Abstract
The pathophysiological processes underlying respiratory diseases like asthma are complex, resulting in an overwhelming choice of potential targets for the novel treatment of this disease. Despite this complexity, asthmatic subjects are uniquely sensitive to a range of substances like adenosine, thought to act indirectly to evoke changes in respiratory mechanics and in the underlying pathology, and thereby to offer novel insights into the pathophysiology of this disease. Adenosine is of particular interest because this substance is produced endogenously by many cells during hypoxia, stress, allergic stimulation, and exercise. Extracellular adenosine can be measured in significant concentrations within the airways; can be shown to activate adenosine receptor (AR) subtypes on lung resident cells and migrating inflammatory cells, thereby altering their function, and could therefore play a significant role in this disease. Many preclinical in vitro and in vivo studies have documented the roles of the various AR subtypes in regulating cell function and how they might have a beneficial impact in disease models. Agonists and antagonists of some of these receptor subtypes have been developed and have progressed to clinical studies in order to evaluate their potential as novel antiasthma drugs. In this chapter, we will highlight the roles of adenosine and AR subtypes in many of the characteristic features of asthma: airway obstruction, inflammation, bronchial hyperresponsiveness and remodeling. We will also discuss the merit of targeting each receptor subtype in the development of novel antiasthma drugs.
Keywords: Adenosine, Adenosine receptors, Asthma, Bronchial hyperresponsiveness, Airway smooth muscle, Airway remodeling, Airway inflammation
1 Adenosine: An Important Signaling Molecule in Asthma
Asthma is a lung disease characterized by airway hyperresponsiveness and inflammation. The pathogenesis of asthma involves the release of a broad array of mediators such as cysteinyl leukotrienes, histamine and cytokines from various cell types, leading to bronchoconstriction, proinflammatory effects, chemoattraction of leukocytes, and airway remodeling (Busse and Lemanske 2001). A number of clinical features distinguish asthmatic subjects from other respiratory diseases and may be considered characteristic of this phenotype (Avital et al. 1995). These include an exacerbation of disease following exposure to beta-adrenoceptor antagonists (Bond et al. 2007), an impairment in the ability to bronchodilate following deep inspiration (Slats et al. 2007), and their bronchoconstrictor sensitivity to a wide range of innocuous stimuli (Cockcroft and Davis 2006; Van Schoor et al. 2002). Various mechanisms have been proposed to account for this bronchial hyperresponsiveness (BHR) phenomenon, and these include increased airway smooth muscle function (An et al. 2007; Gil and Lauzon 2007), altered airway epithelial cell function (Holgate 2007), and the recruitment and activation of numerous inflammatory cells, including dendritic cells, T lymphocytes and eosinophils (Beier et al. 2007; Hammad and Lambrecht 2007; Jacobsen et al. 2007; Kallinich et al. 2007; Lloyd and Robinson 2007; Rosenberg et al. 2007), whose cell-derived products trigger a cascade of events within the lung that lead to airway epithelial cell damage, increased bronchial smooth muscle contractility and airway remodeling.
Asthmatic subjects bronchoconstrict in response to a number of physiological stimuli, such as exercise, distilled water, cold air and hypertonic saline, to which healthy subjects are refractory. Similarly, acidification, pollutants like sulfur dioxide, and chemical substances including adenosine, bradykinin and neuropeptides evoke bronchoconstriction in asthmatics but have little if any effect in nondiseased individuals. These agents are commonly referred to as indirect-acting stimuli, since they do not appear to mediate bronchoconstriction by the direct activation of airway smooth muscle. They are thought to elicit bronchospasm by activating a number of different cell types, including mast cells, vascular smooth muscle cells, vascular endothelial cells, and/or airway nerves (Spina and Page 1996, 2002; Van Schoor et al. 2000). It is therefore of interest that asthmatic subjects are sensitive to such stimuli whilst healthy subjects are invariably unresponsive to these agents (Van Schoor et al. 2000). This suggests that the mechanisms by which these stimuli provoke bronchoconstriction are upregulated in asthma and are characteristic of this phenotype.
Furthermore, airway inflammation appears to be correlated better with BHR to indirect stimuli like adenosine (van den Berge et al. 2001), bradykinin (Polosa et al. 1998; Roisman et al. 1996) and hypertonic saline (Sont et al. 1993) than it is to more direct-acting stimuli like methacholine. Similarly, during an exacerbation of BHR following the deliberate exposure of an asthmatic subject to an environmental allergen (e.g., house dust mite), there is a preferential increase in BHR to an indirect-acting stimulus like bradykinin in contrast to methacholine (Berman et al. 1995). On the other hand, a number of pharmacological drugs used to treat asthma, including nedocromil sodium and ipratropium bromide, suppress airway responsiveness to these indirect-acting stimuli, suggesting the likely involvement of neural reflexes (Van Schoor et al. 2000). Furthermore, it is now recognized that glucocorticosteroids preferentially suppress BHR to adenosine (Ketchell et al. 2002; van den Berge et al. 2001) and bradykinin (Reynolds et al. 2002) compared with methacholine.
It is common for clinicians to use stimuli like methacholine and histamine as provocative inhalation challenge agents to induce bronchoconstriction because these agents are relatively convenient to use. However, whilst there is a separation in airway responsiveness to these agents between asthmatic subjects and healthy individuals, there is also a considerable degree of overlap, and it has been suggested that airway responses to these agents may not be sensitive indicators of the asthma phenotype (Avital et al. 1995; O’Connor et al. 1999). In contrast, asthmatic subjects invariably bronchoconstrict in response to the indirect-acting stimuli described earlier, which provoke little if any response in otherwise healthy individuals or in subjects with other respiratory diseases (Avital et al. 1995; Van Schoor et al. 2000).
A growing body of evidence has emerged in support of the purine nucleoside adenosine in the pathogenic mechanisms of asthma (Spicuzza et al. 2006). This body of evidence is supported by the following reported findings. (a) In asthmatics adenosine levels are elevated in bronchoalveolar lavage (BAL) fluid (Driver et al. 1993), in the circulation following allergen inhalation (Mann et al. 1986a), and in exhaled breath condensate in patients with asthma (Csoma et al. 2005). (b) Adenosine given by inhalation causes a dose-dependent bronchoconstriction in subjects with asthma (Cushley et al. 1983; Polosa 2002; Rorke and Holgate 2002). (c) Inhalational challenge with adenosine monophosphate (AMP), which is metabolized locally by the ectonucleotidase 5′ -nucleotidase to adenosine, increases the release of leukotrienes and other bronchoconstrictive mediators in asthmatics (Bucchioni et al. 2004). (d) Adenosine enhances mast-cell allergen-dependent activation (Polosa et al. 1995); (e) Treatment with dipyridamole, a blocker of adenosine reuptake, significantly enhances the bronchoconstrictor response to inhaled adenosine in subjects with asthma (Crimi et al. 1988). (f) The sensitivity of airways to adenosine and AMP more closely reflects an inflammatory process and the phenotype for allergic asthma than the sensitivity of airways to other known inhalational bronchoprovocative agents, such as methacholine and histamine (de Meer et al. 2002; Holgate 2002; Spicuzza et al. 2003; van den Berge et al. 2001).
1.1 Adenosine Metabolism
The physiological effects of adenosine in asthma via its stimulation of cell-surface adenosine receptors (ARs) and subsequent downstream signaling pathways are a function of the local concentration of adenosine. Adenosine concentrations in unstressed cells and tissue are below 1 µM (estimates 10–100 nM); however, in metabolically stressed inflamed or ischemic tissues, adenosine levels may rise to 100 µM (Fredholm 2007; Hasko and Cronstein 2004). Lower concentrations of adenosine (10–100 nM) activate the high-affinity A1, A2A, and A3 ARs and high adenosine concentrations (10 µM) stimulate low-affinity A2BARs (Fredholm 2007). Factors that determine the net effect of adenosine on specific cell and tissue function are AR expression and coupling to intracellular signaling pathways, all of which are tightly regulated in different tissues and cells.
The local adenosine concentration at its receptor subtypes is determined by several processes, which include extracellular and intracellular adenosine generation, adenosine release from cells, cellular reuptake and metabolism (Fig. 1). These processes are closely intertwined and strictly regulated. For, example, under the hypoxic and inflammatory conditions encountered in asthmatic airways, the increased intracellular dephosphorylation of adenosine 5′-triphosphate (ATP) to adenosine by the cytosolic metabolic enzyme 5′-nucleotidase may be accompanied by a suppression of the activity of the salvage enzyme adenosine kinase, which prevents the rephosphorylation of adenosine to AMP (Deussen 2000). These processes lead to high adenosine concentrations inside the cell and the release of adenosine from the dephosphorylation of AMP into the extracellular space through nucleoside transporters (Hyde et al. 2001; Pastor-Anglada et al. 2001).
Fig. 1.
Metabolism of adenosine. Adenosine is generated mainly by two enzymatic systems: intra/extracellularly localized nucleotidases and cytoplasmic S-adenosylhomocysteine hydrolase (SAHH). In response to hypoxia/cellular damage or other stressful/inflammatory stimuli, ATP is rapidly dephosphorylated by combined effects of adenylate cyclase (AC), phosphodiesterases (PDE) and nucleotidases to form intra/extracellular adenosine. Ecto-5′-nucleotidase (ecto-5′-NT) is one such enzyme that plays an important role in regulating local adenosine production for receptor signaling. Extracellular adenosine can interact with adenosine receptors (AR) that are coupled to heterotrimeric G proteins, which, in turn, couple AR activation to various effector molecules that can regulate second-messenger systems to influence cell and tissue function. Adenosine can also be deaminated to inosine by adenosine deaminase (ADA) that can exist intra- or extracellularly, or it can be transported into and out of the cells via membrane-associated nucleoside transporters. Intracellular adenosine is generated from the dephosphorylation of AMP by a cytosolic form of nucleotidase (cyto-5′-NT) or the hydrolysis of S-adenosylhomocysteine by SAHH. Adenosine can also be phosphorylated back to AMP by adenosine kinase (AK). AMP can also be directly deaminated to inosine monophosphate (IMP) by AMP deaminase. The reaction of phosphorylation predominates when adenosine occurs at a low physiological concentration (<1 µM), whereas ADA is activated at higher concentrations of the substrate (>10 µM). Hypoxanthine is formed after the removal of ribose from inosine by the actions of purine nucleoside phosphorylase (PNP) PNP has only negligible activity towards adenosine and degrades mainly inosine. Hypoxanthine can be salvaged back to IMP by hypoxanthine phosphoribosyltransferase (HPRT), which is again converted to AMP through the purine nucleotide cycle (PNC). Hypoxanthine can also enter the xanthine oxidase (XO) pathway to form xanthine and uric acid sequentially as byproducts
The other major pathway that contributes to high extracellular adenosine concentrations during metabolic stress is release of adenine nucleotides (ATP, adenosine diphosphate (ADP), and AMP) from inflammatory and injured cells. This is followed by extracellular degradation to adenosine by a cascade of ectonucleotidases, which include CD39 (nucleoside triphosphate diphosphohydrolase (NTPDase)) and CD73 (5′-ectonucleotidase) (Eltzschig et al. 2004; Kaczmarek et al. 1996; Resta et al. 1998; Thompson et al. 2004; Zimmermann 1999). Adenosine accumulation is limited by its catabolism to inosine by adenosine deaminase. Inosine is finally degraded to the stable end-product uric acid (Hasko et al. 2000, 2004). Mechanisms of nucleotide release and metabolism, or adenosine release and metabolism, as well as transport mechanisms that account for the increased adenosine levels in exhaled breath condensate after exercise (Csoma et al. 2005), in the circulation following allergen inhalation (Mann et al. 1986a), and in BAL fluid (BAL adenosine concentration of 2.55 ±0.50 µM in asthmatics versus 0.72 ±0.16 µM in normals) (Driver et al. 1993) in human asthmatics, are yet to be determined.
There are several important cell types that are sources of extracellular adenosine. Neutrophils and endothelial cells release large amounts of adenosine at sites of metabolic distress, inflammation and infection (Cronstein et al. 1983; Gunther and Herring 1991; Madara et al. 1993; Rounds et al. 1994). Activated leukocytes are a major source of extracellular adenosine (Mann et al. 1986b). ADP released by platelets can be a significant source of adenosine after dephosphorylation (Marcus et al. 1995). Under conditions of stress including infection, activated macrophages can also serve as a major source of extracellular adenosine via ATP metabolism. Bacterial lipopolysaccharide (LPS) augments the release of ATP from macrophages (Sperlagh et al. 1998). Moreover, T-helper lymphocytes may be an important source of extracellular ATP. The presence of ecto-ATPase and antigen-triggered accumulation of extracellular ATP from T-helper cells has been reported (Apasov et al. 1995). In addition to inflammatory cells, airway epithelial cells and other structural cells in the lung may be important sources of high levels of adenosine in the airways of human asthmatics (Cohn et al. 2004).
1.2 Adenosine-Induced Bronchoconstriction, Airway Inflammation, and Airway Remodeling
In asthmatics, adenosine produces bronchoconstriction, inflammation, and airway plasma exudation, which lead to airway obstruction. Moreover, by acting on ARs, adenosine induces the release of inflammatory mediators that are important in the pathogenesis of airway remodeling in asthmatics. In both humans and animals, adenosine induces increases in BHR in asthmatics but not normal subjects, both in vivo following inhalation (Ali et al. 1994a; Cushley et al. 1983; Dahlen et al. 1983) and in vitro in small airways (Ali et al. 1994b; Bjorck et al. 1992). Adenosine produces bronchoconstriction in airways by directly acting on ARs in bronchial smooth muscle cells or indirectly by inducing the release of preformed and newly formed mediators from mast cells, and by acting on ARs on airway afferent sensory nerve endings (Hua et al. 2007a; Keir et al. 2006; Livingston et al. 2004; Polosa 2002). Multiple mechanisms may be involved in adenosine-induced bronchoconstriction; for example, the effects of adenosine in asthmatic subjects are sensitive to muscarinic receptor antagonists, suggesting that adenosine mediates obstruction indirectly (Crimi et al. 1992; Mann et al. 1985; Polosa et al. 1991), which would be consistent with the preclinical evidence that adenosine can activate afferent nerves in vivo (Hua et al. 2007a; Keir et al. 2006). However, since muscarinic antagonists do not completely abolish bronchoconstriction in response to adenosine, it is plausible to conclude that the “atropine-resistant” component of this response is mediated by direct activation of airway smooth muscle (Brown et al. 2008; Ethier and Madison 2006) and/or indirectly via mediators released from other cell types expressing these receptors.
Adenosine exposure through inhalation increases enhanced pause (Penh), a measure of airway resistance, in allergen-sensitized and -challenged mice (Fan and Mustafa 2002). This increase in enhanced pause due to adenosine was reversed by theophylline with methacholine-mediated enhanced pause being unaffected, suggesting the involvement of ARs (Fan and Mustafa 2002). This finding that adenosine-induced bronchoconstriction is mediated by ARs is supported by an earlier study in a rabbit model of allergic asthma, where adenosine-induced bronchoconstriction was blocked by theophylline (Ali et al. 1992). Following inhalation and its local metabolism to adenosine in the airway, AMP induced bronchoconstriction is attenuated by potent cyclooxygenase inhibitors, H1 receptor and leukotriene receptor antagonists, suggesting that adenosine induces the release of prostaglandins, histamine and leukotrienes in the airways of asthmatics (Phillips and Holgate 1989; Rorke et al. 2002; Rutgers et al. 1999). Another study has shown that inhalation challenge with adenosine, but not methacholine, produces mild airway plasma exudation (Belda et al. 2005). Collectively, these effects of adenosine on airway nerves, contraction of bronchial smooth muscle, release of mast cell mediators, and airway edema produce airflow obstruction.
Adenosine produces inflammation in airways in allergic animals and humans. Animals with increased adenosine concentrations in the lung (adenosine deaminase (ADA)-deficient mice) develop severe pulmonary inflammation, with airway accumulation of eosinophils and activated macrophages, mast cell degranulation, and mucus metaplasia in the airways—features similar to that found in asthmatic bronchi (Blackburn et al. 2000; Chunn et al. 2001). Treatment of these mice with exogenous ADA to reduce adenosine concentrations results in the reversal of these asthmatic features (Chunn et al. 2001). In a mouse model of allergic asthma, inhalation of adenosine has also been shown to cause airway inflammation, as evidenced by an increased release of proin flammatory mediators from eosinophils and mast cells (Fan and Mustafa 2002, 2006; Oldenburg and Mustafa 2005; Tilley et al. 2003). Moreover, in human asthmatics, an inhalational challenge with AMP produced an increase in eosinophils and neutrophils in the sputum (Manrique et al. 2008; van den Berge et al. 2004).
Adenosine-mediated inflammation is not limited to the lung; it also reaches the systemic circulation. In a recent report in a mouse model of asthma activities of eosinophilic peroxidase, myeloperoxidase and beta-hexosaminidase were increased not only in the lung but also in the systemic circulation of allergic mice exposed to adenosine aerosol (Fan and Mustafa 2006). In human asthmatics, adenosine aerosol increases the release of neutrophil chemotactic factor in serum (Driver et al. 1991). Moreover, in a recent study it was demonstrated that adenosine-induced effects on urinary 9α, 11β-prostaglandin (PG) F2 levels (a sensitive biomarker of mast cell de-granulation) were enhanced during repeated low-dose allergen challenge in allergic asthmatics (Ihre et al. 2006). These earlier findings in asthmatics were confirmed by a recent study showing an increase in plasma 9α, 11β-PGF2 levels after adenosine challenge in asthmatics (Bochenek et al. 2008). These studies suggest that following inhalation, adenosine enhances the release of systemic inflammatory mediators from sensitized inflammatory cells. Thus, following inhalation, adenosine not only produces inflammation in the airways of asthmatics but it also induces a systemic inflammatory response that would, in turn, amplify the inflammation locally in the airways of asthmatics.
Adenosine in the lung may also be involved in the airway remodeling process (Cohn et al. 2004). Pathogenic hallmarks of airway remodeling are mucous gland hyperplasia, subepithelial fibrosis, hypertrophy of bronchial smooth muscle, and angiogenesis (Cohn et al. 2004; Jarjour and Kelly 2002). In a recent report, substantial angiogenesis in the tracheas of ADA-deficient mice were seen in association with high levels of adenosine (Mohsenin et al. 2007). ADA replacement enzyme therapy in these mice resulted in a lowering of adenosine levels and reversal of tracheal angiogenesis. Moreover, in lung alveolar epithelial cells and lung fibroblasts, adenosine caused an induction of fibronectin (a matrix glycoprotein highly expressed in injured tissues that has been implicated in wound healing) mRNA and protein expression in a dose- and time-dependent manner (Roman et al. 2006). Furthermore, there appears to be a connection of IL-13 levels to high adenosine levels, ADA activity and airway remodeling (Blackburn et al. 2003). Studies in CC10 IL-13 Tg mice showed that IL-13 induced high levels of adenosine, inflammation, lung collagen content and subepithelial airway fibrosis and reduced ADA activity in the lung. ADA therapy administered to these mice decreased adenosine levels, inflammation, and subepithelial airway fibrosis (Blackburn et al. 2003). Moreover, in ADA-deficient mice, IL-13 was strongly induced. These findings suggest that Il-13 and adenosine stimulate one another to amplify the pathway that contributes to airway inflammation, fibrosis, and remodeling. Similar findings were also seen in the lungs of mice overexpressing the Th2 cytokine IL-4 (Ma et al. 2006).
2 Adenosine Receptors in Asthma
Collectively, the studies presented above suggest a strong role for adenosine not only in the bronchoconstriction of allergic airways but also in the progression and amplification of airway inflammation and airway remodeling. The effects of adenosine as an important signaling molecule in asthma may depend not only on the bioavailability of the nucleoside but also on the expression, density, and affinity of ARs, which are known to be finely modulated by physiological and/or pathological conditions, signaling mechanisms, the local metabolism of adenosine, and the predominant inflammatory cell types in the asthma model, which may be species specific (Chunn et al. 2001; Fan et al. 2003; Sun et al. 2005; Zhong et al. 2006).
Adenosine produces its effects in asthmatics by acting on membrane-bound extracellular ARs on target cells. Four subtypes of ARs (namely A1, A2A, A2B, and A3) have been cloned in humans, are expressed in the lung, and are all targets for drug development for human asthma (Polosa 2002; Rorke and Holgate 2002). These receptors are heptaspanning-transmembrane G-protein-coupled receptors. Three of the AR subtypes (A1, A2A, and A2B) demonstrate 80–95% sequence homology across a wide evolutionary range of species (Fredholm et al. 2001). In contrast, the A3ARs demonstrate significant species variation. Signal transduction by the ARs varies; not only among the subtypes but also for a particular subtype between different cell sources (Fredholm et al. 2001). A1ARs were originally characterized as being coupled to pertussis-toxin-sensitive Gi-coupled signal transduction pathways, but in some cells they are directly associated with, and act through, ion channels. The A2AR subtypes (A2A and A2B) are typically coupled to Gs-linked signal transduction pathways. In some cells, A1AR receptor-mediated inhibition and A2AAR-mediated stimulation of adenylate cyclase may coexist and their functions may be counterregulatory (Fredholm et al. 2001). A summary of the AR subtypes, their signal transduction mechanisms, and selective agonists and antagonists is presented in Table 1.
Table 1.
Characteristics and pharmacology of adenosine receptors
| A1AR | A2AAR | A2BAR | A3AR | |
|---|---|---|---|---|
| Agonists | CPA, CCPA, CHA, S-ENBA | CGS 21680, ATL146e, CV-1808, CVT-3146, MRE0740; MRE0094 | BAY 60-6583 | IB-MECA (CF 101), 2-Cl–IB–MECA (CF102), MRS3558 (CF502) |
| Antagonists | DPCPX, FSCPX, N-0861, BG-9719, BG-9928, WRC-0571; KW 3902, L-97-1, SLV320, EPI-2010 | ZM 241385, KW 6002, SCH 58261, SCH 442416, CSC | IPDX, MRS1754, MRS1706, CVT-6883, CVT-5440 | MRS1220, MRS1191, MRS1523, VUF 5574 |
| Transduction mechanisms | Gi/o, ↓ cAMP; ↑K+, ↓ Ca2+channels, PLA2; Gα16 NF-κB, PLC, PKC, ↑IP3/DAG | Gs/olf, ↑cAMP | Gs/↑cAMP; Gq/PLC, ↑IP3/DAG | Gi/↓cAMP; ↑Ca2+, ERK1/2, Gq/PLC, ↑IP3/DAG; PLD |
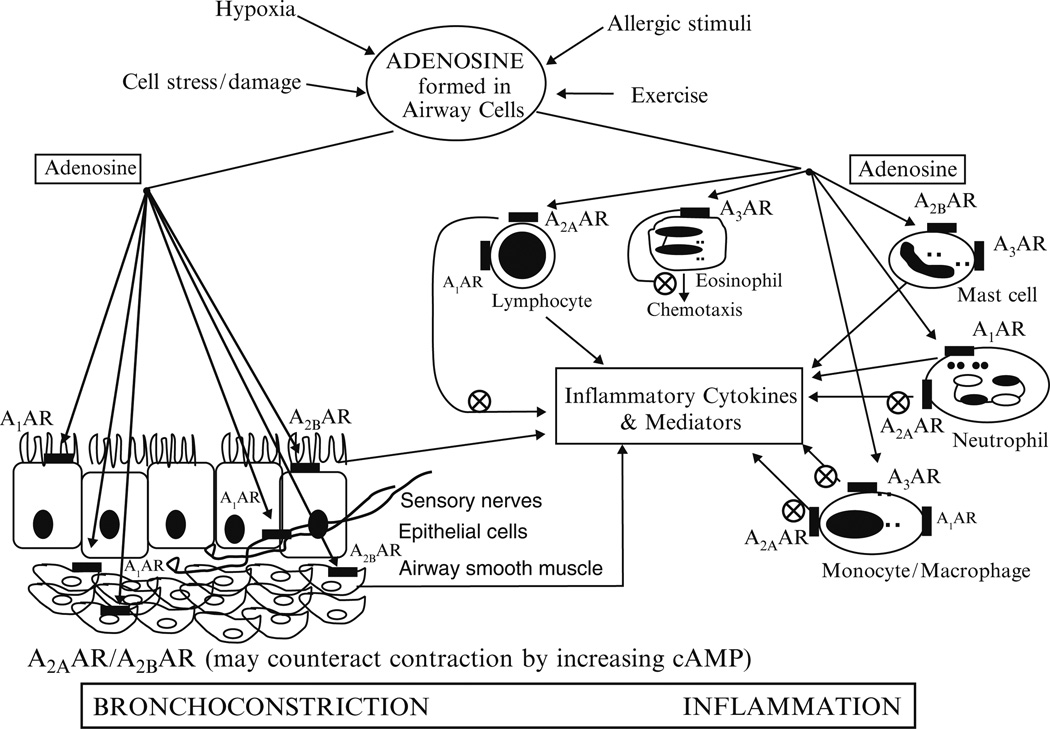
Adenosine receptors have been described on a number of different cell types that are important in the pathophysiology of asthma, including dendritic, antigen-presenting cells, human airway epithelial and bronchial smooth muscle cells, lymphocytes, mast cells, eosinophils, neutrophils, macrophages, fibroblasts and endothelial cells (Thiel et al. 2003; Young et al. 2006; Wilson 2008). Activation of ARs on these different cell types is responsible for inducing the release of mediators and cytokines, leading to BHR, inflammation, edema, and airway remodeling. Activation of ARs on afferent sensory airway nerves contributes to BHR in asthma (Hua et al. 2007a). The contributions of the different AR subtypes to the pathophysiology of asthma will be discussed in the following sections and are presented in Fig. 2. In this review, the pathophysiological role of each AR and its signaling in asthma is discussed. Furthermore, the targeting of ARs with selective agonists or antagonists as therapeutic strategies in the treatment of asthma is also discussed and is presented in Table 2.
Fig. 2.
Adenosine receptors and pathophysiology of asthma. By acting on adenosine receptors (ARs), A1, A2A, A2B, and A3 ARs, adenosine released under conditions of cellular stress as seen in asthmatic airways produces bronchoconstriction and inflammation. The net effect of adenosine on ARs will depend on the relative expression of these receptors on different cell types in asthmatic airways, and is concentration-dependent, as adenosine frequently exhibits opposing effects through the activation of AR subtypes expressed on the same cells coupled to different G proteins and signaling pathways. By acting on A1ARs on bronchial smooth muscle cells and afferent sensory airway nerves, adenosine produces bronchoconstriction. By acting on A1ARs on inflammatory leukocytes such as neutrophils, monocytes, macrophages, and lymphocytes, adenosine produces proinflammatory effects. Activation of A2AARs on the inflammatory cells suppresses the release of proinflammatory cytokines and mediators. Activation of A2AARs coupled to Gs and adenylate cyclase may also lead to bronchial smooth muscle relaxation via the cAMP–PKA (cyclic adenosine monophosphate–protein kinase A) pathway. Activation of A2BARs coupled to Gs and adenylate cyclase induce cytokine release from human bronchial epithelial and smooth muscle cells. Activation of A2BARs on murine bone marrow-derived mast cells (BMMCs) regulates the release of cytokines. The effect of adenosine on A3ARs is species dependent. In mice, rats, and guinea pigs, activation of A3ARs by adenosine produces bronchoconstriction, airway inflammation, mast cell degranulation, and mucus hyperplasia. In humans, activation of A3ARs by adenosine produces anti-inflammatory effects, inhibition of chemotaxis and degranulation of eosinophils and cytokine release from monocytes. Circled times denote inhibition
Table 2.
Comparison of different potential therapeutic approaches targeting adenosine receptors in asthma
| A1AR antagonists | A2AAR agonists | A2BAR antagonists | A3AR agonists | A3AR antagonists | |
|---|---|---|---|---|---|
| Potential effects | Inhibition of bronchoconstriction, mucus hypersecretion, and inflammation | Bronchodilation and inhibition of inflammation | Inhibition of bronchoconstriction, inflammation, and airway remodeling | Inhibition of inflammation | Inhibit bronchoconstriction, inflammation, mucus hyperplasia |
| Disadvantages | No safety concerns reported to date for A1AR antagonists in humans | CV side effects; tachyphylaxis; immune suppression | Reduce airway hydration; bronchoconstriction; inflammation | Tachyphylaxis; immune suppression | Inflammation |
| Latest developments in asthma | L-97–1 (Preclinical); EPI-2010 (Phase II; discontinued, no additional effect with ICSs) | GW328267X (Phase II; discontinued due to CV side effects) | CVT 6883 (Phase I); QAF 805 (Phase Ib) | QAF 805 (Phase Ib) | |
| Pharmaceutical company involved in AR drug discovery | Epigenesis Pharmaceuticals; Endacea, Inc.; Biogen Idec; Merck; Solvay Pharmaceuticals; OSI Pharmaceuticals, Inc. | Glaxo Group Ltd; Pfizer; Novartis | CV Therapeutics; Novartis | Can-Fite Biopharma | Novartis |
AR, Adenosine receptor; CV, cardiovascular; ICSs, inhaled corticosteroids
Adapted with permission from Wilson (2008)
2.1 A1 Adenosine Receptors and Asthma
Until relatively recent times, the A1AR received little attention as an important target in human asthma. However, a number of reports have demonstrated that expression of the A1AR is upregulated in the airways of both animal models of allergic airway inflammation and human asthmatic subjects. Moreover, it is now appreciated that various functions relevant to asthma have also been associated with activation of the A1AR, including bronchoconstriction, leukocyte activation and inflammation, BHR, and mucus secretion.
A pivotal study generated convincing evidence that A1ARs could well play a significant role in the pathophysiology of asthma. The authors demonstrated that airway obstruction in response to aerosol administration of adenosine and allergen was inhibited in a rabbit model of allergic airway inflammation following treatment with antisense oligonucleotides as well as antagonist to this receptor (Ali et al. 1994a, b; Nyce and Metzger 1997). These data suggested that the A1AR not only directly mediates bronchoconstriction following administration of exogenous adenosine, but that endogenous adenosine is an important component of the allergic response.
Although important species differences have been observed with regards to the expression and function(s) of the four AR subtypes, there is evidence that supports similar observations of an increased expression of the A1AR and of A1AR-induced bronchoconstriction in human asthmatic subjects. Firstly, it was demonstrated that adenosine-induced contraction of isolated bronchial tissue in vitro was greater in tissues obtained from asthmatic subjects than healthy subjects, and that this contraction could be significantly inhibited following preincubation with a selective A1AR antagonist (Bjorck et al. 1992). Furthermore, it has been very recently demonstrated for the first time that expression of the A1AR is increased in bronchial biopsies obtained from steroid-naïve mildly asthmatic subjects when compared with healthy subjects (Brown et al. 2008). This increased expression of the A1AR appeared to be predominantly located in the airway epithelium and smooth muscle regions of the tissue, the latter observation thus correlating with the preclinical findings in the rabbit model of allergic asthma. In support of this, it has been demonstrated that activation of the A1AR on human airway smooth muscle cells in vitro results in an increase in intracellular calcium mobilization, which could potentially mediate airway smooth muscle contraction (Ethier and Madison 2006). The finding of increased expression of A1ARs in the airways and increased sensitivity of the airways to adenosine could well be of clinical significance. In asthmatics, the level of adenosine in plasma and exhaled breath condensate is increased following allergen or exercise challenge (Csoma et al. 2005; Mann et al. 1986a; Vizi et al. 2002) and therefore could lead to the activation of A1ARs, thereby contributing toward airway obstruction during an acute exacerbation of asthma.
The report that the expression of A1ARs is increased in bronchial biopsies of asthmatics is confirmed by the findings from another laboratory. In a preliminary study of a small number of human subjects, gene expression for A1ARs is increased approximately 200% in bronchial tissue from small airways obtained from asthmatics (n=3) versus normal subjects (n=3) (Nadeem and Mustafa, unpublished data,West Virginia University). In these studies, expression of A2AARs is decreased while there is little to no change in the expression of A2BARs and A3ARs in bronchial tissue from small airways in asthmatics versus normal subjects. The results of these studies were determined with the use of RT-PCR and confirmed with the use of western blots, with the exception of the A2BAR, which was not tested in western blot studies.
A number of other studies using experimental animals have implicated a role for A1ARs in mediating airway obstruction to adenosine. For example, the A1AR agonist cyclopentyladenosine (CPA) selectively induces airway obstruction only in sensitized guinea pigs (Keir et al. 2006) and allergic rabbits (Ali et al. 1994a; el-Hashim et al. 1996). Further studies with the allergic rabbit model demonstrated that CPA also induced bronchoconstriction and stimulated IP3 generation in airway smooth muscle (Abebe and Mustafa 1998). Allergic rabbits treated with the selective A1AR antagonist L-97-1 ([3-(2-(4-aminophenyl)-ethyl]-8-benzyl-7-(2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl)-1-propyl-3,7-dihydro-purine-2,6-dione]) provided bronchoprotection against inhaled adenosine (Obiefuna et al. 2005). However, atypical (Hannon et al. 2002) and adenosine A1, A2B and A3 ARs (Fan et al. 2003; Hua et al. 2007a) have been suggested to mediate airway obstruction in response to adenosine in the brown Norway rat and mouse, respectively, underlying important species and strain differences.
Expression of the A1AR has also been identified on a number of inflammatory cells. In general, these effects appear to be proinflammatory in nature. Activation of the A1AR on human eosinophils, for example, promotes superoxide release (Ezeamuzie and Philips 1999). Furthermore, the A1AR also mediates the respiratory burst in neutrophils (Salmon and Cronstein 1990), in addition to chemotaxis (Cronstein et al. 1990) and their adherence to endothelial cells (Cronstein et al. 1992). Furthermore, adenosine has been shown to promote monocyte phagocytosis (Salmon et al. 1993) and chemotaxis of immature dendritic cells (Panther et al. 2001), in addition to increasing the release of cytotoxic substances from endothelial cells that increase endothelial cell permeability (Wilson and Batra 2002) via the A1AR.
The effects of adenosine upon inflammatory cells have been determined largely from in vitro experiments, and it should be noted that these effects are concentration dependent, as adenosine frequently exhibits opposing effects through the activation of other AR subtypes expressed on the same cells, since they are coupled to different G proteins. Thus, the relative expression of these receptors on inflammatory cells resident in asthmatic airways and the overall cellular effect of adenosine at the concentration present remain to be determined. It is likely, however, that the pattern of cellular expression for ARs changes following exposure to adenosine, since experimental evidence shows that an increased extracellular level of adenosine somewhat unusually appears to promote AR signaling. This was unequivocally demonstrated in mice partially deficient in ADA that consequently have high levels of adenosine in the lung (Chunn et al. 2001). Besides the severe pulmonary inflammation typical of this phenotype, these mice exhibited an increased transcript level for the A1,A2B and A3 ARs.
In light of the many studies demonstrating the proinflammatory action attributed to activation of the A1ARs, it is perhaps surprising that a preclinical study has purported to document an anti-inflammatory effect of A1AR signaling (Sun et al. 2005). Adenosine deaminase is a ubiquitous enzyme responsible for the inactivation of adenosine, and mice deficient in this protein demonstrate profound pulmonary injury, the presence of elevated levels of macrophages, and increased mucus production. These indices of tissue damage were exacerbated in ADA double-knockout mice also deficient in the expression of A1AR, thereby implicating the loss of an anti-inflammatory pathway mediated by this receptor (Sun et al. 2005). However, the relevance of this model to human asthma or chronic obstructive pulmonary disease is debatable, since two of the principal cell types observed in these diseases, namely eosinophils and neutrophils, respectively, are present in such small numbers (<1.7%). In contrast to these findings, the A1AR antagonist L-97-1 inhibited the recruitment of eosinophils and neutrophils to the airways of allergic rabbits challenged with house dust mite antigen (Nadeem et al. 2006).
Very few studies have specifically addressed the question of whether activation of A1ARs is important in the development of BHR. Animal models of allergic inflammation are characterized by increased sensitivity to inhaled histamine, and interference in A1AR signaling following either treatment with an antisense against this receptor (Nyce and Metzger 1997) or the use of a selective antagonist (Nadeem et al. 2006; Obiefuna et al. 2005) provided some degree of protection against the development of BHR. One can only speculate as to the mechanism by which adenosine, released within the inflammatory milieu of the airways, causes BHR via an A1AR-dependent mechanism. Activation of these receptors on inflammatory cells including mast cells, eosinophils, dendritic cells, and lymphocytes could stimulate the release of other inflammatory mediators that, in turn, increase the sensitivity of the airways. Alternatively, adenosine might stimulate C fibers, thereby lowering the threshold for the activation of afferent input into the nucleus tractus solitarius, and thus facilitating reflex activation of parasympathetic nerves (Chuaychoo et al. 2006; Hong et al. 1998).
The mechanism(s) by which adenosine mediates airway obstruction in vivo in animal models may constitute indirect components. For example, adenosine activates pulmonary C fibers in the rat (Hong et al. 1998) and in the guinea pig (Chuaychoo et al. 2006; Lee et al. 2004), and cholinergic neural pathways in conscious mice (Hua et al. 2007a) via an A1 AR-dependent mechanism. Moreover, the effect of activation of A1ARs by a selective A1AR agonist, CPA, was specific for nodose but not jugular ganglion-derived C fibers (Chuaychoo et al. 2006). The consequence of activating these nerves following the endogenous release of adenosine during an inflammatory response may be airway obstruction, a phenomenon that was abolished in guinea pigs chronically treated with capsaicin in order to chemically inactivate C fibers (Keir et al. 2006). Reflex activation of parasympathetic nerves was further implicated, since vagotomy or treatment with the muscarinic antagonist atropine attenuated bronchospasm induced by CPA (Keir et al. 2006). Moreover, in mice, an adenosine-induced increase in airway resistance was abolished in A1AR knockout mice and following vagotomy in wild type mice, but not in A2A, A2B, or A3 AR knockout mice (Hua et al. 2007a). In conscious mice, the adenosine-induced increase in airway resistance was significantly reduced by the selective A1AR antagonist 1,3-dipropyl–8-cyclopentylxanthine (DPCPX) as well as atropine and bupivacaine, suggesting that the adenosine-induced bronchoconstriction was via the activation of A1ARs on the cholinergic neural pathway. Similarly, the cholinergic-dependent reflex activation of tracheal smooth muscle in situ in response to CPA was mediated by the activation of A1AR (Reynolds et al. 2008).
Finally, in addition to its effects on bronchoconstriction, leukocyte activation and inflammation, and BHR, the A1AR may play an important role in mucus secretion and airway remodeling of human asthma. It has been shown that adenosine is able to induce mucus secretion via activation of the A1AR in the canine trachea in vivo (Johnson and McNee 1985), which has now been confirmed in human bronchial epithelial cells in vitro, where activation of the A1AR was shown to increase the expression of the MUC2 mucin gene (McNamara et al. 2004). Thus, it could be speculated that the reported increased expression of A1AR on asthmatic bronchial epithelium (Brown et al. 2008) promotes adenosine-induced mucin secretion, although the extent to which adenosine contributes to the overall mucus hypersecretion in asthma clearly remains to be determined. Further studies will hopefully precisely define the functional effects of the A1AR expressed in human asthmatic epithelium. With respect to a potential role of A1ARs in airway remodeling, recent reports, albeit not pertaining to the lung per se, suggest that activation of A1ARs may play an important role in angiogenesis and fibrosis, cardinal features of airway remodeling in human asthma (Clark et al. 2007; Cohn et al. 2004; Kalk et al. 2007). For example, activation of A1ARs on human monocytes induces the release of vascular endothelial growth factor (VEGF) (Clark et al. 2007), and an A1AR antagonist with high affinity and high selectivity for the human A1AR, SLV320, significantly reduced levels of collagen I and III in an animal model of myocardial fibrosis (Kalk et al. 2007).
Validation of the A1AR as an important target for human asthma is supported by positive proof of concept (POC) results in patients with asthma for EPI-2010, an antisense (“knockout”) compound that is a respiratory antisense oligonucleotide (RASON) for the human A1AR, in a small clinical trial conducted by EpiGenesis Pharmaceuticals (Cranbury, NJ, USA). EpiGenesis reported that a single dose of EPI-2010 reduced the need for bronchodilator drugs to control asthma symptoms concomitant with a reduction in symptom scores, an effect that was statistically and clinically significant and lasted for one week following a single dose (Ball et al. 2003). However, disappointing results in a Phase II clinical trial with EPI-2010 administered to patients who were taking inhaled corticosteroids (ICSs) were reported (Langley et al. 2005). In this Phase II clinical trial, 146 patients with persistent airway obstruction (forced expiratory volume in 1 S (FEV1) 74.5% predicted, ≥12% reversibility) and currently receiving ICSs were administered EPI 2010 (1, 3, or 9 mg) via nebulizer once or twice weekly for 29 days. In this clinical study there was no significant change in the FEV1 after 29 days of treatment compared to baseline. It was concluded that EPI-2010 showed no additional therapeutic effect in patients currently receiving ICSs. Patients with a stable FEV1 of 74.5% predicted have mild/moderate asthma, depending on the frequency of symptoms and magnitude of variability in the peak expiratory flow rate (PEFR). In patients with mild/moderate asthma treated with ICSs, the FEV1 may be 90–100% of the predicted value when measured between exacerbations and without provocation. Thus, the FEV1 is not a sensitive measure of asthma severity per se, vis-à-vis acute changes in airway function reflected by PEFR variability in ICS-treated patients with mild/moderate asthma. The lack of efficacy for EPI-2010 in this Phase II clinical trial (i.e., that EPI-2010 showed no additional therapeutic effect in patients taking ICSs) was not surprising.
Because of these effects of activation of A1ARs on different cell types to produce bronchoconstriction, inflammation, mucous gland hyperplasia, angiogenesis, and fibrosis, all of which are important in the pathophysiology of human asthma, an A1AR antagonist, L-97-1 (Endacea, Inc.), is in development as a once-daily, oral treatment for human asthma. L-97-1 is a water-soluble, small-molecule A1AR antagonist with high affinity and high selectivity for the human A1AR (Obiefuna et al. 2005). In an animal model of allergic asthma, L-97-1 blocks allergic airway responses, BHR to histamine, and airway inflammation (Nadeem et al. 2006; Obiefuna et al. 2005). A number of A1AR antagonists have been or currently are in clinical trials for a number of different medical indications and, as a class, appear to be safe and well tolerated in humans (Barrett 1996; Bertolet et al. 1996; Dittrich et al. 2007; Doggrell 2005; Gaspardone et al. 1993; Givertz et al. 2007; Gottlieb et al. 2002; Greenberg et al. 2007).
2.2 A2A Adenosine Receptors and Asthma
A2A AR signaling in the pathophysiology of asthma may be critical considering the fact that A2AARs are present on most of the inflammatory cells (including neutrophils, mast cells, macrophages, eosinophils, platelets, and T cells; Lappas et al. 2005; Thiel et al. 2003). Activation of A2AAR on these cell types is almost universally inhibitory, and therefore could modulate inflammatory events in the airways. The anti-inflammatory effects of activation of A2AAR on these cell types include inhibition of chemotaxis, elastase release, phagocytosis, oxidative stress, adherence of neutrophils to endothelial cells, mast cell degranulation, and the release of proinflammatory cytokines (Lappas et al. 2005; Nadeem et al. 2007).
There are a multitude of mechanisms by which an agonist, acting through A2AARs, could suppress inflammation in asthmatic airways. In human neutrophils, stimulation of A2AAR reduces neutrophil adherence to the endothelium, inhibits formyl-Met–Leu–Phe (fMLP)-induced oxidative burst, and inhibits superoxide anion generation (Visser et al. 2000). In monocytes and macrophages, activation of A2AARs inhibits LPS-induced tumor necrosis factor (TNF)-α expression (Bshesh et al. 2002). A2AAR-deficient allergic mice have increased oxidative stress in the lung as well as the airway smooth muscle after ragweed/ovalbumin allergen challenge as compared to their wild type. This oxidative stress is caused by activation of inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase signaling due to A2AAR deficiency (Nadeem et al. 2007, 2008). Moreover, genetic removal of the A2AAR from ADA-deficient mice results in enhanced inflammation (composed largely of macrophages and neutrophils, mucin production in the bronchial airways, and angiogenesis) relative to that seen in the lungs of ADA-deficient mice with the A2AAR, suggesting a protective role of this receptor in pulmonary inflammation when adenosine levels are high (Mohsenin et al. 2007). A2AAR-mediated suppression of inflammation is mainly thought to be mediated by activation of protein kinase A and cyclic AMP response element-binding protein (Allen-Gipson et al. 2005; Bshesh et al. 2002), and inhibition of nuclear factor kappa B (NF-κB) signaling (Bshesh et al. 2002; Lukashev et al. 2004; Nadeem et al. 2007).
Strong anti-inflammatory properties for A2AAR have been shown in an inflammatory disease model using A2AAR gene-deficient mice (Lukashev et al. 2004; Nadeem et al. 2007). Consistent with this, in rat and mouse animal models of allergic asthma, the selective A2AAR agonist CGS 21680 (2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxoamido adenosine) significantly reduced the number of inflammatory cells in the BAL fluid during allergen-induced airway inflammation (Bonneau et al. 2006; Fozard et al. 2002). However, in these rodent animal models of allergic asthma, this selective A2AAR agonist reduced airway inflammation but not BHR. Moreover, in an A2AAR-deficient allergic mouse model, not only was airway inflammation enhanced but BHR was too (Nadeem et al. 2007). The discrepancy in A2AAR-deficient allergic mice on airway reactivity versus the earlier report wherein airway reactivity was not reduced with the A2AAR agonist, CGS 21680, in allergic mice is not apparent and may be due to differences in strains of mice.
Recently, the effects of a new A2AAR agonist, GW328267X, in human asthmatics was reported (Luijk et al. 2008). In this study, treatment with GW328267X delivered as an inhalational treatment did not protect against the late asthmatic response (LAR), expressed as the decline in FEV1 after allergen challenge, or the accompanying increase in airway inflammation (Luijk et al. 2008). However, in an earlier study, GW328267X partially inhibited the early asthmatic response (EAR) and LAR after nasal allergen challenge in patients with allergic rhinitis (Rimmer et al. 2007). There may be several possible explanations for the observed discrepancies between these two human studies. First, this A2AAR agonist is not entirely selective for the A2AAR; it also exhibits some inhibitory effect on A3AR (Luijk et al. 2008). It is possible that inhibition of the A3AR by GW328267X blocked the anti-inflammatory effects of A3AR activation by adenosine, since it is reported that activation of the A3AR in humans produces anti-inflammatory effects, including inhibition of migration of human eosinophils and inhibition of oxidative burst, degranulation and release of inflammatory cytokines in human neutrophils, monocytes, and macrophages (Fishman and Bar-Yehuda 2003). Thus, the inhibitory effect of GW328267X on A3ARs may have counteracted possible beneficial effects of A2AAR activation. Secondly, it is possible that the dose of the GW328267X (inhaled dose, 25 µg twice daily) was subtherapeutic. It was previously determined that higher doses of GW328267X caused cardiovascular side effects (reduction in blood pressure and increase in heart rate) following inhalational delivery (Luijk et al. 2008).
As mentioned above, even following inhalational delivery in small doses, the cardiovascular side effects of A2AAR agonists may limit their clinical development. Moreover, tachyphylaxis and immune suppression may limit the clinical efficacy and safety of A2AAR agonists as antiasthma drugs. For example, with the chronic administration of A2AAR agonists, tachyphylaxis to the bronchodilator and anti-inflammatory effects may occur via the desensitization of Gs-coupled intracellular signaling pathways (Sullivan 2003). This potential effect of A2AAR agonists was evident with the chronic administration of CGS-21680 over a two-week period wherein tachyphylaxis to the blood pressure lowering effect was reported and prevented the development of this A2AAR agonist as an antihypertensive agent (Webb et al. 1993). Furthermore, because A2AAR agonists act via Gs to stimulate adenylate cyclase, they may be associated with an increased risk of sudden death in asthmatics in a similar fashion to that of long-acting β2-agonists (LABAs) (Salpeter et al. 2004). Moreover, activation of A2AARs produces neovascularization (angiogenesis) (Cronstein 2006; Montesinos et al. 1997; Montesinos et al. 2006). Because of this effect of A2AARs on neovascularization/angiogenesis, an A2AAR agonist, MRE-0094, is in Phase II clinical trials as a treatment for wound healing in diabetic foot ulcers (Aderis Pharmaceuticals). However, angiogenesis is a cardinal feature of airway remodeling of human asthma, and despite the report that activation of A2AARs promotes wound healing in bronchial epithelial cells (Allen-Gipson et al. 2005), the effect of A2AAR agonists on angiogenesis may limit their development as anti-asthma drugs.
Further to these clinical considerations for the development of A2AAR agonists as antiasthma drugs, others include their potential to produce antitumor effects and immune suppression (Ohta et al. 2006; Sullivan 2003). Because A2AAR agonists block oxidative and nonoxidative activity of neutrophils, cause functional repression and/or apoptosis of lymphocytes, and inhibit the release of (interleukin) IL-12, which promotes bacterial clearance in infection, these agents may cause immune suppression and predispose to infection (Sullivan 2003). Moreover, in an adenosine-rich tumor microenvironment, activation of A2AARs produces inhibition of antitumor T cells (Ohta et al. 2006). In mice, genetic deletion of the A2AAR or the use of A2AAR antagonists improved inhibition of tumor growth, destruction of metastasis and prevention of neovascularization by antitumor T cells. Despite what should be advantageous effects from the activation of A2AARs (i.e., bronchodilation and anti-inflammatory effects), the potential side effects of hypotension and tachycardia may limit the use of A2AAR agonists as acute rescue antiasthma drugs, and the potential side effects of tachyphylaxis and immune suppression as well as the angiogenesis and antitumor effects produced by A2AAR agonists may limit the use of these molecules as chronic maintenance antiasthma drugs.
2.3 A2B Adenosine Receptors and Asthma
Studies in animals both in vitro and in vivo and human cell lines in vitro have suggested that A2BARs may play an important role in mediating airway reactivity, inflammation, and remodeling in asthma. A2BARs are coupled via both Gs and Gq proteins to intracellular signaling pathways, which results in the release of cytokines and other mediators that are important in the pathophysiology of human asthma (Feoktistov et al. 1999; Zhong et al. 2004). In a human mast cell line (HMC-1), by coupling primarily to Gq, activation of A2BARs by adenosine induces the release of inflammatory cytokines such as IL-4, IL-8 and IL-13 which, in turn, can induce immunoglobulin E (IgE) synthesis by B lymphocytes (Feoktistov et al. 1999; Ryzhov et al. 2004). Moreover, in these HMC-1 cells, the selective A2BAR antagonists IPDX (3-isobutyl–8-pyrrolidinoxanthine) and MRS 1754 ([N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide]) inhibited activation of HMC-1 cells induced by NECA (5′-N-ethylcarboxoamido adenosine), a stable analog of adenosine (Feoktistov et al. 2001). Since HMC-1 cells are derived from a highly malignant, undifferentiated human mastocytoma cancer, the relevance of these findings in this human mast cell line to that in IgE immunologically sensitized human mast cells in allergic asthma is unknown. In the allergic response, antigens bind and crosslink IgE molecules bound to the functional high-affinity receptor for IgE, FcεRI, on mast cells to induce degranulation and the release of a broad spectrum of proinflammatory mediators (Nilsson et al. 1994; Xiang et al. 2001). HMC-1 cells do not express FcεRI (Nilsson et al. 1994). For this reason the reference to HMC-1 cells as human mast cells in allergic conditions, including human asthma, is misleading.
The presence of A2BARs on IgE immunologically sensitized human mast cells has not been reported. However, bone marrow-derived mast cells (BMMCs) from mice do express FcεRI (Hua et al. 2007b). Moreover, as opposed to the HMC-1 cells, A2BARs on murine BMMCs (Hua et al. 2007b), as well as human bronchial epithelial and smooth muscle cells and fibroblasts (Zhong et al. 2003, 2004, 2005), are coupled to Gs and adenylate cyclase, as compared to HMC-1 cells, where they are primarily coupled to Gq and phospholipase C (PLC) (Feoktistov and Biaggioni 1995). Furthermore, as opposed to the HMC-1 cell line, mast cell activation is enhanced in mice deficient for the A2BAR (Hua et al. 2007b). The authors of this study suggested that in mice lacking this Gs-coupled receptor, BMMCs expressing FcεRI have reduced levels of cyclic AMP and an excess of intracellular calcium via store-operated calcium channels following antigen activation, thereby increasing their sensitivity to antigen-mediated degranulation. In addition, these A2BAR-deficient mice display an increased sensitivity to IgE-mediated tachyphylaxis. In a recent study, genetic ablation of the A2BAR had no effect on A3AR-dependent potentiation of antigen-induced degranulation in mouse BMMCs, but abrogated A2BAR-induced release of IL-13 and VEGF. The authors of this study suggest that in the mouse the A3AR regulates mast cell degranulation, whereas the A2BAR regulates mediator release, e.g., IL-13 and VEGF (Ryzhov et al. 2008a)
As mentioned above, as opposed to that seen in HMC-1 cells, by coupling to Gs and adenylate cyclase, adenosine activation of A2BAR increases the release of inflammatory cytokines from human bronchial epithelial cells (HBECs) (Zhong et al. 2006), human bronchial smooth muscle cells (Zhong et al. 2004) and human fibroblasts (Zhong et al. 2005). In human bronchial smooth muscle cells, activation of A2BARs induces the release of IL-6 and the chemokine monocyte chemotactic protein 1 (MCP-1) (Zhong et al. 2004). In HBECs, activation of A2BARs induces the release of IL-19, which in turn induces the release of TNF-α from monocytes, which in turn upregulates the expression of A2BARs on HBECs (Zhong et al. 2006). In human lung fibroblasts, activation of A2BARs induces the release of IL-6, which, in the presence of hypoxia, synergistically induced the differentiation of lung fibroblasts into myofibroblasts (Zhong et al. 2005). These effects of activation of A2BARs by NECA, a stable analog of adenosine, in human bronchial smooth muscle cells (Zhong et al. 2004) and HBECs (Zhong et al. 2006) are blocked by selective antagonists of the A2BAR. Furthermore, in a recent study, genetic ablation of A2BAR abrogated NECA-induced increases in IL-6 release from mouse peritoneal macrophages ex vivo and dramatically reduced the ability of NECA to increase IL-6 plasma levels in vivo (Ryzhov et al. 2008b). Moreover, stimulation of the A2BAR on isolated mouse BMMCs can directly promote the production and secretion of IL-13 and VEGF (Ryzhov et al. 2008a). Taken together, these studies indicate that stimulation of A2BAR is coupled to the release of proinflammatory cytokines, and may play an important role in airway remodeling of asthma.
Although the importance of these in vitro studies to support the role of the A2BAR in vivo in humans with asthma remains to be determined, studies in animal models of allergic asthma support the role of this AR in asthma. In ragweed-sensitized allergic mice, airway challenge with adenosine increased bronchoconstrictor responses and amplified the pulmonary inflammatory response to an allergen challenge (Fan and Mustafa 2002, 2006). This increase in bronchoconstrictor responses and airway inflammation to adenosine was blocked by theophylline and attenuated by a specific antagonist of the A2BAR, which suggests, in part, a role for the A2BAR (Fan and Mustafa 2002; Fan et al. 2003; Mustafa et al. 2007). Moreover, in this allergic mouse model of asthma, adenosine-induced increases in β-hexosaminidase activity (a mast cell marker) were decreased by pretreatment with theophylline (Fan and Mustafa 2006). Furthermore, in another study involving the use of this allergic mouse model of asthma from this same group, aerosolized NECA- and AMP-elicited concentration-dependent increases in Penh were significantly attenuated by CVT-6883, an A2BAR antagonist (Mustafa et al. 2007). In this study, an allergen challenge-induced increase in LAR was inhibited by CVT-6883, and the increase in the number of inflammatory cells in BAL fluid was also inhibited by CVT-6883 or theophylline.
These findings, that the A2BAR antagonist CVT-6883 reduces inflammation in the lung in an animal model of allergic asthma, were demonstrated in another animal model of lung inflammation with a phenotype similar to allergic asthma, albeit not an allergic asthma animal model, ADA-deficient mice (Sun et al. 2006). As previously stated, ADA-deficient mice develop pulmonary inflammation, fibrosis, and enlargement of alveolar airspaces. In CVT-6883-treated ADA-deficient mice there was less pulmonary inflammation, fibrosis, and alveolar airspace enlargement (Sun et al. 2006). Moreover, in ADA-deficient mice, A2BAR antagonism with CVT-6883 significantly reduced elevations in proinflammatory cytokines and chemokines as well as mediators of fibrosis and airway destruction (Sun et al. 2006). These findings in these animal models suggest that A2BAR signaling influences pathways critical for airway reactivity and inflammation.
As opposed to these reports suggesting that activation of A2BARs play an important role in bronchoconstriction and airway inflammation in allergic asthma, recent reports suggest that activation of A2BARs may produce bronchorelaxant and anti-inflammatory effects. In a recent study in guinea pigs, NECA evoked relaxing responses of isolated tracheal preparations precontracted with histamine in normal and sensitized animals, and this effect was reversed by the A2BAR antagonist MRS 1706 (Breschi et al. 2007). Moreover, in vitro desensitization with 100 µM NECA markedly reduced the relaxing effect of NECA, raising the possibility that higher adenosine levels in the lung might desensitize this receptor to cause bronchorelaxation (Breschi et al. 2007). Furthermore, activation of A2BARs may produce anti-inflammatory effects. In A2BAR knockout/reporter gene-knockin mice, there was low-grade baseline inflammation, augmented release of proinflammatory cytokines (including TNF-α and IL-6), as well as leukocyte adhesion to the vasculature (Yang et al. 2006). This finding that TNF-α levels are increased in A2BAR knockout mice was confirmed by a more recent report by the same group (Yang et al. 2008). In a femoral artery injury model that resembles restenosis following angioplasty, A2BAR knockout mice had higher levels of TNF-α, an upregulator of chemokine receptor 4 (CXCR4), and proliferation of vascular smooth muscle cells (Yang et al. 2008).
It is possible that the bronchorelaxant and anti-inflammatory effects of A2BARs described above may be due to an increase in intracellular cyclic AMP levels following activation of A2BARs. It is well known that an increase in intracellular cyclic AMP produces relaxation of bronchial smooth muscle and bronchodilation, suppresses inflammation, and prevents changes in endothelial cells that lead to an increase in endothelial permeability. Given these effects of intracellular cyclic AMP, it is unclear why an approach to the treatment of asthma would be to block these salutary effects of a receptor coupled via Gs to adenylate cyclase (i.e., the A2BAR). It is now reported that the use of A2BAR antagonists may increase endothelial permeability (Lennon et al. 1998). Moreover, in human airway epithelial cells via coupling to Gs and adenylate cyclase, A2BARs play an important role in control of the cystic fibrosis transmembrane conductance regulator (CFTR)-operated Cl− channel (Clancy et al. 1999; Huang et al. 2001). Because of the importance of this Cl− channel in airway hydration, the use of A2BAR antagonists may induce a cystic fibrosis-like phenotype associated with an increased viscosity of mucus in humans, and may therefore limit their development as antiasthma drugs. Thus, although it appears that A2BARs may play an important role in airway remodeling of human asthma, because of their effect on the CFTR-operated Cl− channel in human airway epithelial cells and airway hydration, the safety of A2BAR antagonists in human asthmatics remains to be determined. Moreover, the efficacy of A2BAR antagonists may depend on the relative contribution of this Gs-coupled receptor to adenylate cyclase and increases in intracellular cyclic AMP to produce bronchodilation and anti-inflammatory effects.
With respect to the therapeutic approach to the A2BAR as a target in human asthma, based on the reports that activation of A2BARs in HBECs (Zhong et al. 2006), human bronchial smooth muscle cells (Zhong et al. 2004) and human lung fibroblasts (Zhong et al. 2005) induces the release of mediators important in the pathophysiology of airway remodeling of human asthma, as well as the efficacy of the A2BAR antagonist CVT 6883 in an acceptable animal model of allergic asthma (Mustafa et al. 2007), CVT 6883 has entered Phase I clinical trials as an antiasthma drug (CV Therapeutics, Inc.). Moreover, a combined A2B/A3 AR antagonist, QAF 805 (Novartis), has been tested in humans as an antiasthma drug. This mixed A2B/A3 AR antagonist failed to increase the provocative concentration (PC)20 for AMP (concentration of AMP required to reduce the FEV1 by 20%) versus placebo in 24 AMP-sensitive asthmatics in a placebo-controlled, double-blind, randomized, two-way crossover Phase Ib clinical trial (Pascoe et al. 2007). The results of the clinical trials with CVT 6883 and other selective A2BAR antagonists should more clearly define the role of A2BARs in human asthma, and are eagerly awaited.
2.4 A3 Adenosine Receptors and Asthma
The functional relevance of the A3AR in the pathogenesis of asthma is a matter of debate, primarily due to species differences. In humans, A3ARs have been identified on eosinophils, neutrophils, and monocytes; however, they have not been identified on mast cells (Gessi et al. 2008; Walker et al. 1997). In rats and mice, A3ARs play an important role in adenosine-induced mast cell degranulation, bronchoconstriction, eosinophilia, and mucus production; however, they exhibit poor sensitivity to methylxanthines (Fan et al. 2003; Ramkumar et al. 1993; Tilley et al. 2003; Young et al. 2006). In an A3AR knockout mouse model, a selective A3AR agonist, IB–MECA (N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide) delivered via a nebulizer had no effect on lung mast cell degranulation compared to wild-type mice (Zhong et al. 2003). In murine primary lung mast cells, activation of A3ARs induced mast cell histamine release in association with increases in intracellular calcium mediated through Gi and phosphoinositide 3-kinase signaling pathways (Zhong et al. 2003). Furthermore, in ADA-deficient mice, A3ARs appear to be important in endogenous adenosine-induced lung mast cell degranulation in the absence of antigen stimulation (Zhong et al. 2003). Moreover, in ADA-deficient mice, an increase in eosinophils and mucus production were reversed by a selective A3AR antagonist, suggesting an important role for A3ARs in mediating the lung eosinophilia and mucus hyperplasia in this animal model (Young et al. 2004). Further to these studies, in a murine model of allergic asthma it appears that A3ARs play an important role in adenosine-induced bronchoconstriction (Fan et al. 2003). Finally, in allergen-sensitized guinea pigs, an A3AR antagonist, MRS-1220, significantly inhibited 5′-AMP-induced migration of eosinophils and macrophages into the airways (Spruntulis and Broadley 2001). Taken together, these studies suggest that A3ARs play an important role in adenosine-induced mast cell degranulation as well as eosinophilia, mucus hyperplasia, and bronchoconstriction in mice and guinea pigs, and would support the approach to asthma with an A3AR antagonist.
In humans, expression of A3ARs is elevated in lung biopsies of patients with asthma, and is mostly localized on eosinophils where activation by adenosine via this receptor inhibits chemotaxis (Walker et al. 1997). This initial report describing this anti-inflammatory effect of activation of A3ARs on human eosinophils was reproduced, and the studies were expanded by the same group to show that the activation of A3ARs produced a dose-dependent inhibition in the chemotaxis of human eosinophils to platelet-activating factor, RANTES, and leukotriene B4, and this effect was completely reversed by selective A3AR antagonists (Knight et al. 1997). Moreover, following these reports, another group reported that A3ARs on human eosinophils mediate inhibition of both degranulation and superoxide anion release, and that therapeutic concentrations of theophylline inhibit the human eosinophil partly by acting as an A3AR agonist, thus contributing to the mechanism of the anti-inflammatory action of this drug in vivo (Ezeamuzie 2001; Ezeamuzie and Philips 1999). However, another group studied IB–MECA-induced effects on free radical generation in eosinophils of asthmatics and reported that stimulation of A3ARs does not appear to be a prime mechanism for free radical generation by human peripheral blood eosinophils (Reeves et al. 2000). Taken together, these studies in humans suggest that an A3AR agonist should be considered as a therapeutic option for the treatment of human asthma, as opposed to the studies in mice, rats, and guinea pigs that suggest that the A3AR target to treat asthma should be approached with an A3AR antagonist.
Based on the reports in animals that activation of A3ARs produce mast cell degranulation, bronchoconstriction, eosinophilia and mucus hyperplasia, and that activation of A2BARs on human mast cells may play an important role in human asthma, a combined A2B/A3 AR antagonist QAF 805 (Novartis) is under development as an antiasthma drug (Press et al. 2005; Pascoe et al. 2007). However, as mentioned above, this mixed A2B/A3 AR antagonist has now entered human clinical trials and it failed to increase the PC20 for AMP versus placebo in 24 AMP-sensitive asthmatics in a placebo-controlled, double-blind, randomized, two-way crossover study Phase Ib clinical trial (Pascoe et al. 2007). With respect to the use of A3AR agonists as antiasthma drugs, the use of this new class of drugs for this therapeutic indication may be limited by hypotension, tolerance/tachyphylaxis, and immune suppression (Gessi et al. 2008). Because of the anti-inflammatory and specifically the anti-TNF-α effects of the activation of A3ARs on human monocytes, an A3AR agonist, CF-101, is in Phase IIb clinical trials for the treatment of rheumatoid arthritis (Can-Fite Biopharma). It is reported that CF-101 has an acceptable safety and tolerability profile in humans (van Troostenburg et al. 2004). In this report, bronchospasm was not reported as a side effect of CF-101; however, the patients in this study were taking another anti-inflammatory immune suppressant, methotrexate, and CF-101 has not been tested in humans with asthma. Thus, the safety and efficacy of A3AR agonists as antiasthma drugs are yet to be determined in humans.
3 Conclusions and Future Directions
It is now well accepted that adenosine is an important signaling molecule in the pathogenesis of human asthma, and all AR subtypes are important targets for anti-asthma drug development for humans. A number of AR molecules with good safety profiles and selectivity are now available for testing in humans, in order to determine the role of ARs in human asthma and the therapeutic approach to these AR targets that will produce safe and effective antiasthma therapeutics. Because many patients with asthma are either not controlled or are noncompliant with current antiasthma therapies, a shift in focus towards new mechanisms and novel targets (e.g., adenosine signaling and AR targets) is necessary to discover new classes of drugs that are safe and effective that will not only control the symptoms of asthma, but interrupt the disease of airway remodeling and the progressive loss of lung function, and thus improve not only the quality of life, but the outcome for the patient with asthma.
Acknowledgment
We thank would like toMichael R. Blackburn, Ph.D. (Professor, Department of Biochemistry and Molecular Biology, The University of Texas–HoustonMedical School, Houston, TX, USA) for his critical review of this chapter and his helpful comments.
Abbreviations
- AC
Adenylate cyclase
- ADA
Adenosine deaminase
- ADP
Adenosine diphosphate
- AK
Adenosine kinase
- AMP
Adenosine monophosphate
- AR
Adenosine receptor
- ATP
Adenosine triphosphate
- BAL
Bronchoalveolar lavage
- BHR
Bronchial hyperresponsiveness
- BMMC
Bone marrow-derived mast cell
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CPA
Cyclopentyadenosine
- CXCR4
Chemokine receptor 4
- cyto-5′-NT
Cytosolic form of nucleotidase
- DPCPX
1,3-Dipropyl-8-cyclopentylxanthine
- EAR
Early asthmatic response
- ecto-5′-NT
Ecto-5′-nucleotidase
- FEV1
Forced expiratory volume in 1 s
- fMLP
Formyl-Met–Leu–Phe
- HBEC
Human bronchial epithelial cell
- HPRT
Hypoxanthine phosphoribosyltransferase
- ICS
Inhaled corticosteroids
- IgE
Immunoglobulin E
- IL
Interleukin
- IMP
Inosine monophosphate
- iNOS
Inducible nitric oxide synthase
- LABA
Long-acting beta-adrenoceptor agonist
- LAR
Late asthmatic response
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemotactic protein-1
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-κB
Nuclear factor kappa B
- NTPDase
Nucleoside triphosphate diphosphohydrolase
- PC
Provocative concentration
- PEFR
Peak expiratory flow rate
- PDE
Phosphodiesterase
- PG
Prostaglandin
- PLC
Phospholipase C
- PNC
Purine nucleotide cycle
- PNP
Purine nucleoside phosphorylase
- POC
Proof of concept
- RASON
Respiratory antisense oligonucleotide
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SAHH
S-Adenosylhomocysteine hydrolase
- TNF
Tumor necrosis factor
- VEGF
Vascular endothelium growth factor
References
- Abebe W, Mustafa SJ. A1 adenosine receptor-mediated Ins(1,4,5)P3 generation in allergic rabbit airway smooth muscle. Am J Physiol. 1998;275:L990–L997. doi: 10.1152/ajplung.1998.275.5.L990. [DOI] [PubMed] [Google Scholar]
- Ali S, Mustafa SJ, Metzger WJ. Adenosine-induced bronchoconstriction in an allergic rabbit model: antagonism by theophylline aerosol. Agents Actions. 1992;37:165–167. doi: 10.1007/BF02028098. [DOI] [PubMed] [Google Scholar]
- Ali S, Mustafa SJ, Metzger WJ. Adenosine receptor-mediated bronchoconstriction and bronchial hyper-responsiveness in allergic rabbit model. Am J Physiol. 1994a;266:L271–L277. doi: 10.1152/ajplung.1994.266.3.L271. (Lung Cell Mol Physiol 10) [DOI] [PubMed] [Google Scholar]
- Ali S, Mustafa SJ, Metzger WJ. Adenosine-induced bronchoconstriction and contraction of airway smooth muscle from allergic rabbits with late-phase airway obstruction: evidence for an inducible adenosine A1 receptor. J Pharmacol Exp Ther. 1994b;268:1328–1334. [PubMed] [Google Scholar]
- Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;290:L849–L855. doi: 10.1152/ajplung.00373.2005. [DOI] [PubMed] [Google Scholar]
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apasov S, Koshiba M, Redegeld F, Sitkovsky MV. Role of extracellular ATP and P1 and P2 classes of purinergic receptors in T-cell development and cytotoxic T lymphocyte effector functions. Immunol Rev. 1995;146:5–19. doi: 10.1111/j.1600-065x.1995.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Avital A, Springer C, Bar-Yishay E, Godfrey S. Adenosine, methacholine, and exercise challenges in children with asthma or paediatric chronic obstructive pulmonary disease. Thorax. 1995;50:511–516. doi: 10.1136/thx.50.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HA, Sandrasagra A, Tang L, Scott MV, Wild J, Nyce JW. Clinical potential of respirable antisense oligonucleotides (RASONs) in asthma. Am J Pharmacogenomics. 2003;3:97–106. doi: 10.2165/00129785-200303020-00003. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Cacciari B, Romagnoli R, Merighi S, Varani K, Borea PA, Spalluto G. A3 adenosine receptor ligands: history and perspectives. Med Res Rev. 2000;20:103–128. doi: 10.1002/(sici)1098-1128(200003)20:2<103::aid-med1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Barrett RJ. Realizing the potential of adenosine-receptor-based therapeutics. Proc West Pharmacol Soc. 1996;39:61–66. [PubMed] [Google Scholar]
- Beier KC, Kallinich T, Hamelmann E. T-cell co-stimulatory molecules: novel targets for the treatment of allergic airway disease. Eur Respir J. 2007;30:383–390. doi: 10.1183/09031936.00094406. [DOI] [PubMed] [Google Scholar]
- Belda J, Casan P, Martínez C, Margarit G, Giner J, Homs R, Granel C, Sanchis J. Bronchial plasma exudation after adenosine monophosphate or methacholine challenge. J Asthma. 2005;42:885–890. doi: 10.1080/02770900500371435. [DOI] [PubMed] [Google Scholar]
- Berman AR, Togias AG, Skloot G, Proud D. Allergen-induced hyperresponsiveness to bradykinin is more pronounced than that to methacholine. J Appl Physiol. 1995;78:1844–1852. doi: 10.1152/jappl.1995.78.5.1844. [DOI] [PubMed] [Google Scholar]
- Bertolet BD, Belardinelli L, Franco EA, Nichols WW, Kerensky RA, Hill JA. Selective attenuation by N-0861 (N6-endonorboran-2-yl-9-methyladenine) of cardiac A1 adenosine receptor-mediated effects in humans. Circulation. 1996;93:1871–1876. doi: 10.1161/01.cir.93.10.1871. [DOI] [PubMed] [Google Scholar]
- Beukers MW, Meurs I, Ijzerman AP. Structure–affinity relationships of adenosine A2B receptor ligands. Med Res Rev. 2006;26:667–698. doi: 10.1002/med.20069. [DOI] [PubMed] [Google Scholar]
- Bjorck T, Gustafsson LE, Dahlen S-E. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis. 1992;145:1087–1091. doi: 10.1164/ajrccm/145.5.1087. [DOI] [PubMed] [Google Scholar]
- Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MR, Lee CG, Young HWJ, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek G, Nizankowska E, Gielicz A, Szczeklik A. Mast cell activation after adenosine inhalation challenge in patients with bronchial asthma. Allergy. 2008;63:140–141. doi: 10.1111/j.1398-9995.2007.01565.x. [DOI] [PubMed] [Google Scholar]
- Bond RA, Spina D, Parra S, Page CP. Getting to the heart of asthma: can “beta blockers” be useful to treat asthma? Pharmacol Ther. 2007;115:360–374. doi: 10.1016/j.pharmthera.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Bonneau O, Wyss D, Ferretti S, Blaydon C, Stevenson CS, Trifilieff A. Effect of adenosine A2A receptor activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- Breschi MC, Blandizzi C, Fogli S, Martinelli C, Adinolfi B, Calderone V, Camici M, Martinotti E, Nieri P. In vivo adenosine A(2B) receptor desensitization in guinea-pig airway smooth muscle: implications for asthma. Eur J Pharmacol. 2007;575:149–157. doi: 10.1016/j.ejphar.2007.07.051. [DOI] [PubMed] [Google Scholar]
- Brown RA, Clarke GW, Ledbetter CL, Hurle MJ, Denyer JC, Simcock DE, Coote JE, Savage TJ, Murdoch RD, Page CP, Spina D, O’Connor BJ. Elevated expression of adenosine A1 receptor in bronchial biopsy specimens from asthmatic subjects. Eur Respir J. 2008;31:311–319. doi: 10.1183/09031936.00003707. [DOI] [PubMed] [Google Scholar]
- Bshesh K, Zhao B, Spight D, Biaggioni I, Feokistov I, Denenberg A, Wong HR, Shanley TP. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J Leukoc Biol. 2002;72:1027–1036. [PubMed] [Google Scholar]
- Bucchioni E, Csoma Z, Allegra L, Chung KF, Barnes PJ, Kharitonov SA. Adenosine 5′-monophosphate increases levels of leukotrienes in breath condensate in asthma. Respir Med. 2004;98:651–655. doi: 10.1016/j.rmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M, Pullmann R, Jr, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol. 2006;575:481–490. doi: 10.1113/jphysiol.2006.109371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol. 2001;167:4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]
- Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am J Physiol. 1999;276(2 Pt 1):C361–C369. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- Clark AN, Youkey R, Liu X, Jia L, Blatt R, Day Y-J, Sullivan GW, Linden J, Tucker AL. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ Res. 2007;101:1–9. doi: 10.1161/CIRCRESAHA.107.150110. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118:551–559. doi: 10.1016/j.jaci.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- Crimi N, Palermo F, Oliveri R, Maccarrone C, Palermo B, Vancheri C, Polosa R, Mistretta A. Enhancing effect of dipyridamole inhalation on adenosine-induced bronchospasm in asthmatic patients. Allergy. 1988;43:179–183. doi: 10.1111/j.1398-9995.1988.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Crimi N, Palermo F, Oliveri R, Palermo B, Polosa R, Mistretta A. Protection of nedocromil sodium on bronchoconstriction induced by inhaled neurokinin A (NKA) in asthmatic patients. Clin Exp Allergy. 1992;22:75–81. doi: 10.1111/j.1365-2222.1992.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Cronstein BN. Adenosine receptors and wound healing, revised. Sci World J. 2006;6:984–991. doi: 10.1100/tsw.2006.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- Csoma Z, Huszár E, Vizi E, Vass G, Szabó Z, Herjavecz I, Kollai M, Horváth I. Adenosine level in exhaled breath increases during exercise-induced bronchoconstriction. Eur Respir J. 2005;25:873–878. doi: 10.1183/09031936.05.00110204. [DOI] [PubMed] [Google Scholar]
- Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol. 1983;15:161–165. doi: 10.1111/j.1365-2125.1983.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen S-E, Hansson G, Hedqvist P, Bjorck T, Granstrom E, Dahlen B. Allergen challenge of lung tissue from asthmatics elicits bronchial contraction that correlates with the release of leukotrienes C4, D4, and E4. Proc Natl Acad Sci USA. 1983;80:1712–1716. doi: 10.1073/pnas.80.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meer G, Heederik D, Postma DS. Bronchial responsiveness to adenosine 5′-monophosphate (AMP) and methacholine differ in their relationship with airway allergy and baseline FEV1. Am J Respir Crit Care Med. 2002;165:327–331. doi: 10.1164/ajrccm.165.3.2104066. [DOI] [PubMed] [Google Scholar]
- Deussen A. Metabolic flux rates of adenosine in the heart. Naunyn–Schmiedeberg’s Arch Pharmacol. 2000;362:351–363. doi: 10.1007/s002100000318. [DOI] [PubMed] [Google Scholar]
- Dittrich HC, Gupta DK, Hack TC, Dowling T, Callahan J, Thomson S. The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. J Card Failure. 2007;13:609–617. doi: 10.1016/j.cardfail.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. BG-9928 Biogen Idec. Curr Opin Investig Drugs. 2005;6:962–968. [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Metzger WJ, Mustafa SJ. Bronchial challenge with adenosine causes the release of serum neutrophil chemotactic factor in asthma. Am Rev Respir Dis. 1991;143:1002–1007. doi: 10.1164/ajrccm/143.5_Pt_1.1002. [DOI] [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- el-Hashim A, D’Agostino B, Matera MG, Page CP. Characterization of adenosine receptors involved in adenosine-induced bronchoconstriction in allergic rabbits. Br J Pharmacol. 1996;119:1262–1268. doi: 10.1111/j.1476-5381.1996.tb16031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2006;35:496–502. doi: 10.1165/rcmb.2005-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeamuzie CI. Involvement of A(3) receptors in the potentiation by adenosine of the inhibitory effect of theophylline on human eosinophil degranulation: possible novel mechanism of the anti-inflammatory action of theophylline. Biochem Pharmacol. 2001;61:1551–1559. doi: 10.1016/s0006-2952(01)00613-x. [DOI] [PubMed] [Google Scholar]
- Ezeamuzie CI, Philips E. Adenosine A3 receptors on human eosinophils mediate inhibition of degranulation and superoxide anion release. Br J Pharmacol. 1999;127:188–194. doi: 10.1038/sj.bjp.0702476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Mustafa SJ. Adenosine-mediated bronchoconstriction and lung inflammation in an allergic mouse model. Pul Pharmacol Ther. 2002;15:147–155. doi: 10.1006/pupt.2001.0329. [DOI] [PubMed] [Google Scholar]
- Fan M, Mustafa SJ. Role of adenosine in airway inflammation in an allergic mouse model of asthma. Int Immunopharmacol. 2006;6:36–45. doi: 10.1016/j.intimp.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Fan M, Qin W, Mustafa SJ. Characterization of adenosine receptor(s) involved in adenosine-induced bronchoconstriction in an allergic mouse model. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1012–L1019. doi: 10.1152/ajplung.00353.2002. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726–734. [PubMed] [Google Scholar]
- Feoktistov I, Garland EM, Goldstein AE, Zeng D, Belardinelli L, Wells JN, Biaggioni I. Inhibition of human mast cell activation with the novel selective adenosine A(2B) receptor antagonist 3-isobutyl-8-pyrrolidinoxanthine (IPDX)(2) Biochem Pharmacol. 2001;62:1163–1173. doi: 10.1016/s0006-2952(01)00765-1. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S. Pharmacology and therapeutic applications of A3 receptor subtype. Curr Top Med Chem. 2003;3:463–469. doi: 10.2174/1568026033392147. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Ellis KM, Villela Dantas MF, Tigani B, Mazzoni L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur J Pharmacol. 2002;438:183–188. doi: 10.1016/s0014-2999(02)01305-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Diff. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gao Z-G, Jacobson KA. Emerging adenosine receptor agonists. Expert Opin Emerg Drugs. 2007;12:479–492. doi: 10.1517/14728214.12.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspardone A, Crea F, Iamele M, Tomai F, Versaci F, Pellegrino A, Chiariello L, Gioffre PA. Bamiphylline improves exercise-induced myocardial ischemia through a novel mechanism of action. Circulation. 1993;88:502–508. doi: 10.1161/01.cir.88.2.502. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Varani K, Leung E, Lennan SM, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gil FR, Lauzon AM. Smooth muscle molecular mechanics in airway hyperresponsiveness and asthma. Can J Physiol Pharmacol. 2007;85:133–140. doi: 10.1139/y06-096. [DOI] [PubMed] [Google Scholar]
- Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effect of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50:1551–1560. doi: 10.1016/j.jacc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, Brater C, Thomas I, Havranek E, Bourge R, Goldman S, Dyer F, Gomez M, Bennett D, Ticho B, Beckman E, Abraham WT. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348–1353. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Ignatius T, Banish D, Goldman S, Havranek E, Massie BM, Zhu Y, Ticho B, Abraham WT. Effects of multiple oral doses of an A1 adenosine receptor antagonist, BG 9928, in patients with heart failure. J Am Coll Cardiol. 2007;50:600–606. doi: 10.1016/j.jacc.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Gunther GR, Herring MB. Inhibition of neutrophil superoxide production by adenosine released from vascular endothelial cells. Ann Vasc Surg. 1991;5:325–330. doi: 10.1007/BF02015292. [DOI] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv Immunol. 2007;93:265–278. doi: 10.1016/S0065-2776(06)93007-7. [DOI] [PubMed] [Google Scholar]
- Hannon JP, Tigani B, Wolber C, Williams I, Mazzoni L, Howes C, Fozard JR. Evidence for an atypical receptor mediating the augmented bronchoconstrictor response to adenosine induced by allergen challenge in actively sensitized Brown Norway rats. Br J Pharmacol. 2002;135:685–696. doi: 10.1038/sj.bjp.0704516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hess S. Recent advances in adenosine receptor antagonist research. Expert Opin Ther Pat. 2001;11:1533–1561. [Google Scholar]
- Holgate ST. Adenosine provocation: a new test for allergic type airway inflammation. Am J Respir Crit Care Med. 2002;165:317–319. doi: 10.1164/ajrccm.165.3.2112045a. [DOI] [PubMed] [Google Scholar]
- Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007;28:248–251. doi: 10.1016/j.it.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hong JL, Ho CY, Kwong K, Lee LY. Activation of pulmonary C fibres by adenosine in anaesthetized rats: role of adenosine A1 receptors. J Physiol. 1998;508:109–118. doi: 10.1111/j.1469-7793.1998.109br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Erikson CJ, Chason KD, Rosebrock CN, Deshpande DA, Penn RB, Tilley SL. Involvement of A1 adenosine receptors and neural pathways in adenosine-induced bronchoconstriction in mice. Am J Physiol Lung Cell Mol Physiol. 2007a;293:L25–L32. doi: 10.1152/ajplung.00058.2007. [DOI] [PubMed] [Google Scholar]
- Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007b;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol. 2001;18:53–63. [PubMed] [Google Scholar]
- Ihre E, Gyllfors P, Gustafsson LE, Kumlin M, Dahlén B. Early rise in exhaled nitric oxide and mast cell activation in repeated low-dose allergen challenge. Eur Respir J. 2006;27:1152–1159. doi: 10.1183/09031936.06.00142905. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- Jarjour NN, Kelly EAB. Pathogenesis of asthma. Med Clin North Am. 2002;86:925–936. doi: 10.1016/s0025-7125(02)00087-1. [DOI] [PubMed] [Google Scholar]
- Johnson HG, McNee ML. Adenosine-induced secretion in the canine trachea: modification by methylxanthines and adenosine derivatives. Br J Pharmacol. 1985;86:63–67. doi: 10.1111/j.1476-5381.1985.tb09435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, Bach FH, Robson SC. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg G-W, Hocher B. The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol. 2007;151:1025–1032. doi: 10.1038/sj.bjp.0707319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallinich T, Beier KC, Wahn U, Stock P, Hamelmann E. T-cell co-stimulatory molecules: their role in allergic immune reactions. Eur Respir J. 2007;29:1246–1255. doi: 10.1183/09031936.00094306. [DOI] [PubMed] [Google Scholar]
- Keir S, Boswell-Smith V, Spina D, Page C. Mechanism of adenosine-induced airways obstruction in allergic guinea pigs. Br J Pharmacol. 2006;47:720–728. doi: 10.1038/sj.bjp.0706663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchell RI, Jensen MW, Lumley P, Wright AM, Allenby MI, O’Connor BJ. Rapid effect of inhaled fluticasone propionate on airway responsiveness to adenosine 5′-monophosphate in mild asthma. J Allergy Clin Immunol. 2002;110:603–606. doi: 10.1067/mai.2002.128486. [DOI] [PubMed] [Google Scholar]
- Knight D, Zheng X, Rocchini C, Jacobson M, Bai T, Walker B. Adenosine A3 receptor stimulation inhibits migration of human eosinophils. J Leukoc Biol. 1997;62:465–468. doi: 10.1002/jlb.62.4.465. [DOI] [PubMed] [Google Scholar]
- Langley SJ, Allen DJ, Houghton C, Woodcock A. Efficacy of EPI-2010 (an inhaled respirable anti-sense oligonucleotide: RASON) in moderate/severe persistent asthma. Am J Resp Crit Care Med. 2005;171:A360. [Google Scholar]
- Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- Lee MG, Kollarik M, Chuaychoo B, Undem BJ. Ionotropic and metabotropic receptor mediated airway sensory nerve activation. Pulm Pharmacol Ther. 2004;17:355–360. doi: 10.1016/j.pupt.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AMF, Wong YH. G16-mediated activation of nuclear factor κB by the adenosine A1 receptor involves c-Src, protein kinase C, ERK signaling. J Biol Chem. 2004;279:53196–53204. doi: 10.1074/jbc.M410196200. [DOI] [PubMed] [Google Scholar]
- Livingston M, Heaney LG, Ennis M. Adenosine, inflammation, and asthma: a review. Inflamm Res. 2004;53:171–178. doi: 10.1007/s00011-004-1248-2. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Robinson DS. Allergen-induced airway remodelling. Eur Respir J. 2007;29:1020–1032. doi: 10.1183/09031936.00150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijk B, van den Berge M, Kerstjens HA, Postma DS, Cass L, Sabin A, Lammers JW. Effect of an inhaled adenosine A2A agonist on the allergen-induced late asthmatic response. Allergy. 2008;63:75–80. doi: 10.1111/j.1398-9995.2007.01557.x. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun C-X, Young HW, Elias JA. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J Clin Invest. 2006;116:1274–1283. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5′-Adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JS, Cushley MJ, Holgate ST. Adenosine-induced bronchoconstriction in asthma. Role of parasympathetic stimulation and adrenergic inhibition. Am Rev Respir Dis. 1985;132:1–6. doi: 10.1164/arrd.1985.132.1.1. [DOI] [PubMed] [Google Scholar]
- Mann JS, Holgate ST, Renwick AG, Cushley MJ. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol. 1986a;61:1667–1676. doi: 10.1152/jappl.1986.61.5.1667. [DOI] [PubMed] [Google Scholar]
- Mann JS, Renwick AG, Holgate ST. Release of adenosine and its metabolites from activated human leucocytes. Clin Sci. 1986b;70:461–468. doi: 10.1042/cs0700461. [DOI] [PubMed] [Google Scholar]
- Manrique HA, Gómez FP, Muñoz PA, Peña AM, Barberà JA, Roca J, Rodríguez-Roisin R. Adenosine 5′-monophosphate in asthma: gas exchange and sputum cellular responses. Eur Respir J. 2008;31:1205–1212. doi: 10.1183/09031936.00116207. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Safier LB, Broekman MJ, Islam N, Fliessbach JH, Hajjar KA, Kaminski WE, Jendraschak E, Silverstein RL, von Schacky C. Thrombosis and inflammation as multicellular processes: significance of cell-cell interactions. Thromb Haemost. 1995;74:213–217. [PubMed] [Google Scholar]
- McNamara N, Gallup M, Khong A, Sucher A, Maltseva I, Fahy J, Basbaum C. Adenosine up-regulation of the mucin gene, MUC2, in asthma. FASEB J. 2004;18:1770–1772. doi: 10.1096/fj.04-1964fje. [DOI] [PubMed] [Google Scholar]
- Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L753–L761. doi: 10.1152/ajplung.00187.2007. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang C-K, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (Gs-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2006;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro S, Gao Z-G, Jacobson KA, Spalluto G. Progress in the pursuit of therapeutic adenosine receptor antagonists. Med Res Rev. 2006;26:131–159. doi: 10.1002/med.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Obiefuna PC, Wilson CN, Mustafa SJ. Adenosine A1 receptor antagonist versus montelukast on airway reactivity and inflammation. Eur J Pharmacol. 2006;551:116–124. doi: 10.1016/j.ejphar.2006.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Fan M, Ansari HR, Ledent C, Jamal Mustafa S. Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1335–L1344. doi: 10.1152/ajplung.00416.2006. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Ponnoth DS, Batchelor T, Dey RD, Ledent C, Mustafa SJ. Decreased tracheal relaxation via NADPH oxidase activation in A2A AR deficent allergic mice. Am J Respir Crit Care Med. 2008;177:A487. [Google Scholar]
- Nilsson G, Blom T, Kusche-Gullberg M, Kjellen L, Butterfield JH, Sundstrom C, Nilsson K, Hellman L. Phenotypic characterization of the human mast cell line HMC-1. Scand J Immunol. 1994;39:489–498. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Nyce JW, Metzger WJ. DNA antisense therapy for asthma in an animal model. Nature. 1997;385:721–725. doi: 10.1038/385721a0. [DOI] [PubMed] [Google Scholar]
- Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CN, Mustafa SJ. A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl}-1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther. 2005;315:329–336. doi: 10.1124/jpet.105.088179. [DOI] [PubMed] [Google Scholar]
- O’Connor BJ, Crowther SD, Costello JF, Morley J. Selective airway responsiveness in asthma. Trends Pharmacol Sci. 1999;20:9–11. doi: 10.1016/s0165-6147(98)01280-2. [DOI] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg PJ, Mustafa SJ. Involvement of mast cells in adenosine-mediated bronchoconstriction and inflammation in an allergic mouse model. J Pharmacol Exp Ther. 2005;313:319–324. doi: 10.1124/jpet.104.071720. [DOI] [PubMed] [Google Scholar]
- Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- Pascoe SJ, Knight H, Woessner R. QAF805, an A2b/A3 adenosine receptor antagonist does not attenuate AMP challenge in subjects with asthma. Am J Respir Crit Care Med. 2007;175:A682. [Google Scholar]
- Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, Molina M. Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol Membr Biol. 2001;18:81–85. doi: 10.1080/096876800110033783. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Holgate ST. The effect of oral terfenadine alone and in combination with flurbiprofen on the bronchoconstrictor response to inhaled adenosine 5′-monophosphate in nonatopic asthma. Am Rev Respir Dis. 1989;139:463–469. doi: 10.1164/ajrccm/139.2.463. [DOI] [PubMed] [Google Scholar]
- Polosa R. Adenosine-receptor subtypes: their relevance to adenosine-mediated responses in asthma and chronic obstructed pulmonary disease. Eur Respir J. 2002;20:488–496. doi: 10.1183/09031936.02.01132002. [DOI] [PubMed] [Google Scholar]
- Polosa R, Phillips GD, Rajakulasingam K, Holgate ST. The effect of inhaled ipratropium bromide alone and in combination with oral terfenadine on bronchoconstriction provoked by adenosine 5′-monophosphate and histamine in asthma. J Allergy Clin Immunol. 1991;87:939–947. doi: 10.1016/0091-6749(91)90415-k. [DOI] [PubMed] [Google Scholar]
- Polosa R, Ng WH, Crimi N, Vancheri C, Holgate ST, Church MK, Mistretta A. Release of mast-cell-derived mediators after endobronchial adenosine challenge in asthma. Am J Respir Crit Care Med. 1995;151:624–629. doi: 10.1164/ajrccm/151.3_Pt_1.624. [DOI] [PubMed] [Google Scholar]
- Polosa R, Renaud L, Cacciola R, Prosperini G, Crimi N, Djukanovic R. Sputum eosinophilia is more closely associated with airway responsiveness to bradykinin than methacholine in asthma. Eur Respir J. 1998;12:551–556. doi: 10.1183/09031936.98.12030551. [DOI] [PubMed] [Google Scholar]
- Press NJ, Taylor RJ, Fullerton JD, Tranter P, McCarthy C, Keller TH, Brown L, Cheung R, Christie J, Haberthuer S, Hatto JD, Keenan M, Mercer MK, Press NE, Sahri H, Tuffnell AR, Tweed M, Fozard JR. A new orally bioavailable dual adenosine A2B/A3 receptor antagonist with therapeutic potential. Bioorg Med Chem Lett. 2005;15:3081–3085. doi: 10.1016/j.bmcl.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor, which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- Reeves JJ, Harris CA, Hayes BP, Butchers PR, Sheehan MJ. Studies on the effects of adenosine A3 receptor stimulation on human eosinophils isolated from non-asthmatic or asthmatic donors. Inflamm Res. 2000;49:666–672. doi: 10.1007/s000110050644. [DOI] [PubMed] [Google Scholar]
- Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Reynolds CJ, Togias A, Proud D. Airways hyper-responsiveness to bradykinin and methacholine: effects of inhaled fluticasone. Clin Exp Allergy. 2002;32:1174–1179. doi: 10.1046/j.1365-2745.2002.01443.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Docherty R, Robbins J, Spina D, Page CP. Adenosine induces a cholinergic tracheal reflex contraction in guinea pigs in vivo via an adenosine A1 receptor-dependent mechanism. J Appl Physiol. 2008;105:187–196. doi: 10.1152/japplphysiol.01048.2007. [DOI] [PubMed] [Google Scholar]
- Rimmer J, Peake HL, Santos CM, Lean M, Bardin P, Robson R, Haumann B, Loehrer F, Handel ML. Targeting adenosine receptors in the treatment of allergic rhinitis: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy. 2007;37:8–14. doi: 10.1111/j.1365-2222.2006.02546.x. [DOI] [PubMed] [Google Scholar]
- Roisman GL, Lacronique JG, Desmazes DN, Carre C, Le-Cae A, Dusser DJ. Airway responsiveness to bradykinin is related to eosinophilic inflammation in asthma. Am J Respir Crit Care Med. 1996;153:381–390. doi: 10.1164/ajrccm.153.1.8542147. [DOI] [PubMed] [Google Scholar]
- Roman J, Rivera HN, Roser-Page S, Sitaraman SV, Ritzenthaler JD. Adenosine induces fibronectin expression in lung epithelial cells: implications for airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2006;290:L317–L325. doi: 10.1152/ajplung.00118.2005. [DOI] [PubMed] [Google Scholar]
- Rorke S, Holgate ST. Targeting adenosine receptors: novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Am J Respir Med. 2002;1:99–105. doi: 10.1007/BF03256599. [DOI] [PubMed] [Google Scholar]
- Rorke S, Jennison S, Jeffs JA, Sampson AP, Holgate ST. Role of cysteinyl leukotrienes in adenosine 5′-monophosphate induced bronchoconstriction in asthma. Thorax. 2002;57:323–327. doi: 10.1136/thorax.57.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Rounds S, Hsieh L, Agarwal KC. Effects of endotoxin injury on endothelial cell adenosine metabolism. J Lab Clin Med. 1994;123:309–317. [PubMed] [Google Scholar]
- Rutgers SR, Koeter GH, Van Der Mark TW, Postma DS. Protective effect of oral terfenadine and not inhaled ipratropium on adenosine 5′-monophosphate-induced bronchoconstriction in patients with COPD. Clin Exp Allergy. 1999;29:1287–1292. doi: 10.1046/j.1365-2222.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol. 2008a;180:7212–7220. doi: 10.4049/jimmunol.180.11.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008b;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- Salmon JE, Cronstein BN. Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol. 1990;145:2235–2240. [PubMed] [Google Scholar]
- Salmon JE, Brogle N, Brownlie C, Edberg JC, Kimberly RP, Chen BX, Erlanger BF. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J Immunol. 1993;151:2775–2785. [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a metanalysis. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- Slats AM, Janssen K, van SA, van der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, Sterk PJ. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:121–128. doi: 10.1164/rccm.200612-1814OC. [DOI] [PubMed] [Google Scholar]
- Sont JK, Booms P, Bel EH, Vandenbroucke JP, Sterk PJ. The determinants of airway hyper-responsiveness to hypertonic saline in atopic asthma in vivo. Relationship with sub-populations of peripheral blood leucocytes. Clin Exp Allergy. 1993;23:678–688. doi: 10.1111/j.1365-2222.1993.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Bonfiglio C, Polosa R. Research applications and implications of adenosine in diseased airways. Trends Pharmacol Sci. 2003;24:409–413. doi: 10.1016/S0165-6147(03)00193-7. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006;533:77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Spina D, Page CP. Airway sensory nerves in asthma: targets for therapy? Pulm Pharmacol. 1996;9:1–18. doi: 10.1006/pulp.1996.0001. [DOI] [PubMed] [Google Scholar]
- Spina D, Page CP. Pharmacology of airway irritability. Curr Opin Pharmacol. 2002;2:264–272. doi: 10.1016/s1471-4892(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Spruntulis LM, Broadley KJ. A3 receptors mediate rapid inflammatory cell influx into the lungs of sensitized guinea-pigs. Clin Exp Allergy. 2001;31:943–951. doi: 10.1046/j.1365-2222.2001.01087.x. [DOI] [PubMed] [Google Scholar]
- Sullivan GW. Adenosine A2A receptor agonists as anti-inflammatory agents. Curr Opin Investig Drugs. 2003;4:1313–1319. [PubMed] [Google Scholar]
- Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest. 2005;115:35–43. doi: 10.1172/JCI22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in down-regulation of inflammation and immunity in the pathogenesis of infectious diseases. Microb Infect. 2003;5:515–526. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, Koller BH. Identification of A3 receptor- and mast cell-dependent and -independent components of adenosine-mediated airway responsiveness in mice. J Immunol. 2003;171:331–337. doi: 10.4049/jimmunol.171.1.331. [DOI] [PubMed] [Google Scholar]
- van den Berge M, Kerstjens HA, Meijer RJ, de Reus DM, Koeter GH, Kauffman HF, Postma DS. Corticosteroid-induced improvement in the PC20 of adenosine monophosphate is more closely associated with reduction in airway inflammation than improvement in the PC20 of methacholine. Am J Respir Crit Care Med. 2001;164:1127–1132. doi: 10.1164/ajrccm.164.7.2102135. [DOI] [PubMed] [Google Scholar]
- van den Berge M, Kerstjens HA, de Reus DM, Koeter GH, Kauffman HF, Postma DS. Provocation with adenosine 5′ -monophosphate, but not methacholine, induces sputum eosinophilia. Clin Exp Allergy. 2004;34:71–76. doi: 10.1111/j.1365-2222.2004.01832.x. [DOI] [PubMed] [Google Scholar]
- Van Schoor J, Joos GF, Pauwels RA. Indirect bronchial hyperresponsiveness in asthma: mechanisms, pharmacology and implications for clinical research. Eur Respir J. 2000;16:514–533. doi: 10.1034/j.1399-3003.2000.016003514.x. [DOI] [PubMed] [Google Scholar]
- Van Schoor J, Joos GF, Pauwels RA. Effect of inhaled fluticasone on bronchial responsiveness to neurokinin A in asthma. Eur Respir J. 2002;19:997–1002. doi: 10.1183/09031936.02.01112001. [DOI] [PubMed] [Google Scholar]
- van Troostenburg A-R, Clark EV, Carey WDH, Warrington SJ, Kerns WD, Cohn I, Silverman MH, Bar-Yehuda S, Fong K-LL, Fishman P. Tolerability, pharmacokinetics and concentration-dependent hemodynamic effects of oral CF 101, an A3 adenosine receptor agonist, in healthy young men. Internat J Clin Pharmacol Ther. 2004;42:534–542. doi: 10.5414/cpp42534. [DOI] [PubMed] [Google Scholar]
- Visser SS, Theron AJ, Ramafi G, Ker JA, Anderson R. Apparent involvement of the A2A subtype adenosine receptor in the anti-inflammatory interactions of CGS 21680, cyclopenty-ladenosine, and IB-MECA with human neutrophils. Biochem Pharmacol. 2000;60:993–999. doi: 10.1016/s0006-2952(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Vizi E, Huzzar E, Csoma Z, Boszormenyi-Nagy G, Barat E, Horvath I, Herjavecz I, Kollai M. Plasma adenosine concentration increases during exercise: a possible contributing factor in exercise-induced bronchoconstriction in asthma. J Allergy Clin Immunol. 2002;109:446–448. doi: 10.1067/mai.2002.121955. [DOI] [PubMed] [Google Scholar]
- Walker BAM, Jacobson MA, Knight DA, Salvatore CA, Weir T, Zhou D, Bai TR. Adenosine A3 receptor expression and function in eosinophils. Am J Respir Cell Mol Biol. 1997;16:531–537. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- Webb RL, Sills MA, Chovan JP, Peppard JV, Francis JE. Development of tolerance to the antihypertensive effects of highly selective adenosine A2a agonists upon chronic administration. J Pharmacol Exp Ther. 1993;267:287–295. [PubMed] [Google Scholar]
- Wilson CN, Batra VK. Lipopolysaccharide binds to and activates A(1) adenosine receptors on human pulmonary artery endothelial cells. J Endotoxin Res. 2002;8:263–271. doi: 10.1179/096805102125000470. [DOI] [PubMed] [Google Scholar]
- Wilson CN. Adenosine receptors and asthma in humans. Br J Pharmacol. 2008;155:475–486. doi: 10.1038/bjp.2008.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Ahmed AA, Moller C, Nakayama K, Hatakeyama S, Nilsson G. Essential role of the prosurvival bcl-2 homologue A1 in the mast cell survival after allergic activation. J Exp Med. 2001;194:1561–1569. doi: 10.1084/jem.194.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, Hilaire CS, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A(2B) adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci USA. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol. 2006;35:549–558. doi: 10.1165/rcmb.2006-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzlenko O, Kiec-Kononowicz K. Potent adenosine A1 and A2A receptor antagonists: recent developments. Curr Med Chem. 2006;13:3609–3625. doi: 10.2174/092986706779026093. [DOI] [PubMed] [Google Scholar]
- Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171:338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D. A2B adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2004;30:118–125. doi: 10.1165/rcmb.2003-0118OC. [DOI] [PubMed] [Google Scholar]
- Zhong H, Belardinelli L, Maa T, Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Respir Cell Mol Biol. 2005;32:2–8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- Zhong H, Wu Y, Belardinelli L, Zeng D. A2B adenosine receptors induce IL-19 from bronchial epithelial cells and results in TNF-alpha increase. Am J Respir Cell Mol Biol. 2006;35:587–592. doi: 10.1165/rcmb.2005-0476OC. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nat Med. 1999;5:987–988. doi: 10.1038/12419. [DOI] [PubMed] [Google Scholar]