Abstract
Background
By the preschool years, racial/ethnic disparities in obesity prevalence are already present.
Objective
To examine racial/ethnic differences in early life risk factors for childhood obesity.
Design, Setting, Participants
343 white, 355 black, and 128 Hispanic mother-child pairs in a prospective study.
Main Exposure
Mother’s report of child’s race/ethnicity.
Main Outcome Measures
Risk factors from the prenatal period through age 4 years known to be associated with child obesity.
Results
In multivariable models, compared to their white counterparts, black and Hispanic children exhibited a range of risk factors related to child obesity. In pregnancy, these included higher rates of maternal depression (OR: 1.55 for blacks; 1.89 for Hispanics); in infancy more rapid weight gain (OR: 2.01 for blacks; 1.75 for Hispanics), more likely to introduce solid foods before 4 months of age (OR: 1.91 for blacks; 2.04 for Hispanics), higher rates of maternal restrictive feeding practices (OR: 2.59 for blacks; 3.35 for Hispanics), and after age 2 years, more televisions in their bedrooms (OR: 7.65 for blacks; 7.99 for Hispanics), higher intake of sugar-sweetened beverages (OR: 4.11 for blacks; 2.48 for Hispanics), and higher intake of fast food (OR: 1.65 for blacks; 3.14 for Hispanics). Blacks and Hispanics also had lower rates of exclusive breastfeeding and were less likely to sleep at least 12 hours/day in infancy.
Conclusions
Racial/ethnic differences in risk factors for obesity exist prenatally and in early childhood. Racial/ethnic disparities in childhood obesity may be determined by factors operating at the earliest stages of life.
Keywords: Obesity, Race/Ethnicity, Pregnancy, Infancy, Childhood, Prevention
INTRODUCTION
By the preschool years, racial/ethnic disparities in obesity prevalence are already present. According to the most recent National Health and Nutrition Examination Survey (2003–2006), the prevalence of children ages 2 through 5 years having a BMI ≥ 95th percentile was 10.7% among non-Hispanic white children, 14.9% among non-Hispanic black children, and 16.7% among Mexican-American children.1 These data imply that racial/ethnic disparities in childhood obesity prevalence have their origins in the earliest stages of life.
The life course approach to chronic disease prevention posits that factors may act in the prenatal period into infancy, childhood, and beyond to determine risk of chronic disease.2 These factors can range from the social /built environment through behavior, physiology, and genetics. Factors interact with each other over the life course, with different determinants being more or less important at different life stages. Today we recognize that the prenatal, infancy, and early childhood periods are likely to be key to the development - and thus prevention - of obesity and its consequences in children.
In recent years, investigators have identified several factors in the prenatal, infancy, and early childhood periods that increase the risk of obesity in childhood.3, 4 Few studies,5, 6 however, have examined racial/ethnic differences in the prevalence of obesity-related risk factors during these time periods. Racial/ethnic differences in early life risk factors for obesity might contribute to the high prevalence of obesity among minority preschool age children and beyond. Understanding these differences may help inform the design of clinical and public health interventions and policies to reduce disparities in childhood obesity and its complications.
The purpose of this study was to examine racial/ethnic differences in early life risk factors for childhood obesity. We hypothesized that compared with white children, black and Hispanic children would have higher rates of obesity-related risk factors in early life.
METHODS
Subjects/Study Design
Study subjects were participants in Project Viva, a prospective, pre-birth cohort study.7 Details of recruitment and retention procedures are available elsewhere.7 Of the 2128 women who delivered a live infant, 2109 had information on our main exposure, child race/ethnicity. For this analysis, we excluded participants who were Asian (n=94) or multiracial/other (n=189) in order to examine risk factor differences among white children and children of racial/ethnic groups known to have a high prevalence of childhood obesity. Sample sizes for each time point were 1826 for prenatal and birth measures, 1517 for 6-month measures, 1420 for 1-year measures, 1212 for 2-year measures, 1086 for 3-year measures, and 1058 for 4-year measures.
After obtaining informed consent, we performed in-person study visits with the mother at the end of the first and second trimesters of pregnancy, in the first few days after delivery and at 6 months and 3 years after birth. Mothers completed mailed questionnaires at 1, 2, and 4 years after birth. Human subjects committees of Harvard Pilgrim Health Care, Brigham and Women's Hospital, and Beth Israel Deaconess Medical Center approved the study protocols.
Measurements
Main Exposure
At the 3-year interview, research assistants asked mothers the question, "Which of the following best describes your child’s race or ethnicity?" Mothers had a choice of 1 or more of the following racial/ethnic groups: Hispanic or Latina, white or Caucasian, black or African American, Asian or Pacific Islander, American Indian or Alaskan Native, and other (please specify). For the participants who chose the "other" race/ethnicity, we compared the specified responses to US census definitions for the other 5 race and ethnicities and reclassified them where appropriate.
Outcome Measures
The main outcomes were risk factors during the prenatal, infancy, and early childhood periods that are associated with childhood obesity in the medical literature.
Gestational weight gain (GWG)
We calculated total GWG by subtracting pre-pregnancy weight from the last prenatal weight. Studies have shown that excessive weight gain during pregnancy is associated with increased risk of offspring obesity in childhood and adolescence.8, 9 In this analysis, we expressed GWG in categories based on the recommendations of the Institute of Medicine.10
Gestational Diabetes (GDM)
Mothers in the study were routinely screened for gestational diabetes at 26–28 weeks of gestation with a non-fasting oral glucose challenge test. We categorized women with 2 or more abnormal fasting glucose tolerance test results as having GDM, based on published criteria.11 Previous studies have found that GDM exposure is associated with higher adiposity among children.12, 13
Smoking during pregnancy
We asked mothers at both first and second trimester visits about their cigarette smoking habits before and during pregnancy. A recent meta-analysis of 14 studies has shown that smoking during pregnancy is associated with a 50% increased odds of childhood obesity.14
Maternal depression
Mothers completed a validated 10-item Edinburgh Postpartum Depression Scale (EPDS) during mid-pregnancy that queried history of depression and current depressive symptoms.15 We used the cut point of 13 or more to indicate probable depression during pregnancy16. Previous studies have found that maternal stressors are associated with childhood obesity.17, 18
Fetal growth and rapid infant weight gain
We calculated birth weight for gestational age z-score as a measure of fetal growth.19 As a measure of infant weight gain, we calculated the difference between weight-for-age z-scores at 6 months and at birth. Both fetal growth and rapid early infancy weight gain are associated with later BMI in childhood or adulthood.20–23
Cord blood adipokines
We collected cord blood samples from the umbilical vein after delivery of the infant, and measured concentrations of leptin and adiponectin, as described previously.24, 25 In Project Viva, lower cord blood leptin levels was found to be associated with higher BMI at age 3 years while higher cord blood adiponectin levels predicted an increase in central adiposity.26
Infant feeding
Research assistants asked mothers if they had initiated breastfeeding. At 6 and 12 months, we asked mothers if they were exclusively breastfeeding, mixed breast and formula feeding, weaned, or formula-feeding only. If they had stopped breastfeeding, we asked them the children’s age at cessation. A recent meta-analysis examining duration of breastfeeding demonstrated a decrease in odds of later obesity for each additional month of breastfeeding.27
At 6 months, we also measured timing of introduction of solids (<4 months, 4–5 months and ≥6 months). Previous studies have found that introduction of solids < 4 months is a risk factor for increased infant weight gain.28, 29
Maternal control of infant feeding
At 1 year, we measured mothers’ reports of restricting their children’s food intake and pressuring their children to eat more food using a modified Child Feeding Questionnaire (CFQ).30 Restriction was measured by the question: “I have to be careful not to feed my child too much.” We derived a continuous pressure to eat score from 5 questions of the modified CFQ. We have previously reported the internal reliability of this score.31 Maternal feeding restriction is associated with greater odds of child obesity at age 3.32 In some studies, mothers’ pressure on their children to eat has been associated with disinhibited eating33 increased child energy intake, and body weight.34, 35
Daily sleep during infancy
At 6 months, 1-year, and 2-years postpartum, we asked mothers to quantify the average amount of daily sleep their children obtained over the past month. We calculated a weighted average of sleep duration from 6 months to 2 years. We have recently shown in the Project Viva cohort that average daily sleep duration from 6 months to 2 years is inversely associated with obesity at 3 years.36
Daily television viewing during infancy
At 6 months, 1-year, and 2-years postpartum, we asked mothers to report the number of hours their children watched TV/videos on an average weekday and weekend day in the past month. We calculated a weighted average of TV/video viewing from 6 months to 2 years. At age 4 years, we also asked mothers if their child had a TV in their bedroom. Previous studies have found that greater hours spent watching TV at age 3 years was associated with obesity at age 7 years,4 and having a TV in a child’s bedroom is a risk factor for child obesity.6
Sugar-Sweetened Beverages, Fast Food, and Family Dinner
We used a validated semi-quantitative child food frequency questionnaire completed by each mother when the child was 2 years old to estimate daily sugar-sweetened beverage intake.37 We defined a sugar-sweetened beverage as soda (not sugar-free), flavored milks, and fruit drinks (Hi-C, Kool-Aid, lemonade). Previous studies have found that consumption of sugar-sweetened beverages is associated with childhood obesity;38 although the relationship between 100% fruit juice and obesity is still controversial.39 Thus, we excluded orange juice and other 100% fruit juice intake. At age 3 years, we asked mothers to report their child’s weekly servings of fast food using a question adapted from a longitudinal study of adults.40 A similar, modified question was validated by association with childhood obesity in a study of 9–14 year olds.41 Greater intake of fast food has been found to be associated with poorer diet quality among children42, 43 and with higher BMI among adolescents.41 At age 4 years, we asked mothers to report how often their child ate supper or dinner together with family members. Eating dinner with family members is associated with healthful dietary intake patterns and with BMI in cross-sectional analyses of 9–14 year olds.44, 45
Other Measures
We measured length/height and weight of children using a calibrated stadiometer and scale. We calculated age- and sex-specific anthropometric measures using US national reference data46. Mothers reported their pre-pregnancy weight and height as well as fathers’ weight and height, from which we calculated their BMIs. We also collected information about maternal age, education, parity, marital status, immigration status, and household income.
Statistical Analysis
We first examined bivariate relationships of child race/ethnicity with other covariates and our main outcomes. We then used multivariable linear and logistic regression models to assess the independent effects of child race/ethnicity on our main outcomes. Our first model was adjusted for child sex only. We then additionally adjusted the multivariable models for demographic and socioeconomic factors including maternal age, education, parity; and household income. Because maternal pre-pregnancy BMI and paternal BMI could be confounders of the relationship between race/ethnicity and obesity-related risk factors, we additionally adjusted the multivariable models for these variables. It is also possible that adjustment for parental BMI may be over-adjustment given shared family behaviors. Thus, we separately present models with and without such adjustment. We report regression estimates (β) or odds ratios and 95% confidence intervals for child race/ethnicity. In the regression models, the referent group was white children. We performed data analyses with SAS version 9.1.
RESULTS
Table 1 shows characteristics of the 1826 mother-child pairs from Project Viva included in our analyses. Consistent with national statistics, we found that compared with white children, black and Hispanic children had lower birth weights for gestational age but higher BMI z-scores and higher prevalence of obesity at age 3 years (Table 1). Table 2 shows the overall and race/ethnicity-specific prevalence of obesity-related risk factors.
Table 1.
Selected Parent and Child Characteristics Overall and According to Child Race/Ethnicity. Data from 1826 Mother-Child Pairs from Project Viva.
| Maternal, Paternal, and Household Characteristics |
Overall (N=1826) |
White, non- Hispanic (N=1343) |
Black, non- Hispanic (N=355) |
Hispanic or Latino (N=128) |
|---|---|---|---|---|
| % or Mean (SD) | ||||
| Maternal age, years (±SD) | 31.9 (5.2) | 32.9 (4.4) | 29.4 (6.3) | 28.8 (5.8) |
| Education, Some college or more (%) | 88% | 94% | 73% | 69% |
| Parity, Multipara, (%) | 53% | 50% | 61% | 58% |
| Marital Status, Married/cohabiting, (%) | 92% | 97% | 76% | 81% |
| Household income > $40,000, (%) | 85% | 93% | 57% | 52% |
| Non-US born, (%) | 17% | 10% | 35% | 45% |
| Maternal pre-pregnancy BMI categories (%) | ||||
| < 25 kg/m2 | 62% | 68% | 44% | 53% |
| 25 – < 30 kg/m2 | 22% | 20% | 26% | 31% |
| ≥ 30 kg/m2 | 16% | 12% | 30% | 17% |
| Paternal pre-pregnancy BMI categories (%) | ||||
| < 25 kg/m2 | 36% | 35% | 37% | 37% |
| 25 – < 30 kg/m2 | 48% | 50% | 39% | 48% |
| ≥ 30 kg/m2 | 16% | 15% | 24% | 15% |
| Child Characteristics | ||||
| Boy (%) | 52% | 51% | 54% | 55% |
| Birth and Early Childhood Anthropometric Characteristics | ||||
| Birth weight, kg (±SD) | 3.48 (0.58) | 3.55 (0.55) | 3.30 (0.64) | 3.34 (0.61) |
| Gestational age at birth (weeks) | 39.5 (1.9) | 39.6 (1.7) | 39.1 (2.2) | 39.1 (2.1) |
| Birth weight for gestational age z-score | 0.20 (0.97) | 0.29 (0.95) | −0.08 (0.97) | −0.03 (0.98) |
| Weight-for-age z-score at age 6m | 0.34 (0.97) | 0.33 (0.96) | 0.43 (1.01) | 0.18 (1.01) |
| Body mass index z-score at age 3 | 0.48 (1.02) | 0.45 (0.98) | 0.59 (1.11) | 0.63 (1.28) |
| BMI ≥ 95th percentile at age 3 | 9.6% | 8.5% | 12.4% | 18.5% |
Table 2.
Early Life Risk Factors for Childhood Obesity Overall and According to Child Race/Ethnicity
| Early Life Risk Factors for Childhood Obesity | Overall | White, non- Hispanic |
Black, non- Hispanic |
Hispanic or Latino |
|---|---|---|---|---|
| % or Mean (SD) | ||||
| Institute of Medicine gestational weight gain category (%) | ||||
| Adequate | 34% | 34% | 33% | 38% |
| Inadequate | 15% | 13% | 20% | 18% |
| Excessive | 51% | 53% | 47% | 44% |
| Gestational diabetes (%) | 5% | 5% | 6% | 11% |
| Mother smoked during early pregnancy (%) | 13% | 12% | 14% | 22% |
| Maternal antenatal depression (%) | 9% | 7% | 15% | 18% |
| Fetal growth | ||||
| Large for gestational age | 14% | 16% | 9% | 7% |
| Small for gestational age | 6% | 4% | 10% | 9% |
| Cord blood adipokines | ||||
| Leptin (ng/mL) | 9.0 (6.7) | 8.7 (6.4) | 10.8 (8.1) | 8.8 (5.8) |
| Adiponectin (µg/mL) | 28.7 (6.8) | 28.8 (6.6) | 28.1 (7.7) | 28.5 (6.4) |
| Rapid infant weight gain | ||||
| Highest quartile of change in weight-for-age 0 – 6m | 25% | 22% | 39% | 34% |
| Infant feeding | ||||
| Did not initiate breastfeeding (%) | 14% | 14% | 13% | 13% |
| Not exclusively breastfed until 6m (%) | 79% | 76% | 88% | 90% |
| Total duration of breastfeeding, months (±SD) | 5.7 (4.6) | 6.0 (4.6) | 4.6 (4.2) | 4.6 (4.2) |
| Introduction of solids < 4m (%) | 17% | 14% | 32% | 36% |
| Maternal restriction of child feeding at 1y (%) | 13% | 9% | 29% | 38% |
| Highest quartile of pressure to eat score at 1y (%) | 22% | 19% | 33% | 40% |
| Infant sleep | ||||
| Average daily sleep duration 6m – 2y, < 12 hours (%) | 34% | 30% | 62% | 59% |
| Television viewing | ||||
| Average daily TV viewing 6m – 2y, ≥ 2 hours (%) | 17% | 15% | 32% | 11% |
| Television in room where child sleeps at 4y (%) | 15% | 7% | 51% | 56% |
| Diet patterns | ||||
| Any (vs. none) sugar sweetened beverage intake at 2y (%) | 51% | 45% | 82% | 74% |
| Any (vs. none) fast food intake at 3y (%) | 70% | 66% | 83% | 88% |
| Family meals consumption per day at 4y, (±SD) | 0.82 (0.26) | 0.83 (0.25) | 0.75 (0.31) | 0.83 (0.26) |
In crude models adjusted only for child sex, we observed racial/ethnic differences in almost every risk factor examined (Tables 3 and 4). Socioeconomic factors confounded the observed relationships between race/ethnicity and obesity-related risk factors. Adjustment for socioeconomic factors minimally (~10%) attenuated our observed crude estimates for some risk factors (e.g. gestational weight gain, birth weight for gestational age, infant weight gain), modestly (~20–30%) attenuated the estimates for other risk factors, particularly those in early childhood such as television viewing and dietary practices, and substantially (>30%) attenuated others including maternal smoking during pregnancy, antenatal depression, initiation and total duration of breastfeeding, and having a TV in the room where a child sleeps. Adjustment for maternal and paternal pre-pregnancy BMI further attenuated our observed estimates. In particular, while we observed marked racial/ethnic differences in total breastfeeding duration in crude models, the relationship between race/ethnicity and total breastfeeding duration disappeared after adjustment for maternal and paternal BMI (Table 3).
Table 3.
Crude and Multivariable Adjusted* Effect Estimates (95% Confidence Interval) for Associations of Child Race/Ethnicity with Risk Factors for Childhood Obesity (Continuous Outcomes).
| Risk Factors for Childhood Obesity | Black, non-Hispanic | Hispanic or Latino | ||||
|---|---|---|---|---|---|---|
| Effect Estimate (95% Confidence Interval) | ||||||
| Crude |
SES Adjusted |
Parental BMI Adjusted |
Crude |
SES Adjusted |
Parental BMI Adjusted |
|
| Fetal growth | ||||||
| Birth weight for gestational age z-score | −0.38 (−0.49, −0.27) | −0.36 (−0.49, −0.24) | −0.39 (−0.52, −0.27) | −0.33 (−0.50, −0.15) | −0.28 (−0.46, −0.10 | −0.30 (−0.48, −0.11) |
| Cord blood adipokines† | ||||||
| Leptin (per 10 ng/mL increment) | 0.30 (0.18, 0.43) | 0.33 (0.20, 0.47) | 0.31 (0.17, 0.44) | 0.11 (−0.07, 0.30) | 0.13 (−0.07, 0.34) | 0.09 (−0.11, 0.30) |
| Adiponectin (per 10 µg/mL increment) | −0.05 (−0.18, 0.08) | −0.12 (−0.26, 0.02) | −0.12 (−0.26, 0.03) | −0.003 (−0.21, 0.21) | −0.11 (−0.33, 0.11) | −0.17 (−0.40, 0.06) |
| Infant feeding | ||||||
| Total duration of breastfeeding, months | −1.41 (−2.05, −0.77) | −0.48 (−1.19, 0.23) | −0.08 (−0.80, 0.65) | −1.40 (−2.45, −0.36) | −0.19 (−1.28, 0.89) | −0.13 (−1.23, 0.97) |
| Diet patterns | ||||||
| Family meals consumption/day at 4y | −0.07 (−0.12, −0.03) | −0.08 (−0.13, −0.03) | −0.08 (−0.13, −0.02) | 0.004 (−0.08, 0.09) | −0.01 (−0.09, 0.08) | 0.0002 (−0.09, 0.09) |
Crude models are adjusted for child sex only. SES Models are additionally adjusted for maternal age, education, parity; and household income. Parental BMI models adjust for maternal and paternal pre-pregnancy body mass index. Reference group for all models are white children.
Models predicting cord blood adipokines are additionally adjusted for birth weight for gestational age z-score.
Table 4.
Crude and Multivariable Adjusted* Odds Ratios (95% Confidence Interval) for Associations of Child Race/Ethnicity with Risk Factors for Childhood Obesity (Dichotomous Outcomes).
| Risk Factors for Childhood Obesity | Black, non-Hispanic |
Hispanic or Latino |
||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | ||||||
| Crude |
SES Adjusted |
Parental BMI Adjusted |
Crude |
SES Adjusted |
Parental BMI Adjusted |
|
| Excessive gestational weight gain † | 0.85 (0.67, 1.08) | 0.80 (0.61, 1.05) | 0.68 (0.52, 0.91) | 0.76 (0.52, 1.10) | 0.70 (0.47, 1.05) | 0.66 (0.44, 0.99) |
| Gestational diabetes | 1.18 (0.70, 1.98) | 1.43 (0.80, 2.55) | 0.91 (0.50, 1.68) | 2.57 (1.39, 4.76) | 3.48 (1.75, 6.92) | 3.08 (1.51, 6.25) |
| Mother smoked during early pregnancy | 1.15 (0.79, 1.66) | 0.52 (0.34, 0.82) | 0.47 (0.30, 0.74) | 2.08 (1.29, 3.34) | 0.94 (0.54, 1.62) | 0.89 (0.51, 1.55) |
| Maternal antenatal depression | 2.31 (1.47, 3.62) | 1.51 (0.92, 2.49) | 1.55 (0.93, 2.58) | 2.96 (1.55, 5.62) | 1.88 (0.94, 3.78) | 1.89 (0.94, 3.81) |
| Fetal growth | ||||||
| Large for gestational age | 0.51 (0.34, 0.76) | 0.40 (0.26, 0.62) | 0.33 (0.21, 0.53) | 0.40 (0.20, 0.81) | 0.35 (0.17, 0.72) | 0.35 (0.17, 0.75) |
| Small for gestational age | 2.20 (1.42, 3.42) | 1.63 (0.97, 2.75) | 1.41 (0.79, 2.52) | 2.05 (1.07, 3.93) | 1.48 (0.72, 3.03) | 1.55 (0.73, 3.29) |
| Rapid infant weight gain | ||||||
| Highest quartile of change in weight-for-age 0 – 6m | 2.35 (1.83, 3.39) | 2.27 (1.50, 3.44) | 2.01 (1.30, 3.10) | 1.93 (1.08, 3.45) | 1.71 (0.91, 3.20) | 1.75 (0.91, 3.35) |
| Infant feeding | ||||||
| Did not initiate breastfeeding | 0.93 (0.66, 1.31) | 0.41 (0.27, 0.63) | 0.38 (0.25, 0.59) | 0.88 (0.51, 1.51) | 0.35 (0.19, 0.65) | 0.31 (0.16, 0.60) |
| Not exclusively breastfed until 6m | 2.35 (1.57, 3.52) | 1.82 (1.17, 2.82) | 1.50 (0.96, 2.37) | 2.80 (1.39, 5.66) | 2.29 (1.07, 4.90) | 2.84 (1.19, 6.74) |
| Introduction of solids < 4m | 3.09 (2.13, 4.50) | 2.14 (1.41, 3.26) | 1.91 (1.23, 2.97) | 3.33 (1.95, 5.67) | 2.27 (1.27, 4.06) | 2.04 (1.11, 3.77) |
| Maternal restriction of child feeding at 1y | 4.21 (2.75, 6.43) | 2.99 (1.83, 4.87) | 2.59 (1.55, 4.35) | 6.18 (3.29, 11.6) | 4.22 (2.13, 8.37) | 3.35 (1.59, 7.05) |
| Highest quartile of pressure to eat score at 1y | 2.09 (1.42, 3.07) | 2.07 (1.34, 3.21) | 2.14 (1.36, 3.36) | 2.74 (1.50, 5.02) | 2.66 (1.40, 5.05) | 2.32 (1.19, 4.52) |
| Infant sleep | ||||||
| Average daily sleep duration 6m - 2y < 12 hours | 3.83 (2.52, 5.83) | 3.66 (2.31, 5.79) | 3.70 (2.30, 5.95) | 3.45 (1.68, 7.11) | 2.98 (1.40, 6.37) | 2.53 (1.16, 5.50) |
| Television viewing | ||||||
| Average daily TV viewing 6m - 2y ≥ 2 hours | 2.69 (1.69, 4.29) | 2.00 (1.19, 3.36) | 1.54 (0.89, 2.67) | 0.70 (0.21, 2.35) | 0.44 (0.12, 1.58) | 0.44 (0.12, 1.63) |
| Television in room where child sleeps at 4y | 14.27 (9.42, 21.63) | 8.19 (5.13, 13.08) | 7.65 (4.70, 12.44) | 17.45 (8.90, 34.21) | 8.03 (3.81, 16.96) | 7.99 (3.72, 17.14) |
| Diet patterns | ||||||
| Any sugar-sweetened beverage intake at 2y | 5.66 (3.66, 8.76) | 4.58 (2.83, 7.39) | 4.11 (2.52, 6.69) | 3.61 (1.74, 7.51) | 2.66 (1.21, 5.84) | 2.48 (1.12, 5.51) |
| Any fast food consumption at 3y | 2.49 (1.61, 3.86) | 2.00 (1.25, 3.20) | 1.65 (1.01, 2.71) | 3.76 (1.59, 8.92) | 2.79 (1.14, 6.82) | 3.14 (1.19, 8.31) |
Crude models are adjusted for child sex only. SES Models are additionally adjusted for maternal age, education, parity; and household income. Parental BMI models additionally adjust for maternal and paternal pre-pregnancy body mass index. Reference group for all models are white children. Models predicting maternal risk factors are not adjusted for paternal BMI.
Models predicting excessive gestational weight gain are additionally adjusted for total length of gestation.
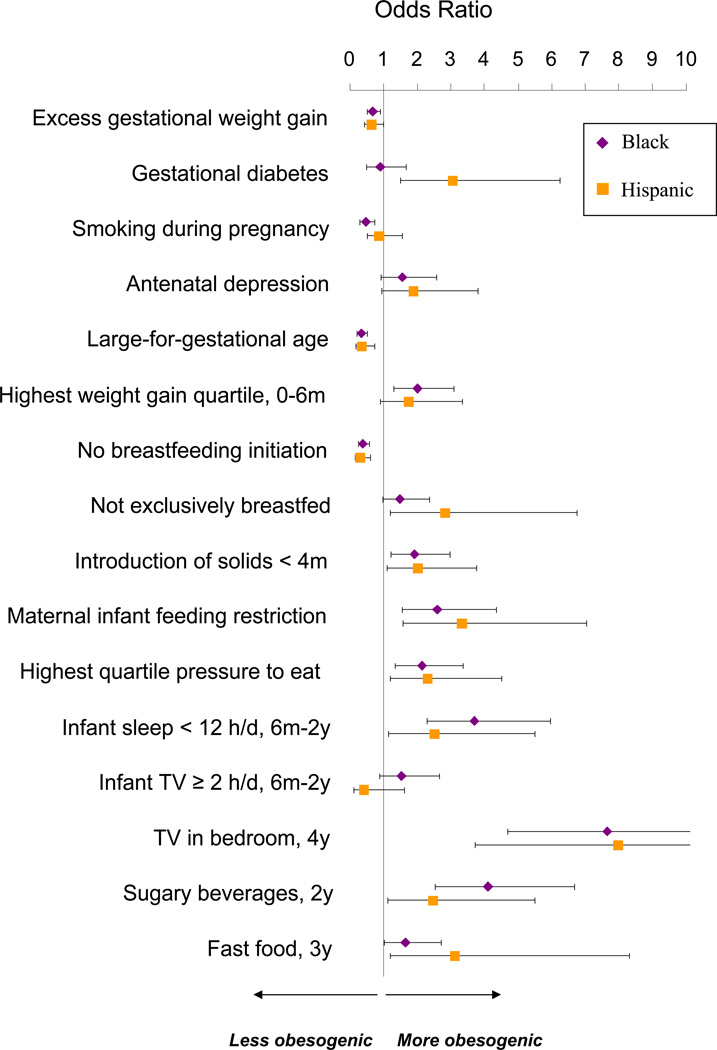
In fully adjusted multivariable models, we observed several differences between black and Hispanic children compared with white children in a range of risk factors related to childhood obesity. Black and Hispanic children had lower fetal growth indices but grew very rapidly in the first 6 months of life (Tables 3 and 4). Although black and Hispanic mothers were more likely to initiate breastfeeding, they were less likely to exclusively breastfeed their infants to 6 months of age, and were more likely to introduce solid foods prior to 4 months of age (Table 4 and Figure). Black and Hispanic mothers were also more likely to exert greater control over their infants’ feeding by restricting and pressuring their children to eat (Table 4). Between 6 months to 2 years of age, black and Hispanic children were sleeping less than their white counterparts. After age 2 years, black and Hispanic children were much more likely to have a TV in their bedroom and had higher consumption of sugar-sweetened beverages and fast food (Table 4 and Figure).
Figure.
Odds of each childhood obesity risk factor in black and Hispanic participants, relative to whites. Data from 1826 Mother-Infant Pairs in Project Viva. Odds ratios adjusted for maternal age, education, parity, pre-pregnancy BMI; paternal BMI; household income; and child sex. Models predicting maternal risk factors are not adjusted for paternal BMI. Bars show 95% confidence limits.
Some risk factors were associated with only one minority group. Hispanic, but not Black mothers had elevated odds of gestational diabetes (Table 4). Black children had higher cord blood leptin levels, viewed greater hours of TV from 6 months to 2 years of age, and consumed fewer meals together with their families. In contrast to these risk factors that might raise the risk of obesity, black and Hispanic women had lower odds of excess gestational weight gain. Black women also had lower odds of smoking during pregnancy. (Table 4 and Figure).
DISCUSSION
In this pre-birth, prospective cohort of mother-child pairs, we found racial/ethnic differences in many early life risk factors for childhood obesity. Although adjustment for socioeconomic status and maternal and paternal obesity attenuated many of the observed relationships, independent of socioeconomic status and parental obesity, black and Hispanic children had increased odds of rapid infant weight gain, greater maternal control of infant feeding, shorter sleep duration during infancy, more televisions in bedrooms, higher sugar-sweetened beverage intake, and higher intake of fast food compared to white children.
Our findings are consistent with previous studies of racial/ethnic differences in pregnancy-related risk factors for childhood obesity.16, 47–49 Consistent with a national study by Chu et al.47, black and Hispanic women in our study were more likely than white women to begin their pregnancies already overweight or obese and gained less weight during pregnancy. Similar to a previous study in New York City,48 we found that Hispanic women had a higher risk of gestational diabetes. In contrast to other studies, however, we did not find that black women had an increased risk of gestational diabetes.50 Few studies have examined racial/ethnic differences in smoking during pregnancy49 and in maternal antenatal depression.16 In our study, the observed racial/ethnic differences in both smoking during pregnancy and antenatal depression were largely explained by socioeconomic factors including maternal education and household income.
Our findings extend to infancy and early childhood previous studies of older children that have found higher prevalence of obesity-related risk factors among racial/ethnic minorities. Previous studies have found higher levels of TV viewing and more televisions in bedrooms,6 higher consumption of sugar sweetened beverages51, increased fast food consumption,52 and lower levels of physical activity among black and Hispanic youth compared to white youth53, 54. In addition, previous studies have observed racial/ethnic differences in maternal feeding practices and beliefs.55, 56 To our knowledge, no published studies have examined racial/ethnic differences in rapid infant weight gain, sleep duration in infancy, or fast food consumption in early childhood.
Overall, our findings suggest that racial/ethnic disparities in childhood obesity may be determined by factors operating in pregnancy, infancy, and early childhood. These factors may include differences in behaviors such as diet patterns and physical activity or differences in access to and utilization of pregnancy- or infancy-related health care. It is also possible that cumulative disadvantage or “weathering”,57 even before conception, among racial/ethnic minority mother-child pairs may also explain the observed disparities. Further studies should examine these possibilities. Our study also implies that interventions to modify early life risk factors may have substantial impact on reducing disparities in childhood obesity prevalence and its related co-morbidities. To date, however, few interventions have been developed to target the prenatal, infancy, and early childhood periods in an attempt to prevent obesity. A recent systematic review of effective programs to prevent or treat obesity among children under the age of 6 yielded only seven published interventions of 9- to 70-month-old children.58 Even fewer interventions exist involving the pre- and postpartum period.59 Completed trials are few, and none has evaluated an intervention lasting from pregnancy through the postpartum period. In addition, many national programs60 and funding initiatives61 with the purpose of preventing childhood obesity have largely focused on children over the age of 3 years. Our findings support the recent recommendations by the National Heart, Lung, and Blood Institute that interventions early in life are needed particularly among vulnerable, high-risk populations, including ethnically diverse groups.62
Strengths of our study included having prospectively collected data on a wide range of risk factors extending from pregnancy to early childhood and the ability to adjust for several important confounding factors including parental obesity. The study also had several potential limitations. First, most of our measures were from self report, including pre-pregnancy maternal weight, smoking, breastfeeding, and infant sleep, and loss to follow-up was not random. These factors could have introduced bias towards the null. Second, the educational and income levels of our study population were relatively high. As some of the associations between race/ethnicity and obesity risk factors appeared to depend on socioeconomic status, our results may not be generalizable to more socio-economically disadvantaged populations. Although we studied many risk factors for childhood obesity that had plausible hypotheses, we did not measure others such as lifestyle or cultural determinants of dietary and sedentary practices. Third, we did not have enough power to examine potentially important interactions between race/ethnicity and socioeconomic status.
In summary, we found that from pregnancy to the preschool period, many risk factors for child obesity are more prevalent among blacks and Hispanics than among whites. These differences may very well explain the observed racial/ethnic differences in prevalence of obesity in young children. While more studies are needed to understand the underlying reasons for the development of these disparities, our findings provide a strong rationale for testing comprehensive interventions in early life to reduce disparities in obesity prevalence.
Acknowledgment
Funding: This study was supported by a grant from the National Center on Minority Health and Health Disparities (MD 003963).
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to disclose.
Dr. Taveras had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Taveras EM
Acquisition of data: Gillman MW, Rifas-Shiman SL.
Analysis and interpretation of data: Taveras EM, Rifas-Shiman SL, Kleinman K, Rich-Edwards J, Gillman MW
Drafting of manuscript: Taveras EM
Critical revision of the manuscript for important intellectual content: Taveras EM, Rifas-Shiman SL, Kleinman K, Rich-Edwards J, Gillman MW.
Statistical analysis: Rifas-Shiman SL.
Obtained funding: Taveras EM, Gillman MW.
Administrative, technical, or material support: Taveras EM, Rifas-Shiman SL.
Study supervision: Taveras EM, Gillman MW.
References
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Kuh D, Ben-Shlomo Y. A life course approach to chronic disease epidemiology: tracing the origins of ill-health from early to adult life. 2nd edition. London: Oxford University Press; 2004. [Google Scholar]
- 3.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring) 2008;16(7):1651–1656. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimbro RT, Brooks-Gunn J, McLanahan S. Racial and ethnic differentials in overweight and obesity among 3-year-old children. Am J Public Health. 2007;97(2):298–305. doi: 10.2105/AJPH.2005.080812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennison BA, Erb TA, Jenkins PL. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics. 2002;109(6):1028–1035. doi: 10.1542/peds.109.6.1028. [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal Gestational Weight Gain and Offspring Weight in Adolescence. Obstet Gynecol. 2008;112(5):999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr. 2008;87(6):1818–1824. doi: 10.1093/ajcn/87.6.1818. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Nutrition During Pregnancy. Washington, D.C: National Academy Press; 1990. [Google Scholar]
- 11.Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 13.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9):2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 14.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32(2):201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 16.Rich-Edwards JW, Kleinman K, Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60(3):221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gundersen C, Lohman BJ, Garasky S, Stewart S, Eisenmann J. Food Security, Maternal Stressors, and Overweight Among Low-Income US Children: Results From the National Health and Nutrition Examination Survey (1999–2002) Pediatrics. 2008;122(3):e529–e540. doi: 10.1542/peds.2008-0556. [DOI] [PubMed] [Google Scholar]
- 18.Davis M, Young L, Davis SP, Moll G. Parental depression, family functioning and obesity among African American children. J Cult Divers. 2008;15(2):61–65. [PubMed] [Google Scholar]
- 19.Oken E, Kleinman KP, Rich-Edwards JW, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77:1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 22.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65(Suppl 3):65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6(2):143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 24.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased Serum Leptin in Bulimia Nervosa. J Clin Endocrinol Metab. 2000;85(12):4511–4514. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 25.Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 26.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord Blood Leptin and Adiponectin as Predictors of Adiposity in Children at 3 Years of Age: A Prospective Cohort Study. Pediatrics. 2009;123(2):682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Peterson KE. Association of infant child care with infant feeding practices and weight gain among US infants. Arch Pediatr Adolesc Med. 2008;162(7):627–633. doi: 10.1001/archpedi.162.7.627. [DOI] [PubMed] [Google Scholar]
- 29.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80(6):1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 30.Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36(3):201–210. doi: 10.1006/appe.2001.0398. [DOI] [PubMed] [Google Scholar]
- 31.Taveras EM, Scanlon KS, Birch L, Rifas-Shiman SL, Rich-Edwards JW, Gillman MW. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics. 2004;114(5):e577–583. doi: 10.1542/peds.2004-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taveras EM, Rifas-Shiman SL, Scanlon KS, Grummer-Strawn LM, Sherry B, Gillman MW. To what extent is the protective effect of breastfeeding on future overweight explained by decreased maternal feeding restriction? Pediatrics. 2006;118(6):2341–2348. doi: 10.1542/peds.2006-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carper JL, Orlet Fisher J, Birch LL. Young girls' emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite. 2000;35(2):121–129. doi: 10.1006/appe.2000.0343. [DOI] [PubMed] [Google Scholar]
- 34.Klesges RC, Coates TJ, Brown G, et al. Parental influences on children's eating behavior and relative weight. J Appl Behav Anal. 1983;16(4):371–378. doi: 10.1901/jaba.1983.16-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klesges RC, Malott JM, Boschee PF, Weber JM. The effects of parental influences on children's food intake, physical activity, and relative weight. Int J Eat Dis. 1986;5:335–346. [Google Scholar]
- 36.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008;162(4):305–311. doi: 10.1001/archpedi.162.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blum RE, Wei EK, Rockett HR, et al. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3:167–172. doi: 10.1023/a:1022350023163. [DOI] [PubMed] [Google Scholar]
- 38.Welsh JA, Cogswell ME, Rogers S, Rockett H, Mei Z, Grummer-Strawn LM. Overweight among low-income preschool children associated with the consumption of sweet drinks: Missouri, 1999–2002. Pediatrics. 2005;115(2):e223–e229. doi: 10.1542/peds.2004-1148. [DOI] [PubMed] [Google Scholar]
- 39.Nicklas TA, O'Neil CE, Kleinman R. Association between 100% juice consumption and nutrient intake and weight of children aged 2 to 11 years. Arch Pediatr Adolesc Med. 2008;162(6):557–565. doi: 10.1001/archpedi.162.6.557. [DOI] [PubMed] [Google Scholar]
- 40.Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study)15-year prospective analysis. Lancet. 2005;365(9453):36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 41.Taveras EM, Berkey CS, Rifas-Shiman SL, et al. The association of fried food consumption away from home with body mass index and diet quality in older children and adolescents. Pediatrics. 2005;116(4):e518–e524. doi: 10.1542/peds.2004-2732. [DOI] [PubMed] [Google Scholar]
- 42.French SA, Story M, Neumark-Sztainer D, Fulkerson JA, Hannan P. Fast food restaurant use among adolescents: associations with nutrient intake, food choices and behavioral and psychosocial variables. Int J Obes Relat Metab Disord. 2001;25(12):1823–1833. doi: 10.1038/sj.ijo.0801820. [DOI] [PubMed] [Google Scholar]
- 43.Guthrie JF, Lin BH, Frazao E. Role of food prepared away from home in the American diet, 1977–78 versus 1994–96: changes and consequences. 2002;34(3):140–150. doi: 10.1016/s1499-4046(06)60083-3. [DOI] [PubMed] [Google Scholar]
- 44.Gillman MW, Rifas-Shiman SL, Frazier AL, et al. Family dinner and diet quality among older children and adolescents. Arch Fam Med. 2000;9:235–240. doi: 10.1001/archfami.9.3.235. [DOI] [PubMed] [Google Scholar]
- 45.Taveras EM, Rifas-Shiman SL, Berkey CS, et al. Family dinner and adolescent overweight. Obes Res. 2005;13(5):900–906. doi: 10.1038/oby.2005.104. [DOI] [PubMed] [Google Scholar]
- 46.National Center for Health Statistics. CDC Growth Charts, United States. 2000 Available at: http://www.cdc.gov/growthcharts/.
- 47.Chu SY, Callaghan WM, Bish CL, D'Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol. 2009 doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- 48.Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH. Ethnicity and gestational diabetes in New York City, 1995–2003. Bjog. 2008;115(8):969–978. doi: 10.1111/j.1471-0528.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 49.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92(11):1801–1808. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorpe LE, Berger D, Ellis JA, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health. 2005;95(9):1536–1539. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giammattei J, Blix G, Marshak HH, Wollitzer AO, Pettitt DJ. Television watching and soft drink consumption: associations with obesity in 11- to 13-year-old schoolchildren. Arch Pediatr Adolesc Med. 2003;157:882–886. doi: 10.1001/archpedi.157.9.882. [DOI] [PubMed] [Google Scholar]
- 52.Bowman SA, Gortmaker SL, Ebbeling CB, Pereira MA, Ludwig DS. Effects of fast-food consumption on energy intake and diet quality among children in a national household survey. Pediatrics. 2004;113:112–118. doi: 10.1542/peds.113.1.112. [DOI] [PubMed] [Google Scholar]
- 53.Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(12):938–942. doi: 10.1001/jama.279.12.938. [DOI] [PubMed] [Google Scholar]
- 54.Kimm SY, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. NEJM. 2002;347(10):709–715. doi: 10.1056/NEJMoa003277. [DOI] [PubMed] [Google Scholar]
- 55.Baughcum AE, Burklow KA, Deeks CM, Powers SW, Whitaker RC. Maternal feeding practices and childhood obesity. A focus group study of low-income mothers. Arch Pediatr Adolesc Med. 1998;152:1010–1014. doi: 10.1001/archpedi.152.10.1010. [DOI] [PubMed] [Google Scholar]
- 56.Baughcum AE, Powers SW, Johnson SB, et al. Maternal feeding practices and beliefs and their relationships to overweight in early childhood. J Dev Behav Pediatr. 2001;22(6):391–408. doi: 10.1097/00004703-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 58.Bluford DA, Sherry B, Scanlon KS. Interventions to prevent or treat obesity in preschool children: a review of evaluated programs. Obesity (Silver Spring) 2007;15(6):1356–1372. doi: 10.1038/oby.2007.163. [DOI] [PubMed] [Google Scholar]
- 59.Dodd JM, Crowther CA, Robinson JS. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: a systematic review. Acta Obstet Gynecol Scand. 2008;87(7):702–706. doi: 10.1080/00016340802061111. [DOI] [PubMed] [Google Scholar]
- 60.American Heart Association and The William J. Clinton Foundation. Alliance for a Healthier Generation. http://www.americanheart.org/downloadable/heart/1222278448727About%20the%20Alliance%20for%20a%20Healthier%20Generation.pdf. [Google Scholar]
- 61.The Robert Wood Johnson Foundation. Childhood Obesity Framing Document. http://www.rwjf.org/programareas/ChildhoodObesityFramingDoc.pdf. [Google Scholar]
- 62.Pratt CA, Stevens J, Daniels S. Childhood obesity prevention and treatment: recommendations for future research. Am J Prev Med. 2008;35(3):249–252. doi: 10.1016/j.amepre.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]