Abstract
The dopamine D3 receptor (D3R) has been investigated as a potential target for medication development to treat substance use disorders (SUDs) with a particular focus on cocaine and methamphetamine. Currently, there are no approved medications to treat cocaine and methamphetamine addiction and thus developing pharmacotherapeutics to compliment existing behavioral strategies is a fundamental goal. Novel compounds with high affinity and D3R selectivity have been evaluated in numerous animal models of drug abuse and favorable outcomes in nonhuman primate models of self-administration and relapse have provided compelling evidence to advance these agents into the clinic. One approach is to repurpose drugs that share the D3R mechanism and already have clinical utility, and to this end buspirone has been identified as a viable candidate for clinical trials. A second, but substantially more resource intensive and risky approach involves the development of compounds that exclusively target D3R, such as GSK598809 and PG 619. Clinical investigation of these drugs or other novel D3R-selective agents will provide a better understanding of the role D3R plays in addiction and whether or not antagonists or partial agonists that are D3R selective are effective in achieving abstinence in this patient population.
Keywords: cocaine, methamphetamine, buspirone, psychostimulant abuse, medication development
1. Overview of the challenge in medications development for substance use disorders: focus on cocaine and methamphetamine
Major challenges exist for developing medications to treat substance use disorders (SUDs) in general, and psychostimulant addiction, in particular. Despite a considerable global burden [1], the negative health and societal consequences of cocaine and methamphetamine abuse have continued unabated. Based on decades of research, it is clear that therapeutic strategies that include medications are necessary to reduce, and ultimately, eliminate illicit drug taking. Nevertheless, translation from mechanistic target identification to preclinical testing in animal models of self-administration and relapse through clinical evaluation of novel or repurposed molecules has progressed at a snail’s pace. The reasons for failure to deliver effective medications to treat psychostimulant addictions have been described and debated (e.g., [2]). The lack of consistent efforts in drug development for this patient population in the private sector has historically been a barrier to success. Further, the multi-billion dollar price tag [3] coupled with the high risk of developing psychiatric medications has contributed to the recent exodus of big pharma from development of drugs that act on the central nervous system (CNS). This disengagement from psychiatric drug development will undoubtedly further slow this process because of downstream effects on smaller pharmaceutical and biotech firms that have traditionally relied on the resources of big pharma in the latter stages of drug development [4]. Translation from animal to clinical studies, low medication compliance during the conduct of clinical trials, placebo effects, and the current FDA perspective to demonstrate complete abstinence, as opposed to reduction in drug use [5], all provide formidable challenges to the successful development of medications to treat SUDs [2, 6].
Nevertheless, scientific advances have led to the identification of “druggable” targets, and medicinal chemistry efforts have, in turn, resulted in the development of small molecules that show promise in preclinical models of addiction. In this commentary, we present the dopamine D3 receptor (D3R) as a uniquely suited target for drug development, with D3R antagonists and partial agonists showing promise in models of cocaine and methamphetamine abuse. We briefly summarize recent advances in the discovery of small molecules that bind with high affinity and selectivity to D3R and have properties in preclinical models that forecast successful translation to the clinic. We highlight selected novel agents and in addition, present promising data on a repurposed molecule, buspirone, as a candidate for clinical trials.
2. Why the Dopamine D3 Receptor (D3R) may be a uniquely suited target for cocaine abuse and addiction
Although both cocaine and methamphetamine are psychostimulants and bind to all three monoamine transporters (norepinephrine, dopamine, serotonin), their mechanisms of action differ. Cocaine blocks neurotransmitter transport into the cell, but cannot itself be transported, whereas methamphetamine serves as a substrate, competing for the neurotransmitter both at the membrane and vesicular transporters, ultimately facilitating the release of neurotransmitter into the synapse. Although all three transporters are affected by chronic use of these drugs and the neurotoxicity associated with methamphetamine is certainly one consequence of this, it is the dopamine transporter that appears to be most closely linked to the psychomotor stimulant and euphoric effects produced by these agents.
Chronic exposure to cocaine and/or methamphetamine causes long lasting molecular and cellular neuroadaptations of the mesencephalic dopaminergic system that may ultimately contribute to the addict’s inability to stop taking drugs, despite serious negative consequences [7–10]. Indeed, an increased extracellular concentration of dopamine has been implicated as a critical factor in morphological changes that can lead to changes in neural plasticity and behavior. These changes may contribute to excessive activation of all dopamine receptor subtypes [11]. Specifically, increased expression and function of D3R upon exposure to psychostimulant drugs has led to further investigation into the role of D3R in cocaine and methamphetamine addiction [12–14]. In addition, the restricted high density localization of D3Rs in neurocircuits that play a critical role in emotional and cognitive functions as well as increases in D3R in the ventral striatum of human cocaine fatalities [15, 16] has further heightened interest in D3R. More recently, PET studies using the D3R-preferential ligand [11C]-(+)PHNO in methamphetamine polydrug abusers showed upregulation of D3R but not D2R in this subject population [17]. These studies, coupled with preclinical studies that will be briefly summarized below suggest that normalization of D3R function may reduce vulnerability to relapse in psychostimulant abuse.
3. D3R selective antagonists and partial agonists
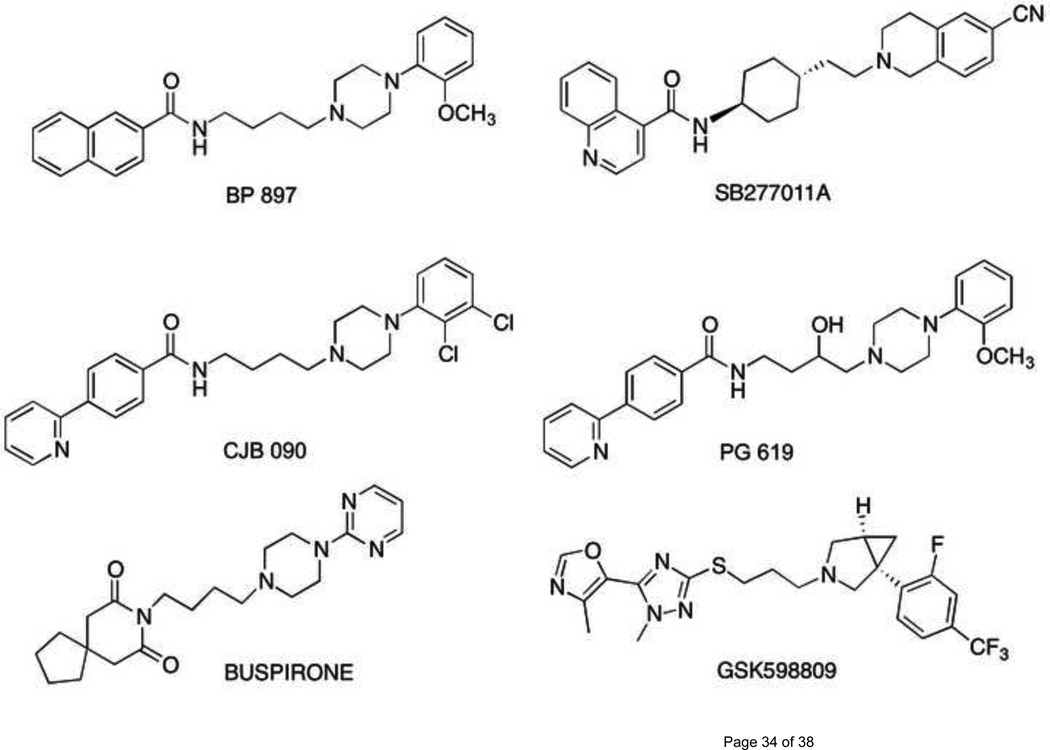
A seminal report describing the D3R-selective partial agonist/antagonist BP 897 (Fig. 1) (hD3R and hD2R Ki=0.92 and 61 nM, respectively) showed inhibition of conditioned cue-controlled cocaine-seeking behavior in rats without producing rewarding effects of its own [18]. Many subsequent studies [19] expanded these results in other models of cocaine and methamphetamine abuse and laid the groundwork for establishing a role of D3R in psychomotor stimulant associated cues and drug seeking.
Figure 1.
Chemical structures of D3R selective ligands
The similarly potent and D3R selective antagonists SB277011A and NGB 2904 also demonstrated efficacy in these animal models [14, 20], and have provided both critical tools for further characterization of D3R in addiction and pharmacophoric templates for the evolution of subsequent generations of D3R selective agents [14, 21]. Structure-activity relationships have been derived through extensive medicinal chemistry efforts, resulting in highly potent and D3R selective agents with varying intrinsic activities (for review see [22, 23].)
Recently, the human D3R was crystallized in complex with the antagonist eticlopride, [24] which has further illuminated structural components of the receptor-binding domain, and will undoubtedly provide the basis for novel ligand discovery [25, 26]. However, discovering small molecules that bind with high affinity and selectivity to the D3R is only the initial hurdle in drug development. The successful molecule must also possess appropriate biopharmaceutical properties (ADME) that provide adequate levels of an efficacious medication for treating addicted patients and preventing relapse. Although numerous animal models developed to mirror human addiction predict that these agents will be effective, clinical trials testing the efficacy of D3R selective antagonists and partial agonists in SUDs are still on the horizon.
4. Utilization of preclinical nonhuman primate models in addiction research
Effective preclinical models are essential towards identifying the in vivo profile of novel D3R partial agonists and antagonists as well as enhancing the field’s understanding of the role of D3Rs in psychostimulant addiction. Since the cloning of D3Rs in 1990 [27], animal studies have been crucial to elucidating how these receptors function in situ. Over the past 15 years, a number of factors influencing the in vivo selectivity and efficacy of D3R selective compounds have been elucidated through employing various behavioral pharmacology assays in both rodents and nonhuman primates. While rodents offer beneficial characteristics such as the ability to make genetic modifications (e.g. knock-out mice) important to investigating specific roles of D3Rs in the behavioral effects of drugs of abuse [28, 29], ongoing research strongly support the use of nonhuman primates in this field of research.
When considering translational research, nonhuman primates are an advantageous animal model for preclinical research due to phylogenetic similarities and 95% overall shared gene homology to humans [30]. Furthermore, the ability to conduct within-subject longitudinal assessments in monkeys with an extensive history of self-administration is an essential attribute to modeling the human condition, as addicts have typically abused cocaine for many years. With respect to dopamine D2-like receptors, nonhuman primate imaging studies show similar neurobiological changes in response to long-term drug self-administration to that of humans [31–33]. Moreover, recent reports using the D3R-preferring ligand [11C]-PHNO [34] have shown that rhesus macaques demonstrate comparable regional binding to humans [35, 36] suggesting similarities in D3R distribution and availability. In addition, monkeys can self-administer cocaine for years, which provides a truly chronic model of addiction. All of these factors lend advantages toward employing nonhuman primates in preclinical behavioral pharmacology studies aimed toward evaluating potential compounds for treating drug addiction. The focus of this commentary is on the evaluation of D3R-selective agents, first with unconditioned behaviors, and then in drug self-administration and relapse models in nonhuman primates as a means to further progress candidate compounds to the clinic.
5. Unconditioned behaviors for understanding in vivo profiles of novel D3R compounds
Although in vitro assays aid in identifying receptor-selectivity and efficacy to guide medicinal chemistry efforts to obtain highly selective and potent drug-like molecules, in vivo assessments are necessary to corroborate in vitro findings in order to validate structure-activity relationships. Seminal reports by Collins, Woods and colleagues [37, 38] demonstrated, in rodents, that a dopamine D2R/D3R agonist will produce an inverted-U function on drug-elicited behavior in which low-doses would elicit yawning and higher doses would produce less yawning and concomitantly induce hypothermia. Through a series of elegant antagonist studies, it was shown that D3Rs mediate the ascending limb of the drug-elicited yawning curve whereas D2Rs were implicated in the actions described on the descending limb in which yawning was lower and hypothermia was observed. While many neurotransmitter systems contribute to yawning (for review see [39]), this simple behavioral assay has been shown to be pharmacologically sensitive to D3R-selective compounds, thus making it a suitable framework for determining the selectivity and efficacy of novel compounds in vivo [37, 38].
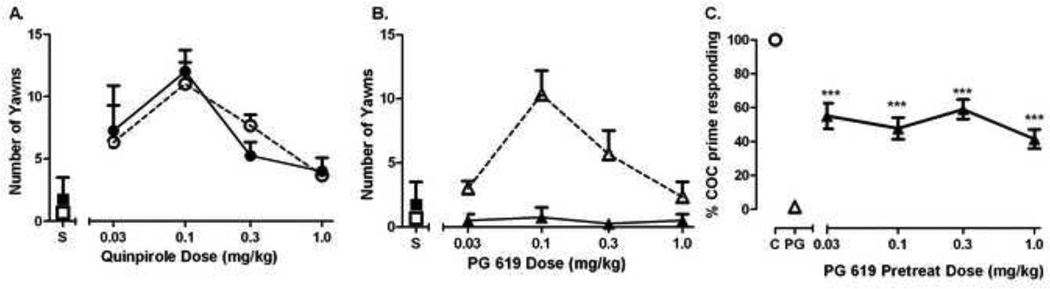
D3R- and D2R-elicited yawning and hypothermia, respectively, have recently been validated in nonhuman primates (Fig. 2) and employed to understand how a pharmacological history can functionally alter D3Rs (for methods see [40]). Differential effects of the profile of D3R partial agonists in drug-naïve rhesus monkeys compared to monkeys with an extensive history of cocaine self-administration was recently reported [40]. For these studies, monkeys were placed in primate chairs in a quiet room with a video camera. Quinpirole, PG 619 (both 0.03–1.0 mg/kg) or saline (1.0 ml) was administered intravenously and yawning measured for 30 minutes beginning immediately after the injection. Scoring of yawns was done by researchers blind to the drug condition. While the D3R-preferrential agonist quinpirole dose-dependently elicits yawning in a similar fashion in all age-matched monkeys irrespective of drug history, the partial agonist PG 619 (hD3R and hD2R Ki= 2.8 and 284 nM, respectively) [41] (Fig 1) elicited yawning comparable to that of the D2R/D3R receptor agonist quinpirole only in cocaine-history monkeys [40, 42] (Fig. 2A, 2B) suggesting functional sensitivity following cocaine self-administration. We also extended this finding to the partial agonist CJB 090 (hD3R and hD2R Ki= 0.5 and 25 nM, respectively; Fig. 1), showing that it would elicit yawns only in monkeys with an extensive cocaine history [43, 44]. These findings suggest that in individuals currently abusing cocaine, a partial agonist may function as a full agonist in vivo. Interestingly, such consequences appear to be long lasting, as rhesus monkeys exposed to cocaine in utero show enhancements in quinpirole-elicited yawning some 13 years later, in adulthood [45]. An even more promising outcome was that in the same monkeys in which the D3R-partial agonist PG 619 (0.1 mg/kg) elicited yawning, it did not induce reinstatement of cocaine seeking (Fig. 2C, open triangle). When given in combination with cocaine, a range of PG 619 doses attenuated cocaine-induced reinstatement (Fig. 2C, filled triangles). There is still much research to be conducted to understand the functional role of D3R in reinstatement. All doses of PG 619 were equally effective in decreasing cocaine-elicited reinstatement to approximately 50% (Fig. 2C), while CJB 090, a drug that also elicited yawning in these monkeys, did not affect cocaine-induced reinstatement [46]. Factors such as those presented above, which more closely resemble the treatment population, are critical to comprehensively identify pharmacological mechanisms of D3R partial agonists and antagonists to facilitate a faster progression from bench to bedside.
Figure 2.
A. Effects of quinpirole (circles) and B. PG 619 (triangles) on drug-elicited yawning in cocaine-naïve (solid lines, filled symbols, n=4) versus monkeys with an extensive history of cocaine self-administration (dashed lines, open symbols, n=3). Quinpirole elicits yawning equivalently in both groups, but PG 619 only elicits yawns in monkeys with a history of cocaine self-administration. C. Effects of PG 619 on cocaine-primed (0.1 mg/kg, i.v.) reinstatement in cocaine-experienced monkeys (n=4). Compared to rates of cocaine-elicited reinstatement, PG 619 (0.1 mg/kg, i.v.) did not reinstate responding (left abcissa), but attenuated cocaine-primed reinstatement when administered 10-min prior to the noncontingent cocaine prime (right abcissa). Figure modified from [40].
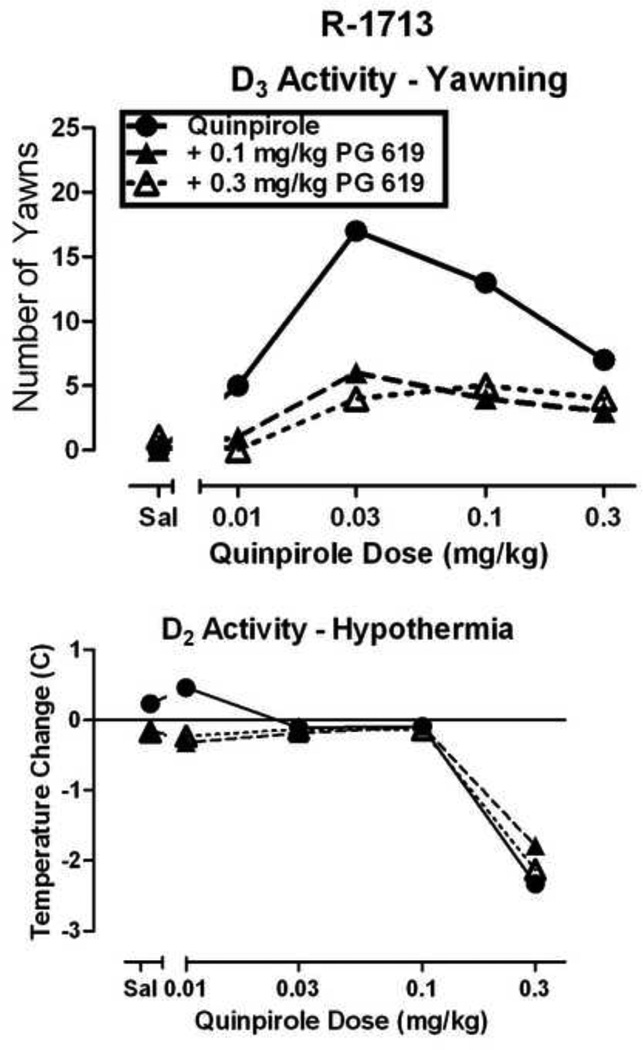
One advantageous use of a model involving unconditioned behaviors is to initially identify mechanisms of action of compounds and to determine appropriate dose ranges for self-administration studies. For example, quinpirole robustly elicits yawning in monkeys and the shape of the quinpirole dose-response curve is characterized as an inverted-U shaped function (Fig. 2A) [40]. Just as has been reported in rodents [38], the descending limb of the quinpirole dose-response curve is associated with hypothermia in monkeys (see Fig. 3). Administration of the D3R-selective partial agonist PG 619 attenuated quinpirole-elicited yawning but did not affect quinpirole-induced hypothermia, suggesting a primarily D3R mediated effect (Fig. 3).
Figure 3.
Effects of PG 619 on quinpirole-elicited yawning (top) and hypothermia (bottom) in a drug-naïve rhesus monkey. Quinpirole (0.01–0.3 mg/kg, i.m., 30-min cumulative administration) dose-dependently elicits yawning and hypothermia. Pretreatment with PG 619 (0.1–0.3 mg/kg, 10-min PT, i.m.) attenuates quinpirole-elicited yawning while having no effect on hypothermia thus showing low efficacy D2R activity in vivo.
6. Operant behavioral models for evaluating D3R-selective agents: drug discrimination, self-administration and reinstatement
A successful medication for addiction would ideally decrease psychostimulant intake as well as prevent relapse in order to completely extinguish use. Typically, preclinical drug self-administration and reinstatement models are used to test this aspect of the medications development process.
Historically, self-administration and drug discrimination procedures have been employed to assess the abuse liability of a candidate treatment agent as well as evaluating its efficacy in reducing the behavioral effects of drugs of abuse. Several rodent and nonhuman primate reports have demonstrated the ability of D3R-selective partial agonists and antagonists to attenuate the discriminative stimulus effects of cocaine [42, 46–50] suggesting a therapeutic potential for this strategy in treating cocaine abuse. Much attention has been directed toward developing optimal intravenous self-administration conditions to model human patterns of drug taking, while also providing a framework sensitive to evaluating the efficacy of D3R selective partial agonists and antagonists in reducing drug-taking behavior. Initially, second-order schedules of reinforcement which model behavior related to drug seeking were represented as the gold standard for examining candidate treatment agents. Under a second-order schedule of reinforcement, responding is maintained by the presentation of a conditioned stimulus, such as a light paired with a positive reinforcer, under one reinforcement schedule, with behavior leading to the primary reinforcer maintained under another reinforcement schedule. In one of the examples described below [18], the reinforcement schedule was a fixed-interval (FI) 15-min schedule leading to a cocaine injection, but the completion of each fixed-ratio (FR) resulted in presentation of a conditioned stimulus [FI 15-min (FR 10: S)]. A particular advantage of this schedule is the ability to measure extensive behavior leading up to the presentation of a reinforcer (either drug or food) in the absence of the reinforcer. For example, monkeys were trained to self-administer cocaine under a second-order schedule [FR 5 (FI 6-min:S)] in which cocaine-seeking behavior occurred for 30 min in the absence of a single cocaine injection [42]. In one of the earliest reports using a second-order schedule to evaluate D3R-selective compounds, Pilla and colleagues [18] implicated the partial agonist BP 897 as an efficacious treatment for cocaine abuse due to its profile in reducing cocaine-seeking behavior. The partial agonist CJB 090 was also effective in decreasing cocaine-maintained responding under a second-order schedule of reinforcement; however these effects were non-selective, as food-maintained responding was also affected [42]. Hence, a critical component for evaluating potential pharmacotherapies must include effects on non-drug-related behaviors.
Progressive ratio (PR) schedules of reinforcement have also been utilized for examining the potential of D3R partial agonist and antagonist to attenuate the reinforcing efficacy of cocaine and methamphetamine. For this procedure, animals must complete increasing fixed-ratio requirements for each successive drug reinforcer and the terminal fixed-ratio completed is defined as the breakpoint, an index of the relative reinforcing strength of that drug. An effective pharmacotherapy would ideally result in lower breakpoints compared to baseline performance. Prior rodent reports have shown that the D3R antagonist SB277011A significantly results in lower breakpoints of both cocaine and methamphetamine self-administration [51, 52]. Likewise, CJB 090 and the D3R antagonist PG01037 have also been reported to dose-dependently reduce PR methamphetamine self-administration, specifically in rats with extended access to self-administration suggesting potential utility of D3R-selective compounds in treatment populations with excessive drug intake [53].
Although drug addiction typically occurs in the context of other reinforcers, animal models frequently study only one reinforcer – either drug or food – in the same animal. Human drug taking also occurs in settings with multiple discriminative stimuli and alternative reinforcers, so it is essential that our animal models attempt to incorporate these aspects of drug-taking behavior. Recently, concurrent choice schedules in which monkeys choose between food reinforcement or self-administering intravenous drug have been utilized to examine potential pharmacotherapies for drug addiction [54]. This model allows for the observation of how potential treatments affect the relative reinforcing strength of a drug of abuse compared to a natural reinforcer. Such a model can better ensure that the treatment drug does not completely disrupt all behavior, but rather reallocates behavior from the drug of abuse to a non-drug reinforcer. Furthermore, this self-administration schedule is amenable to chronic treatments with D3R partial agonists and antagonists to understand if tolerance develops to a therapeutic effect and if repeated treatment will enhance the outcome.
In addition to reducing self-administration of the drug of abuse, D3R partial agonists and antagonists have consistently shown potential in preventing relapse to use. Reinstatement models involve drug-maintained responding that is extinguished by substituting saline. Once a new low-level of responding occurs, the behavior is reinstated by administering either a non-contingent injection of the self-administered drug (drug-primed reinstatement), reintroducing discriminative stimuli associated with the self-administration setting (cue-induced reinstatement), exposing the animal to an acute stressor (stress-induced reinstatement) or administering another drug from the same pharmacological class. Using this model, novel compounds can be evaluated for their ability to reinstate drug-seeking behavior or attenuate the effects of cocaine and/or the conditioned stimuli associated with cocaine reinforcement. A D3R-mediated mechanism for drug-primed reinstatement has been proposed as the D3R-selective antagonist PG01037 attenuated cocaine-primed reinstatement in squirrel monkeys [47]. Mixed results have been reported with the compounds of interest in Fig. 1 as the partial agonist CJB 090 did not attenuate cocaine-primed reinstatement [46], but PG 619 blocked cocaine-primed reinstatement without reinstating responding when administered alone (Fig. 2C) [40]. D3Rs are also implicated in mediating stress-induced reinstatement of cocaine as the D3R antagonist SB-277011A attenuated foot-shock induced cocaine seeking in rats [55]
The advantages of developing a D3R partial agonist are: (1) it should have no or low abuse liability; (2) should be able to block the behavioral effects of cocaine or methamphetamine; and (3) may actually produce agonist-like effects in subjects with an extensive cocaine or methamphetamine history, which may improve compliance for taking the medication. It is clear from the brief description of research involving D3R partial agonists that the schedule of drug availability can impact the effectiveness of the drug to decrease cocaine self-administration. What are the implications that D3R partial agonists appear most effective on self-administration maintained under second-order schedules of reinforcement? It implies that conditioned stimuli in the environment are selectively attenuated by D3R partial agonists. The finding that PG 619 attenuated cocaine-induced reinstatement [40] supports the role of these compounds in Pavlovian-associated processes.
Additional studies evaluating the behavioral characteristics of an effective pharmacotherapy under conditions in which alternative reinforcers are available in the context of varying doses of cocaine or methamphetamine have been reported, It will be important to also extend the characterization of D3R partial agonists to models involving cocaine and methamphetamine discrimination. It has been hypothesized that subjective effects of drugs mediate their continued use and drug discrimination is an excellent model of subjective effects [56, 57]. Importantly, the discriminative stimulus effects of drugs can be differentiated from the reinforcing effects, indicating multiple CNS pathways may be involved in drug effects leading to addiction [58, 59]. Studies examining the effects of D3R partial agonists on the discriminative stimulus effects of cocaine and methamphetamine are ongoing. Hypothetically, an ideal profile would be one in which the compound partially substituted for the training drug (between 20–80% drug-appropriate responding) and could also attenuate the discriminative stimulus effects of cocaine and methamphetamine.
Only when the complete behavioral profile is evaluated – unconditioned behaviors, drug-food choice, reinstatement, and drug discrimination – can behavioral pharmacologists inform medicinal chemists as to what in vitro profiles would be most relevant for the development of a novel and effective pharmacotherapy. Ongoing and future studies will evaluate the oral bioavailability of D3R partial agonists, such as PG 619, and the efficacy of such compounds with chronic administration. Such assessment in the behavioral models discussed above will aid in progressing candidate compounds toward clinical development
7. Buspirone: new look at an old drug
Despite converging lines of evidence that indicate D3R antagonists or partial agonists may be effective medications for the treatment of SUDs, this hypothesis has not been tested in the clinic. One approach to test the D3R hypothesis while developing novel molecules is to evaluate approved drugs that may also engage the target of interest. Independent reports published in the 1990s demonstrated that buspirone (Buspar®), characterized as a potent and selective 5HT1A partial agonist [60], is also a high affinity ligand at both D3R and D4R. These reports [61, 62] were not focused on buspirone per se, and were published prior to studies linking these dopamine receptor subtypes to SUDs. While the efficacy of buspirone at these receptors was not examined in these studies, the reported affinities at recombinant human D3R and D4R (Ki values of 3.5 and 78 nM, respectively) fall within the range of values reported at 5HT1A receptors. Buspirone has been approved as an anxiolytic for more than 25 years, and if the engagement of 5HT1A receptors is required for its anti-anxiety actions, then both D3R and D4R might also be occupied at pharmacologically relevant doses. A corollary of this hypothesis is that if D3R antagonists are effective for the treatment of SUDs, then buspirone, like other compounds sharing this profile, should exhibit effectiveness in relevant preclinical models of drug abuse.
Recent studies [63] have confirmed the high affinity of buspirone at hD3R and hD4R (Ki, 98 and 29 nM, respectively) relative to D2R (484 nM) receptors. The affinities at D3R and D4R reported in this study are higher [62] and lower [61] than those previously reported. However, using two different radioligands ([3H]YM 09151-2 and [3H]NGD 94-1), Tallman et al. [61] reported significant differences in the apparent affinities of a number of compounds, including buspirone, at D4R indicating that its apparent affinity may depend, in part, on the radioligand used. Both the high affinity of buspirone at recombinant human D3R and D4R and its antagonist profile (demonstrated using an in vitro assay of dopamine (agonist)-induced coupling to β-arrestin [64] at both receptors triggered a series of studies to further explore its effects on both cocaine self-administration [63] and in a model of relapse used by the Addictions Treatment Discovery Program (ATDP) at NIDA.
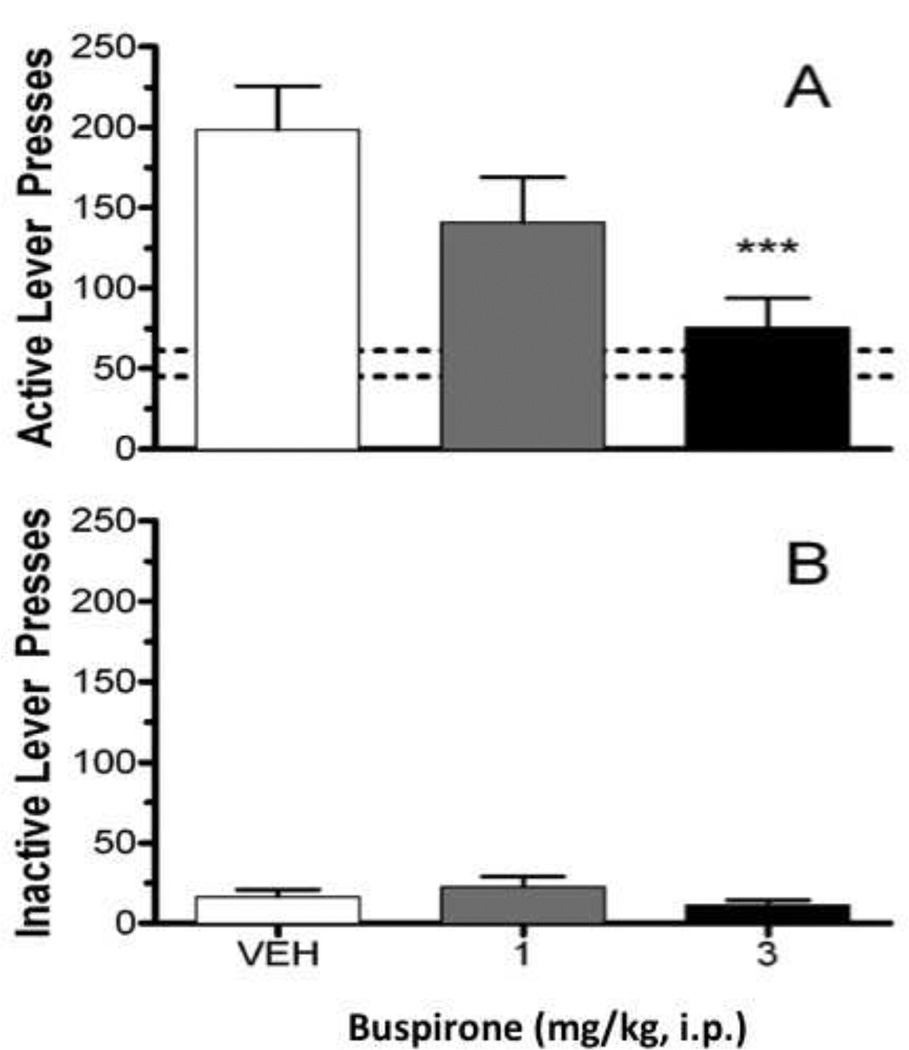
Buspirone exhibited a pharmacological profile in both self-administration and relapse studies that is generally consistent with the actions reported for D3R antagonists [14, 52, 65]. In cocaine self-administration studies using rhesus monkeys, buspirone essentially abolished responding for cocaine (0.003–0.1 mg/kg/inj) at doses (0.1–0.32 mg/kg, i.m.) that did not consistently affect responding for food [63]. Dose extrapolation between humans and non-human primates can be problematic, especially when comparing different routes of administration. Nonetheless, the doses of buspirone that suppressed cocaine self-administration fall within the range prescribed for the treatment of anxiety (20–60 mg/day, orally). Buspirone also has been studied in models of relapse that measure the reinstatement of drug seeking behaviors provoked by stress, cues, or a small priming dose of abused drug (reviewed in [66]). Buspirone produces a dose-dependent (1–3 mg/kg, i.p.) and statistically significant blockade (manifested as a reduction in active lever presses) of methamphetamine-primed [Fig. 4] and cue-induced reinstatement (NIDA ATDP program files). These doses of buspirone do not significantly affect inactive lever presses, suggesting this effect does not result from a generalized disruption of behavior. Moreover, these same doses block stress-induced reinstatement to cocaine, but did not significantly affect cocaine-primed reinstatement (NIDA ATDP program files). These doses of buspirone (administered orally) have been reported to produce anti-anxiety like actions in the “thirsty rat” conflict test [67]. While speculative, a significant effect of buspirone might be manifested by either using higher doses of buspirone or a different priming dose of cocaine.
Figure 4.
Effect of buspirone on methamphetamine-primed reinstatement. Animals were trained and experiments performed essentially as described by Beardsley and colleagues [84]. Buspirone (as the HCl salt) was administered 30 min. prior to testing. The priming dose of methamphetamine was 1 mg/kg, i.p., administered 30 min. prior to testing. Panel A: Active lever presses during methamphetamine prime-induced reinstatement as a function of buspirone dose. Rats received either vehicle or the indicated dose of buspirone. Panel B: Inactive lever presses during methamphetamine prime-induced reinstatement as a function of buspirone dose. Bars represent mean values (±SEM) from 12 male Long-Evans hooded rats/group. The dashed horizontal lines indicate the range of means of active lever presses across all groups during the last session of extinction. *** p<0.001 compared to vehicle.
Buspirone is rapidly and extensively metabolized in humans after oral administration [68], and the 6-hydroxyl metabolite is thought to significantly contribute to its anti-anxiety actions [60]. Thus, 6-hydroxybuspirone is a potent 5HT1A receptor ligand, and following clinically relevant doses of buspirone, peak plasma concentrations (Cmax) are more than an order of magnitude higher than the parent, whilst total plasma exposures (AUC) of this metabolite are approximately 40-fold higher than the parent [69, 70]. This metabolite, as well as 5-hydroxybuspirone (a metabolite with plasma concentrations approximating that of the parent after oral administration [68]) are, like buspirone, D3R and D4R antagonists [63]. The affinity of 6-hydroxybuspirone at D4R is similar to buspirone (40 versus 29 nM, respectively), whereas its affinity at D3R is ~8-fold lower than the parent drug [63]. Thus, with reported Cmax values in excess of 100 nM [69, 70], 6-hydroxybuspirone is likely to more completely engage D4R compared with D3R at pharmacologically relevant doses. The lower affinities of 5-hydroxybuspirone at both D3R and D4R, taken together with plasma concentrations similar to the parent suggest that this metabolite may not meaningfully engage either of these receptors at pharmacologically relevant concentrations. The high affinity, antagonist properties of 6-hydroxybuspirone at D4R may be important because recently emerging evidence indicates that D4R antagonists can also attenuate drug-seeking and reinstatement behaviors ([71], J. Bergman, personal communication) and reduce stimulant induced dopamine increases in the nucleus accumbens [72]. The relative contributions of D3R and D4R antagonism to the ability of buspirone to block cocaine self administration as well as methamphetamine prime- and cue-induced reinstatement are not known. However, from among the more than 120 compounds that have been examined in the NIDA ATDP primate model of cocaine self-administration over the past two decades, buspirone and the selective D4R antagonist NGD 94-1 (J. Bergman, personal communication) emerge as among only a handful of compounds that can essentially abolish cocaine self-administration (flattening the inverted U-shaped dose response curve of cocaine) at doses that do not consistently affect food-maintained responding (J. Acri, personal communication). Thus, the D4R antagonist actions of buspirone (and the 6-hydroxyl metabolite) may contribute to its effects on cocaine self-administration in primates. Both buspirone and 6-hydroxybuspirone are also high affinity ligands at 5HT1A receptors [60], and because 5HT1A receptors have been implicated in many aspects of stimulant abuse (reviewed in [73]), it could be argued that its actions at these receptors also contribute to the anti-addictive properties of buspirone. However, the structurally related 5HT1A partial agonist gepirone does not mimic the effects of buspirone on intravenous self administration behavior in nonhuman primates [74]. In addition, using a cocaine-food choice paradigm in monkeys, the 5-HT1A agonist 8-OH-DPAT shifted the cocaine dose-response curve to the left [75], an effect opposite to that observed with buspirone [63]. Buspirone is also a D2R antagonist, albeit with low affinity [63, 76] relative to D3R, D4R and 5HT1A receptors (e.g., a >15-fold lower affinity at D2R compared to D4R) [63]. While a role of D2R in these actions of buspirone cannot be definitively ruled out, its low affinity (and the very low affinities of both the 5-and 6-hyroxyl metabolites for D2R (> 4 µM) [63] indicates that D2R occupancy would be only transient at doses that produce the actions described here.
There has been one double-blind, placebo-controlled, 12 week study of buspirone in a cohort of cocaine abusers [77]. No remarkable effects of buspirone were reported, but the small trial size (<20 arm) makes interpretation of these data problematic. Moreover, buspirone is not reinforcing, and this patient population might have been even less inclined to be medication compliant than is generally encountered in clinical trials [78]. Based on preclinical studies and the need for more definitive data in human drug abusers, NIDA’s clinical trial network (CTN) is planning a study of buspirone for relapse prevention in adults with cocaine dependence. This trial is scheduled to begin enrollment in August, 2012. Although buspirone is clearly not D3R-selective, examining its efficacy in treating human subjects who are cocaine dependent will be instructive, and may guide medication development strategy in the future.
8. GSK 598809 – a novel and highly D3R-selective antagonist
For more than a decade, GlaxoSmithKline launched a tour de force to identify novel and high affinity D3R–selective molecules that has provided some of the most useful tools in elucidating the role of D3R in SUDs. The first description of SB 277011A (hD3R and hD2R Ki=10 and 1000 nM, respectively, [79, 80] Fig. 1.) and the development of structure-activity relationships of this class of molecules has been followed by this research group and many others since its first publications in 2000 [79, 80]. SB 277011A became the most reported D3R selective antagonist for in vivo studies with >60 publications to date describing its actions, especially in animal models of addiction. Both its poor bioavailability and predicted short half life precluded translation to human studies [80], but these preclinical studies provided the momentum to optimize this lead [22, 81, 82] until success was realized with GSK598809 (Fig. 1), which entered Phase 1 clinical trials with a cognate analogue GSK618334 in 2007 (http://clinicaltrials.gov/ct2/results?term=GSK598809 last reviewed April 24, 2012). GSK598809 is now being used in human laboratory studies and was recently reported to verify the pharmacological specificity of 11C-PHNO as a PET ligand for D3R in human volunteers [83]. Additional studies in human volunteers for smoking cessation are underway. Unfortunately, as GlaxoSmithKline terminated their D3R drug discovery program, it is uncertain how far clinical investigation of GSK598809 will be taken, and thus moving other candidates through the pipeline is necessary to fully examine the D3R as a viable target for SUD treatment.
9. Summary
Taken together, the behavioral models of addiction described above have facilitated an understanding of D3R mechanisms involved in psychostimulant abuse and to ultimately identify potential compounds to move along the medication development pipeline. Preclinical data obtained in multiple animal models of cocaine and methamphetamine self administration and relapse, especially in nonhuman primates, support the D3R as a viable target for SUD medication development. Nevertheless, translation of these studies into human clinical trials has been hampered by the reticence of pharmaceutical companies to develop medications for SUDs and an exodus from neuropsychiatric medications development. More recently, although labs in both academia and the NIH continue to pursue the design, synthesis and in vivo investigation of D3R-selective agents, translation to the clinic is limited by the lack of resources and expertise required to bring molecules from bench to bedside. Repurposing drugs such as buspirone, which has a pharmacological profile that includes D3R antagonism is one approach being pursued by NIDA, and may guide future clinical studies. However, to truly translate the D3R hypothesis, selective D3R antagonists and partial agonists must ultimately be evaluated in human cocaine and methamphetamine abusers. Achieving this objective will continue to challenge researchers in this field.
Acknowledgements
AHN would like to acknowledge the members of her lab, past and present, and her many collaborators that have moved our D3R program forward. In addition, we would like to thank Dr. Emilio Merlo-Pich for participating in the 2011 ACNP mini-symposium that inspired this commentary and for his substantial contributions to the D3R field while at GSK. Funding from this work has come from the NIDA-Intramural Research Program, with support from the NIDA ATDP. BLB and MAN would like to acknowledge the support of Dr. Jane Acri and funding by NIDA grant R01 DA12460 (MAN) and F31 DA033106 (BLB).
Abbreviations
- SUD
substance use disorder
- CNS
central nervous system
- D2R
dopamine D2 receptor
- D3R
dopamine D3 receptor
- D4R
dopamine D4 receptor
- 11C-PHNO
[11C]-(+)-propyl—3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b]-[1,4]-oxazine-9-ol
- ADME
absorption, distribution, metabolism, excretion
- AUC
area under the curve
- ATDP
Addiction Treatment Discovery Program
- CTN
Clinical Trials Network
- PET
positron emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: AHN, BLB, PS, DS JB and MAN declare no conflicts of interest in the present work.
References
- 1.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. The Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Skolnick P. New Medications for Substance Use Disorders: Challenges and Opportunities. Neuropsychopharmacology. 2012;37:290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 4.Ratner M. Big pharma upheavals cast shadow across biotech sector. Nat Biotech. 2012;30:119–120. doi: 10.1038/nbt0212-119. [DOI] [PubMed] [Google Scholar]
- 5.Winchell C, Rappaport BA, Roca R, Rosebraugh CJ. Reanalysis of Methamphetamine Dependence Treatment Trial. CNS Neuroscience & Therapeutics. 2012;18:367–368. doi: 10.1111/j.1755-5949.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker RE, Greig NH. Lost in Translation: Neuropsychiatric Drug Development. Science Translational Medicine. 2010;2:61rv6. doi: 10.1126/scitranslmed.3000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestler EJ, Hope BT, Widnell KL. Drug addiction: A model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Sanna PP, Bloom FE. Neuroscience of Addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British Journal of Pharmacology. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collo G, Bono F, Cavalleri L, Plebani L, Merlo Pich E, Millan MJ, et al. Pre-synaptic dopamine D3 receptor mediates cocaine-induced structural plasticity in mesencephalic dopaminergic neurons via ERK and Akt pathways. Journal of Neurochemistry. 2012;120:765–778. doi: 10.1111/j.1471-4159.2011.07618.x. [DOI] [PubMed] [Google Scholar]
- 11.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine Receptors: From Structure to Function. Physiological Reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 12.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- 13.Neisewander JL, Fuchs RA, Tran-Nguyen LTL, Weber SM, Coffey GP, Joyce JN. Increases in Dopamine D3 Receptor Binding in Rats Receiving a Cocaine Challenge at Various Time Points after Cocaine Self-Administration: Implications for Cocaine-Seeking Behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- 14.Heidbreder CA, Newman AH. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of the New York Academy of Sciences. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staley JK, Mash DC. Adaptive Increase in D3 Dopamine Receptors in the Brain Reward Circuits of Human Cocaine Fatalities. The Journal of Neuroscience. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staley JK, Talbot JZ, Ciliax BJ, Miller GW, Levey AI, Kung M-P, et al. Radioligand binding and immunoautoradiographic evidence for a lack of toxicity to dopaminergic nerve terminals in human cocaine overdose victims. Brain Research. 1997;747:219–229. doi: 10.1016/s0006-8993(96)01196-1. [DOI] [PubMed] [Google Scholar]
- 17.Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher Binding of the Dopamine D3 Receptor-Preferring Ligand [11C]-(+)-Propyl-Hexahydro-Naphtho-Oxazin in Methamphetamine Polydrug Users: A Positron Emission Tomography Study. The Journal of Neuroscience. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Ladona FJ, Cox BF. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Rev. 2003;9:141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xi Z-X, Gardner EL. Pharmacological Actions of NGB 2904, a Selective Dopamine D3 Receptor Antagonist, in Animal Models of Drug Addiction. CNS Drug Reviews. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boeckler F, Gmeiner P. Dopamine D3 receptor ligands—Recent advances in the control of subtype selectivity and intrinsic activity. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2007;1768:871–887. doi: 10.1016/j.bbamem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Micheli F. Recent Advances in the Development of Dopamine D3 Receptor Antagonists: a Medicinal Chemistry Perspective. Chem Med Chem. 2011;6:1152–1162. doi: 10.1002/cmdc.201000538. [DOI] [PubMed] [Google Scholar]
- 23.Lober S, Hubner H, Tschammer N, Gmeiner P. Recent advances in the search for D3- and D4-selective drugs: probes, models and candidates. Trends in Pharmacological Sciences. 2011;32:148–157. doi: 10.1016/j.tips.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Chien EYT, Liu W, Zhao Q, Katritch V, Won Han G, Hanson MA, et al. Structure of the Human Dopamine D3 Receptor in Complex with a D2/D3 Selective Antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tschammer N, Elsner J, Goetz A, Ehrlich K, Schuster S, Ruberg M, et al. Highly Potent 5-Aminotetrahydropyrazolopyridines: Enantioselective Dopamine D3 Receptor Binding, Functional Selectivity, and Analysis of Receptor-Ligand Interactions. Journal of Medicinal Chemistry. 2011;54:2477–2491. doi: 10.1021/jm101639t. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H, Schlessinger A, et al. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nat Chem Biol. 2011;7:769–778. doi: 10.1038/nchembio.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 28.Glickstein SB, DeSteno DA, Hof PR, Schmauss C. Mice Lacking Dopamine D2 and D3 Receptors Exhibit Differential Activation of Prefrontal Cortical Neurons during Tasks Requiring Attention. Cerebral Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- 29.Chourbaji S, Brandwein C, Vogt MA, Dormann C, Mueller R, Drescher KU, et al. Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharmacological Research. 2008;58:302–307. doi: 10.1016/j.phrs.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Hacia JG, Makalowski W, Edgemon K, Erdos MR, Robbins CM, Fodor SPA, et al. Evolutionary sequence comparisons using high-density oligonucleotide arrays. Nat Genet. 1998;18:155–158. doi: 10.1038/ng0298-155. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schyler DJ, et al. Deceased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 32.Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, et al. Dopamine Type 2/3 Receptor Availability in the Striatum and Social Status in Human Volunteers. Biological Psychiatry. 2004;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 34.Gallezot J-D, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, et al. Affinity and selectivity of [11C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- 35.Willeit Mu, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, et al. High-Affinity States of Human Brain Dopamine D2/3 Receptors Imaged by the Agonist [11C]-(+)-PHNO. Biological Psychiatry. 2006;59:389–394. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 37.Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, et al. Dopamine Agonist-Induced Yawning in Rats: A Dopamine D3 Receptor-Mediated Behavior. Journal of Pharmacology and Experimental Therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins G, Newman A, Grundt P, Rice K, Husbands S, Chauvignac C, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins G, Eguibar JR. Neuropharmacology of Yawning. In: OW, editor. The Mystery of Yawning in Physiology and Disease. Basel, Karger: 2010. pp. 90–106. Front Neurol Neurosci. [Google Scholar]
- 40.Blaylock BL, Gould RW, Banala A, Grundt P, Luedtke RR, Newman AH, et al. Influence of Cocaine History on the Behavioral Effects of Dopamine D3 Receptor-Selective Compounds in Monkeys. Neuropsychopharmacology. 2011;36:1104–1113. doi: 10.1038/npp.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi J-K, et al. Heterocyclic Analogues of N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with Functionalized Linking Chains as Novel Dopamine D3 Receptor Ligands: Potential Substance Abuse Therapeutic Agents. Journal of Medicinal Chemistry. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- 42.Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of Two Novel D3-Selective Compounds, NGB 2904 [N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB-090-[N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the Reinforcing and Discriminative Stimulus Effects of Cocaine in Rhesus Monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- 43.Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl}arylcarboxamides as novel dopamine D3 receptor antagonists. Bioorganic & Medicinal Chemistry Letters. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- 44.Newman AH, Grundt P, Nader MA. Dopamine D3 Receptor Partial Agonists and Antagonists as Potential Drug Abuse Therapeutic Agents. Journal of Medicinal Chemistry. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton L, Czoty P, Gage H, Nader M. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2010;210:481–488. doi: 10.1007/s00213-010-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achat-Mendes C, Platt D, Newman A, Spealman R. The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology. 2009;206:73–84. doi: 10.1007/s00213-009-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 Receptor Mechanisms in the Abuse-Related Behavioral Effects of Cocaine: Studies with Preferential Antagonists in Squirrel Monkeys. Journal of Pharmacology and Experimental Therapeutics. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baladi MG, Newman AH, France CP. Dopamine D3 Receptors Mediate the Discriminative Stimulus Effects of Quinpirole in Free-Feeding Rats. Journal of Pharmacology and Experimental Therapeutics. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine's reinforcing and discriminative stimulus effects in rhesus monkeys. Drug and Alcohol Dependence. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- 50.Lamas X, Negus S, Mello N, Nader M. Effects of the putative dopamine D<sub>3</sub> receptor agonist 7-OH-DPAT in rhesus monkeys trained to discriminate cocaine from saline. Psychopharmacology. 1996;124:306–314. doi: 10.1007/BF02247435. [DOI] [PubMed] [Google Scholar]
- 51.Xi Z-X, Gilbert JG, Pak AC, Ashby CR, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost–variable-payoff fixed-ratio cocaine self-administration in rats. European Journal of Neuroscience. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higley AE, Kiefer SW, Li X, Gaal J, Xi Z-X, Gardner EL. Dopamine D3 receptor antagonist SB-277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. European Journal of Pharmacology. 2011;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addiction Biology. 15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negus SS. Rapid Assessment of Choice between Cocaine and Food in Rhesus Monkeys: Effects of Environmental Manipulations and Treatment with d-Amphetamine and Flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- 55.Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Hagan JJ, et al. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuster CR, Balster R. The discriminative stimulus properties of drugs. In: Thompson T, Dews PB, editors. Advances in behavioral pharmacology. New York: Academic; 1977. pp. 85–138. [Google Scholar]
- 57.Holtzman SG. Drug discrimination studies. Drug and Alcohol Dependence. 1985;14:262–282. doi: 10.1016/0376-8716(85)90061-4. [DOI] [PubMed] [Google Scholar]
- 58.Ator N. Relation between discriminative and reinforcing effects of midazolam, pentobarbital, chlordiazepoxide, zolpidem, and imidazenil in baboons. Psychopharmacology. 2002;163:477–487. doi: 10.1007/s00213-002-1076-4. [DOI] [PubMed] [Google Scholar]
- 59.Martelle J, Nader M. A within-subject assessment of the discriminative stimulus and reinforcing effects of self-administered cocaine in rhesus monkeys. Psychopharmacology. 2009;203:343–353. doi: 10.1007/s00213-008-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong H, Dockens RC, Pajor L, Yeola S, Grace JE, Stark AD, et al. 6-Hydroxybuspirone Is a Major Active Metabolite of Buspirone: Assessment of Pharmacokinetics and 5-Hydroxytryptamine1A Receptor Occupancy in Rats. Drug Metabolism and Disposition. 2007;35:1387–1392. doi: 10.1124/dmd.107.015768. [DOI] [PubMed] [Google Scholar]
- 61.Tallman JF, Primus RJ, Brodbeck R, Cornfield L, Meade R, Woodruff K, et al. I. NGD 941: Identification of a Novel, High-Affinity Antagonist at the Human Dopamine D4 Receptor. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1011–1019. [PubMed] [Google Scholar]
- 62.Kula NS, Baldessarini RJ, Kebaian JW, Neumeyer JL. S-(+)-aporphines are not selective for human D3 dopamine receptors. Cell Mol Neurobiol. 1994;14:185–191. doi: 10.1007/BF02090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, et al. Modification of cocaine self-administration by buspirone (buspar (R)): potential involvement of D3 and D4 doamine receptors. Int J Neuropsychopharmacology. 2012 doi: 10.1017/S1461145712000661. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banala AK, Levy BA, Khatri SS, Furman CA, Roof RA, Mishra Y, et al. N-(3-Fluoro-4-(4-(2-methoxy or 2,3-dichlorophenyl)piperazine-1-yl)butyl)arylcarboxamides as Selective Dopamine D3 Receptor Ligands: Critical Role of the Carboxamide Linker for D3 Receptor Selectivity. Journal of Medicinal Chemistry. 2011;54:3581–3594. doi: 10.1021/jm200288r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song R, Yang R-F, Wu N, Su R-B, Li J, Peng X-Q, et al. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addiction Biology. 2012;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weissman B, Barrett JE, Brady L, Witkin JM, Mendelson WB, Paul SM, et al. Behavioral and neurochemical studies on the anticonflict actions of buspirone. Drug Devel Res. 1984;4:83–93. [Google Scholar]
- 68.Gammans R, Mayol R, LaBudde J. Metabolism and dispotion of buspirone. Am J Med. 1986;80:41–51. doi: 10.1016/0002-9343(86)90331-1. [DOI] [PubMed] [Google Scholar]
- 69.Dockens RC, Salazar DE, Fulmor IE, Wehling M, Arnold ME, Croop R. Pharmacokinetics of a Newly Identified Active Metabolite of Buspirone After Administration of Buspirone Over Its Therapeutic Dose Range. The Journal of Clinical Pharmacology. 2006;46:1308–1312. doi: 10.1177/0091270006292250. [DOI] [PubMed] [Google Scholar]
- 70.Dockens RC, Tran AQ, Zeng J, Croop R. Pharmacokinetics of 6-hydroxybuspirone and its enantiomers administered individually or following buspirone administration in humans. Biopharmaceutics & Drug Disposition. 2007;28:393–402. doi: 10.1002/bdd.566. [DOI] [PubMed] [Google Scholar]
- 71.Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, et al. Blockade of Dopamine D4 Receptors Attenuates Reinstatement of Extinguished Nicotine-Seeking Behavior in Rats. Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feldpausch DL, Needham LM, Stone MP, Althaus JS, Yamamoto BK, Svensson KA, et al. The Role of Dopamine D4 Receptor in the Induction of Behavioral Sensitization to Amphetamine and Accompanying Biochemical and Molecular Adaptations. Journal of Pharmacology and Experimental Therapeutics. 1998;286:497–508. [PubMed] [Google Scholar]
- 73.Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: Focus on 5-HT1A-receptors. Progress in Neurobiology. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Gold L, Balster R. Effects of buspirone and gepirone on i.v. cocaine self-administration in monkeys. Psychopharmacology. 1992;108:289–294. doi: 10.1007/BF02245114. [DOI] [PubMed] [Google Scholar]
- 75.Czoty PW, McCabe C, Nader MA. Effects of the 5-HT(1A) agonist (+/−)-8-hydroxy-2-(di-n-propylamino)tetraline (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav Pharmacol. 2005;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Riblet LA, Tayor DP, Eison MS, Stanton HC. Pharmacology and neurochemistry of buspirone. J Clin Psychiatry. 1982;43:11–18. [PubMed] [Google Scholar]
- 77.Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 78.Czobor P, Skolnick P. The secrets of a successful clinical trial: compliance, compliance, and compliance. Mol Interv. 2011;11:107–110. doi: 10.1124/mi.11.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, et al. Design and Synthesis of trans-N-[4-[2-(6-Cyano-1,2,3,4-tetrahydroisoquinolin-2- yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): A Potent and Selective Dopamine D3 Receptor Antagonist with High Oral Bioavailability and CNS Penetration in the Rat. Journal of Medicinal Chemistry. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- 80.Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological Actions of a Novel, High-Affinity, and Selective Human Dopamine D3 Receptor Antagonist, SB-277011-A. Journal of Pharmacology and Experimental Therapeutics. 2000;294:1154–1165. [PubMed] [Google Scholar]
- 81.Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, et al. 1,2,4-Triazol-3-yl-thiopropyl-tetrahydrobenzazepines:  A Series of Potent and Selective Dopamine D3 Receptor Antagonists. Journal of Medicinal Chemistry. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- 82.Micheli F, Andreotti D, Braggio S, Checchia A. A specific and direct comparison of the trifluoromethyl and pentafluoro sulfanyl groups on the selective dopamine D3 antagonist 3-(3-{[4-methyl-5-(4-methyl-1,3-oxazol-5-yl)-4H-1,2,4-triazol-3-yl]thio}propyl)-1-phenyl-3-azabicyclo[3.1.0]hexane template. Bioorganic & Medicinal Chemistry Letters. 2010;20:4566–4568. doi: 10.1016/j.bmcl.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 83.Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging Dopamine D3 Receptors in the Human Brain with Positron Emission Tomography, [11C]PHNO, and a Selective D3 Receptor Antagonist. Biological Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 84.Beardsley PM, Shelton KL, Hendrick E, Johnson KW. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. European Journal of Pharmacology. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]