This unit describes methods for culturing, storage, and maintenance of the Gram-negative, anaerobic Bacteroides species Members of the genus Bacteroides play important roles in human and animal health (Smith et al.,2006). They are the predominate members of the indigenous intestinal flora in humans where they contribute to normal intestinal development, physiology and function. Largely due to their proximity to the human host, several Bacteroides species can be opportunistic pathogens and they are frequently isolated from a range of anaerobic infections including intraabdominal and pelvic abscesses, soft tissue, female genital tract, and bacteremia. Their ability to successfully colonize mammals is due in part to their extended aerotolerance, simple nutritional requirements, and ability to utilize diverse carbohydrate substrates for carbon and energy. All of these factors are important considerations when culturing these organisms. Due to a combination of innate and acquired mechanisms the Bacteroides species are resistant to a wide array of commonly used antibiotics which can complicate antimicrobial therapy of anaerobic infections. Although there has been considerable interest in the mechanisms responsible for the transmissible, acquired antibiotic resistance, it is their innate resistance to aminoglycosides and vancomycin that has proven to be important for development of selective media for their isolation (see Smith et al., 2006).
The single most important consideration when culturing the Bacteroides is that they are obligate anaerobes and they will not divide in the presence of oxygen. That said, they are one of the most aerotolerant anaerobic species known and a recent report (Baughn and Malamy, 2004) has suggested that they may actually slowly divide in the presence of nanomolar concentrations of oxygen. Thus, while it is appropriate that this unit include a discussion of anaerobic culturing techniques, the methods discussed here may not be suitable for many other anaerobic bacteria since they are not as rigorous in their exclusion of oxygen at all stages of media preparation and inoculation. In this regard, it is important to note that anaerobic culturing requires some specialized equipment. It is our intention to describe sufficient equipment options so that those new to the field will be able to easily initiate Bacteroides culturing without a large initial investment in expensive equipment. However, this unit is not intended to be a comprehensive treatise on anaerobic culturing methods and the reader will be directed to several excellent reviews and manuals for detailed descriptions of anaerobic techniques.
CAUTION: Bacteroides are considered Biosafety Level 1 (BSL-1) organisms in the USA although in some European countries the are BSL-2. These organisms are not known to consistently cause disease in healthy adult humans, and are of minimal potential hazard to laboratory personnel and the environment. However, given that some procedures described in this unit require the use of syringes for the transfer of cells, it is recommended that the standards for BSL-2 be followed. See UNIT 1A.1 and other pertinent resources (APPENDIX 1B) for more information.
STRATEGIC PLANNING
Culturing Bacteroides in Liquid Media
Bacteroides cultures must be incubated anaerobically to allow growth, but since they are aerotolerant many manipulations can be done conveniently on the bench top. The choice of whether to work on the bench top or in an anaerobic chamber/glove box depends on the needs of the experiment. Culturing performed entirely in the glove box will result in more consistent growth patterns with shorter lag periods which are necessary for some physiological studies but may be of no consequence when simply inoculating a series of overnight cultures for DNA isolation.
Another important consideration when growing cultures in broth is the type of tube or growth vessel to be used. For experiments in which the culture will remain in the anaerobic chamber or jar, any type of closure that maintains sterility will suffice. However, it often is desirable to grow cultures outside of the chamber due to limited space or the need to frequently monitor growth. In these situations tubes or flasks which can be tightly sealed with a butyl rubber stopper are used. Thus, for long term incubation of culture tubes outside of the glove box the culture tubes must be inoculated in the chamber and then sealed with a sterile rubber stopper (#00 for 13 × 100 mm tubes and #1 for 16 × 150 mm tubes). Screw cap tubes with a rubber cap liner are useful culture tubes and will maintain an anaerobic atmosphere for a short time when removed from the anaerobic chamber. Another type of tube suitable for anaerobic culturing outside of the glove box is the Hungate tube (see internet resources). These are standard screw cap tubes that have a specialized cap (with a 9 mm opening) that is used to hold a flanged, septum type butyl rubber stopper in place in the top of the tube. When using Hungate tubes inoculation and sampling are done directly through the stopper with a syringe or the cap is taken off and the rubber stopper removed with a hemostat. Another advantage of Hungate tubes is that they can be autoclaved with the caps securely fastened (see Support Protocol 5). [*AQ: We have added the preceding sentence based on your earlier response to our queries. Please confirm it is okay as written or edit as appropriate. –TD]
Culturing Bacteroides On Solid Media
Solid media are always prepared outside of the anaerobic chamber for the convenience of pouring agar plates on the bench top and to avoid introducing high levels of moisture into the chamber. The plates are poured in a traditional manner (APPENDIX 4A) and then allowed to dry prior to use or transfer into the anaerobic chamber for storage. Generally resazurin, a redox indicator, is used in media and it will turn from pink to the reduced colorless form about two hours after being put into the chamber. Plates should not be stored outside of the chamber for more than several hours.
In general, spreading or streaking of agar plates is accomplished as described in Appendix 4A with the following modifications. The inoculation of agar plates either by spread plating or streaking usually is performed outside of the anaerobic chamber and plates are transferred into the chamber for incubation. In this regard the procedures for using anaerobic jars are no different that those used with an anaerobic glove box.
Some genetic or physiological studies require that the organisms not be exposed to oxygen at any time due to their extreme oxygen sensitivity or reduced viability. In these cases it is necessary to perform all plating in the chamber. For spread plating experiments, we have made a large number of bent glass rods which are sterilized in beakers covered with aluminum foil and stored in the chamber until needed. Commercially available sterile plastic spreaders also can be used. Streak plates are done with disposable plastic inoculating loops. Several packs of sterile loops are brought into the chamber and used as needed.
When storing or incubating plates in an anaerobic glove box it is important to protect them from the drying atmosphere created by the silica gel desiccant. Plates will dry out in several days but this can be avoided by placing cultures in plastic bags for both storage and during incubation.
Bacteroides are robust organisms and grow rapidly in complex media. In most applications we expect to see colonies forming on plates within 24 hours and they will be large enough to count in 36–48 hours. Bacteroides colonies are distinctive with a smooth rounded morphology, frosty surface and dull white to pale beige color.
Antibiotics Used In the Culturing of Bacteroides
Antibiotics not only play an important role in the management of infectious diseases but they also are critical tools used during the course of genetic and physiological studies of microbes. For example, antibiotic selection of plasmid cloning vectors or for gene disruption techniques are essential applications of antibiotics in modern microbiology but care must be taken in choosing suitable drugs that will work with the Bacteroides. These anaerobes are inherently resistant to a wide range of antibiotics typically used in genetic engineering. Most anaerobes including the Bacteroides are naturally resistant to high levels of aminoglycoside antibiotics and to common β-lactam antibiotics such ampicillin. Thus some of these drugs such as gentamicin and kanamycin would not work well in genetic tools but they are typically used to select for Bacteroides from mixed populations that contain facultative anaerobes. Other drugs such as rifampicin, fusidic acid, and nalidixic acid are effective against Bacteroides but resistant mutants are available for use in different experimental genetic protocols.
The Bacteroides also have acquired antimicrobial resistance in the form of antibiotic resistance genes associated with plasmids and transposons (Hecht, 2006 and Smith et al., 1998). These acquired resistance genes have been identified in clinical isolates and they are commonly employed in most of our genetic tools. The most frequently used drugs are tetracycline (tetQ) and erythromycin (ermF) one or the other of which are found on most of the cloning/shuttle vectors. These drug resistance markers also have been used successfully in transposon mutagenesis strategies and for targeted gene inactivation. The drug cefoxitin (3rd generation cephalosporin) also has found some use in genetic engineering applications since the discovery of a gene, cfxA, encoding a β-lactamase which degrades it. The carbapenem drugs one of the “drugs of choice” in treating Bacteroides infections and resistance is rare (Hecht, 2006). Although though several carbapenem resistance genes encoding metalo-β-lactamases have been identified, they have not found widespread use as genetic tools.
Long Term Storage of Bacteroides
Two approaches are used to store Bacteroides strains; they are either flash frozen and stored at −70°C or grown in chopped meat medium and stored in the dark at room temperature. Chopped meat cultures remain viable for more than 10 years but there is some variation in survival depending on the phenotype of the strains. Due to the good longevity and rapid recovery of cells from chopped meat cultures they also are used as “working stocks” for short- or mid-term storage. These cultures will last for months depending on the amount of material taken for each subculturing.
The method of choice for long term storage is to maintain frozen cultures at −70°C. These frozen stocks are prepared using either glycerol or skim milk as the cryoprotectant and they remain viable for at least 20 years without significant loss in viability. The glycerol stocks are easy to prepare and the medium has a longer shelf life compared to the skim milk media but the skim milk is a superior cryoprotectant for Bacteroides. [*AQ: If known, please briefly mention the reason skim milk is a superior cryoprotectant. Also provide a reference for more information, if available. –TD]
Basic Protocol 1
PREPARING LIQUID CULTURES OF BACTERIODIES IN AN ANAEROBIC GLOVE BOX
The following is a typical protocol for starting cultures from a working stock culture in an anaerobic glove box
Materials
Bacteroides strain in chopped meat medium
BHIS or TYG media, 5 ml in 13×100 mm screw cap tubes
Glove box (Support Protocol 3)
Disposable sterile pipette - 1 ml
Disposable antiseptic wipes (Allesiance Antiseptic Towelettes)
6" hemostat
- Prepare a clean work space in the glove box and gather the materials together.It is good practice to bring all glassware, plasticware, pipettes, and other materials into the glove box at least four hours (preferably overnight) prior to use. This is especially important for plasticware which tends to bind oxygen and releases it slowly which can lead to oxidation of the media.
- Arrange two tubes of BHIS media in a rack and fully loosen the caps. Make sure the tubes are well separated in the rack so that there is room to maneuver with the neoprene gloves.TYG medium is a versatile, complex medium that can be substituted for BHIS in situations where glucose is not desirable (also see Critical Parameters and Troubleshooting).
Gently mix the contents of the chopped meat culture, then swab the top of the tube with an antiseptic wipe take off the screw cap and carefully remove the stopper with the hemostat. Place the stopper upside down in a clean location.
- Using a 1 ml pipette remove 0.6 ml from the culture, then lift the cap to one BHIS tube, dispense 0.4 ml of the culture, and then repeat with the second BHIS tube adding 0.2 ml to it.Growth of cultures from chopped meat stocks can vary depending on the age of the culture, types of antibiotics present in the media (Table 1), or other factors. Thus usually two amounts of inoculum are used to optimize the results. If equal growth is observed in both tubes then the one that received the lower inoculum volume is used for subsequent operations.An alternate method to prepare cultures from chopped meat stocks in Hungate tubes is to simply wipe the top of the tube with an antiseptic and then insert a 1 ml syringe (20 gauge needle) into the tube. Remove the indicated amount of culture to transfer to the new tubes‥One also can inoculate broth cultures from plates. The only difference is that disposable inoculating loops are used to pick colonies and then swirled in the tubes to dislodge the cells. These tubes can be vortexed to further break up the cell clumps and ensure even growth in the tube
Screw the caps into place and then replace the stopper on the chopped meat culture making sure that it is well seated in the tube.
- Place the inoculated tubes at 37°C in the glove box incubator. Dense growth (A550 > 0.8) of the cultures should be observed following overnight incubation.Growth will be slower if antibiotics (Table 1) are added to the medium and the generation time varies greatly with different media.
Table 1.
Commonly Used Antibiotics In Genetic Studies
| Antibiotic | Resistance Gene |
Stock Solution (mg/ml) | Working Concentration (µg/ml) |
|---|---|---|---|
| Antibiotics used in plasmid and transposon vectors | |||
| Cefoxitin | cfxA | 20 in dH20, filter-sterilize | 20–25 |
| Chloramphenicol | cat | 25 in 95% ethanol | 25 |
| Clindamycin | ermF | 10 in dH20, filter-sterilize | 5 |
| Erythromycin | ermF | 50 in 95% ethanol | 10–20 |
| Tetracycline** | tetQ | 10 in 70% ethanol | 4–10 |
| Other useful antibiotics | |||
| Gentamicin | 50 in dH20, filter-sterilize | 50–200 | |
| Nalidixic Acid* | 20 in 0.1 M NaOH | 40–50 | |
| Rifampicin** | 20 in 100% methanol | 20 | |
| Fusidic Acid | 25 in 95% ethanol | 50 | |
| Trimethoprim | add powder to cooled media | 100 |
in the sodium salt form, make stock in dH20 and filter-sterilize
light sensitive and unstable
Basic Protocol 2
PREPARING LIQUID CULTURES OF BACTERIODIES IN AN ANAEROBIC JAR
The following is a typical protocol for starting cultures from a petri plate followed by incubation in an anaerobic jar
Materials
Bacteroides strain streaked on BHIS or TYG plate
BHIS or TYG media, 5 ml in 13 × 100 mm screw cap tubes in rack
Inoculating loop
Bunsen Burner
Anaerobic jar (Support Protocols 1 and 2)
Dispense freshly prepared BHIS media into sterile culture tubes. Gather other materials and cultures.
Sterilize loop, cool, and pick up cells from a large colony.
Remove cap from culture tube, flame lip of tube, and swirl loop in media.
- Replace cap on tube, mix well and place in round test tube rack.Ensure that the caps on the culture tubes are sufficiently loose to allow exchange of gasses.
Place rack in jar and then process jar as described below using GasPak™ gas generating envelopes or as described for the vented jars.
Place jar upright in 37°C incubator.
ESTABLISHING AND MAINTAINING AN ANAEROBIC ENVIRONMENT
There are two fundamental conditions that must be met for reproducible culturing of Bacteroides. That is one must establish a relatively low redox potential in the media and oxygen must be removed from the atmosphere. The redox potential is generally controlled by using freshly prepared media to which a reducing agent, usually cysteine, has been added. Due to the limited capacity of reducing agents, media should be used on the day of preparation unless it can be stored under anaerobic conditions. Immediately upon inoculation of either solid or liquid media, the cultures must be placed under appropriate anaerobic conditions with oxygen concentrations <0.5%. The atmosphere most commonly used for culturing Bacteroides contains 5 or 10% CO2 with the balance made up of N2; however, depending on the system used to generate anaerobic conditions, the atmosphere may also contain 5% or 10% H2 which is utilized by catalyst systems in the removal of oxygen. The hydrogen reacts with oxygen in the presence of a palladium catalyst to form water effectively removing the oxygen from the atmosphere. Investigators should not use H2 concentrations greater than 10% since these are explosive and must be carefully monitored.
The four most common systems used to establish the anaerobic conditions needed for Bacteroides culturing are sealed Brewer’s jars, vented anaerobe jars, anaerobic chambers, and the gassing cannula. These methods apply similar principals of oxygen removal but differ in the implementation of those principals. Each method will be discussed briefly below but the reader is encouraged to review the references provided and the Internet Resources for a more detailed account of the techniques and their theoretical basis.
Support Protocol 1
Sealed Brewer’s Jars
The routine use of sealed anaerobic jars for cultivation of anaerobes was made possible by the development of a self-contained, disposable H2/CO2 generation system and it still remains one of the most cost effective methods in anaerobic microbiology (Brewer and Allgeier, 1966). In these commercially available systems hydrogen is produced when water activates a sodium borohydride tablet; the hydrogen then reacts with oxygen and the palladium catalyst present in the lid of the jar. Carbon dioxide (4–10%) is generated from a sodium bicarbonate/citric acid tablet and an anaerobic atmosphere suitable for growth of Bacteroides species is produced in about 90 minutes.
Materials
Cultures (rack of culture tubes or petri plates)
Polycarbonate GasPak™ Jar or equivalent with lid
Palladium Catalyst (in wire mesh basket attached to jar lid)
GasPak™ Anaerobic System Envelope
GasPak™ Anaerobic Indicator
10 ml tap or dH2O
10 ml pipette or syringe
37°C incubator
NOTE: GasPak™ is a trademark for the Becton, Dickinson and Company (BD). The Oxoid Company also supplies Brewer type jars and anaerobic gas generating envelope systems that work equally well for the growth of Bacteroides species and the Mitsubishi Gas Chemical-America Company supplies gas generating envelopes.
- Fill the wire mesh basket with 2–3 grams of palladium catalyst pellets and attach it to the jar lid.The palladium catalyst must be rejuvenated after each use by heating at 160–170°C for at least two hours. After rejuvenation the pellets should be stored desiccated or in a dry environment. Catalyst pellets can become contaminated by hydrogen sulfide over time and must be replaced. Replacement is indicated when the anaerobic indicator fails to turn colorless within 6 hours.GasPak™ envelopes are now available that have a disposable palladium catalyst sachet attached to the envelope. This avoids the need to monitor the palladium catalyst but greater expense will be incurred.
- Cut off the corner of a GasPak™ envelope and place it upright in the jar together with the rack of petri plates or tubes. Add 10 ml of water to the gas generation packet with a pipette or syringe.When placing any liquid cultures in the jar, the caps of the culture tubes must be loose to allow adequate circulation of the atmosphere. Also, avoid stacking petri plates directly under the catalyst basket since extreme heat is generated during the reaction.Two jar sizes are available. The smaller 2.5 L jar can accommodate one rack of 12 standard petri plates and requires one GasPak™ envelope. The 9.5 liter jar uses three envelopes and can accommodate 36 standard petri plates.
- Open an anaerobic indicator strip and place it in the jar. The strip is saturated with methylene blue and turns from blue to colorless in the absence of oxygen.The strip should begin to turn colorless within 60 minutes of closing the jar and it should be completely colorless within a few hours.
- Quickly place the lid on top of the jar and secure with the lid clamp. Place the jar in a 37°C incubator for 24 to 48 hours. The jar must remain upright at all times in order to avoid spillage of the water.Within an hour of incubation the user can check progress of the anaerobic system by examination of the indicator strip visible through the clear polycarbonate jar and by the appearance of condensation on the inside of the jar indicating the formation of water vapor as the free oxygen is removed. The use of the clear polycarbonate jars also is advantageous since cultures can be inspected periodically without having to open the jar.
Support Protocol 2
Vented Anaerobe Jars
The use of vented anaerobe jars is essentially the same in principal as the sealed Brewer’s jars except that instead of using a gas generating system the atmosphere of the jar is evacuated and replaced with an anaerobic gas mixture, usually consisting of N2 85%, CO2 10% and H2 5%. Any oxygen remaining in the atmosphere is removed by reaction with hydrogen in the presence of a palladium catalyst. The advantage to this method is that an anaerobic atmosphere is generated much more quickly due to the considerably lower concentrations of oxygen remaining after replacement of the jar atmosphere with the gas mixture. The use of vented jars is somewhat more cumbersome and requires a slightly greater initial investment than the sealed Brewer’s jars, but the supply costs remain relatively low since it does not require purchase of the gas generating envelopes.
Materials
Cultures (rack of culture tubes or petri plates)
Polycarbonate GasPak™ Jar or equivalent with lid
Palladium Catalyst (in wire mesh basket attached to jar lid)
Standard vacuum pump with gauge, tubing, and three way stopcock
Gas Mixture cylinder (85%N2, 10%CO2, and 5%H2) and regulator
GasPak™ Anaerobic Indicator
37°C incubator
NOTE: An automatic evacuation/replacement system, Anoxomat, (see Internet Resources) that precisely evacuates and fills jars by a microprocessor controlled pumping unit is now available. The standard Anoxomat configuration has one gas connection and one jar connection but options are available for connecting to up to 5 jars at one time.
- Fill the wire mesh basket with 2–3 grams of palladium catalyst pellets and attach it to the jar lid.The same considerations for care and maintenance of the palladium catalyst exist as stated above.
- Place all cultures in the jar, open an anaerobic indicator strip, place it in the jar, close the lid and secure with the lid clamp.The jars used in this method are essentially the same as those described above except that they are vented with a hose nipple to which is attached a short length of rubber tubing and a screw clamp to control air flow. Commercially available jars all have a vent option.
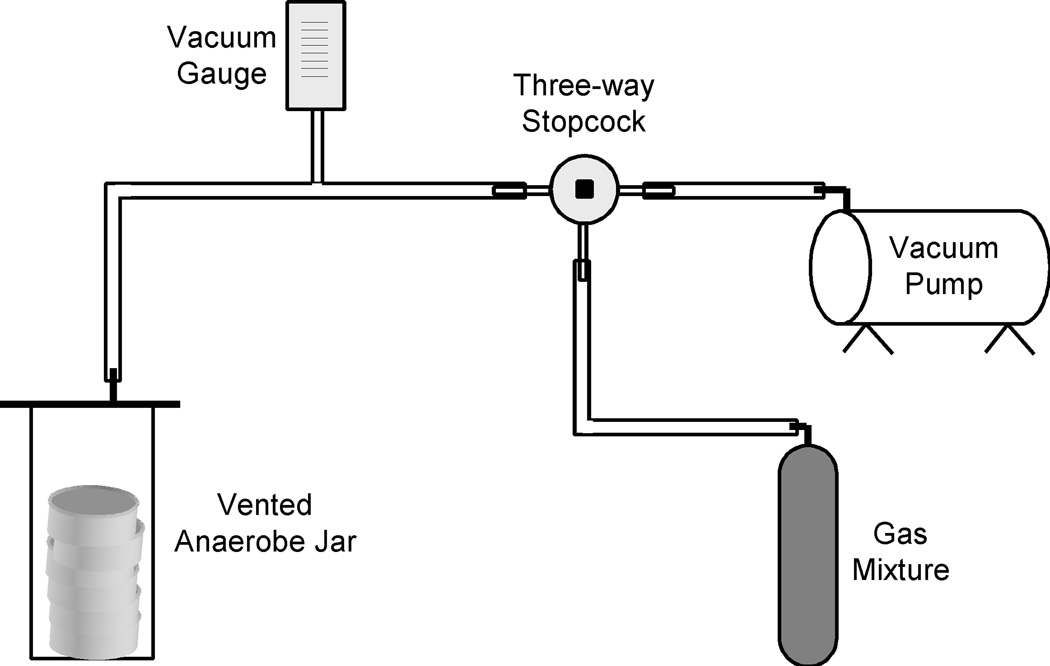
- Attach the jar to a vacuum pump via the vent nipple and ensure the screw clamp is fully open. Evacuate the jar to approximately 25" Hg. Using the stopcock direct the air flow away from the vacuum pump to the gas cylinder and fill the jar slowly with gas being careful not to overfill the jar. Repeat the evacuation and fill again.The anaerobe jars are designed to hold a vacuum but cannot hold positive pressure. Thus there is little danger of overfilling a jar to the point where it will burst however, close attention must be paid to the gas replacement step so that the jar lid does not become damaged.It is generally convenient to construct a small gassing manifold which has the stopcock valve and tubing fixed to a wooden platform together with a vacuum gauge to monitor pressure in the jar (Figure1). The idea is to design the manifold so that the user can control direction of the air flow at the proper time between the vacuum pump and the gas cylinder. The vacuum gauge should be connected to the manifold by a T connector and should be located between the jar and the first stopcock valve. The manifold greatly simplifies the evacuation and filling process and ensures that the jars will not be overfilled with gas.
Finally evacuate one more time and then use the stopcock to direct airflow to the Gas mix cylinder and proceed to fill the jar with the anaerobic gas mixture. Close the screw clamp and disconnect the jar from the vacuum pump assembly.
Incubate the jar at 37°C for 24 to 28 hours. Inspect the anaerobic indicator after several hours to ensure proper functioning of the catalyst system.
Figure 1.
Typical gassing manifold for the filling of vented anaerobic jars.
Support Protocol 3
Anaerobe Chamber
The anaerobic chamber or anaerobic glove box is a self-contained, closed system providing the investigator with an anaerobic lab bench where nearly any experiment can be performed under anaerobic conditions. The only disadvantage of the anaerobic chamber is in the initial cost of the investment but the experimental benefits far outweigh the cost for a laboratory routinely engaged in anaerobic microbiology. Anaerobic chambers accommodate an incubator, allowing the culture of plates, flasks, and tubes without the need for jars. There also is shelving space to allow the storage of media and other materials, thus eliminating the need to freshly prepare media before use. Cultures also can be stored in the anaerobic chamber on plates but care must be taken to ensure that they do not dry out.
As with the vented anaerobic jars, the glove box atmosphere contains 5% to 10% CO2, 5% to 10% H2, and the balance will be nitrogen or argon. An anaerobic atmosphere with less than 5ppm of O2 is maintained in the glove box by circulation through wire screen filters containing palladium catalyst. All materials (cultures, media, tubes etc) enter the chamber through a double door airlock which is purged with anaerobic gas and thus functions to limit the amount of oxygen that enters the glove box. Most modern anaerobic chambers possess an automated airlock that simplifies their use and makes if difficult to accidentally introduce large amounts of air into the chamber by inappropriate opening of the airlock doors. Once one becomes at ease with inconvenience of working with the neoprene gloves they will find the anaerobic chamber to be a convenient, efficient way to culture the Bacteroides. There are several types of chambers available, from rigid to flexible, and gloved to gloveless (see Internet Resources). By far the most adaptable and cost effective are flexible vinyl glove boxes manufactured by Coy Laboratory Products Inc. and based on the original design by Aranki and Freter (Aranki et al., 1969). Only use of the flexible glove box will be considered in this unit.
Materials
Coy flexible glove box (2 person) with 37°C forced air incubator
Nitrogen gas cylinder and regulator
Gas Mixture cylinder (80%N2, 10%CO2, and 10%H2) and regulator
Cultures, media, and other materials
Palladium catalyst, 4 packs (wire screen enclosure containing ~165 g catalyst)
Silica gel desiccant type IV, 2 trays each containing ~ 2.5 kg desiccant
Oxygen/Hydrogen meter
-
1.Gather materials together and open the outer airlock door. Place the catalyst, trays of desiccant and other materials inside the airlock. Close the outer airlock door and initiate the purging cycle to lower the oxygen level in the entry lock.Caution! Hot liquids should not be brought through the airlock since they will boil over during the vacuum cycle. Allow them to cool to at least 60°C.
-
2.The purging cycle will evacuate and replace the airlock atmosphere with N2 twice and then following a final evacuation the air lock is filled with the anaerobic gas mixture. When the cycle is complete check the front panel of the airlock to determine that the anaerobic indicator light is on and then open the inner airlock door. Remove all items from the airlock and then place in any outgoing materials before closing the inner door.The anaerobic indicator light is used to signal the status of the airlock and is activated following the purging cycle and remains lit until the outer airlock door is opened. The indicator light should always be checked prior to opening the inner airlock door in order to prevent accidental contamination of the chamber atmosphere with oxygen.
-
3.Place the catalyst packs into the two catalyst fan boxes and set the trays of desiccant on the shelving unit. Check the oxygen/hydrogen meter to ensure safe, efficient operation of the glove box.Four catalyst packs (2 in each fan box) are needed in very active chambers and they should be exchanged about twice a week. Humidity can get high in active chambers due to oxygen removal so the silica gel desiccant should be changed twice a week or more according to the level of humidity as shown by the indicator in the desiccant.To rejuvenate the catalyst heat in an oven at 160° for 2 hours then store with a desiccant until needed. The life expectancy of the catalyst pellets is about one year but can vary depending of the level of usage and exposure to hydrogen sulfide which poisons the catalyst. Refer to Critical Parameters and Troubleshooting for instruction on how to determine if the catalyst is still active. Assesing changes in the the color indicator present in the media is not a useful indicator of the catalyst state as there are many possible causes to such a change. [*AQ: We have added the preceding sentence in response to your comments. Please confirm or edit as needed. -TD] Desiccant is dried before use in a 110°C oven and stored in an airtight container.A chamber using a 10% hydrogen gas mixture should maintain internal levels of hydrogen between 4–6%. Higher hydrogen levels suggest a decrease in catalyst efficiency and lower hydrogen levels indicate a recent exposure to oxygen or infrequent use of the chamber. The hydrogen in the chamber atmosphere is only replaced during entry through the airlock and the levels can become low over time in an inactive chamber. If hydrogen levels fall below 1–2% additional gas mixture should be added through the airlock.
-
5.Place bacterial cultures in the incubator and other materials on the shelving unit.When first bringing cultures into the glove box loosen the caps to allow circulation of the anaerobic atmosphere in the tubes or flasks. Likewise, fresh media should be stored overnight with the caps loosened to ensure that all oxygen diffuses out of the media and that there is full exchange of the head space of the media vessels with the anaerobic atmosphere.The redox indicator resazurin (final concentration 0.0001%) is routinely incorporated into many of our culture media. This allows easy monitoring of the media to ensure that it is fully reduced prior to inoculation and it provides a backup check on the atmosphere in the glove box. The indicator is colorless when fully reduced at −110 mV and red when oxygen is present and the redox potential goes above −50 mV.
-
6.
Open the outer door and remove items from air lock. Recycle the airlock leaving it in the anaerobic state if not planning additional use of the glove box.
Support Protocol 4
Anaerobic Gassing Cannula
Not every anaerobic culturing situation that arises can be handled in a glove box or with anaerobic jars. There will be times that air must be excluded from a culture vessel on the bench top during the course of specialized media preparation, inoculation, or sampling. These circumstances require the use of a gassing cannula attached to a source of oxygen-free gas. Oxygen is excluded by directing a stream of anaerobic gas into the culture vessel thus maintaining anaerobiosis.
The basic use of a gassing cannula is described here in order to support other protocols described in this unit. The reader is directed to the Holdeman et al., 1977, Bryant (1977) and Breznak and Costilow (1994) for more detailed descriptions of using gassing cannulas and the preparation of prereduced media.
Materials
Gassing cannula with 6", 16 gauge blunted needle bent at 90° (Popper & Sons, Inc.)
Gas cylinder (90% N2, 10% CO2, 10% H2) and regulator
Bunsen burner
Tube of chopped meat media (see below)
Test tube rack
Culture plate containing isolated colonies
Inoculating loop
6" hemostat
-
1.Open the regulator for the gas mixture and adjust the gas flow so that a gentle stream of gas flows from the end of the cannula with a force great enough to put a small dent into the Bunsen burner flame.The gassing cannula is a 2 ml Luer-Lok glass syringe. The syringe barrel is filled with cotton and then sterilized. The syringe is then attached to the gas source via 1/4" rubber tubing.It is not desirable to have such a strong flow of gas from the cannula that the culture media will splatter or bubble up from the vessel.
-
2.
Flame the top of the chopped meat media tube and quickly remove the rubber stopper with the hemostat, being careful to not contaminate the rubber stopper. Place the tube in the rack.
-
3.
Sterilize the cannula by heating the bent needle in the Bunsen burner until it is red, then place it in the tube of media so that it rests on the lip of the tube.
-
4.
Sterilize the inoculating loop and then pick a colony from the plate. Transfer the colony to the tube of media by gently stirring the loop in the media. Antibiotics (Table 1) and other additions to the media can be made while the tubes are under the flow of gas.
-
5.Retrieve the rubber stopper, briefly flame it, and reseal the tubes quickly by pulling up on the cannula as the stopper is being pushed down in place. Once the tube is closed twist the stopper to ensure a good seal and the turn off the gas.Care must be taken when closing tubes and other culture vessels with the rubber stoppers. Do not use too much force or push down excessively since the tubes will break and injury can result. Use a twisting motion to ensure a good seal with minimal force.
-
7.
Incubate the tube at 37°C.
Basic Protocol 3
LONG-TERM STORAGE OF BACTERIODIES AT ROOM TEMPERATURE
Inoculate chopped meat media stock cultures with a single well isolated colony from an agar plate.
- Incubate the culture 36–48 hours at 37°C. Store the cultures in the dark at room temperature.Due to the inherent turbidity of the chopped meat media it is not always easy to determine if adequate growth has occurred; thus, cultures are usually incubated for the prescribed length of time to ensure adequate growth. Cultures grown in this way will remain viable for about 10 years.The inoculation of chopped meat cultures can be done as described above in the gassing cannula protocol or they can be inoculated from plates in the anaerobic chamber as described under the inoculation of liquid cultures protocol.The growth in chopped meat cultures is not as vigorous as in some other media due to the low levels of carbohydrate present in the media, but it is this very fact that makes chopped meat a good storage medium. The low carbohydrates result in low acid production which consequently does not stress the cells.
[*CE: The Chapter Edtior really wanted to include the following protocol in Reagents and Solutions as a recipe. It seems far too complex to me; however, if you want to incorporate it there, please feel free to do so. –TD]
Support Protocol 5
CHOPPED MEAT MEDIUM
This medium is used for long-term and short-term storage of Bacteroides strains; thus, it needs to be well reduced and free of any oxidized components. To ensure the most consistent results this media is prepared under more stringent anaerobic conditions during all steps.
Materials
Gas cylinder (either a gas mix or 100% N2) and regulator
Gassing cannula (Support Protocol 4)
Bunsen burner
500 g extra lean ground beef
1 M NaOH
Tryptone, 30 g
Yeast extract, 5 g
KH2PO4, 2.5 g
K2HPO4, 2.5 g
Hemin solution, 10 ml
Cysteine (free base Sigma), 1 g
Cheese cloth
Hungate tubes (Bellco Biotechnology) 16 × 125 mm with septum stoppers and screw caps
In a 2 liter beaker put one liter dH2O, a stir bar, 500 g extra lean ground beef, and 25 ml of 1 M NaOH. Bring to a boil with slow stirring and let boil for about five minutes.
- Cover the beaker with aluminum foil and place at 4° overnight or until fat solidifies. Remove ALL of the fat and discard it.The fat comes to the surface and is easily removed.
Strain the meat broth through several layers of cheese cloth, retain both the broth and the meat particles. Spoon off any remaining fat floating on the surface of the strained broth.
Pour the broth into a graduated cylinder and bring up volume to one liter with dH2O.
Pour the broth into a 2 l flask and add 30 g tryptone, 5 g yeast extract, 2.5 g KH2PO4, 2.5 K2HPO4, 10 ml hemin solution, and 1 ml resazurin solution.
- Using the gassing cannula, bring the broth to a boil under a stream of N2 or N2 + CO2 gas and boil for about 2 minutes. Cool the broth on ice while still gassing. When cooled stopper flask tightly, secure the stopper with laboratory tape and then put the flask into the anaerobe chamber.Boiling the media under a stream of oxygen-free gas will drive off all oxygen from the media and as it cools under the stream of anaerobic gas there will be little or no oxygen contamination. This mixture is high in organic material and can rapidly boil over if care is not taken to adjust the flame as the boil begins.
- While the broth is cooling prepare about 100 Hungate culture tubes in racks by adding ground beef meat particles so that they are evenly distributed among the tubes. Place chopped meat tubes, rubber stoppers and screw caps into the anaerobe chamber and allow them to equilibrate for a short time.Placing the meat into the tubes can be facilitated by pressing the tubes into the meat and then using a 1 ml disposable pipette or glass rod to push the meat to the bottom of the tube.
Add 1.0 g of cysteine and 20 ml of NaHCO3 (10% solution) to the meat broth and mix by swirling.
When cysteine is dissolved dispense 10 ml of broth into each tube, then secure a stopper into place, and screw on the cap. Ensure that the cap is tightened to prevent leakage of the anaerobic atmosphere during autoclaving.
- Remove tubes from chamber and autoclave for 30 minutes.The sealed Hungate tubes with the tightened cap are designed to be autoclaved directly in the test tube racks. These tubes will easily withstand the internal pressure resulting from the autoclave temperatures and they will maintain their anaerobic atmosphere.
Basic Protocol 4
FROZEN GLYCEROLSTOCK CULTURES OF BACTEROIDES
Materials
BHIS medium (see reagents and solutions)
Glycerol
2 ml Cryogenic vials (Nalgene)
Prepare 70 ml of BHIS medium without cysteine, resazurin or NaHCO3. Add 30 ml of glycerol, filter sterilize and store in a 125 ml bottle.
Inoculate a BHIS plate containing the appropriate antibiotics (Table 1) with 0.1 ml of an overnight culture. Incubate overnight or up to 48 hours.
Label cryogenic tubes and then cover label with clear tape.
Pipette 2 ml of BHIS/glycerol onto the surface of the plate, harvest cells from plate using a glass rod or rubber spatula and place in cryogenic tube.
Flash freeze in a dry ice/ethanol bath and store at −70°C.
Basic Protocol 5
FROZEN SKIM MILK STOCK CULTURES OF BACTEROIDES
[*AQ: Please provide a brief introduction to this protocol. Include a comparison of the advantages and disadvantages of chopped meat stocks vs. those prepared in skim milk medium. Done above in strategic planning]
Materials
Bacto Skim milk (Difco)
2 ml Cryogenic vial (Nalgene)
Add 150 g Bacto Skim milk to 1 liter dH2O. Stir about 30 min or until powder is dissolved. Autoclave for 10 min (longer sterilization times will result in curdled milk)
Store medium at 4° until needed.
Inoculate a BHIS plate containing the appropriate antibiotics (Table 1) with 0.1 ml of an overnight culture. Incubate overnight and up to 48 hours.
Label cryogenic tube and then cover label with clear tape.
Pipette 2 ml of Skim milk media on to the lawn of cells and harvest with a glass rod or rubber spatula.
Place cell mixture into the cryogenic tube.
Flash freeze in a dry ice/ethanol bath and store at −70°C.
Basic Protocol 6
DETERMINATION OF THE GROWTH CURVE
The growth curve is an essential protocol used to compare basic physiological properties of different Bacteroides species or mutant strains. These basic features are often best determined under defined conditions; thus a minimal, defined medium is best suited for these studies. The Bacteroides have simple nutritional needs and the defined medium of Varel and Bryant (1974) has been used for several decades with only slight modification. In this protocol the basic defined medium with glucose as the carbohydrate source and ammonia as nitrogen source will be described but this medium can be easily modified to meet any nutritional requirements that an experiment demands.
It is possible to perform growth rate measurements with a variety of culture tubes using this protocol. The only stipulation is that if screw cap tubes are to be used they must be returned to the incubator inside of the anaerobic glove box between OD readings. This is necessary since screw cap tubes will not maintain an anaerobic atmosphere for extended periods.
Materials
Anaerobic glove box
Spectronic 20 or equivalent spectrophotometer for reading absorbance of culture tubes
Chopped meat stock culture
13 × 100 mm sterile screw cap tubes in rack
125 ml side arm flask (sterile, aluminum foil cap)
sterile #3 rubber stopper (in covered beaker)
disposable 1 and 10 ml pipettes
antiseptic wipes
100 ml bottle of defined medium
-
1.
Gather materials together in the anaerobic glove box and prepare work space by placing all items within easy reach.
-
2.
Arrange four tubes in the rack, completely loosen caps, and dispense 5 ml of media into the tubes immediately replacing the caps.
-
3.
Gently mix the chopped meat culture, clean the top with an antiseptic wipe, unscrew cap and remove the stopper with a hemostat.
-
4.Remove 1 ml of broth and add 0.5 ml, 0.3 ml, and 0.2 ml respectively to the media. Tighten the caps and mix the tube. Place in the 37°C incubator overnight. The fourth tube of media will be used to zero the spectrophotometer during the growth curve determination.Initial growth of the inoculum in defined media can be variable when coming from a stock culture thus it is necessary to use several inoculum amounts to ensure adequate growth.
-
5.
Carefully remove the foil from the top of the flask and place upside down in a clean location. Add 50 ml of media using a 10 ml pipette, replace the foil cap and place in the incubator.
-
6.
The following day remove flask and tubes from incubator and inspect tubes for growth.
-
7.
Choose the tube with the most growth (most turbid). If all tubes have equal growth then pick the tube with the lowest initial inoculum size and place it in a rack. Loosen the cap, remove 1 ml and add to the flask.
-
8.
Using a hemostat, carefully grasp a sterile #3 stopper from the beaker and seal the flask. Twist the stopper to ensure a tight seal and then remove from the anaerobic chamber together with the tube of sterile media.
-
7.
Set the spectrophotometer to A550 and zero with the tube of sterile media. Record the initial OD from the side arm and then place the flask in a 37°C water bath.
-
8.
Continue to record OD readings as needed until growth has completed about 20–30 hours.
REAGENTS AND SOLUTIONS
Supplemented Brain Heart Infusion medium (BHIS)
This medium is used for routine culturing of Bacteroides and allows for rapid reproducible growth.
Ingredients per liter:
| Brain Heart Infusion Broth powder (Difco) | 37 g |
| Cysteine (free base, Sigma) | 1.0 g |
| Hemin solution | 10 ml |
| Resazurin solution (0.1%, optional) | 1 ml |
| NaHCO3 solution (sterile 10%) | 20 ml |
| H2O | 970 ml |
| Agar - as required | 15 g |
Add all dry components to a flask containing the water, swirl until dissolved and then add the hemin and resazurin solutions. Aliquot the media to 125 ml bottles (or any appropriate size bottle) and autoclave immediately for 20 min. When media has cooled to about 60°C place in the anaerobic chamber for storage.
Add 2 ml of NaHCO3 solution to each 100 ml of media prior to use and then aliquot media into sterile screw cap tubes as needed. Do not add NaHCO3 prior to autoclaving as it will be converted to CO2 and lost from the media. Antibiotics (Table 1) that are required must be added just prior to inoculation of tubes.
For solid media prepare as above, autoclave and then place in a 55°C water bath. When the medium is cool, add the sterile NaHCO3 and antibiotics if needed. Pour plates on the bench top or in a Biosafety hood. Allow the plates to dry for several hours and then use immediately or place in the anaerobic chamber for storage.
Defined Minimal Medium (Varel and Bryant, 1974)
Ingredients per liter:
| Mineral 3B solution | 50 ml |
| Glucose (20% in H2O, filter sterilized) | 25 ml (0.5%, 28 mM) |
| L-cysteine (free base) | 1 g |
| Hemin solution | 10 ml |
| L-methionine (0.2%, in H2O, filter sterilized) | 10 ml |
| FeSO4 solution | 1.5 ml |
| NaHCO3 (10% sterile solution) | 20 ml |
| H2O | 885 ml |
| Agar when required | 15 g |
Mix together components except for NaHCO3 and adust the pH to 7.1. Autoclave for 20 min. Cool in water bath at 50–55°C before adding 2o ml of sterile 10% NaHCO3.
Other carbohydrates can be substituted for glucose and 1 ml of 0.01 % vitamin B12 solution can be substituted for methionine.
[*CE: Please retain the recipe for TYG medium even though it is not directly cited in the text. See Critical Parameters, Media. –TD]
Tryptone Yeast Extract Glucose (TYG) Medium
Ingredients per liter:
| Tryptone | 20 g |
| Yeast Extract | 10 g |
| Glucose | 5 g |
| Cysteine (free base) | 1 g |
| Salts solution "A" | 40 ml |
| Hemin solution | 10 ml |
| Resazurin solution (0.1%) | 1 ml |
| Agar (if required) | 15 g |
| NaHCO3 (10% solution) | 20 ml |
All of the components except NaHCO3 are added to 930 ml of H2O. Then adjust pH to 7.0, and autoclave for 20 min. When media has cooled to 55°C add sodium bicarbonate solution.
10% NaHCO3
Dissolve 50 g into 500 ml H2O, filter sterilize.
Hemin solution
For 200 ml:
Dissolve 100 mg of hemin in 2 ml of 1 M NaOH and then bring up volume to 200 ml with dH2O. Store in an amber bottle at 4°C.
0.1% Resazurin
Dissolve 0.1 g of resazurin (Sigma Chemical Co.) in 100 ml distilled water.
FeSO4 solution
For 100 ml:
Dissolve 0.278 g of FeSO4 · 7H2O in 100 ml distilled water. Add two drops of concentrated HCl.
Mineral 3B solution
Ingredients per liter:
| KH2PO4 | 18 g |
| NaCl | 18 g |
| MgCl2·6H2O | 0.4 g |
| CaCl2·2H2O | 0.52 g |
| CoCl2·6H2O | 0.02 g |
| MnCl2·4H2O | 0.2 g |
| NH4Cl | 10.0 g |
| Na2SO4 | 5.0 g |
Dissolve salts in 800 ml dH2O, then bring volume up to 1 liter. Autoclave for 20 min.
Salts Solution “A”
Ingredients per liter:
| CaCl2 · 2H2O | 0.26 g | |
| MgSO4 · 7H2O | 0.48 g | |
| KH2PO4 | 1 g | |
| K2HPO4 | 1 g | |
| NaCl | 2 g |
Mix CaCl2 · 2H2O and MgSO4 · 7H2O in 300 ml H2O until dissolved. Then add 500 ml H2O and add remaining salts. When dissolved add 200 mlH2O and store at 4°C.
COMMENTARY
Background Information
In 1989 the genus Bacteroides was restricted and included just species associated with the human intestinal tract forming a phylogenetically and physiologically uniform group of Gram-negative, non-sporeforming, non-motile, anaerobic rods. The current Bacteroides species are B. fragilis, B. thetaiotaomicron, B. ovatus, B. uniformis, B. vulgatus, B. distasonis, B. eggerthii, B. caccae, B. merdae and B. stercoris. The Bacteroides are the most abundant of the indigenous intestinal flora making up to 30% of the total cultivable microbes in the gut. Although these organisms are capable of limited survival outside the mammalian host, they are best considered as obligate symbionts where they contribute to the normal intestinal development and physiology. During the past few years interest in the Bacteroides has been rekindled by new discoveries of their ability to directly communicate with the host and influence development of the gut (Hooper et al., 2001). Other studies have shown that they also may have a fundamental role in the normal development of the immune system where it is thought that they modulate CD4 T cell expansion in the spleen (Mazmanian et al., 2005). These host associated organisms also are potential opportunistic pathogens when they are translocated to normally sterile sites such at the peritoneal cavity and B. fragilis is the most commonly isolated anaerobic pathogen in such situations. The Bacteroides do not possess a wide array of virulence factors and it is becoming more clear that the same properties that allow them to evade the immune system and cause infection also permit them to successfully colonize the gut as normal flora.
The intestinal tract is a unique environment which has shaped the growth and culturing characteristics of Bacteroides. This is an anaerobic environment with carbon dioxide levels up to 10% and oxygen averaging less than 1%. Other gases such as hydrogen and methane also are present, indicating that the gut atmosphere is the product of a complex anaerobic metabolism. This is a highly reducing environment and the redox potential usually runs in the range of −150 to −350 mV. The pH varies along the length of the tract from slightly acid along the ascending colon to neutrality or mildly alkaline conditions at the distal colon. The temperature is constant at about 37°C and there is a constant influx of nutrients. It is not surprising that this is a highly competitive environment and home to some 500 species of bacteria. The Bacteroides have proven to be the most successful microbes in the human gut and the renewed interest in their interactions with the host promises to reveal new insight into their roles in human health. For example a colonic microflora with increased numbers of Firmicutes and a smaller population of Bacteroides has been associated with obesity in animal models (Turnbaugh et al. 2006).
Critical Parameters and Troubleshooting
Anaerobic conditions
Most problems encountered with culturing the Bacteroides can be attributed to a lack of adequate anaerobic technique. Anaerobic culturing is as much a philosophy as it is a technique and the researcher needs to scrutinize all aspects of an experiment to ensure that reasonable consideration has been given to maintaining anaerobiosis at all stages of growth. Even low levels of oxygen contamination can inhibit growth of these organisms and influence experimental outcome. Thus it is important to use anaerobic indicators whenever possible as these will allow the investigator to eliminate aerobiosis as a source of error when troubleshooting an experiment. The three indicators described in the unit, methylene blue strips (BD), resazurin, and the oxygen/hydrogen meter, will cover all routine culturing needs.
The anaerobic techniques described in this unit require an active palladium catalyst for the removal of oxygen from the atmosphere and it is this catalyst that is the source of most problems. The catalyst must be heated regularly as described in the protocols above in order to drive off the moisture on the catalyst surface. It also is important to replace the catalyst on a regular schedule (every 9 months to a year) since it eventually becomes permanently poisoned from volatile sulfides produced by bacterial metabolism. Although the Bacteroides do not produce large amounts of H2S it is still useful to have in the glove box a solution of 90 mM lead acetate or trays of activated charcoal to adsorb the sulfides.
A simple test will determine if the catalyst is still active. Rejuvenate the catalyst by heating at 160°C for two hours and let cool. Then using the gassing cannula direct a stream of an anaerobic gas mix containing H2 over the surface of the catalyst. The catalyst pellets should immediately become hot and if the stream of gas is maintained the pellets will begin to glow red from the heat. If the pellets do not heat up then the catalyst must be replaced.
A second common source of oxygen contamination results from air leaks in the culturing equipment. The rubber gaskets that seal anaerobic jar lids to the jar must be inspected frequently to make sure they are free of defects or debris. Likewise the gasket around the airlock entry of an anaerobic chamber must be intact to maintain anaerobiosis.
Anaerobic glove box
The anaerobic glove box has become the method of choice for cultivation of anaerobes in most research laboratories so it may be beneficial to review some of the important features and maintenance requirements. The clear, flexible, vinyl glove box is the most practical for the average laboratory and unless space is of paramount importance always choose a 2-person (4 glove) chamber for the extra storage space and convenience of having an extra pair of hands for some procedures. The flexible style is preferred for a number of reasons but the fact that these chambers are always under a slight positive pressure is a great advantage for detecting leaks and maintaining anaerobiosis when there is a leak. Leaks are immediately detected whenever the chamber deflates more quickly than usual.
Two required accessories for the glove box are a 37°C incubator and an oxygen/hydrogen gas meter. The incubator will tend to hold down the number of entries and exits from the chamber since cultures can be readily monitored. However, if a smaller glove box is purchased and one does not wish to give up the “bench space” for an incubator, it is possible to use anaerobic jars in conjunction with the glove box. Simply bring the jars with a fully charged catalyst into the chamber add the cultures, secure the lid and remove for incubation. Only the smaller size jars will fit into the entry lock. The oxygen/hydrogen meter is useful to keep watch on oxygen levels but more importantly it readily allows the investigator to monitor hydrogen. Changes is hydrogen levels can be indicative of a degenerating catalyst or a slow leak in the glove box. In our experience we have found that redox indicators in the media are more sensitive to changes in oxygen in the chamber than the meter but the meter is important to monitor aerobiosis following accidental contamination with oxygen.
All anaerobic chambers will eventually leak and the frequency of problems is directly related to the rate of use. The majority of problems occur with the neoprene gloves and the seams where the arms are joined to the body of the chamber. The gloves endure the most abuse and eventually wear out or get punctured. Likewise the arm holes endure a significant amount of stress and the seams can sometimes split. Repairs on the vinyl chambers are relatively simple and anaerobic conditions can be maintained in the chamber for all repairs.
Media
Media formulation is straightforward for the Bacteroides and a range of media are used for their cultivation. One versatile medium not employed in any of our protocols but added to the Reagents and Solutions section is Tryptone Yeast Extract Glucose medium which is used by many laboratories for their routine culturing needs. This is a complex, rich medium to which any carbohydrate of choice can be added and it was initially designed for analysis of sugar utilization in anaerobes. Thus it can readily replace BHIS in most situations.
Although the Bacteroides have relatively simple growth requirements, several parameters must be met to permit abundant growth. First, these are saccharolytic organisms and require a source of fermentable carbohydrate. Simple sugars are readily assimilated but these organisms also have sophisticated enzyme systems for the utilization of complex polysaccharides such as starch, pectin, and hemicellulose. It is this capacity to adapt to a wide range of carbohydrates that is thought to be a key to their success in the gut.
Another growth requirement is for a source of hemin. Although it is possible to cultivate these organisms for an extended period without hemin, the typical generation time is only about 1/10 of normal growth. Thus, Bacteroides are essentially heme auxotrophs and must be provided with a source of hemin, protoporphyrin IX, or blood in the media. Among other things hemin is required for synthesis of the b-type cytochrome important for succinate production and anaerobic electron transport.
The Bacteroides also require a source of vitamin B12. Cobalamin is an important cofactor for many enzymes but the ability of L-methionine to be substituted for B12 suggests that a key enzyme in methionine synthesis is the most critical defect in the absence of B12. The addition of NaHCO3 has two functions; it provides a ready source of CO2 which is a growth requirement for these organisms and it is good buffer against a CO2 atmosphere.
The preferred nitrogen source is ammonia and the Bacteroides have very little capacity to gain nitrogen for growth from single amino acids. They can however readily utilize peptides for growth if no ammonia is present but the growth yield is decreased. These nitrogen requirements are well suited for the gut environment where there are little or no free amino acids but ammonia is plentiful. Finally, Bacteroides require a reduced form of sulfur. That is they cannot utilize sulfate as the sole source of sulfur. In laboratory media the source of sulfur generally comes from cysteine which is added to the media as a reducing agent but they also have the ability to utilize H2S and to a lesser extent they can use thioglycolate.
Time Considerations
The Bacteroides are robust organisms and can double at a relatively high rate under optimal conditions. Generation times for B. fragilis and B. thetaiotaomicron in BHIS media is approximately 40–60 minutes but the presence of antibiotics can have a significant affect even in resistant organisms. In defined media growth rate will increase from 1.75–2 hours when glucose is the carbon/energy source. When inoculating a BHIS plate with a frozen or chopped meat stock of Bacteroides, one should expect to see colonies within 36 hours.
Acknowledgement
Much of the work described here has been supported by PHS grant AI40588 to CJS.
Footnotes
Internet Resources
Sites containing information about anaerobic systems.
Anoxomat: http://www.anoxomat.com/english/standardpage.php[?ArtikelID=46
Anaerobe systems: http://www.anaerobesystems.com
Belco Biotechnology source of Hungate Tubes: http://www.bellcoglass.com/product_detail.php?product_id=276
Sites for commercially available gas packs.
BD GasPak: http://www.bd.com/ds/productCenter/BblGaspakProductsAndAccessories.asp
Mitsubishi Anaerobe systems: http://www.mgc-a.com/Pages/anaeropac.html
Oxoid Anaerobic systems: http://www.oxoid.com/us/index.asp?mpage=ipsearch&c=US
Sites for anaerobic chambers.
Coy Laboratories: http://www.coylab.com/anaerobic_chamber.html
Bactron: http://www.shellab.com/bactron.html
Concept series: http://www.biotrace.com/content.php?hID=2&nhID=75
MACS: http://www.800ezmicro.com/productImages.asp?mb=01&ez=9
Literature Cited
- Aranki A, Syed SA, Kenney EB, Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl. Microbiol. 17:568–578. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn AD, Malamy MH. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature. 2004;427:441–444. doi: 10.1038/nature02285. [DOI] [PubMed] [Google Scholar]
- Brewer JH, Allgeier DL. Safe Self contained Carbon Dioxide Hydrogen Anaerobic System. Appl. Microbiol. 1966;14:985–988. doi: 10.1128/am.14.6.985-988.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak JA, Costilow RN. Physiochemical factors in Growth. In: Gerhards P, Murray RGE, Wood WA, Krieg NR, editors. Methods for General and Molecular Bacteriology. Washington DC: American Society for Microbiology; 1994. pp. 137–154. [Google Scholar]
- Bryant MP. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Hecht DW. Anaerobes: Antibiotic resistance, clinical significance, and the role of susceptibility testing. Anaerobe. 2006;12:115–121. doi: 10.1016/j.anaerobe.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Holdeman LV, Cato EP, Moore WEC. Anaerobic laboratory manual. 4th ed. Blacksburg: Anaerobe Laboratory, Virginia Polytechnic Institute and State University; 1977. [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Jouseimies Somer H, Summanen P, Citron D, Baron E, Wexler H, Finegold S. Wadsworth KTL Anaerobic Bacteriology Manual. 6th Ed. Belmont, CA: Star Publishing Company; 2002. [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Rocha ER, Paster BJ. The medically important Bacteroides spp. in health and disease. In: Dworkin M, et al., editors. The Prokaryotes. Vol. 7. Springer Verlag, NY: 2006. pp. 381–427. [Google Scholar]
- Smith CJ, Tribble GD, Bayley D. Genetic elements of the Bacteroides: a moving story. Plasmid. 1998;40:12–29. doi: 10.1006/plas.1998.1347. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley1 RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Varel VH, Bryant MP. Nutritional features of Bacteroides fragilissp. fragilis. Appl. Microbiol. 1974;28:251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]