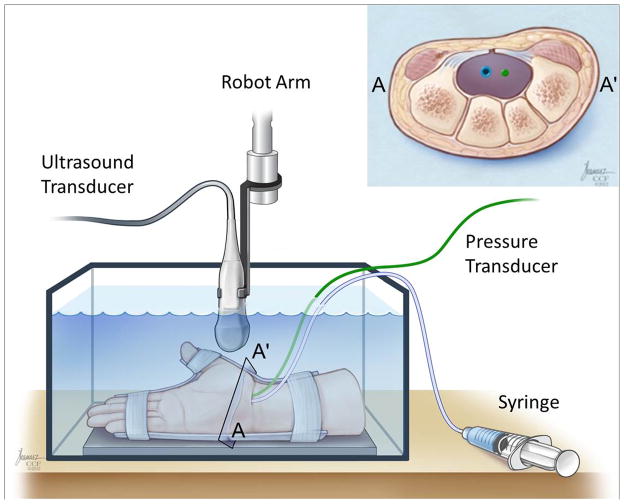
Cross-sectional images of the carpal tunnel were acquired using an ultrasound system (Acuson S2000, Siemens Medical Solutions USA, Inc., Mountain View, CA). A linear array transducer (18L6 HD, Siemens Medical USA Inc., Mountain View, CA) and the specimen were submerged in a tank of room-temperature water (Figure 1). The ultrasound transducer was positioned in the water at the level of the hook of the hamate and the ridge of the trapezium using a six-axis robot (Denso Corporation, Kariya, Aichi, Japan). A distance of approximately 1.5 cm between the ultrasound transducer and the volar side of the specimen provided an adequate water interface for ultrasound imaging and ensured that the ultrasound transducer would not make contact with specimen during experimentation. The ultrasound system was operated in 2D mode with tissue harmonic imaging and tissue equalization. The imaging frequency was 12 MHz and with an image field depth of 4.5 cm. Ultrasound videos were recorded at a rate of 30 fps.
Figure 1.
A schematic of the experimental set-up and a cross-section of the evacuated carpal tunnel with pressure transducer and catheter tubing in the tunnel. A–A′ indicates to the location of cross-section at the hook of the hamate level.
Carpal tunnel pressure was regulated by manual infusion of dyed water through the catheter tubing using a syringe and measured by the pressure transducer. The carpal tunnel pressure was increased to 120 mmHg at a rate of 3.33 mmHg/s and no leakage of dyed water was observed from the skin incisions. A maximum pressure of 120 mmHg was chosen because carpal tunnel pressure can surpass 100 mmHg during hand activities, even after CTR.16–18 A custom LabVIEW (National Instrument, Austin, TX) program was used to record the pressure measurements from the pressure transducer at a frequency of 100 Hz and provided synchronization of the pressure data with the ultrasound video clips. After completing the experiment, each specimen was dissected to confirm that the TCL was divided completely.
Data analysis
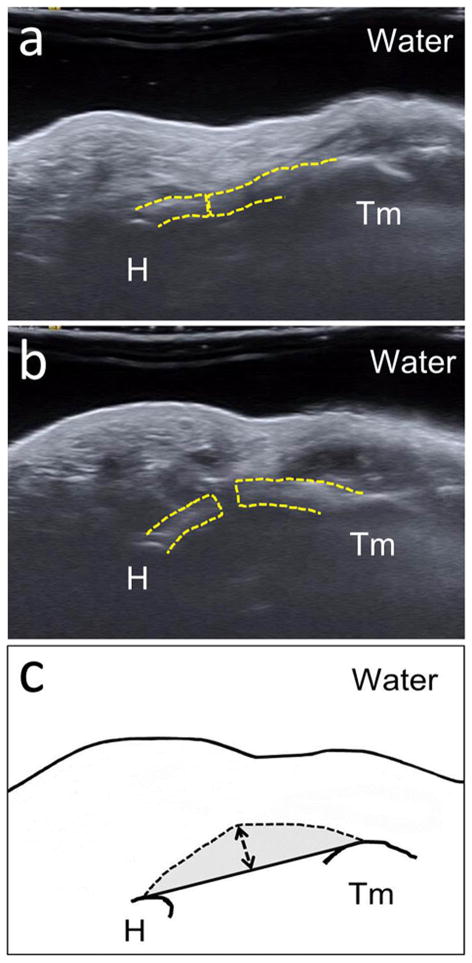
Each ultrasound video clip was decomposed into individual frames corresponding to discrete pressure levels in 10 mmHg increments from 10 to 120 mmHg (Figure 2a–b). The baseline pressure of each specimen was variable, ranging ~ 2 to 4 mmHg. Therefore, the baseline pressure level was excluded because inter-specimen comparisons could not be made. The outcome measures at 10 mmHg were used as references for the quantification of relative changes at the other pressure levels because physiological carpal tunnel pressure is approximately 10 mmHg.12 A custom LabVIEW program was developed to automatically track two region of interest boxes with the centroid of each box placed at the most volar aspect of the hook of the hamate and the ridge of the trapezium. The regions of interest were automatically tracked based on pixel intensity by initially defining a region of interest and then using this same region for tracking in subsequent images. The centroid coordinates of each region of interest box were extracted for each pressure level. The volar aspect of the TCL was manually traced one investigator using the multi-point selection tool in ImageJ (v1.43, National Institutes of Health, USA).
Figure 2.
Ultrasound images showing the changes of a released carpal tunnel with separation of the TCL (dotted line) at 10mmHg (a) and 120 mmHg (b) at the level of the hook of hamate (H) and the ridge of the trapezium (Tm). The schematic (c) shows the following carpal arch parameters: carpal arch width (CAW): solid line between the bones, carpal arch length (CAL): dashed line, carpal arch height (CAH): dashed line with arrows and carpal arch area (CAA): gray shading. The carpal arch radius of curvature (CAR), not labeled in this figure, is based on a circular fit of the volar boundary of the TCL.
Using the coordinates of the hook of the hamate, the ridge of the trapezium, and the volar boundary of the TCL, a custom MATLAB program (Mathworks, Natick, MA) was used to calculate multiple parameters including: carpal arch height (CAH), carpal arch length (CAL), carpal arch width (CAW), radius of curvature of the carpal arch (CAR), and carpal arch area (CAA) (Figure 2c). CAH was defined as the longest perpendicular distance between the volar boundary of the TCL and a line connecting the hook of the hamate and the ridge of the trapezium. CAL was calculated as the length of the volar TCL boundary, between the hamate and the trapezium, while assuming a linear interpolation between the two cutting edges of the TCL. The distance between the hook of the hamate and the ridge of the trapezium defined the CAW. All data points on the volar boundary of the TCL between the hamate and the trapezium were least-squares fitted to a circle. The radius of the fitted circle was defined as the CAR. Finally, CAA was calculated as the area enclosed by the volar boundary of the TCL and the line connecting the hook of the hamate and the ridge of the trapezium.
Statistical analysis
One-way repeated measures ANOVAs (SigmaStat, Systat Software, San Jose, CA) were used to analyze each parameter (CAH, CAL, CAW, CAR, CAA) with respect to pressure. A post-hoc Tukey test was used for all pairwise comparisons. Tests were considered significant if the p value was below 0.05. Additionally, stepwise linear regression analyses were performed among pressure and the change in CAA (ΔCAA).
3 Results
Seven specimens (n=7) were used for data analysis. Two tested specimens were excluded from data analysis because for one specimen the ultrasound images were out of plane and for the other specimen the pressure transducer became caught in the surrounding soft tissue. Post-experiment dissection confirmed complete release of the TCL for each specimen. Generally, as carpal tunnel pressure increased the carpal arch of the carpal tunnel changed from a flat to a more convex shape (Figure 3). The data did not pass normality testing, therefore repeated measures ANOVA on ranks was used to analyze each parameter. All measured carpal tunnel parameters were significantly affected by increasing pressure (p<0.05).
Figure 3.
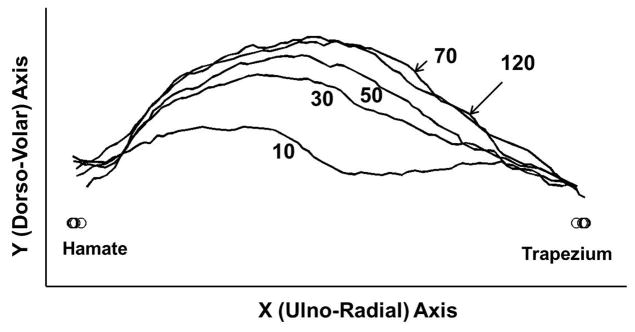
The volar boundary of the TCL at various pressure levels for a representative specimen.
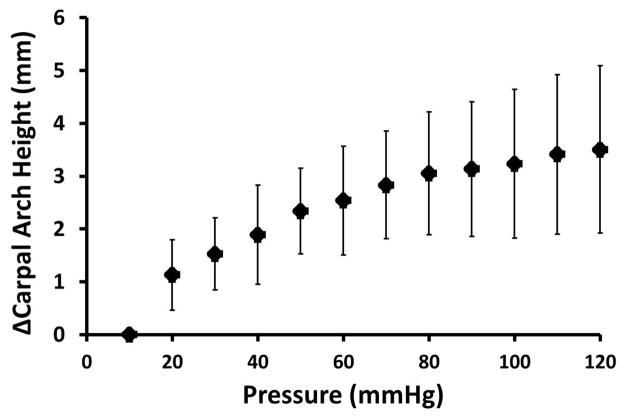
CAH was significantly dependent on pressure (p<0.001), with pressures at and above 70 mmHg having significantly larger CAHs than that at 10 mmHg (p<0.05). CAH increased by a maximum of 3.5 ± 1.6 mm at a pressure of 120 mmHg (Figure 4). CAW was significantly affected by pressure (p<0.05), though the change was relatively small. The CAW decreased from 26.6 ± 1.4 mm to 26.1 ± 1.7 mm as pressure changed from 10 to 120 mmHg. CAL was also significantly affected by pressure (p<0.05) increasing slightly from 27.6 ± 1.5 mm at 10 mmHg to 28.5 ± 1.7 mm at 120 mmHg. Because the transected TCL at 10 mmHg fell down and formed a concave carpal tunnel instead of a convex carpal tunnel, the pressure level of 10 mmHg was excluded from the statistical analysis on CAR. A significant difference occurred for CAR among pressure levels (p<0.05). Specifically, as pressure ranged from 20 to 120 mmHg, the CAR decreased from 30.4 ± 14 mm to 19.7 ± 3.1 mm, respectively, meaning that the carpal arch became more convex. Pairwise comparisons revealed that the CAR at 120 mmHg was significantly smaller (p<0.05) than CARs at 20 mmHg, 30 mmHg (27.6 ± 7.9 mm), and 50 mmHg (24.4 ± 4.9 mm).
Figure 4.
Change in the carpal arch height with increasing carpal tunnel pressure.
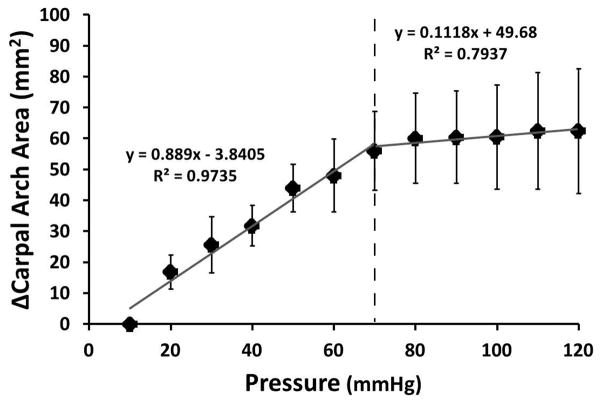
A significant difference occurred for CAA among pressure levels (p<0.001). CAA at 10 mmHg was significantly smaller than those at and above 70 mmHg (p<0.05). Generally, CAA increased at a linear rate of 0.889 mm2/mmHg and then began to plateau at 70 mmHg (ΔCAA = 56.0 ± 12.8 mm2). Within the plateau region, CAA increased only by 6.4 mm2 at a rate of 0.112 mm2/mmHg. The total ΔCAA at 120 mmHg was 62.4 ± 20.2 mm2 (Figure 5).
Figure 5.
Change in the carpal arch area with increasing carpal tunnel pressure. The linear regression lines provided show that the rate of increasing area changes at 70 mmHg (dashed line).
4 Discussion
We found that the pressure-morphology relationship of the carpal tunnel is influenced by the inherent compliance of the carpal tunnel. After CTR, the carpal tunnel pressure decreases and morphological changes of the carpal tunnel have been reported.5–8 This may be an indication that a released carpal tunnel will respond differently to increases in carpal tunnel pressure than a carpal tunnel with an intact TCL. In our results, the carpal arch area at and above 70 mmHg was significantly larger than that of 10 mmHg. We found that the carpal arch area increased by 62.4 mm2, with 90% of the increase in area reached at a pressure of 70 mmHg. This demonstrates a high compliance of the carpal arch at low pressure ranges. The pressure-area relationship of the carpal tunnel with an intact TCL was investigated previously.15 It was found that carpal tunnel area increased as pressure increased, especially the carpal arch area which increased at a rate of 0.1 mm2/mmHg within the pressure range of 10 to 75 mmHg. Our study showed that the carpal arch area changed at a much higher rate of 0.9 mm2/mmHg in a similar pressure range. This implies that the structural compliance for the released carpal tunnel is nine times greater than that for a carpal tunnel with an intact TCL. The high compliance of a released carpal tunnel is clinically important to the understanding of how the carpal tunnel adapts to increases in carpal tunnel pressure. For example, it has been shown that carpal tunnel pressure is highly dependent on the wrist posture16, 19 and the tension of the flexor tendons during hand activities. The relative increases in carpal tunnel compliance and cross-sectional area, after release, may be potential contributing factors to the relief of the carpal tunnel pressure during hand activities.
Increases in carpal arch area can be sensitive to carpal tunnel pressure, but carpal arch area has also been shown to be closely related with other morphological parameters through geometrical modeling.22 According to the model, an independent decrease in CAW or an independent increase in CAL results in an increase of CAA. Similarly, CAH is also dependent on carpal arch width and length.22 In our study, a decrease in CAW of about 0.5 mm and an increase in CAL of 0.9 mm resulted in an increase in CAA by more than 60 mm2 and an increase in CAH by more than 3 mm when the pressure was 120 mmHg. The increases of CAA and CAH in the current study differed from those predicted by the model22 because the carpal arch width and length did not change independently. Rather, a synergistic effect of combined CAW narrowing and CAL elongation occurred.
As pressure increased, the radius of curvature of the carpal arch (CAR) was negatively correlated, decreasing by about 10.7 mm when pressure changed from 20 to 120 mmHg. This indicates that the carpal arch increased in convexity and circularity, corresponding to previous studies.5–8 In addition to pressure, the radius of curvature can be dependent on changes in carpal arch width and length. As the width decreased, the carpal arch became more circular because of the decrease in the radius. Similarly, as the length increased it permitted the height to increase leading to a decrease in the radius of curvature. Overall, the decrease in the radius of curvature of the released carpal tunnel supports why the area of the carpal arch increased with pressure. Therefore, the carpal arch area was dependent on multiple morphological parameters including changes in the width, length, height, and radius of curvature of the TCL boundary.
There are a few limitations in this study. Firstly, a small number of specimens (n = 7) were used in this study for data analysis. However, seven specimens were sufficient for determining that each parameter was significantly affected by carpal tunnel pressure. For example, the power to detect the difference for CAH and CAA, our main parameters of high clinical importance, was 99.9% and 98%, respectively. Secondly, the carpal tunnel contents were removed to prevent their physical contact with the pressure transducer, which could have interfered with pressure measurements. From a biomechanical viewpoint, the experimental variables of carpal tunnel pressure and its outcome measures would not be dependent on whether the carpal tunnel contents were removed or not. Thirdly, the volar boundary of the TCL was used instead of the dorsal boundary to calculate all of the parameters. The volar boundary has been shown to be reliably identifiable in ultrasound images23 and in the current study the tissue-water interface made the dorsal boundary appear thicker and more difficult to trace. Consequently, using the volar boundary to calculate the carpal arch area and height resulted in the inclusion of the TCL thickness and did not represent the amount of space available to the carpal tunnel contents. However, the relative changes reported for these parameters mitigate this limitation as the TCL thickness does not affect the calculation. Also, the injection of water into the tunnel tended to penetrate into the soft tissues via the transected TCL, potentially negating the pressure inside the tunnel. However, we observed that this water penetration occurred at relatively high pressure levels greater than 150 mmHg, therefore data analyses was limited to a maximum pressure of 120 mmHg. Finally, the creep behavior of the carpal tunnel was not considered in this study. In comparison to other imaging modalities such as magnetic resonance imaging and computed tomography, ultrasound imaging allowed for dynamic data collection within a relatively short time period (36 seconds), thus minimizing the influence of the viscoelastic effect on the tunnel.
In conclusion, we investigated the pressure-morphology relationship of the carpal tunnel after carpal tunnel release and found that the released carpal tunnel had a greater compliance than previously reported for a carpal tunnel with an intact TCL. The edges of the transected TCL separated permitting the elongation of the carpal arch length under the condition of increasing pressure. This increase in carpal arch length, along with the decrease in carpal arch width, contributed to the height and convexity increases of the carpal arch. Together, these sequential parameter changes led to an increase in carpal arch area. Therefore, transection of the TCL impacted the changes of the carpal tunnel morphology in response to pressure and contributed to the high compliance of a released carpal tunnel.
Acknowledgments
This publication was made possible by Grant Number R21AR062753 from NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Kline SC, Moore JR. The transverse carpal ligament. An important component of the digital flexor pulley system. J Bone Joint Surg Am. 1992;74:1478–1485. [PubMed] [Google Scholar]
- 2.Garcia-Elias M, An KN, Cooney WP, 3rd, et al. Stability of the transverse carpal arch: an experimental study. J Hand Surg Am. 1989;14:277–282. doi: 10.1016/0363-5023(89)90021-x. [DOI] [PubMed] [Google Scholar]
- 3.Fuss FK, Wagner TF. Biomechanical alterations in the carpal arch and hand muscles after carpal tunnel release: a further approach toward understanding the function of the flexor retinaculum and the cause of postoperative grip weakness. Clin Anat. 1996;9:100–108. doi: 10.1002/(SICI)1098-2353(1996)9:2<100::AID-CA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Kung J, Budoff JE, Wei ML, et al. The origins of the thenar and hypothenar muscles. J Hand Surg Br. 2005;30:475–476. doi: 10.1016/j.jhsb.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Richman JA, Gelberman RH, Rydevik BL, et al. Carpal tunnel syndrome: morphologic changes after release of the transverse carpal ligament. J Hand Surg Am. 1989;14:852–857. doi: 10.1016/s0363-5023(89)80089-9. [DOI] [PubMed] [Google Scholar]
- 6.Ablove RH, Peimer CA, Diao E, et al. Morphologic changes following endoscopic and two-portal subcutaneous carpal tunnel release. J Hand Surg Am. 1994;19:821–826. doi: 10.1016/0363-5023(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Kim TK, Yoon ES, Dhong ES. Postoperative morphologic analysis of carpal tunnel syndrome using high-resolution ultrasonography. Ann Plast Surg. 2005;54:143–146. doi: 10.1097/01.sap.0000143799.88513.47. [DOI] [PubMed] [Google Scholar]
- 8.Kato T, Kuroshima N, Okutsu I, Ninomiya S. Effects of endoscopic release of the transverse carpal ligament on carpal canal volume. J Hand Surg Am. 1994;19:416–419. doi: 10.1016/0363-5023(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 9.Gartsman GM, Kovach JC, Crouch CC, et al. Carpal arch alteration after carpal tunnel release. J Hand Surg Am. 1986;11:372–374. doi: 10.1016/s0363-5023(86)80144-7. [DOI] [PubMed] [Google Scholar]
- 10.Brooks JJ, Schiller JR, Allen SD, Akelman E. Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech (Bristol, Avon) 2003;18:685–693. doi: 10.1016/s0268-0033(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 11.Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63:380–383. [PubMed] [Google Scholar]
- 12.Luchetti R, Schoenhuber R, De Cicco G, et al. Carpal-tunnel pressure. Acta orthopaedica Scandinavica. 1989;60:397–399. doi: 10.3109/17453678909149305. [DOI] [PubMed] [Google Scholar]
- 13.Tung WL, Zhao C, Yoshii Y, et al. Comparative study of carpal tunnel compliance in the human, dog, rabbit, and rat. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28:652–656. doi: 10.1002/jor.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li ZM. Gender difference in carpal tunnel compliance. Journal of Musculoskeletal Research. 2005;9:153–159. [Google Scholar]
- 15.Li ZM, Masters TL, Mondello TA. Area and shape changes of the carpal tunnel in response to tunnel pressure. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29:1951–1956. doi: 10.1002/jor.21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss BC, Agee JM. Dynamics of intracarpal tunnel pressure in patients with carpal tunnel syndrome. J Hand Surg Am. 2010;35:197–206. doi: 10.1016/j.jhsa.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Okutsu I, Ninomiya S, Hamanaka I, et al. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. J Bone Joint Surg Am. 1989;71:679–683. [PubMed] [Google Scholar]
- 18.Seradge H, Jia YC, Owens W. In vivo measurement of carpal tunnel pressure in the functioning hand. J Hand Surg Am. 1995;20:855–859. doi: 10.1016/S0363-5023(05)80443-5. [DOI] [PubMed] [Google Scholar]
- 19.Okutsu I, Ninomiya S, Yoshida A, et al. Measurement of carpal canal and median nerve pressure in patients with carpal tunnel syndrome. Techniques in hand & upper extremity surgery. 2004;8:124–128. doi: 10.1097/01.bth.0000127362.60294.92. [DOI] [PubMed] [Google Scholar]
- 20.Smith EM, Sonstegard DA, Anderson WH., Jr Carpal tunnel syndrome: contribution of flexor tendons. Archives of physical medicine and rehabilitation. 1977;58:379–385. [PubMed] [Google Scholar]
- 21.Tanzer RC. The carpal-tunnel syndrome; a clinical and anatomical study. J Bone Joint Surg Am. 1959;41-A:626–634. [PubMed] [Google Scholar]
- 22.Li ZM, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131:081011. doi: 10.1115/1.3148469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen ZL, Li ZM. Ultrasound assessment of transverse carpal ligament thickness: a validity and reliability study. Ultrasound in medicine & biology. 2012;38:982–988. doi: 10.1016/j.ultrasmedbio.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]