Abstract
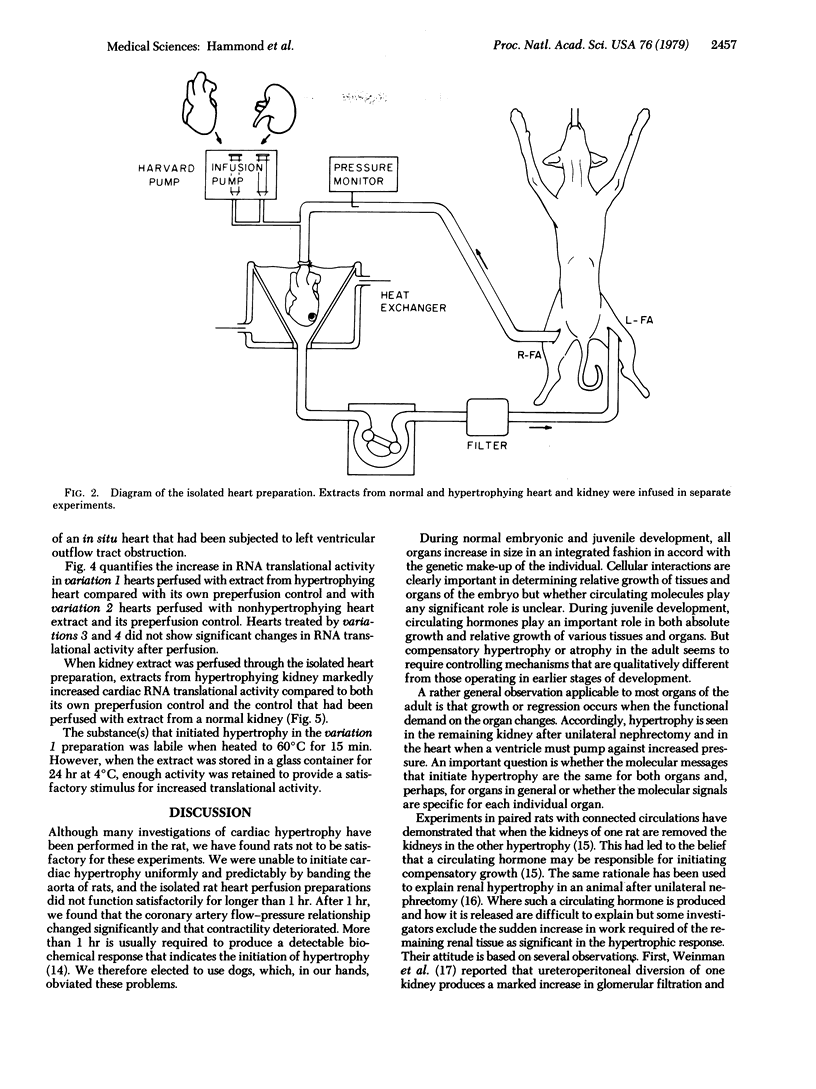
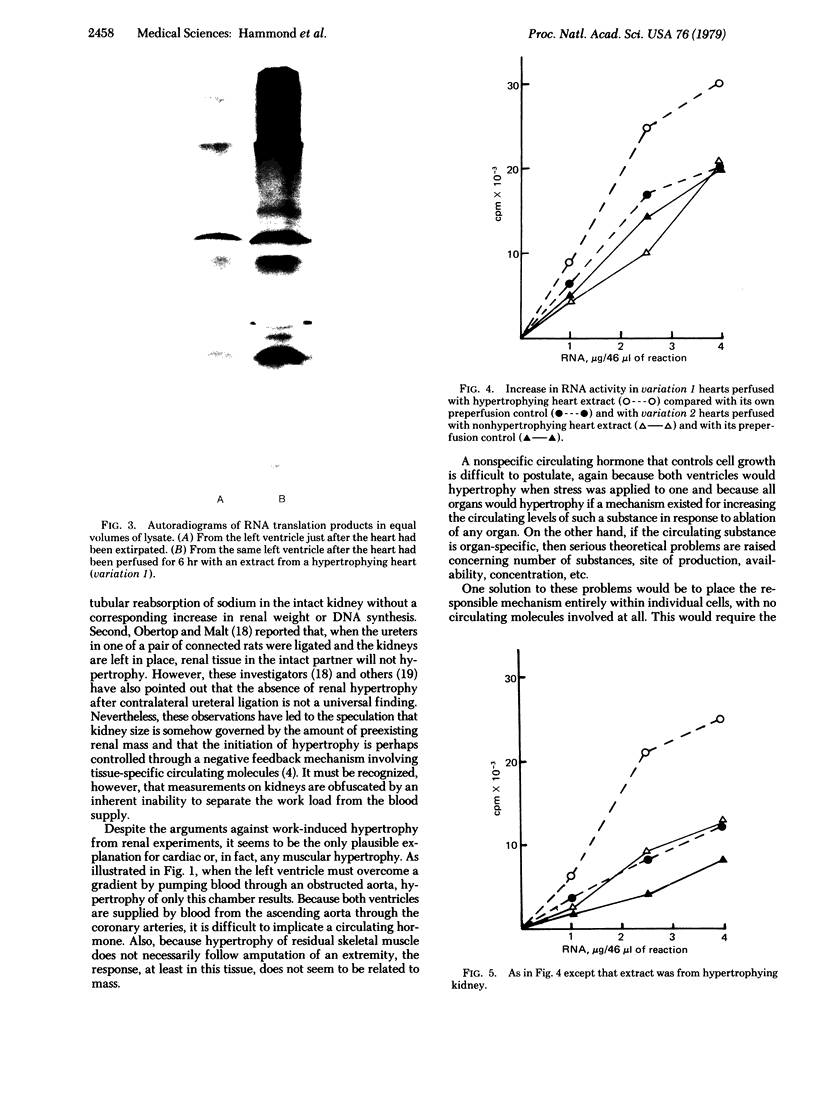
When chronically provoked to increased physiologic activity, organs increase in mass through augmented protein protein synthesis. This process of compensatory hypertrophy can involve cell division as well as cell growth. To test for molecules that might regulate organ size, by inducing hypertrophy, we performed a series of experiments using isolated, perfused, canine hearts in which the left ventricle was beating but performed no work. Hypertrophying hearts and kidneys as well as normal control organs were extracted and the extracts were perfused through isolated heart preparations. Before and after perfusion, RNA was extracted from fragments of the isolated hearts and translated in cell-free media containing [35S]methionine. Incorporation of methionine into protein was measured by liquid scintillation spectrometry. When perfused through normal hearts, extracts from hypertrophying heart and kidney were able to increase greatly the translational ability of RNA extracted from the normal hearts; corresponding perfusates from nonhypertrophying hearts and kidneys had no effect. Our results indicate that molecules that initiate hypertrophic organ growth are extractable, are generated by the cells of the organ under stress, and are probably similar in heart and kidney and perhaps in many other organs as well.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badeer H. S. Metabolic basis of cardiac hypertrophy. Prog Cardiovasc Dis. 1968 Jul;11(1):53–63. doi: 10.1016/s0033-0620(68)80011-8. [DOI] [PubMed] [Google Scholar]
- Badeer H. S. Pathogenesis of cardiac hypertrophy in coronary atherosclerosis and myocardial infarction. Am Heart J. 1972 Aug;84(2):256–264. doi: 10.1016/0002-8703(72)90341-9. [DOI] [PubMed] [Google Scholar]
- Bishop S. P., Altschuld R. A. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol. 1970 Jan;218(1):153–159. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Dicker S. E., Shirley D. G. Compensatory hypertrophy of the contralateral kidney after unilateral ureteral ligation. J Physiol. 1972 Jan;220(1):199–210. doi: 10.1113/jphysiol.1972.sp009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. L., Nadal-Ginard B., Talner N. S., Markert C. L. Myocardial LDH isozyme distribution in the ischemic and hypoxic heart. Circulation. 1976 Apr;53(4):637–643. doi: 10.1161/01.cir.53.4.637. [DOI] [PubMed] [Google Scholar]
- Kavanau J. L. A MODEL OF GROWTH AND GROWTH CONTROL IN MATHEMATICAL TERMS, II. COMPENSATORY ORGAN GROWTH IN THE ADULT. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1658–1673. doi: 10.1073/pnas.46.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton B., Schiff M., Jr, Bloom N. Compensatory renal growth: evidence for tissue specific factor of renal origin. J Urol. 1969 May;101(5):648–652. doi: 10.1016/s0022-5347(17)62395-4. [DOI] [PubMed] [Google Scholar]
- Meerson F. Z. The myocardium in hyperfunction, hypertrophy and heart failure. Circ Res. 1969 Jul;25(1 Suppl):1–163. [PubMed] [Google Scholar]
- NORMAN T. D. The pathogenesis of cardiac hypertrophy. Prog Cardiovasc Dis. 1962 Mar;4:439–463. doi: 10.1016/s0033-0620(62)80020-6. [DOI] [PubMed] [Google Scholar]
- Obertop H., Malt R. A. Lost mass and excretion as stimuli to parabiotic compensatory renal hypertrophy. Am J Physiol. 1977 May;232(5):F405–F408. doi: 10.1152/ajprenal.1977.232.5.F405. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. II. Quantification and immunoprecipitation of polysomes. J Biol Chem. 1972 May 25;247(10):3296–3304. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M., Zak R. Biochemical and cellular changes in cardiac hypertrophy. Annu Rev Med. 1972;23:245–262. doi: 10.1146/annurev.me.23.020172.001333. [DOI] [PubMed] [Google Scholar]
- Sanford C. F., Griffin E. E., Wildenthal K. Synthesis and degradation of myocardial protein during the development and regression of thyroxine-induced cardiac hypertrophy in rats. Circ Res. 1978 Nov;43(5):688–694. doi: 10.1161/01.res.43.5.688. [DOI] [PubMed] [Google Scholar]
- TANNER J. M. REGULATION OF GROWTH IN SIZE IN MAMMALS. Nature. 1963 Aug 31;199:845–850. doi: 10.1038/199845a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Van Vroonhoven T. J., Soler-Montesinos L., Malt R. A. Humoral regulation of renal mass. Surgery. 1972 Aug;72(2):300–305. [PubMed] [Google Scholar]




