Abstract
In humans, most drug use is initiated during adolescence and adolescent users are more likely to become drug-dependent than adult users. Repeated, high levels of use are required for the transition from use to addiction. Individual levels of drug use are thought to result from a balance between the pleasant or rewarding and the unpleasant or aversive properties of the drug. Repeated high levels of drug use are required for the transition from drug use to dependence. We hypothesized that diminished aversive effects of drugs of abuse during adolescence might be one reason for higher rates of use and addiction during this phase. We therefore tested adolescent and adult CD rats in single-dose cocaine conditioned taste aversion (CTA) at a range of doses (10–40 mg/kg), and examined whether various behavioral markers of addiction vulnerability were correlated to outcome in cocaine CTA. We found that adolescents are indeed less susceptible to cocaine CTA. In fact, age was the predominant predictor of CTA outcome, predominating over measures of novelty-seeking, anxiety, and stress hormone levels, which are all known to be related to drug intake in other models. Furthermore, we found that adolescent rats are also less susceptible to conditioned taste aversion to a low dose of a non-addictive substance, lithium chloride. These results suggest that one explanation for elevated drug use and addiction among adolescents is reduced aversive or use-limiting effects of the drugs. This contributes to our understanding of why adolescence is a particularly vulnerable period for development of drug abuse.
Keywords: Cocaine, Conditioned taste aversion, Rat, Adolescent, Linear correlation, Anxiety, Novelty-seeking, Corticosterone, CRF, Vulnerability
1. Introduction
Most drug abuse in humans begins during adolescence (Chen and Kandel, 1995). Furthermore, adolescents are more likely to progress from use to dependence than adults (Chen et al., 1997) and do so much faster than adults (Clark et al., 1998; Myers and Andersen, 1991). Many have hypothesized that this rapid progression means that adolescence is a critical period of developmental vulnerability, in which the interaction of the drug with a still-developing brain has particularly strong addictive effects.
In addition to age, several other factors play a role in determining which drug users will progress to drug dependence. Personality characteristics, such as high anxiety (Costello et al., 2003), increased novelty seeking/risk taking (Conway et al., 2003; Piazza et al., 1991; Zilberman et al., 2003) and elevated impulsivity (Shoal and Giancola, 2003) are predictive of which individuals are most at risk for development of addiction. Circulating stress hormone levels may also predict development of addiction. Sons of substance abusing fathers, considered “at-risk” for development of substance abuse themselves, have reduced cortisol levels compared to sons of non-drug-abusing fathers (Tarter et al., 1995). Furthermore, low levels of circulating cortisol are predictive of increased aggressive behavior and reduced self-control (Shoal et al., 2003) which are positively correlated with addiction (Shoal and Giancola, 2003).
Another factor in determining whether a user will progress to dependence is the quality of the user's early experience with the drug. Someone who initially finds a drug aversive or not enjoyable is less likely to repeat using it. People who have experienced panic attack associated with cannabis use are more likely to be ex-users (i.e., people who no longer use the drug at all) than current users (who use it regularly or occasionally) (Thomas, 1996). Conversely, someone who likes a drug on his first experience with it may be more likely to abuse that drug. In a study examining a group of poly-drug experienced drug addicts, the degree of liking a drug on the first occasion, as defined in a self-report scale, was the factor most significantly related to subsequent abuse of the drug (Haertzen et al., 1983).
Rodents provide a useful model system for studying behavioral and neurological responses to drugs of abuse. Adolescence in rats is typically defined as occurring between 28 and 42 days of age (Spear, 2000), because behavioral changes, such as peer affiliation and separation from parents, and hormonal and physiological changes, such as puberty and a growth spurt, typically occur during this period. In rats, adolescence has been shown to influence many behavioral responses to drugs of abuse. Adolescent male rats (aged 27 days) are more likely to acquire self-administration of nicotine+acetaldehyde (at concentrations typically found in cigarette smoke) than adult male rats (aged 90 days), although this same study found no effect of age on cocaine self-administration (Belluzzi et al., 2005). Young adult female rats (aged 54 days) self-administer greater amounts of nicotine than older female rats (aged 80 days) (Levin et al., 2003). In addition to higher levels of self-administration compared to adults, adolescent rats also exhibit reduced evidence of aversive drug effects. Several lines of evidence suggest that the potentially aversive effects of the initial drug exposure in rats are age-dependent. Infurna and Spear showed that conditioned taste aversion to amphetamine is reduced in adolescent (35 days old) compared to either juvenile (18 days old) or adult rats (52 days old) (Infurna and Spear, 1979). Nicotine taste aversion after drinking a nicotine-containing solution is also reduced in adolescent compared to adult rats (Shram et al., 2006; Wilmouth and Spear, 2004). Other affective responses to drugs of abuse are also age-dependent. Adolescent rats are more sensitive to the social facilitating effects of ethanol, but less sensitive to the social inhibitory effects of high ethanol doses (Varlinskaya and Spear, 2004a). Adolescent rats are also less sensitive to hangover-induced anxiety after alcohol administration (Doremus et al., 2003; Varlinskaya and Spear, 2004b). Adolescent rats (age 36–40 days) are also less susceptible to physiologic symptoms of nicotine withdrawal than adult rats (aged 73–77 days) (O'Dell et al., 2004). These examples, although they are distributed across different drug classes and behavioral models, demonstrate that adolescents are less sensitive to use-limiting effects of drugs of abuse, and therefore may tolerate higher levels than adults which may facilitate development of addiction.
Several researchers have attempted to model human personality traits in rodents and to assess their relationship to drug intake. For instance, rats that exhibit elevated locomotion in a novel environment are said to model humans who are “novelty seekers.” These rats are also more likely to self-administer amphetamine (Piazza et al., 1991) and nicotine (Suto et al., 2001). Impulsivity is also a predictor of drug intake in rodents as it is in humans. Female rats which exhibit high impulsivity scores in a delay-discounting task acquire self-administration of cocaine more quickly than low-impulsive rats (Perry et al., 2005). Impulsivity measured in a delay-of-reward task also predicts the level of ethanol self-administration (Poulos et al., 1995).
Stress hormone levels in rodents have also been associated with drug-related behavioral responses. Rats which have an elevated corticosterone response to novelty are also more likely to self-administer amphetamine (Piazza et al., 1991). Drug-induced elevation of corticosterone is required for acquisition of amphetamine self-administration (Campbell and Carroll, 2001; Deroche et al., 1997; Goeders and Guerin, 1996; Piazza et al., 1991). This is somewhat counter-intuitive considering the data presented above from human studies. Humans with lower cortisol levels are more at-risk for drug addiction, while rats with an elevated stress hormone response to novelty are more likely to self-administer. It is currently unclear whether the resting level of stress hormones, or the stress- or drug-stimulated level of hormones predicts development of drug abuse. Rodents are a useful model in which to resolve this question, because we can accurately measure hormone levels under controlled conditions before and after drug exposure.
Emerging evidence from animal models suggests that repeated high levels of consumption are required for users to progress to addiction (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). However, as evidenced by bell-shaped dose–response curves for almost all self-administered drugs, most drugs of abuse become aversive (or at least less desired) at high levels (Caine et al., 2002; Doron et al., 2006; Kitamura et al., 2006). We hypothesized that one reason for increased drug use in adolescence might be a lack of sensitivity to aversive effects.
The aversive effects of cocaine are modulated in rats by corticotropin releasing factor (CRF), the central neuropeptide which integrates many behavioral and hormonal responses to stressful stimuli and stimulates the peripheral hormonal response to stress via the hypothalamic–pituitary–adrenal (HPA) axis. Blockade of central CRF receptors enhances the aversive effects of cocaine in conditioned taste aversion (CTA) (Heinrichs et al., 1998). CRF also mediates secretion of adrenal stress hormones by neural stimuli which activate the HPA axis, including cocaine and other addictive drugs (Borowsky and Kuhn, 1991).
The HPA axis is regulated differently in adolescent and adult rodents. Basal corticosterone levels are elevated in adolescent compared to adult mice (Laviola et al., 1999). This elevated baseline is accompanied by an attenuated percentage increase in corticosterone levels in response to psychostimulants (Adriani and Laviola, 2000). Thus, although adolescents operate at a higher baseline, their HPA axis response to psychostimulants may be proportionately blunted. It is unclear whether the percentage change or the peak level achieved is more important for development of drug addiction, but either of these types of developmental variation could contribute to developmental variation in the effects of cocaine.
In the present study, we have examined whether age, locomotor response to novelty, anxiety-like behavior, and circulating stress hormone levels are correlated with degree of aversion in a rat's initial experience with cocaine. We hypothesized that younger rats and those with behavioral traits that have previously been suggested to predict increased self-administration of psychostimulants (anxiety, locomotor response to novelty, and stress hormone level) would show reduced cocaine CTA. We observed, however, that of these predictors, age was the predominant factor.
2. Methods
2.1. Subjects
Male rats of the CD strain (a Sprague–Dawley derivative) were obtained from Charles River Laboratories (Raleigh, NC) and habituated to our housing colony for 7 days before the start of each experiment. Naïve rats were used in each experiment. “Adolescent” rats were 28 days of age, “adult” rats were 64–66 days of age at the beginning of each experiment. Twenty-eight days of age is early adolescence in rats; 65 days is early adulthood (Spear, 2000). Rats were housed 2 or 3 per cage with food and water available ad libitum and maintained on an 11:13 h light/dark cycle (lights on at 06.30). All procedures were performed under the approval of Duke University's Animal Care and Use Committee.
2.2. Conditioned taste aversion
Rats were water deprived for 24 h and then placed in individual cages and allowed to drink from a pre-weighed water bottle for 15 min. They were returned to the home cage with ad libitum access to water for 24 h. The following day, they were water deprived for 24 h, and then given 15 min access to 0.2% saccharin solution. After the 15-min access, they were injected intraperitoneally with the indicated dose of cocaine, lithium chloride (LiCl), or saline and returned to their home cages with ad libitum access to drinking water for 24 h. On the final day, the rats were water deprived for 24 h before being given 15 min access to two bottles–one containing water and the other containing 0.2% saccharin solution. The water bottle was always placed on the right side of the cage and saccharin on the left, to generate both a place and taste association. The intake of all fluids was measured by weighing the bottles before and after each session. The dependent measure of interest is the “percent saccharin choice” on the test day, defined as:
Rats that consumed less than 1 mL of total solution on either the water day, saccharin day, or test day were excluded from further analysis. Twelve rats (out of 138 total) were dropped for this reason from various treatment groups (Adults: 1 saline; 1 cocaine 10 mg/kg; 2 cocaine 40 mg/kg; 1 LiCl 19 mg/kg; 1 LiCl 76 mg/kg. Adolescents: 3 before drug treatment; 2 LiCl 76 mg/kg; 1 cocaine 20 mg/kg). CTA experiments were performed between 1 and 3 pm.
2.3. Elevated plus maze
The elevated plus maze task is used to measure anxiety-related behavior (Pellow et al., 1985; Pellow and File, 1986). The maze is made of sealed wood arranged in a “+” shape which is elevated 90 cm above the floor. Two of the arms (north and south) are open and are 50 × 10 cm. The other two arms (east and west) are enclosed with walls that are 36 cm high on the two long sides. The areas surrounding the EPM were made visually neutral by white curtains on all four sides. The room was dimly lit by incandescent light so that the brightness on the open arms was approximately 7 lx, and in the closed arms approximately 3.5 lx. Rats were allowed to habituate to the room by being placed in a Hamilton–Kinder open field apparatus in the same room for 5 min. Locomotor activity, reported as distance traveled in inches, was recorded during this time by infrared beam breakage. The rats were then moved immediately to the plus maze, where a video camera recorded their behavior from above for 5 min using “nightshot” recording mode. Videos were later scored for entries into and time spent in each arm. Percentage of time in the open arms is calculated as:
A rat was considered to be in an arm when all four paws were in that arm. Otherwise, he was considered to be in the center.
2.4. Corticosterone analysis
Blood was collected from the saphenous (leg) vein for analysis of corticosterone levels (Hem et al., 1998). Home cages containing the rats were placed on a heating pad on medium setting for 5–10 min to stimulate blood flow. Rats were then removed individually from the cage and restrained in a Decapicone (Braintree Scientific). The hind leg was shaved using a scalpel blade, then coated with petroleum jelly. A 25-gauge needle was inserted into the vein and quickly removed to allow blood flow outside the skin. Blood was collected in Sarstedt Multivette tubes coated with EDTA and placed on ice. Bleeding was stopped by direct pressure with an alcohol prep pad. The rats were restrained for a total of about 2 min for this procedure. In separate experiments, as indicated, blood was collected from the trunk after decapitation under isoflurane anesthesia and immediately placed on ice. Pilot experiments showed that ACTH and corticosterone levels are similar in blood collected by either method. Since circulating corticosterone levels vary diurnally, all collections were performed between 09.00 and 12.00.
After either blood collection method, the serum was obtained by centrifugation at 3000×g and frozen at −80 °C until analysis of corticosterone levels. Corticosterone levels were determined using the Rat Corticosterone Coat-a-count radioimmunoassay kit from Diagnostic Products Corporation (Los Angeles, CA). Kit instructions were followed exactly.
2.5. Drug injections
Cocaine HCl and lithium chloride (purchased from Sigma) were dissolved in sterile saline (purchased from Sigma). Both drugs were injected intraperitoneally in a volume of 1 mL/kg.
2.6. Experimental design for prescreen experiments
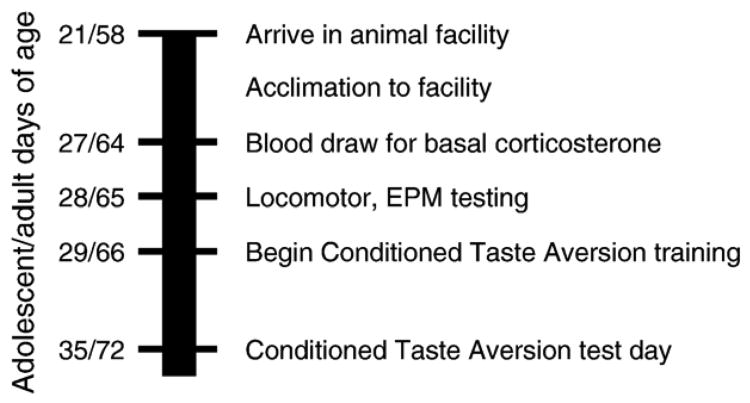
Rats were received at 21 (adolescent group) and 57–59 days of age (adult group) and allowed to habituate to our colony room. On day 27/64 (adolescent/adult group), blood from their saphenous veins was collected for analysis of basal corticosterone levels as described above. On day 28/65, they were placed in the locomotor box for 5 min in a dimly-lit room which also contained the EPM. After recording of behavior in the locomotor box, they were immediately placed on the elevated plus maze and videotaped for 5 min as described above. Beginning on day 29/66, they were tested in conditioned taste aversion as described above. (See timeline, Fig. 1.) A total of 70 rats were used for these experiments.
Fig. 1.
Time line for prescreen experiments. (See Methods.) To the left of the bar are the ages (in days) of adolescent and adult rats (adolescent/adult) at each phase of the experiment. To the right of the bar are the manipulations performed at those days.
To assess the effect of the blood-draw procedure on performance in the EPM, we conducted preliminary experiments in which rats were subjected to the full prescreen (with blood draw) vs. an abbreviated prescreen (open field activity and the EPM). There was no effect of the prior blood draw procedure on locomotor or EPM behavior in these experiments (data not shown). We are aware that performing the locomotor test immediately before the EPM does affect behavior in the EPM (Hogg, 1996; Pellow et al., 1985). Without prior open field exposure, almost all rats spend all of their time in the closed arms. We therefore included the locomotor test immediately before the EPM to increase individual variation in the EPM and create a normal distribution of behavior, which allows us to perform correlation analyses using classical statistical methods.
2.7. Statistics
Analysis of variance (ANOVA) methods were used to test for age, dose, and age×dose interaction effects on percent saccharin choice in CTA experiments. Main and interaction effects were judged significant at the 0.05 alpha level. Post-hoc comparisons among individual effect levels were Bonferroni corrected for multiple comparisons to achieve an experiment-wise 0.05 alpha level.
Pearson's product moment correlation coefficient was used to assess correlation between prescreen variables (percent time in open arms in EPM, distance traveled in open field, and ng/mL corticosterone in serum) and CTA outcome. Prior to analysis, percent saccharin choice was expressed as square root of arcsin of % saccharin choice to more closely approximate a normal distribution. Data were analyzed using Microsoft Excel, Statview and JMP 5.1 software.
3. Results
3.1. Cocaine conditioned taste aversion
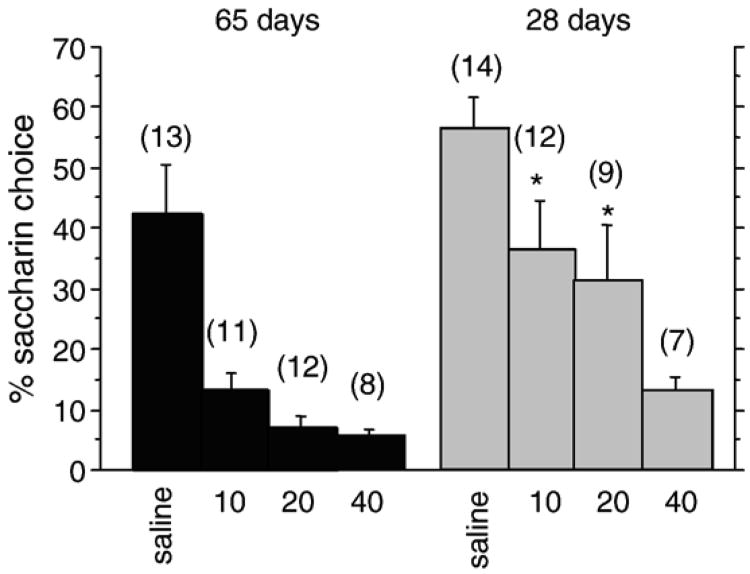
As shown in Fig. 2, pairing cocaine with saccharin-flavored solution caused a dose-dependent reduction in subsequent saccharin choice (age×dose ANOVA, dose effect: F(3,78)=15.773; p<0.0001). Post-hoc testing using Fisher's PLSD revealed that all three cocaine doses resulted in percent saccharin choice that was significantly reduced compared to saline (p<0.0001). There was also a significant effect of age (F(1,78)= 12.890, p= 0.0006), suggesting that CTA was greater in adults than in adolescents. This was confirmed using Fisher's PLSD (p<0.0001). The age×dose interaction effect did not reach significance, however, post-hoc tests revealed that the percent saccharin choice was greater in adolescent than in adult rats at 10 and 20 mg/kg cocaine (at 10 mg/kg: F(1,78)=6.78, p=0.011; at 20 mg/kg: F(1,78) = 6.77, p=0.011). In contrast, after association of saline with saccharin, there was no significant difference between the two age groups. This suggests that there is no age difference in the rats' baseline preferences for saccharin. Also, after association of saccharin with the highest cocaine dose examined, 40 mg/kg, there was also no significant effect of age. Overall, these results suggest that adolescents are more susceptible than adults to the aversive effects of cocaine at low to moderate doses, yet high dose cocaine can be similarly aversive in both ages.
Fig. 2.
Cocaine-conditioned taste aversion. Percent saccharin choice (see Methods) for adult (65 days old) and adolescent (28 days old) rats treated with the indicated doses (mg/kg, i.p.) of cocaine. *, significantly different from adults at same dose, p<0.05. Number per group is indicated in parentheses above each bar.
3.2. Lithium chloride conditioned taste aversion
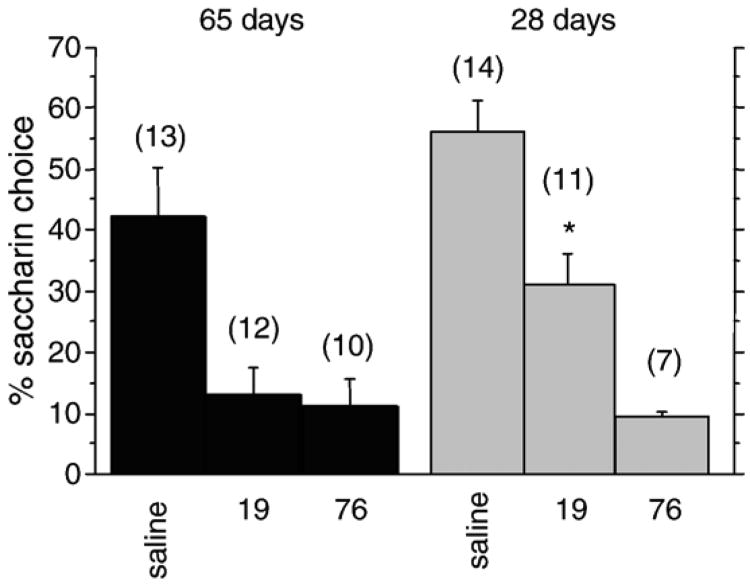
To test whether reduced aversion in adolescence was unique to psychostimulants, we tested CTA to LiCl in adolescent and adult rats at doses which generated similar levels of aversion to the cocaine doses we examined (Jahng et al., 2004) (Fig. 3). At 19 and 76 mg/kg cocaine, there was a significant age×dose interaction effect (F(1,36)=4.43, p=0.0423). There was also a significant dose effect (F(1,36)=5.957, p=0.0197). Post-hoc tests revealed that adolescents exhibited greater saccharin choice than adults at 19 mg/kg (31% vs. 13%; F(1,61)=4,54, p=0.037), but not at 76 mg/kg (9.5% vs. 11%). Thus, adolescents exhibit reduced aversive effects of the lower LiCl dose than adults, similar to our results with cocaine. This result suggests that adolescent rats are generally less sensitive to conditioned taste aversion than adults, regardless of whether the substance is addictive.
Fig. 3.
Lithium chloride-conditioned taste aversion. Percent saccharin choice for adult and adolescent rats at the indicated doses of lithium chloride (mg/kg, i.p.). *, significantly different from adults at same dose, p<0.05. Number per group is indicated in parentheses above each bar.
3.3. Prescreen experiments
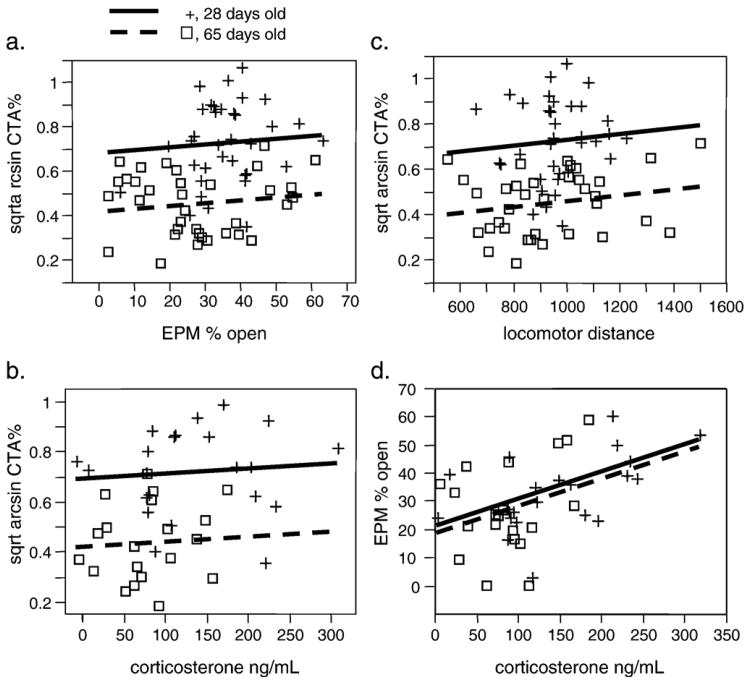
To test whether behaviors known to predict drug intake also predict aversion, we conducted several prescreen tests (basal corticosterone analysis, locomotor activity, and elevated plus maze) as described in methods before testing adolescent and adult rats in cocaine CTA. For these experiments, we performed CTA at 10 mg/kg in all rats, the dose from the dose–response curve in which we observed the largest interindividual variability. In these experiments, age was overwhelmingly the predominant factor in predicting saccharin choice among these factors (F(1,80)=63.181; p<0.0001). There was no correlation between basal corticosterone, open arm time in the EPM, or locomotor response to novelty and CTA outcome in either age group (Fig. 4a, b, and c). When all four factors were included in a multiple regression model, age was the only significant effect (t=5.28; p<0.0001). This replicates our finding of enhanced cocaine CTA in adolescent rats from the previous experiment (Fig. 2), and demonstrates that parameters that have been shown to predict drug intake (anxiety, locomotor response to novelty, corticosterone level) do not predict degree of taste aversion.
Fig. 4.
Correlations between prescreened parameters and conditioned taste aversion outcome (expressed as the square root of the arcsine of the percent saccharin choice, see Methods). (a) Percent open arm time in the EPM is not significantly correlated with CTA outcome. (b) Basal corticosterone levels are not significantly correlated with CTA outcome. (c) Locomotor distance in a novel open field is not significantly correlated with CTA outcome. (d) Basal corticosterone is significantly correlated with percent open arm time in the EPM (R2=0.22, p<0.01). Solid lines and + symbols, adolescent rats (28 days old); dashed lines and open boxes, adult rats (65 days old). Lines represent the least-squares fit for each age. Age effect, p<0.01.
One explanation for the lack of correlation between our prescreened parameters and CTA outcome could be a lack of statistical power. However, there was a significant correlation between basal corticosterone level and open arm time in the EPM (Fig. 4d; R2 =0.22, p<0.01). Thus, we did have sufficient statistical power to detect correlations in this data set. Interestingly, animals with the highest basal corticosterone levels spent the most time in the open arms. This result suggests that these two manifestations of central CRF activity are related to each other, although they are not related to cocaine CTA.
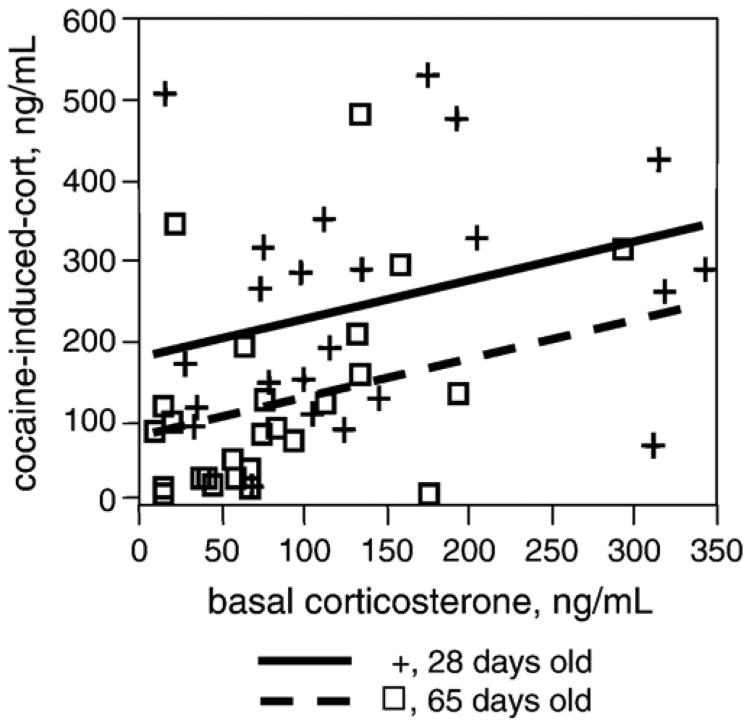
We were surprised by the lack of correlation between endocrine and behavioral manifestations of CRF activity and CTA outcome, since the latter is known to be modulated by CRF (Heinrichs et al., 1998). Therefore, we also tested whether basal corticosterone might predict cocaine-induced elevation of corticosterone. In a separate group of adolescent and adult rats, we sampled blood for basal corticosterone at 28 and 65 days of age. On the following day, we injected the rats intraperitoneally with 15 mg/kg cocaine, decapitated them, and collected trunk blood 45 min later. This time point was chosen because it occurs after the peak corticosterone levels induced by cocaine (30 min) and before levels return to baseline (60 min) and would therefore potentially detect interindividual variation in the duration of corticosterone elevation. Prolonged duration of corticosterone elevation after a stressor, rather than the peak level, has been shown to be a predictor of self-administration of psychostimulants (Piazza et al., 1991). In these experiments, shown in Fig. 5, we found a modest but significant correlation between basal and cocaine induced corticosterone levels (R2 =0.16, p=0.01). Thus, basal corticosterone is correlated to the HPA axis response to cocaine.
Fig. 5.
Basal corticosterone is significantly correlated with cocaine-induced corticosterone levels. R2=0.16, p<0.01. Solid lines and + symbols, adolescent rats (28 days old); dashed lines and open boxes, adult rats (65 days old). Lines represent the least-squares fit for each age. Age effect, p<0.05.
Age also significantly affected the increase in corticosterone after cocaine in this experiment. The level of circulating corticosterone after cocaine administration was greater in adolescents than in adults (F(1,47)=10.478; p=0.0022, Fig. 5). Thus, age is a significant predictor of both the HPA axis response to cocaine and the aversiveness of cocaine in CTA.
4. Discussion
These experiments suggest that adolescence is a time of relative insensitivity to aversive properties of both a psychostimulant and a non-addictive substance. This characteristic has the potential to contribute significantly to adolescent addiction. If adolescents experience fewer aversive effects of cocaine, they could be more likely to take it repeatedly and at higher doses, conditions which are necessary for the progression from drug use to dependence (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). These results extend those reported previously showing that adolescents are also less sensitive to the aversive effects of amphetamine (Infurna and Spear, 1979) and nicotine (Shram et al., 2006; Wilmouth and Spear, 2004). The finding with LiCl suggests that this inconsistency reflects affective processing in general, not just responses to addictive drugs.
Age was also a powerful predictor of cocaine-induced circulating corticosterone release. Adolescent rats' corticosterone was higher than adult rats' after cocaine administration. Our interpretation of this result contrasts with a previous report (Adriani and Laviola, 2000). There are multiple explanations for this discrepancy. First, the previous report examined animals in a novel environment, while we examined animals in their home cages. In our preliminary experiments, we observed that the rise in corticosterone after exposure to the novel environment is so large that it obscures the drug-induced rise in corticosterone in our rats. Another source of discrepancy is that the previous group reported the percentage change from baseline, rather than the absolute level, which we reported. This group also reported, as we do, that adolescents have higher corticosterone levels at baseline than adults. This higher baseline contributed to a reduced percentage increase compared to adults. Currently, it is unknown whether the percentage change or the absolute level is important, therefore we have presented the data in its simplest form.
The increased corticosterone in adolescent rats suggests a role for CRF mechanisms in the diminished CTA in adolescents. Recent findings suggest that corticosterone responses to stress are prolonged in prepubertal rats due to enhanced activation of CRF neurons in the paraventricular nucleus of the hypothalamus (Romeo et al., 2006; Viau et al., 2005). This finding supports a role for enhanced activation of at least one population of CRF containing neurons. The exaggerated HPA axis response coupled with reduced taste aversion in adolescents is consistent with Heinrichs et al.'s observation that CRF is protective against cocaine taste aversion and suggests that increased CRF may mediate the reduced CTA observed in adolescence.
These results contribute to our understanding of adolescence as a unique developmental period. Adolescence has long been recognized as a period of increased risk-taking which is necessary for the transition from the dependence of childhood to the independence of adulthood (Spear, 2000). Our results now suggest that it is also a period of relative protection from the negative affective responses to stressors, possibly due to increased central CRF signaling. Adolescence is a time of increased impulsivity, which can be characterized by decision making involving choices of high risks and low benefits (Chambers et al., 2003). In mice, impulsivity is greater in adolescents in a delayed-reward task (Adriani and Laviola, 2003). Continuing to drink a substance which is associated with illness could be a manifestation of these characteristics in the CTA task. The combined features of increased impulsivity, increased risk-taking, and protection from aversion make adolescence an evolutionarily adaptive period in which to try new things, emerge from maternal care, and become an independent adult.
Another explanation for the age difference in taste aversion could be that the adolescents failed to learn the association between saccharin and the drug's aversive effects. One previous report has shown in mice that adolescents are less likely to exhibit a conditioned place preference for amphetamine, which may be due to a failure to learn the intended association (Adriani and Laviola, 2003). Spear and Brake (1983) reviewed studies on adolescent learning and concluded that periadolescent rats are hyperactive, and therefore perform better than adults in simple active avoidance tasks where their hyperactivity is an advantage. In more complex learning tasks, where hyperactivity is not advantageous, periadolescents perform more poorly than adults. CTA would be an example of a task in which hyperactivity is not an advantage to performance. Thus, one could argue that adolescents exhibited less aversion to the lower doses of cocaine and lithium chloride due to a learning deficit, rather than a reduced aversion to the substances.
The similar age effect in CTA for cocaine and LiCl supports the idea that reduced CTA is a manifestation of reduced cognitive processing of the aversion, since the mechanisms of the two substances are quite different. The aversive effects of LiCl are thought to arise because it causes nausea (Coil et al., 1978). Aversive effects of cocaine, however, are thought to be mediated by its anxiogenic properties (Ettenberg and Geist, 1991; Freeman et al., 2005). Since we obtained similar results with the two substances, it suggests that the cognitive processing of aversive stimuli, not the ability to detect the stressor, differs between adolescents and adults. In fact, neuronal processing of stress is reduced in adolescent compared to adult rats. Amygdala c-fos activation after restraint stress is reduced in adolescent compared to adult rats (Kellogg et al., 1998; Romeo et al., 2006; Viau et al., 2005). This reduced activation in adolescents may also occur in response to cocaine and LiCl and lead to the results we observed.
Another explanation for our observation that CTA is reduced in adolescents compared to adults is suggested by studies examining the development of amygdalo-cortical connections, which undergo extensive maturation during adolescence. Fibertracing experiments have demonstrated that fibers projecting from the amygdala to the cortex begin sprouting around postnatal day 16 in rats, and do not reach maturity until approximately day 65 (Cunningham et al., 2002). Thus, if the aversive properties of cocaine and LiCl stimulate the amygdala, yet the amygdala's fibers do not stimulate the cortex in adolescents, then higher processing centers will not receive the information.
Regardless of the mechanism, a lack of sensitivity to or awareness of aversiveness could contribute to the enhanced use of addictive substances observed during adolescence. If human adolescents fail to learn the link between drug taking and adverse life consequences or undesirable pharmacological side effects, then they are more likely to engage in repeated drug-taking behavior.
It is possible, but not likely, that the age differences we observed in CTA could reflect an age-specific difference in liking the sweet taste of saccharin. If the adolescents liked saccharin more than the adults they might be less willing to give it up after association with the aversive substance. This age difference in saccharin preference is potentially suggested by the data obtained on the pre-association days (not shown). Adolescents drank water and saccharin in equal amounts on the two days, but adults drank significantly more water than saccharin. Thus, adolescents may have greater affinity for sweetness than adults and be less likely to inhibit drinking it after association with an aversive substance. This interpretation contrasts, however, with the percent saccharin choice we observed after saline administration. Both adolescent and adult rats chose saccharin at approximately 50%. This interpretation also contrasts with data summarized by Spear (2000) which suggests that adolescents may be either anhedonic compared to adults, or indifferent to sweet taste. For these reasons, we suspect that the age differences we observed in CTA are not due to differences in their response to saccharin flavor.
Neither the locomotor response to novelty, open arm time in the EPM, nor circulating corticosterone levels predicted outcome in the CTA task with cocaine at 10 mg/kg. This result indicates that these characteristics may be more predictive of rewarding and reinforcing effects of drugs of abuse (Conway et al., 2003; Costello et al., 2003; Piazza et al., 1991; Shoal et al., 2003; Tarter et al., 1999; Zilberman et al., 2003) than of the initial aversive effects examined here.
The predictive power of age for both CTA outcome and cocaine-induced corticosterone secretion indicates that both are developmentally regulated. Although the phenomena that we measured are interrelated, our findings suggest that interindividual variation in these measures is not dictated by the same factors. Different CRF-containing brain regions could contribute differently to each of the behavioral and endocrine measures. CRF in the amygdala is thought to mediate anxiety in the EPM (Koob et al., 1993; Shepard et al., 2000), whereas CRF release in the hypothalamus stimulates the HPA axis cascade, leading to peripheral corticosterone release in a process affected by interindividual variation in testosterone levels (Viau et al., 2005). The brain region(s) involved in CTA is not known. Heinrichs et al. administered CRF antagonists into the lateral ventricle, which could allow the drug to target receptors in multiple brain regions (Heinrichs et al., 1998) and therefore does not suggest a specific anatomic substrate.
These studies demonstrate the feasibility of examining multiple behavioral and endocrine measures in adolescent rodents. We have demonstrated that the stress of having blood drawn does not affect anxiety-related outcome in the EPM, and that we can use a manipulation (locomotor testing) that is known to affect EPM behavior to our advantage. Furthermore, these tests can be done quickly during the brief period of adolescence in the rat to allow for examination of intra and inter-age effects. This screening approach will facilitate future studies of how individual differences in early life may influence adult behavior.
We have shown that adolescents are less susceptible to at least one use-limiting effect of cocaine. Others have shown that adolescents are, in some behavioral paradigms, differentially sensitive to the rewarding properties of drugs of abuse (Adriani and Laviola, 2003; Shram et al., 2006, but see also Schramm-Sapyta et al., 2004). In addition, neurochemical responses to drugs of abuse are dependent upon both age and personality factors (Stansfield and Kirstein, 2005). This balance between rewarding and aversive properties most likely affects which individuals at critical developmental stages will repeat drug use to the point of becoming addicted.
Adolescence is a time of increased risk-taking which is evolutionarily adaptive (Spear, 2000). These studies now suggest that perceived aversive consequences of risk-taking may be reduced in adolescence compared to young adulthood as well. Alternatively, the ability to learn from an aversive experience may be reduced, rather than the severity of the aversive experience. The lack of correlation between the individual characteristics we prescreened and cocaine CTA suggests that developmental stage may contribute to the initial response to substances of abuse more than these individual characteristics. The individual behaviors we examined may be more predictive of use-promoting effects of drugs of abuse and the progression to addiction, rather than use-limiting effects. Future studies will focus on these effects.
Acknowledgments
The authors wish to thank Dr. David Walker and Dr. Melissa Glenn for helpful comments on the manuscript, and Saba Chaudhry for assistance in preparing the figures. This work was supported by DA14931 to CMK.
References
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and D-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–46. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with D-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. Monoamine mediation of cocaine-induced hypothalamo–pituitary–adrenal activation. J Pharmacol Exp Ther. 1991;256:204–10. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, et al. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–88. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology (Berl) 2001;154:311–8. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–7. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB, Davies M. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug Alcohol Depend. 1997;46:53–67. doi: 10.1016/s0376-8716(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–21. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Coil JD, Hankins WG, Jenden DJ, Garcia J. The attenuation of a specific cue-to-consequence association by antiemetic agents. Psychopharmacology (Berl) 1978;56:21–5. doi: 10.1007/BF00571403. [DOI] [PubMed] [Google Scholar]
- Conway KP, Kane RJ, Ball SA, Poling JC, Rounsaville BJ. Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug Alcohol Depend. 2003;71:65–75. doi: 10.1016/s0376-8716(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–44. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants: II. Cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–7. [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doron R, Fridman L, Gispan-Herman I, Maayan R, Weizman A, Yadid G. DHEA, a neurosteroid, decreases cocaine self-administration and reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301013. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–61. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Konaklieva MI, Riley AL. Assessment of the contributions of Na+ channel inhibition and general peripheral action in cocaine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;80:281–8. doi: 10.1016/j.pbb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–48. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11:147–65. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Klaassen A, Koob GF, Schulteis G, Ahmed S, De Souza EB. Corticotropin-releasing factor receptor blockade enhances conditioned aversive properties of cocaine in rats. Psychopharmacology (Berl) 1998;136:247–55. doi: 10.1007/s002130050563. [DOI] [PubMed] [Google Scholar]
- Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim. 1998;32:364–8. doi: 10.1258/002367798780599866. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–5. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Lee JH, Lee S, Lee JY, Kim GT, Houpt TA, et al. N(omega)-nitro-l-arginine methyl ester attenuates lithium-induced c-Fos, but not conditioned taste aversion, in rats. Neurosci Res. 2004;50:485–92. doi: 10.1016/j.neures.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience. 1998;83:681–9. doi: 10.1016/s0306-4522(97)00408-9. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, et al. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 1993;172:277–89. doi: 10.1002/9780470514368.ch14. discussion 290-5. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–9. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Myers DP, Andersen AR. Adolescent addiction. Assessment and identification. J Pediatr Health Care. 1991;5:86–93. doi: 10.1016/0891-5245(91)90096-9. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–74. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88:2088–92. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–4. [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, et al. Stress history and pubertal development interact to shape hypothalamic–pituitary–adrenal axis plasticity. Endocrinology. 2006;147:1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berl) 2004 doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–95. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR. Negative affectivity and drug use in adolescent boys: moderating and mediating mechanisms. J Pers Soc Psychol. 2003;84:221–33. [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2003;42:1101–7. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Neurochemical effects of cocaine in adolescence compared to adulthood. Brain Res Dev Brain Res. 2005;159:119–25. doi: 10.1016/j.devbrainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–80. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Blackson T, Brigham J, Moss H, Caprara GV. The association between childhood irritability and liability to substance use in early adolescence: a 2-year follow-up study of boys at risk for substance abuse. Drug Alcohol Depend. 1995;39:253–61. doi: 10.1016/0376-8716(95)01175-6. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, et al. Etiology of early age onset substance use disorder: a maturational perspective. Dev Psychopathol. 1999;11:657–83. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Thomas H. A community survey of adverse effects of cannabis use. Drug Alcohol Depend. 1996;42:201–7. doi: 10.1016/s0376-8716(96)01277-x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague–Dawley rats. Alcohol Clin Exp Res. 2004a;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004b;1021:459–61. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–46. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Zilberman ML, Tavares H, el-Guebaly N. Relationship between craving and personality in treatment-seeking women with substance-related disorders. BMC Psychiatry. 2003;3:1. doi: 10.1186/1471-244X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]