Abstract
Stress causes decreased cell accumulation in early periimplantation embryos and the placental trophoblast stem cells derived from them. Benzopyrene and many other stressors activate stress enzymes that lead to suppressed stem cell accumulation through diminished proliferation and increased apoptosis. Trophoblast stem cells proliferate and a subpopulation of early postimplantation trophoblast cells differentiate to produce the first placental hormones that arise in the implanting conceptus. These hormones mediate antiluteolytic effects that enable the continuation of a successful implantation. The normal determination and differentiation of placental trophoblast stem cells is dependent upon a series of transcription factors. But, these transcription factors can also be modulated by stress through the activity of stress enzymes. This review enumerates and analyzes recent reports on the effects of benzopyrene on placental function in terms of the emerging paradigm that placental differentiation from stem cells can be regulated when insufficient production of stem cells is caused by stress. In addition, we review the other effects caused by benzopyrene throughout placental development.
Keywords: microarray, placental differentiation, placental stem cell, preimplantation embryo, toxicology
INTRODUCTION
Stress on the earliest placenta can have profound effects on immediate loss of the developing placenta and conceptus in the first trimester and on development of diseases of placental insufficiency later in the second and third trimesters [Bose et al. 2006; Huppertz 2008]. Our goal here is to define the two key effects of stress, decreased cell accumulation and modulation of differentiation of placental trophoblast stem cells (TSC). We will emphasize the kinds of stress effects induced by components of cigarette smoke such as Benzo(a)pyrene and nicotine, but also describe general stressors from experimental and clinical studies. These include maternal stress hormones such as epinephrine and cortisol, and experimental stressors such as hyperosmolar sorbitol that are used by stress enzymologists to understand the impact of cellular stress on homeostatic and developmental responses.
Why the clinical interest in this period of development? Stress due to malnutrition in vivo [Kwong et al. 2000] or stress during in vitro fertilization (IVF) that occurs only during preimplantation development [Ecker et al. 2004] can cause post-natal effects including hypertension and learning anomalies. Also, two-thirds of all fertilized human embryos are lost and most of the loss occurs in the early postimplantation period [Cross et al. 1994]. Since molecular and biological events are linked between late preimplantation and early postimplantation [Rappolee 2007; Huppertz 2008], the embryos can be used to test a wide variety of stressors for time- and dose-dependence and then these embryos can be reimplanted and tested for long-term consequences and their mechanisms.
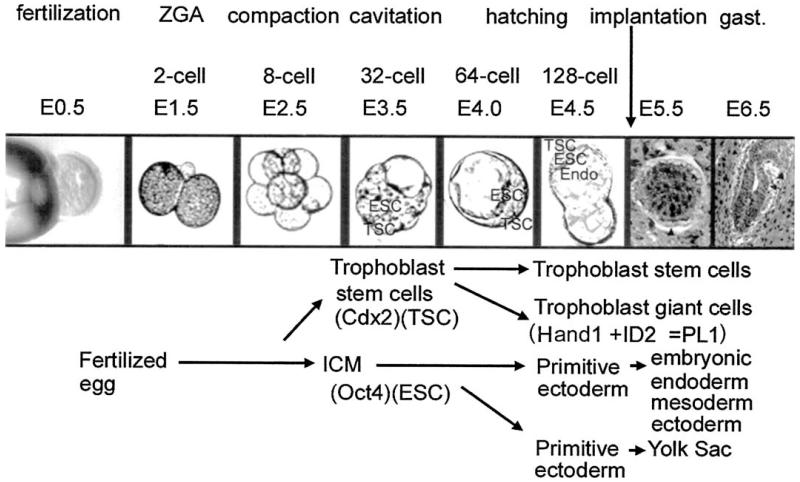
Preimplantation embryos live free of maternal tissue between ovulation from the ovary and implantation into the uterus. As they travel through the lumen of the oviduct and uterus, they can be removed, perturbed during serum-free culture and then reimplanted to test for effects of perturbations on later placental, fetal, and postnatal development (Fig. 1).
FIGURE 1.
Preimplantation development. Preimplantation development takes place between fertilization and implantation and encompasses essential events such as zygotic genome activation, epithelialization/compaction, and the determination of the TSC and ESC lineages of stem cells for the placenta and the embryo. Preimplantation development encompasses the first seven cell divisions and results in the production of determined stem cells for the embryo and extraembryonic yolk sac endoderm and placental lineages. Soon after implantation a subpopulation of TSC differentiates to trophoblast giant cells to produce the first placental hormone placental lactogen (PL)l that contributes to sustaining the corpus luteum and the life of the conceptus. This requires upregulation of heart and mesoderm induced (Hand)1 and downregulation of the related basic helix loop helix transcription factor Inhibition of Differentiation (ID)2.
Thus, preimplantation embryos provide a model where time- and dose-dependent molecular mechanisms can be tested in some embryos while others are re-implanted to correlate and link these mechanisms to long-term effects. This kind of easy testing for direct effects in isolated embryos cannot be done in vivo on oocytes in the ovary or in postimplantation conceptus in the uterus, because of primary effects on the gestational female.
In addition, the preimplantation blastocyst at 3.5 days after fertilization (E3.5) carries the first embryonic and placental trophoblast stem cells (ESC and TSC, respectively) that are the candidate lineages for carrying the long-term effects of toxic stress. The ESC are derived from the inner cell mass (ICM) of the blastocyst and the TSC are derived from the outer trophectodermal epithelium adjacent to the ICM. Therefore, late preimplantation embryos provide an experimental model for studying stress mechanisms and their effects on the potency versus differentiation of TSC.
THE TRANSCRIPTION FACTOR SEQUENCE NECESSARY TO DETERMINE AND DIFFERENTIATE THE EARLIEST STEPS IN THE PLACENTAL TSC LINEAGE DURING NORMAL DEVELOPMENT
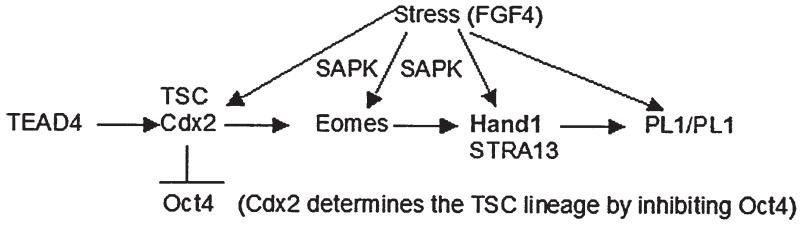
Four transcription factors have been shown to act in sequence to establish the placental lineage (Fig. 2) from the two-cell stage embryo at E1.0 to early post implantation development at E6.0. The first transcription factor required for placental determination is the TEA DNA-binding domain family member (Tead)4, whose expression is activated at the two cell stage zygotic genome activation and is necessary for mammalian Caudal type homeobox transcription factor (Cdx)2 [Yagi et al. 2007]. In turn the Cdx2 transcription factor is necessary to induce Eomesodermin (Eomes) [Strumpf et al. 2005], and Cdx2 function at E3.5 is sufficient to suppress Oct4 which distinguishes the placental lineage from the embryonic lineage [Niwa et al. 2005]. Eomes is necessary to express the transcription factor heart and neural crest derivatives (Hand) expressed (Hand)1 [Russ et al. 2000]. Hand1 is necessary to induce the expression of placental lactogen (PL)1 (also known as chorionic somatomammotropin hormone, Csh1) in embryos [Sahgal et al. 2005] and is sufficient to induce PL1 in cultured TSC [Hughes et al. 2004], and PL1 is detected in maternal serum by E6.0 [Ogren et al. 1989]. Evidence for stress modulation of several of these transcription factors is discussed below. As suggested in Figure 2, many of the transcription factors required for the earliest stages of placental TSC determination and differentiation can be regulated by stress.
FIGURE 2.
TSC differentiation and transcription factors. Normal TSC differentiation is a function of the sequential expression and function of four transcription factors, the last three being dependent upon the previous one. Null mutants indicate that each transcription factor in the series from TEAD4 to Cdx2 to Eomes to Hand1 is necessary for embryonic survival and for determination and development of the first differentiated placental lineage. Cdx2 has an additional role in being positively autoregulatory by its own promoter and negatively regulatory of the Oct4 promoter. Cdx2 thus is a key branchpoint in the determination of TSC identity and suppression of ESC cell identity in the outer cells of the preimplantation blastocyst at E3.5 (see Fig. 1). Hand1 and stimulated by retinoic acid (STRA)13 are both sufficient to induce PL1 when transgenes are overexpressed in TSC. Although Cdx2 is dependent on TEAD4, Eomes is dependent on Cdx2, and Hand1 is dependent on Eomes, the first three are transiently expressed during embryo and TSC development and only Hand1 (and STRA13) persist in maintaining the differentiated state of some TSC progeny subpopulations.
EXAMPLES OF STRESS THAT DIMINISH PLACENTAL TSC ACCUMULATION AND ALTER FUNCTION IN THE EARLY PLACENTA
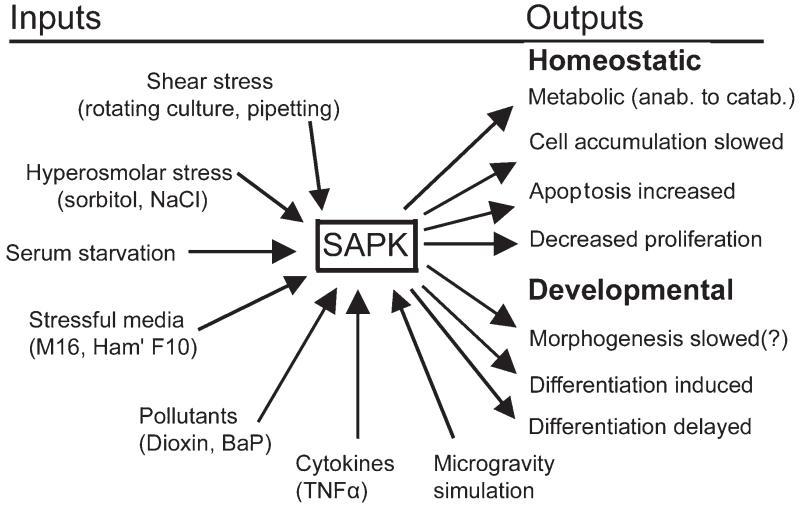
Many types of cellular stressors affect embryos and TSC, including shear stress, culture stress, cytokines, hyperosmolar stress, pollutants such as dioxins and benzo(a)pyrene (BaP), and microgravity simulation (Fig. 3). All these stress stimuli that have been tested cause a significant decrease in the rate of TSC accumulation. Ultimately an embryo that has less TSC will produce fewer differentiated placental progeny and insufficient antiluteolytic hormones. Thus stress during periimplantation development leads to a higher probability of implantation failure.
FIGURE 3.
SAPK mediates homeostatic and developmental outcomes in TSC and embryos after stimulation by several stressors. It is not yet understood how dose- and time-dependent effects are enzymatically integrated by stem cells in the embryo. But, recent results suggest that for two stressors, hyperosmolar sorbitol and benzopyrene, homeostatic events occur over a broad range of concentrations, but cell accumulation and developmental (differentiation) effect occur at higher doses that begin at a shared common minimum threshold dose. [Wang et al. 2005, 2009; Xie et al. 2006b, 2007a, 2007b, 2008; Zhong et al. 2007; submitted].
Malnutrition, embryo culture, hypoxia, and lipopolysaccharide (LPS) are pathophysiological stressors of embryos and TSC. In addition, stress response molecular mechanisms may be incorporated in signaling in normal, inductive developmental pathways stimulated by Wnt ligands, retinoic acid, or removal of fibroblast growth factor (FGF)4 from cultured TSC. Vignettes of several pathophysiological and normal physiological and developmental responses that may incorporate stress enzymes are enumerated below.
Malnutrition and embryo culture
Decreased cell accumulation is a standard response to stress [Ip and Davis 1998]. Maternal malnutrition uniquely occurring between E0.5–E4.0 leads to a decreased cell accumulation rate in the trophoblast lineage and the embryonic lineage [Kwong et al. 2000]. Cell accumulation rates are lower during embryo culture in media or with experimental stressors such as hyperosmolar sorbitol [Xie et al. 2006a, 2007b; Zhong et al. 2007]. In highly stress-activated protein kinase/jun kinase (SAPK/JNK, but shortened to SAPK here, also known as Mapk8/9) SAPK-activating media such as Hams F12 [Wang et al. 2005], 15% of cells are TUNEL positive. The least SAPK-activating medium, KSOMaa, can be made to be equivalent to Hams F12 for decreased cell accumulation rates by adding 200 mM sorbitol for 24 h of blastocyst culture [Xie et al. 2007b]. But, as little as 50 mM sorbitol is sufficient to significantly decrease total cell accumulation after 24 h of blastocyst culture [Xie et al. 2006b]. The fraction of cells in cell cycle (brdU positive) is about 50% lower in Hams F12 than KSOMaa. In fact, it is estimated that about 50% of decreased cell accumulation in Hams F12 is due to apoptosis and 50% is due to decreased entry into the cell cycle. Thus a standard sign of stress is decreased cell accumulation rate as the homeostatic response to stress diverts energy from macromolecular synthesis.
Hypoxia
Physiological stressors include hypoxia due to smoking or reproduction at high altitudes. During persistent hypoxia, intercellular communication is induced that functions to regulate maternal immune function, increase maternal supply of food, gas exchange, and vascular enhancement. This occurs via placental hormones that are induced by hypoxia and are necessary for the survival of the rodent conceptus when gestation occurs under hypobaric conditions [Ain et al. 2004]. Placental prolactin-like protein (PLP)A regulates the production of NK cells that regulate trophoblast expansion into the mesometrial chamber and regulates placental interaction with the uterine mesometrial vasculature. Thus PLPA is an adaptive response to gestational hypoxic stress.
In cultured TSC, hypoxia [Genbacev et al. 2001] or normal differentiation emulated by FGF4 removal [Maltepe et al. 2005], induces hypoxia inducible factor (HIF) and modulates TSC differentiation. Thus transcription factors such as HIF exhibit the “developmental plasticity” whereby they are regulated by normal and pathophysiological stressors as shown for other placental transcription factors in Figure 2.
Lipopolysaccharide (LPS)
The cell walls from gram-negative bacteria stimulate the toll-like receptor (TLR)4, an event that typically activates nuclear factor kappa beta (NFkB)-inducing kinase and SAPK [Akira 2000; O’Neill 2000]. Several signaling enzymes mediate the effect of LPS-induced toll-like receptor (TLR)4 activation, p38 mitogen-activated protein kinase (MAPK, also known as Mapk14) [Matsuzawa et al. 2005], SAPK [Matsuguchi et al. 2003], and NFkB in a PKC-dependent manner [Rolls et al. 2007]. TLR4 is most prominent in syncytiotrophoblast and myofibroblasts [Ma et al. 2006, 2007]. LPS induces cytokines in syncytiotrophoblast in a TLR4-dependent manner in primary syncytiotrophoblasts [Ma et al. 2006, 2007] and in a first trimester placental cell line [Svinarich et al. 1996].
Although no expression of TLR receptor has been reported in TSC [Liu et al. 2009] or ESC, Zampetaki et al. [2006] reported that TLR4 expression in mouse ESC is induced by epigenetic modification, modulation of histone acetylation, and CpG islands, focused mainly on the TLR4 promoter. Similar modulation of histone acetylation in TSC drives these cells to differentiate into syncytiotrophoblasts [Maltepe et al. 2005] that express TLR4 in mice [Ma et al. 2006, 2007]. In agreement with these data in cell lines, TLR4 is detected in the first trimester but higher expression is detected in the term placenta [Beijar et al. 2006]. Taken together the data suggest that TLR4 is induced during normal TSC differentiation, or is induced by other stimuli in certain differentiated lineages of TSC, and may act through stress enzymes to mediate sublethal functions such as induction of cytokines.
Potential physiological functions of stress response pathways occur when stress enzymes function downstream of developmental signaling mechanisms. This occurs in the non-canonical pathway downstream of Wnt ligand and retinoic acid signaling.
Wnt pathway
The Wnt-frizzled pathway regulates the canonical catenin function in the nucleus but also induces the non-canonical SAPK/JNK function during physiological and developmental responses [Weston and Davis 2002; Yamanaka et al. 2002]. Wnt signaling is important in the development and vascularization of the labyrinthine placenta [Monkley et al. 1996; Ishikawa et al. 2001; Cross et al. 2006].
Retinoic acid pathway
The retinoic acid pathway is important in early development [Rossant et al. 1991; Balkan et al. 1992], and SAPK is involved with neural crest migration and differentiation of the extraembryonic endodermal and embryonic neuronal lineages [Kanungo et al. 2000; Li et al. 2001; Wang et al. 2001; Yu et al. 2003; Lee et al. 2004]. Thus SAPK may mediate many more functions downstream of normal inductive stimuli in addition to the multitude of stress stimuli illustrated in Figure 3.
MATERNAL STRESS HORMONES HAVE EFFECTS ON THE IMPLANTING EMBRYO AND PLACENTAL TSC
The implanting embryo and its TSC must coordinate molecular mechanisms mediating adhesion, invasion, and endocrine integration of maternal and fetal/placental function. Maternal malnutrition (discussed above) is one maternal stressor, but emerging evidence suggests that two maternal stress-induced hormones regulate the implanting embryos and TSC.
Epinephrine receptors are detected in the mouse preimplantation embryo, and expression is higher in the ICM than the trophectoderm [Cikos et al. 2005]. Epinephrine is sufficient to reduce cell accumulation in cultured embryos [Cikos et al. 2007] and much of this reduction in cell number is likely in TSC. Epinephrine has been detected in luminal fluids of the oviduct and uterus [Levin and Phillips 1983], suggesting that maternal epinephrine could diminish accumulation rates of stem cells in embryos in vivo. Further functional studies are required to distinguish whether the effects of epinephrine are pathologic in vivo and whether these effects are detected in the ICM and/or TSC.
Maternal cortisol has a negative effect on fetal development and the placental enzyme 11betahydroxysteroid dehydrogenase inactivates the hormone and protects the fetus from it [Yang 1997; Burton and Waddell 1999]. Maternal cortisol can decrease placental TSC proliferation rates in vitro and in vivo [Mandl et al. 2006]. Taken together, these data suggest that maternal stress hormones such as epinephrine and cortisol can downregulate the size of the placenta and lead to loss of the conceptus, or sublethal runting of the embryo or fetus. It will be of interest to understand which enzymes mediate the effects of maternal stress hormones and whether these enzymes are upstream of some of the lethal and sublethal homeostatic and developmental outcomes illustrated in Figure 3.
Experimental evidence in animal models suggests that maternal stress is teratogenic [Chernoff et al. 1988; Colomina et al. 1995]. Epidemiological studies also support the hypothesis that maternal stress is associated with spontaneous abortion in the first trimester [Fenster et al. 1995; Neugebauer et al. 1996; Maconochie et al. 2007]. Thus, experimental and epidemiological evidence suggest that maternal hormones could lead to a pathological cellular stress in the TSC, as well as other stem cells of the implanting embryo.
Cellular Stress Effects and Enzymatic Control of Stress Effects
Many of the stressors discussed above induce intracellular enzyme cascades that mediate molecular and biological effects. The mouse and human kinomes share 510 protein kinases and contain 30 and 8 unique kinases, respectively [Caenepeel et al. 2004]. A small fraction of these kinases are induced by cellular stresses that generally use cellular energy stores in response to stress and decrease cell accumulation rates, either by decreased proliferation and/or increased apoptosis. SAPK is one of a handful of protein kinases that are activated by a broad range of physiological, pathophysiological, and pathological stressors in the embryo and its TSC (Fig. 3).
Biochemistry and cell biology of stress enzymes, SAPK
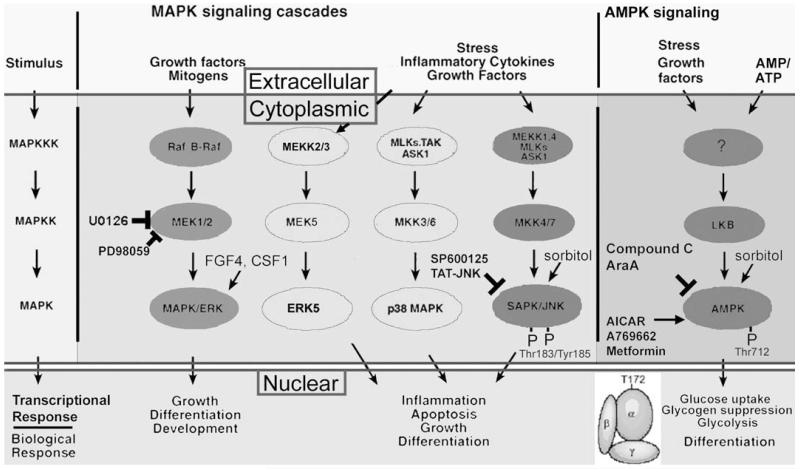
SAPK is a single subunit stress enzyme in the mitogen-activated protein kinase (MAPK) family (Fig. 4). This family contains a subfamily of MAPK/ERK that is usually positive and mitogenic and another stress enzyme subfamily-p38MAPK that can be mitogenic or mediate decreased mitogenesis during stress. ERK5 is another enzyme in the MAPK superfamily that can be mitogenic or mediate stress-induced differentiation in embryonic stem cells [Rappolee 2007]. There are three SAPKs, JNK1/2/3 (also named Mapk8/9/10). SAPK is part of a cascade of 4 enzymes that amplify stress signals from damage in the nucleus or cytoplasm [Ip and Davis 1998]. Cytoplasmic SAPK is activated by dual phos-phorylation by the MAP2kinases, MKK4 and MKK7 (also known as Mapk2k4/7). Upon activation, SAPK transits to the nucleus where it phosphorylates and activates members of the AP1 family of transcription factors. We have found that SAPK mediates biological changes in stressed TSC and embryos correlated with AP1 activation and attenuation of c-myc [Xie et al. 2007b; Zhong et al. 2007].
FIGURE 4.
Stress and the Kinome. Stress may influence a substantial fraction of the 510 enzymes shared by the human and mouse protein kinase kinome, but several members of the MAPK family (SAPK/JNK, p38MAPK, and ERK5) and the two genes encoding the alpha catalytic subunit of AMPK are important in numerous examples of the stress response of oocytes, preimplantation embryos, and TSC. The AMPK and MAPK superfamilies are similar in being activated by a number of stimuli including many of those listed in Figure 2, but AMPK has been characterized as an energy cellular sensor, primarily being rapidly activated by increases in the cellular AMP/ATP ratio that indicates cellular energy depletion. SAPK/JNK and AMPK are blocked by chemical and polypeptide inhibitors (SP600125 and TAT-JNK/LJNKl1, and Compound C and AraA, respectively). Activation of endogenous SAPK/JNK and AMPK is completely correlated with phosphorylation by upstream kinases of Thr183/Tyr185 and Thr 172, respectively. The amplifying cascade of protein kinases results in cytoplasmic effects such as metabolic control through management of rate limiting enzymes in anabolic and catabolic processes, and by nuclear control of transcription factors that mediate more profound and far-reaching changes in cell potency and proliferation states.
Related SAPK and p38MAPK mediate transcriptional responses in stressed embryos that are ~90% unique [Maekawa et al. 2005; Xie et al. 2008], so we anticipate that the SAPK and AMP-activated protein kinase AMPK (also known as Prkaa1/2) will also mediate different parts of the global mRNA response to BaP stress.
Biochemistry and cell biology of AMPK
AMPK is a heterotrimer of enzymatic (α1, α2), glycogen-negative regulatory (β1, β2), and AMP/ATP ratio positive regulatory (γ1, γ2, γ3) subunits [Hardie 2003, 2004] (Fig. 4). Activated AMPK is detected by antibodies to the Thr172 phosphorylation site on AMPKα1/α2. AMPK is an energy sensor activated by conditions such as exercise and diabetes when AMP is high and ATP depleted, and when glycogen depleted.
SAPK and AMPK mediate some of the stress effects in the early embryo and TSC discussed below. But, further research is needed to fully understand the role of these two enzymes and the subset of other enzymes in the kinome that contributes to pathophysiological and developmental outcomes early in development.
MECHANISMS OF DIMINISHED PLACENTAL TSC ACCUMULATION AND ALTERED PLACENTAL FUNCTION
Early embryonic and placental runting are contributors to second and third trimester diseases of placental insufficiency [Pedersen et al. 2008; Bottomley and Bourne 2009]. In preimplantation embryos and TSC, cellular stresses such as hyperosmolar stress, culture media, shear stress, simulated microgravity, and stress due to toxins such as benzopyrene, lead to diminished cell accumulation. For hyperosmolar stress, shear stress, simulated microgravity, and culture media stress, SAPK is activated [Wang et al. 2005, 2009; Xie et al. 2006b, 2007a, 2007b; Rappolee 2007; Zhong et al. 2007] and causal both for decreased entry into S phase and for apoptosis [Xie et al. 2006a, 2007b; Zhong et al. 2007]. Thus SAPK is important in mediating decreased cell accumulation via two mechanisms.
It is also likely that p38MAPK, AMPK, and other stress-induced enzymes contribute to the decreasing cell accumulation, mediated through increased apoptosis or decreased entry into S phase, as these enzymes have these functions in adult somatic cells [Ip and Davis 1998; Kuan et al. 1999; Jones et al. 2005].
Interestingly, spatial (Fig. 5) and temporal (Fig. 6) effects of 24 h of hyperosmolar stress on TSC are consistent with a prioritization of stress-induced TSC differentiation to produce the first differentiated placental progeny that appear soon after implantation [Liu et al. 2009]. It will be important to follow up these studies to determine if SAPK, AMPK, and other stress enzymes regulate overlapping and/or distinct parts of the global stress response and to determine if similar stress responses occur at lower doses of stress.
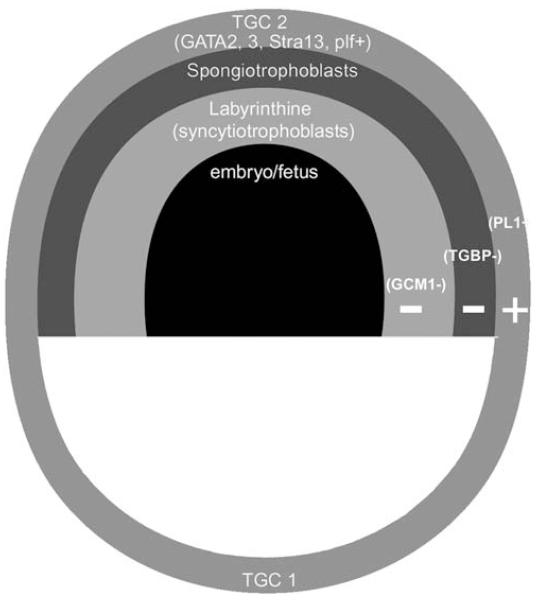
FIGURE 5.
Spatial confines of TSC differentiation. TSC differentiation is affected by stress globally on an mRNA level in a manner suggesting activation of early differentiation of lineages occurring spatially early after implantation. PL1 transcripts that are markers of placental lineage (primary trophoblast giant cells) that initially contact maternal vasculature are induced by stress [Liu et al. 2009]. Markers for later placental lineages such as the labyrinthine (glial cells missing; GCM) and spongiotrophoblast (TGBP1) are not detected. The secondary trophoblast giant cell lineage arising soon after the primary trophoblast giant cell lineage is detected as a partial phenotype (GATA2, STRA13, and proliferin, but not PLII and PLPA).
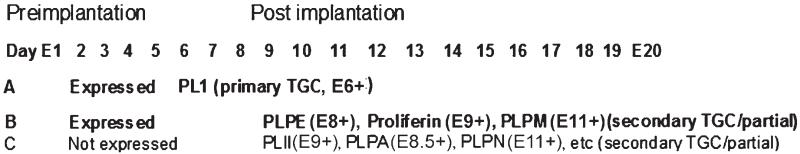
FIGURE 6.
Temporal bounds of TSC differentiation. TSC differentiation is affected by stress globally on an mRNA level in a manner suggesting activation of early differentiation of lineages occurring temporally early after implantation. Consistent with the data for induction of PL1 protein, stress-induced PL1 mRNA (Liu et al. 2009). Similar to PL1, mRNA transcripts for other early placental hormones prolactin like protein- (PLP)M, PLPE and proliferin were also induced, but later placental hormone mRNA such as PLII, PLPA, PLPN, and others were not induced [Yamaguchi et al. 1994; Wiemers et al. 2003a; 2003b; Lin et al. 1997a; 1997b; Fassett et al. 2000; Fang et al. 1999].
CIGARETTE SMOKE IMPAIRS HUMAN REPRODUCTION AND IS CLOSELY ASSOCIATED WITH MAJOR PREGNANCY COMPLICATIONS
It has been well documented that cigarette smoke has adverse effects on reproduction. Cigarette smoking impairs female fertility. Several studies have reported the negative effects of maternal smoking on the success rate of in vitro fertilization and gamete intrafallopian transfer procedures [Mattison 1982; Baird and Wilcox 1985; Harrison et al. 1990; Rosevear et al. 1992; Augood et al. 1998]. Maternal cigarette smoking also has serious adverse effects on the outcome of pregnancy. These include intrauterine growth retardation (IUGR), low birth weight, spontaneous abortion, and preterm labor [Everson et al. 1988; Salafia and Shiverick 1999; Shiverick and Salafia 1999; Andres and Day 2000; Kolas et al. 2000; Wang et al. 2002]. Interestingly, several studies have shown the protective effects of maternal cigarette smoking on preeclampsia, a major pregnancy complication that has similar pathological changes of placenta as IUGR. A review by England and Zhang [2007] analyzed 48 epidemiologic studies, and concluded that smoking during pregnancy reduces the risk of preeclampsia by up to 50% in a dose-response manner. A protective effect was consistently found in both nulliparous and multiparous, singleton, and multifetal pregnancies, and for mild and severe preeclampsia [England and Zhang 2007]. However, the underlying mechanism through which smoking reduces the risk of preeclampsia is unclear. The regulation of smoking on angiogenesis, endothelium function, and immune system are suggested to be the target for future studies.
WHAT IS BENZO[A]PYRENE (BAP) AND HOW IS IT PATHOGENIC FOR THE PLACENTA?
Benzo[a]pyrene (BaP) is a five-ring polycyclic aromatic hydrocarbon (PAHs) that is mutagenic and highly carcinogenic [Brookes 1977; Aust et al. 1980]. BaP is a product of incomplete combustion and an important compound of cigarette smoke and urban pollution. BaP induces cytochrome P4501A (CYP1A1) by binding to the AHR (aryl hydrocarbon receptor) in the cytosol [van Cantfort and Gielen 1981; Solhaug et al. 2005; Sanyal and Li 2007]. Activated CYP1A1, together with other enzymes, catalyze BaP into benzopyrene diol epoxide (BPDE), which binds to several classes of macromolecules (DNA, RNA, and protein) to produce macromolecular adducts [Shamsuddin and Gan 1988; Ginsberg and Atherholt 1990; Mukherjee et al. 2008]. BPDE-DNA adducts can be detected by ELISA or immunocytochemistry means using a polyclonal antibody against BPDE [Sanyal et al. 2007]. BPDE-DNA adducts disrupt DNA replication and are mutagenic.
Human placenta activates cytochrome p-450 (CYP1A1) expression and activity in response to maternal cigarette smoke exposure. BPDE-DNA adducts accumulate on the placenta and affect placental lineage proliferation and differentiation. They can also cross the placenta barrier and are toxic to the developing conceptus [Everson et al. 1988; Arnould et al. 1997; Sanyal et al. 2007]. BaP-DNA adducts in placenta and conceptus are considered to be a biomarker for maternal exposure to cigarette smoke or pollution [Sanyal et al. 2007].
BAP INHIBITS PLACENTAL TSC LINEAGE PROLIFERATION
BaP has been implicated as an environmental endocrine disruptor and growth disregulator and acts by altering gene expression through the aryl hydrocarbon receptor (AhR). BaP can act to silence estrogen effects at estrogen response elements of promoters and can decrease testosterone levels associated with dysregulated spermatogenesis [Inyang et al. 2003; Hockings et al. 2006]. The induction of cytochrome p450 1A1(CYP1A1) is one of the most sensitive biomarkers of exposure to AhR agonists [van Cantfort and Gielen 1981]. BaP significantly inhibited proliferation of human placental cell lines characterized by G2/M cell cycle phase arrest. Anti-proliferation effect of BaP involved activation of p53, p21/CIP1, transforming growth factor (TGF)β and suppression of c-myc, and epidermal growth factor receptor (EGFR) [Zhang et al. 1995; Zhang and Shiverick 1997; Drukteinis et al. 2005]. Phosphoinositol–3-kinase (PI-3K)/Akt/extracellular receptor kinase (ERK), SAPK/JNK, and p38 mitogen-activated protein kinase (MAPK) have been reported to be involved in the alterations of cellular proliferation in response to BaP treatment in human embryo lung fibroblasts [Solhaug et al. 2004; 2005; Du et al. 2006; Gao et al. 2006]. However, the roles of stress enzymes and pathways in mediating BaP-induced changes of proliferation in placental lineages have not been reported.
BAP AFFECTS PLACENTAL CELL LINEAGE DIFFERENTIATION THROUGH REGULATION OF STRESS ENZYMES
It has been clearly demonstrated that BaP inhibits proliferation of placental cell lines, but it is still unclear if BaP affects the differentiation of placental cell lines, especially TSC. In addition, the molecular mechanisms involved in alternating the placental cell lineages proliferation and differentiation upon BaP exposure are almost unknown. Thus, the identification of signaling molecule and related pathways involved in BaP-induced placental lineage proliferation and differentiation is essential. Data from our laboratory showed BaP induced two major stress enzymes, SAPK and AMPK phosphorylation in a dose and time dependent manner. BaP also induced loss of inhibitor of differentiation (ID)2, a transcription factor [Xie et al. submitted] that maintains TSC potency. The stress enzyme AMPK is found to be necessary for the loss of ID2 induced by BaP. In addition, BaP induced transcription heart and mesoderm inducer (Hand)1 expression that will eventually lead to induction of placental hormone PL1. Thus the regulation determined of Hand1 and ID2 by SAPK and AMPK appear to be similar in TSC whether stimulated by hyperosmolar stress or BaP (Fig. 7). However it remains to be determined whether the differentiation induced by BaP is as strong as that induced by hyperosmolar stress, or whether BaP in vivo has similar effects.
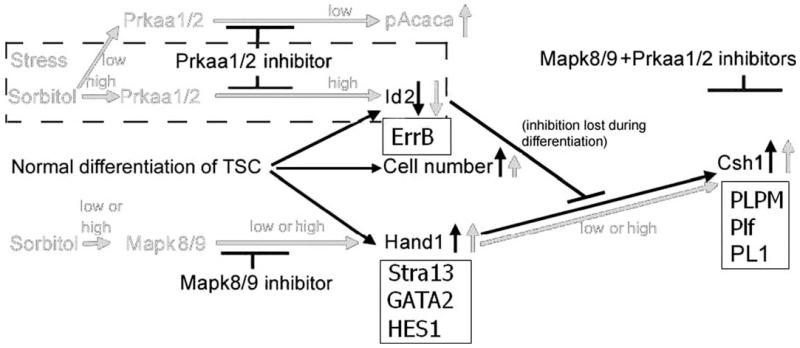
FIGURE 7.
TSC differentiation, stress, and mRNA and protein levels. TSC differentiation is affected by stress globally on the mRNA level and functionally on the protein level. The black lines show the mechanism where ID2 is lost and Hand1 derepressed to activate the PL1 promoter, the first marker of differentiation. Stress-induced PL1 protein requires the activity of SAPK which stabilizes and induces Hand1, and AMPK which induces proteasome-dependent ID2 loss [Zhong et al. submitted]. The dash-lined box shows that the kinetics of AMPK induction and ID2 loss, and AMPK-dependence of ID2 loss, are the same in E3.5 embryos and TSC. The solid lined boxes show that like differentiation-inhibiting ID2 protein, inhibitory ErrB mRNA is also downregulated by stress, like Hand1 protein, positively-acting STRA13, Gata2, and HES1 mRNA are upregulated by stress, and like PL1 hormone PLPM, proliferin, and PLPM hormone mRNA transcripts are also upregulated by stress [Liu et al. 2009]. These studies were carried out using hyperosmolar stress, but BaP also induces AMPK-dependent ID2 loss in TSC [Xie et al. (in press); Toft and Linzer 2000; Ma and Linzer 2000].
As mentioned above, maternal cigarette smoke and environmental pollution increased the risk of many major pregnancy complications including IUGR and preeclampsia. It has been well demonstrated that all of these pregnancy complications share common changes in placenta pathology, impaired angiogenesis, and shallow endometrial invasion by placental cells. It is important to investigate if BaP has an effect on placental angiogenesis. Angiogenesis is finely controlled by a set of proteins, including angiogenic factors and angiogenic inhibitors. The appropriate balance between angiogenic factors and angiogenic inhibitors guarantees the angiogenesis of the placenta.
The application of microarray technology allows a shift in focus from one gene or protein at a time to the study of thousands of genes that change simultaneously in response to a given stimulus. The effects of BaP exposure on development and diseases have been explored with the use of microarrays. Ramos et al. [2007] have identified eight discrete classes of genes altered by BaP challenge in a murine meta-nephrous organ culture system. These genes are involved in cellular differentiation and proliferation, apoptosis, stress response, transformation, extracellular remodeling, and transcriptional control. Similar microarray profiles have been obtained from BaP-treated fetal lung fibroblasts, human mammary epithelium cells, and human bronchial epithelia cells [Belitskaya-Levy et al. 2007; Keshava et al. 2009; Sohn et al. 2008]. However, to date there is no such research addressing BaP induced changes in the array profiles of TSC.
EFFECTS OF BAP ON THE IMPLANTING EMBRYO, PLACENTA, AND VASCULARIZATION
As components of cigarette smoke, BaP and nicotine also cause placental defects in animal models. Before implantation into the uterus, cultured E3.5 mouse blastocysts metabolize BaP to BPDE that binds DNA [Filler and Lew 1981]. Embryos treated with BaP in vitro activate stress enzymes such as SAPK [Xie et al. 2008], and display a significantly reduced rate of implantation [Iannaccone et al. 1984] and elevated loss soon after reimplantation. The placenta can also activate BaP to induce harmful metabolites [Manchester et al. 1988; Madhavan and Naidu 2000], and presumably BaP activates SAPK in the placenta as it does in TSC in preimplantation embryos, and adult somatic cells [Du et al. 2006; Xie et al. 2008].
Relevance of the doses of BaP studied
BaP levels were tested by gas chromatograph in ovarian follicular fluid of women in IVF therapy exposed to mainstream smoke and found to average 1.8 ng/mL [Neal et al. 2007]. For women who smoked 12–24 cigarettes/day, BaP was 4–10 ng/mL in follicular fluid and twice as high in serum. However, other BaP metabolites increased this range by as much as 10 fold (40–100 ng/mL). This is equivalent to ~0.4 uM/0.8 uM/1.2 uM BaP metabolites for 1/2/3 pack/day smokers, respectively. This dose range of BaP has homeostatic effects at the low end and developmental effects at the high end as recently reported for TSC [Xie et al. (in press)].
EFFECTS OF BENZOPYRENE ON 2ND AND 3RD TRIMESTER PLACENTAL INSUFFICIENCY AND CLINICAL RAMIFICATIONS OF SMOKING
Clinical observations indicate that maternal cigarette smoking has significant detrimental effects on fetoplacental development. In pregnant women who have continued to smoke, benzo[a]pyrene, a compound of cigarette smoke, is metabolically activated to diol-epoxide derivative: benzo[a]pyrene-trans-7, 8-dihydrodiol-9,10-epoxide, which accumulates in the placenta and affects placental lineageproliferation and differentiation. However, studies on the placentas of smoking mothers show that metabolism of BaP into different metabolites, and the production of toxic DNA adducts from metabolites in vitro by human placenta, were variable and unrelated to the extent of smoke exposure. The metabolic characteristic of human placenta for xenobiotic exposure substrates is based on the expression and function of diverse enzymes, hence such metabolism exhibited inter-individual variation for toxic metabolite production or detoxification of the substrates in response to maternal smoke exposure [Sanyal and Li 2007]. In addition, Arnould et al. [1997] have shown that in pregnant women who have continued to smoke, the accumulation of BPDE-DNA adducts in the placenta, are seen in smaller quantities in the umbilical cord blood, probably because of the metabolic capacity of the placenta and the transfer of BaP from the mother to the fetus. In placental choriocarcinoma JEG-3 cell line, BaP inhibits cell proliferation, which is correlated with disruption of expression of significant regulators of trophoblast growth [Zhang and Shiverick 1997].
Several epidemiologic studies suggest that maternal smoking and environmental air pollutants including second-hand smoke can compromise fetal growth [Sanyal et al. 1994; Perera et al. 2004]. PAHs such as BaP are widespread air contaminants released by transportation vehicles, power generation, and other combustion sources such as maternal smoking. Therefore the effect contributed by maternal smoking may be additive to that produced by the environment. This fact is highlighted by the study by Perera et al. [2005], who analyzed BPDE-DNA adducts in maternal (n=170) and umbilical cord blood (n=203) obtained at delivery from nonsmoking women. These authors found no independent fetal growth effects of either PAH-DNA adducts or environmental tobacco smoke (ETS), but adducts in combination with in utero exposure to ETS were associated with decreased fetal growth. Specifically, a doubling of adducts among ETS-exposed subjects corresponded to an estimated average 276-g (8%) reduction in birth weight (p=0.03) and a 1.3-cm (3%) reduction in head circumference (p=0.04). These results confirmed the authors’ earlier findings [Rauh et al. 2004] in an inner-city minority population that there was no main effect of BaP-DNA adducts on birth outcomes but there was a significant interaction between the two pollutants such that the combined exposure to high ETS and high adducts had a significant multiplicative effect on birth weight (p=0.04) and head circumference (p=0.01) after adjusting for ethnicity, sex of newborns, maternal body mass index, dietary PAHs, and gestational age.
The carcinogenic effect of BaP was assessed in one study. Manchester and Jacoby [1984] measured monooxygenase activities toward BaP and ethoxyresorufin in placentas from 18 abnormal infants and compared these with activities in placentas from 64 concurrently studied normal infants for the presence or absence of major somatic anomalies. They found that placentas from the abnormal infants had significantly lower monooxygenase activities and higher apparent Kms toward ethoxyresorufin (10(−5) M), indicating that induction of specific cytochrome P-450 systems occurred less frequently among placentas from abnormal infants. The reasons for this association could not be ascertained from their study and no specific maternal condition or environmental exposure associated with lack of monooxygenase induction was identified.
There are no studies in the literature linking BaP to preterm labor, however, several studies exist in the literature that suggest preterm labor is more prevalent in mothers who smoke compared to those that do not [Nabet et al. 2007; Voigt et al. 2007; Vahdaninia et al. 2008; Wills and Coory 2008]. One such study estimated that additional costs due to neonates born prematurely because of smoking in Germany in 2002 amounted to 43 million Euros. However, given that most of the women who smoke cigarettes during pregnancy also drink alcohol, and both are additive in causing preterm labor and small for gestational age infants, the individual effect of each may be difficult to tease out in these epidemiological studies [Odendaal et al. 2009].
Similarly, there are no studies in the literature linking BaP with preeclampsia. However, a number of previous studies have reported an inverse association between maternal smoking and preeclampsia [Jeyabalan et al. 2008; Engel et al. 2009]. Jeyabalan et al. [2008] presented data that suggests that cigarette smoke exposure may decrease the risk of preeclampsia in part by moderating the anti-angiogenic phenotype observed in the syndrome. Controversy exists whether smokers who develop preeclampsia have worse maternal and fetal outcomes than nonsmokers who develop preeclampsia [Pipkin 2008]. However, smoking may only be protective against preeclampsia when there is no pregestational hypertension or chronic hypertension and the apparent protection conferred by maternal smoking may be restricted to young women [Engel et al. 2009].
SUMMARY AND FUTURE STUDIES
It is clear that many types of physiological, nonphysiologic, and environmental stressors can have immediate catastrophic effects leading to lethality for the conceptus, or lesser effects leading to diseases of placental insufficiency such as preeclampsia and IUGR, or long-term effects on neonates and adults. Stress enzymes are likely to mediate the integration of immediate cellular metabolic responses of cells to stress, as well as more profound but immediate decisions concerning rate of entry into S phase or commitment to apoptosis. What has emerged in recent years is that stress also affects oocytes, preimplantation embryos, and their constituent ESC and placental TSC that ramify into long term effects lasting for the life of the offspring.
Several areas of research require increased effort. One area is the connection of early embryonic and TSC stress stimulation with later effects at birth including neonatal and placental weight, litter size, and amount of resorption. Also a connection needs to be made with levels of stress and stress enzyme-mediated effects at the end of the period of embryonic stress with postnatal, lifelong effects.
Much more information is needed about the distinct, integrated, and shared roles of stress enzymes in the immediate and long-term effects of stress. Of the 510 protein kinases shared in the human and mouse kinome only a few have been studied for their role in stress responses. Although the major players in integrating stress responses are a small fraction of the 510 protein kinases, they are many more than have been studied to date. Biosystems approaches using noncandidate assays for testing whole transcriptome, proteome, phosphoproteome, and kinome effects will continue to play an important role in understanding the integration of stress effects in embryos and TSC.
A third major area of research will be to understand stress responses that more closely emulate lower dose ranges of the stressors studied to date. High “demonstration” doses are used to illustrate paradigms for single variable stressors such as hyperosmolar stress and toxic stressors such as BaP, but follow up studies using longer exposures to lower doses are needed. Early results suggest that lessons learned at higher doses are likely to be similar and proportional at lower doses, but this is not assured for all effects.
ACKNOWLEDGMENTS
Supported by a grant to DAR from NICHD, NIH (R01 HD40972A is a second grant that supports us).
Abbreviations
- TSC
trophoblast stem cells
- ESC
embryonic stem cells
- TSC
trophoblast stem cells
- ICM
inner cell mass
- IVF
in vitro fertilization
- Cdx
Caudal type homeobox transcription factor
- Eomes
Eomesodermin
- Hand
heart and neural crest derivatives
- PL
placental lactogen
- Csh1
chorionic somatomammotropin hormone
- BaP
benzo(a)pyrene
- LPS
lipopolysaccharide
- FGF
fibroblast growth factor
- SAPK/JNK (SAPK) or Mapk8/9
stress-activated protein kinase/jun kinase
- PLP
placental prolactin-like protein
- HIF
hypoxia inducible factor
- TLR
toll-like receptor
- NFkB
nuclear factor kappa beta
- MAPK or Mapk14
mitogen-activated protein kinase
- AMPK or Prkaa1/2
AMP-activated protein kinase
- IUGR
intrauterine growth retardation
- BaP
Benzo[a]pyrene
- PAHs
polycyclic aromatic hydrocarbon
- AHR/AhR
aryl hydrocarbon receptor
- BPDE
benzopyrene diol epoxide
- CYP1A1
cytochrome p-450
- EGFR
epidermal growth factor receptor
- ERK
extracellular receptor kinase
- ID
inhibitor of differentiation
- ETS
environmental tobacco smoke
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Daniel A. Rappolee, C.S. Mott Center for Human Growth and Development, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility; Departments of Reproductive Sciences and Physiology; Karmanos Cancer Institute; Institute for Environmental Health and Safety, Wayne State University School of Medicine, Detroit, MI, USA and Department of Biological Sciences, University of Windsor, Windsor, Ontario, Canada
Awoniyi O. Awonuga, C.S. Mott Center for Human Growth and Development, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, Wayne State University School of Medicine, Detroit, MI, USA
Elizabeth E. Puscheck, C.S. Mott Center for Human Growth and Development, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, Wayne State University School of Medicine, Detroit, MI, USA
Sichang Zhou, C.S. Mott Center for Human Growth and Development, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility and Departments of Reproductive Sciences and Physiology, Wayne State University School of Medicine, Detroit, MI, USA.
Yufen Xie, C.S. Mott Center for Human Growth and Development, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, Wayne State University School of Medicine, Detroit, MI, USA.
REFERENCES
- Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci USA. 2004;101(47):16543–16548. doi: 10.1073/pnas.0406185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. Toll-like receptors: lessons from knockout mice. Biochem Soc Trans. 2000;28(5):551–556. doi: 10.1042/bst0280551. [DOI] [PubMed] [Google Scholar]
- Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- Arnould JP, Verhoest P, Bach V, Libert JP, Belegaud J. Detection of benzo[a]pyrene-DNA adducts in human placenta and umbilical cord blood. Hum Exp Toxicol. 1997;16(12):716–721. doi: 10.1177/096032719701601204. [DOI] [PubMed] [Google Scholar]
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13(6):1532–1539. doi: 10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- Aust AE, Falahee KJ, Maher VM, McCormick JJ. Human cell-mediated benzo(a)pyrene cytotoxicity and mutagenicity in human diploid fibroblasts. Cancer Res. 1980;40(11):4070–4075. [PubMed] [Google Scholar]
- Baird, Wilcox AJ. Cigarette smoking associated with delayed conception. JAMA. 1985;253(20):2979–2983. [PubMed] [Google Scholar]
- Balkan W, Colbert M, Bock C, Linney E. Transgenic indicator mice for studying activated retinoic acid receptors during development. Proc Natl Acad Sci USA. 1992;89(8):3347–3351. doi: 10.1073/pnas.89.8.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 2006;27(2-3):322–326. doi: 10.1016/j.placenta.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Belitskaya-Levy I, Hajjou M, Su WC, Yie TA, Tchou-Wong KM, Tang MS, Goldberg JD, Rom WN. Gene profiling of normal human bronchial epithelial cells in response to asbestos and benzo(a)pyrene diol epoxide (BPDE) J Environ Pathol Toxicol Oncol. 2007;26(4):281–294. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i4.50. [DOI] [PubMed] [Google Scholar]
- Bose P, Kadyrov M, Goldin R, Hahn S, Backos M, Regan L, Huppertz B. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the anti-phospholipid syndrome. Placenta. 2006;27(8):869–875. doi: 10.1016/j.placenta.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bottomley C, Bourne T. Dating and growth in the first trimester. Best Pract Res Clin Obstet Gynaecol. 2009;23(4):139–152. doi: 10.1016/j.bpobgyn.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Brookes P. Mutagenicity of polycyclic aromatic hydrocarbons. Mutat Res. 1977;39(3-4):257–283. doi: 10.1016/0165-1110(77)90008-2. [DOI] [PubMed] [Google Scholar]
- Burton PJ, Waddell BJ. Dual function of 11beta-hydroxysteroid dehydrogenase in placenta: modulating placental glucocorticoid passage and local steroid action. Biol Reprod. 1999;60(2):234–240. doi: 10.1095/biolreprod60.2.234. [DOI] [PubMed] [Google Scholar]
- Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, Manning G. The mouse kinome: discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci USA. 2004;101(32):11707–11712. doi: 10.1073/pnas.0306880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff N, Miller DB, Rosen MB, Mattscheck CL. Developmental effects of maternal stress in the CD-1 mouse induced by restraint on single days during the period of major organogenesis. Toxicology. 1988;51(1):57–65. doi: 10.1016/0300-483x(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Cikos S, Rehak P, Czikkova S, Vesela J, Koppel J. Expression of adrenergic receptors in mouse preimplantation embryos and ovulated oocytes. Reproduction. 2007;133(6):1139–1147. doi: 10.1530/REP-07-0006. [DOI] [PubMed] [Google Scholar]
- Cikos S, Vesela J, Il’kova G, Rehak P, Czikkova S, Koppel J. Expression of beta adrenergic receptors in mouse oocytes and preimplantation embryos. Mol Reprod Dev. 2005;71(2):145–153. doi: 10.1002/mrd.20256. [DOI] [PubMed] [Google Scholar]
- Colomina MT, Albina ML, Domingo JL, Corbella J. Effects of maternal stress on methylmercury-induced developmental toxicity in mice. Physiol Behav. 1995;58(5):979–983. doi: 10.1016/0031-9384(95)00140-e. [DOI] [PubMed] [Google Scholar]
- Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation. 2006;74(7):393–401. doi: 10.1111/j.1432-0436.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266(5190):1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Drukteinis JS, Medrano T, Ablordeppey EA, Kitzman JM, Shiverick KT. Benzo[a]pyrene, but not 2,3,7,8-TCDD, induces G2/M cell cycle arrest, p21CIP1 and p53 phosphorylation in human choriocarcinoma JEG-3 cells: a distinct signaling pathway. Placenta. 2005;26(Suppl A):S87–95. doi: 10.1016/j.placenta.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Du HJ, Tang N, Liu BC, You BR, Shen FH, Ye M, Gao A, Huang C. Benzo[a]pyrene-induced cell cycle progression is through ERKs/cyclin D1 pathway and requires the activation of JNKs and p38 mapk in human diploid lung fibroblasts. Mol Cell Biochem. 2006;287(1-2):79–89. doi: 10.1007/s11010-005-9073-7. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci USA. 2004;101(6):1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Janevic TM, Stein CR, Savitz DA. Maternal smoking, preeclampsia, and infant health outcomes in New York City, 1995-2003. Am J Epidemiol. 2009;169(1):33–40. doi: 10.1093/aje/kwn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12:2471–2483. doi: 10.2741/2248. [DOI] [PubMed] [Google Scholar]
- Everson RB, Randerath E, Santella RM, Avitts TA, Weinstein IB, Randerath K. Quantitative associations between DNA damage in human placenta and maternal smoking and birth weight. J Natl Cancer Inst. 1988;80(8):567–576. doi: 10.1093/jnci/80.8.567. [DOI] [PubMed] [Google Scholar]
- Fang Y, Lepont P, Fassett JT, Ford SP, Mubaidin A, Hamilton RT, Nilsen-Hamilton M. Signaling between the placenta and the uterus involving the mitogen-regulated protein/proliferins. Endocrinology. 1999;140(11):5239–5249. doi: 10.1210/endo.140.11.7142. [DOI] [PubMed] [Google Scholar]
- Fassett JT, Hamilton RT, Nilsen-Hamilton M. Mrp4, a new mitogen-regulated protein/proliferin gene; unique in this gene family for its expression in the adult mouse tail and ear. Endocrinology. 2000;141(5):1863–1871. doi: 10.1210/endo.141.5.7479. [DOI] [PubMed] [Google Scholar]
- Fenster L, Schaefer C, Mathur A, Hiatt RA, Pieper C, Hubbard AE, Von Behren J, Swan SH. Psychologic stress in the work-place and spontaneous abortion. Am J Epidemiol. 1995;142(11):1176–1183. doi: 10.1093/oxfordjournals.aje.a117576. [DOI] [PubMed] [Google Scholar]
- Filler R, Lew KJ. Developmental onset of mixed-function oxidase activity in preimplantation mouse embryos. Proc Natl Acad Sci USA. 1981;78(11):6991–6995. doi: 10.1073/pnas.78.11.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A, Liu BC, Huang CS, Shi XL, Jia XW, You BR, Ye M. ERK and JNK/AP-1 pathways involved in benzo(a)pyrene induced cell cycle changes in human embryo lung fibroblasts. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006;24(2):72–76. [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Kaelin W, Fisher SJ. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev Biol. 2001;233(2):526–536. doi: 10.1006/dbio.2001.0231. [DOI] [PubMed] [Google Scholar]
- Ginsberg GL, Atherholt TB. DNA adduct formation in mouse tissues in relation to serum levels of benzo(a)pyrene-diolepoxide after injection of benzo(a)pyrene or the diol-epoxide. Cancer Res. 1990;50(4):1189–1194. [PubMed] [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway-new players upstream and downstream. J Cell Sci. 2004;117(Pt 23):5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Harrison KL, Breen TM, Hennessey JF. The effect of patient smoking habit on the outcome of IVF and GIFT treatment. Aust N Z J Obstet Gynaecol. 1990;30(4):340–342. doi: 10.1111/j.1479-828x.1990.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res. 2006;66(4):2224–2232. doi: 10.1158/0008-5472.CAN-05-1619. [DOI] [PubMed] [Google Scholar]
- Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol. 2004;271(1):26–37. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- Iannaccone PM, Fahl WE, Stols L. Reproductive toxicity associated with endometrial cell mediated metabolism of benzo[a]-pyrene: a combined in vitro, in vivo approach. Carcinogenesis. 1984;5(11):1437–1442. doi: 10.1093/carcin/5.11.1437. [DOI] [PubMed] [Google Scholar]
- Inyang F, Ramesh A, Kopsombut P, Niaz MS, Hood DB, Nyanda AM, Archibong AE. Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod Toxicol. 2003;17(5):527–537. doi: 10.1016/s0890-6238(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol. 1998;10(2):205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128(1):25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Powers RW, Durica AR, Harger GF, Roberts JM, Ness RB. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am J Hypertens. 2008;21(8):943–947. doi: 10.1038/ajh.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kanungo J, Potapova I, Malbon CC, Wang H. MEKK4 mediates differentiation in response to retinoic acid via activation of c-Jun N-terminal kinase in rat embryonal carcinoma P19 cells. J Biol Chem. 2000;275(31):24032–24039. doi: 10.1074/jbc.M002747200. [DOI] [PubMed] [Google Scholar]
- Keshava C, Divi RL, Einem TL, Richardson DL, Leonard SL, Keshava N, Poirier MC, Weston A. Chlorophyllin significantly reduces benzo[a]pyrene-DNA adduct formation and alters cytochrome P450 1A1 and 1B1 expression and EROD activity in normal human mammary epithelial cells. Environ Mol Mutagen. 2009;50(2):134–144. doi: 10.1002/em.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolas T, Nakling J, Salvesen KA. Smoking during pregnancy increases the risk of preterm births among parous women. Acta Obstet Gynecol Scand. 2000;79(8):644–648. [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22(4):667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127(19):4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Lee YN, Malbon CC, Wang HY. G alpha 13 signals via p115RhoGEF cascades regulating JNK1 and primitive endoderm formation. J Biol Chem. 2004;279(52):54896–54904. doi: 10.1074/jbc.M407581200. [DOI] [PubMed] [Google Scholar]
- Levin RJ, Phillips JC. Rat endometrial bioelectric activity in vivo and in vitro: effects of adrenaline. J Physiol. 1983;336:465–478. doi: 10.1113/jphysiol.1983.sp014591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Molkentin JD, Colbert MC. Retinoic acid inhibits cardiac neural crest migration by blocking c-Jun N-terminal kinase activation. Dev Biol. 2001;232(2):351–361. doi: 10.1006/dbio.2001.0203. [DOI] [PubMed] [Google Scholar]
- Lin J, Poole J, Linzer DI. Three new members of the mouse prolactin/growth hormone family are homologous to proteins expressed in the rat. Endocrinology. 1997a;138(12):5541–5549. doi: 10.1210/endo.138.12.5626. [DOI] [PubMed] [Google Scholar]
- Lin J, Poole J, Linzer DI. Two novel members of the prolactin/growth hormone family are expressed in the mouse placenta. Endocrinology. 1997b;138(12):5535–5540. doi: 10.1210/endo.138.12.5636. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu W, Sun T, Wang F, Puscheck E, Brigstock D, Wang QT, Davis R, Rappolee DA. Hyperosmolar Stress Induces Global mRNA Responses in Placental Trophoblast Stem Cells that Emulate Early Post-implantation Differentiation. Placenta. 2009;30(1):66–73. doi: 10.1016/j.placenta.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma GT, Linzer DI. GATA-2 restricts prolactin-like protein A expression to secondary trophoblast giant cells in the mouse. Biol Reprod. 2000;63(2):570–574. doi: 10.1095/biolreprod63.2.570. [DOI] [PubMed] [Google Scholar]
- Ma Y, Krikun G, Abrahams VM, Mor G, Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta. 2007;28(10):1024–1031. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Mor G, Abrahams VM, Buhimschi IA, Buhimschi CS, Guller S. Alterations in syncytiotrophoblast cytokine expression following treatment with lipopolysaccharide. Am J Reprod Immunol. 2006;55(1):12–18. doi: 10.1111/j.1600-0897.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage-results from a UK-population-based case-control study. BJOG. 2007;114(2):170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- Madhavan ND, Naidu KA. Purification and partial characterization of peroxidase from human term placenta of non-smokers: metabolism of benzo(a)pyrene-7, 8-dihydrodiol. Placenta. 2000;21(5-6):501–509. doi: 10.1053/plac.2000.0537. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Yamamoto T, Tanoue T, Yuasa Y, Chisaka O, Nishida E. Requirement of the MAP kinase signaling pathways for mouse preimplantation development. Development. 2005;132(8):1773–1783. doi: 10.1242/dev.01729. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ. Hypoxia-inducible factordependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132(15):3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- Manchester D, Jacoby E. Decreased placental monooxygenase activities associated with birth defects. Teratology. 1984;30(1):31–37. doi: 10.1002/tera.1420300105. [DOI] [PubMed] [Google Scholar]
- Manchester DK, Weston A, Choi JS, Trivers GE, Fennessey PV, Quintana E, Farmer PB, Mann DL, Harris CC. Detection of benzo[a]pyrene diol epoxide-DNA adducts in human placenta. Proc Natl Acad Sci USA. 1988;85(23):9243–9247. doi: 10.1073/pnas.85.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl M, Ghaffari-Tabrizi N, Haas J, Nohammer G, Desoye G. Differential glucocorticoid effects on proliferation and invasion of human trophoblast cell lines. Reproduction. 2006;132(1):159–167. doi: 10.1530/rep.1.00976. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Masuda A, Sugimoto K, Nagai Y, Yoshikai Y. JNK-interacting protein 3 associates with Toll-like receptor 4 and is involved in LPS-mediated JNK activation. EMBO J. 2003;22(17):4455–4464. doi: 10.1093/emboj/cdg438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6(6):587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Mattison DR. The effects of smoking on fertility from gametogenesis to implantation. Environ Res. 1982;28(2):410–433. doi: 10.1016/0013-9351(82)90139-6. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Mukherjee JJ, Gupta SK, Kumar S. Inhibition of benzopyrene diol epoxide-induced apoptosis by cadmium(II) is AP-1-independent: role of extracelluler signal related kinase. Chem Biol Interact. 2008;172(1):72–80. doi: 10.1016/j.cbi.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabet C, Lelong N, Ancel PY, Saurel-Cubizolles MJ, Kaminski M. Smoking during pregnancy according to obstetric complications and parity: results of the EUROPOP study. Eur J Epidemiol. 2007;22(10):715–721. doi: 10.1007/s10654-007-9172-8. [DOI] [PubMed] [Google Scholar]
- Neal MS, Zhu J, Holloway AC, Foster WG. Follicle growth is inhibited by benzo-[a]-pyrene, at concentrations representative of human exposure, in an isolated rat follicle culture assay. Hum Reprod. 2007;22(4):961–967. doi: 10.1093/humrep/del487. [DOI] [PubMed] [Google Scholar]
- Neugebauer R, Kline J, Stein Z, Shrout P, Warburton D, Susser M. Association of stressful life events with chromosomally normal spontaneous abortion. Am J Epidemiol. 1996;143(6):588–596. doi: 10.1093/oxfordjournals.aje.a008789. [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123(5):917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2000;2000(44):re1. doi: 10.1126/stke.442000re1. [DOI] [PubMed] [Google Scholar]
- Odendaal HJ, Steyn DW, Elliott A, Burd L. Combined effects of cigarette smoking and alcohol consumption on perinatal outcome. Gynecol Obstet Invest. 2009;67(1):1–8. doi: 10.1159/000150597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren L, Southard JN, Colosi P, Linzer DI, Talamantes F. Mouse placental lactogen-I: RIA and gestational profile in maternal serum. Endocrinology. 1989;125(5):2253–2257. doi: 10.1210/endo-125-5-2253. [DOI] [PubMed] [Google Scholar]
- Pedersen NG, Wojdemann KR, Scheike T, Tabor A. Fetal growth between the first and second trimesters and the risk of adverse pregnancy outcome. Ultrasound Obstet Gynecol. 2008;32(2):147–154. doi: 10.1002/uog.6109. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH, Andrews H, Ramirez J, Qu L, Tang D. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Health Perspect. 2004;112(5):626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Rauh V, Lester K, Tsai WY, Tu YH, Weiss L, Hoepner L, King J, Del Priore G, et al. Relationships among polycyclic aromatic hydrocarbon-DNA adducts, proximity to the World Trade Center, and effects on fetal growth. Environ Health Perspect. 2005;113(8):1062–1067. doi: 10.1289/ehp.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin FB. Smoking in moderate/severe preeclampsia worsens pregnancy outcome, but smoking cessation limits the damage. Hypertension. 2008;51(4):1042–1046. doi: 10.1161/HYPERTENSIONAHA.107.106559. [DOI] [PubMed] [Google Scholar]
- Ramos KS, Steffen MC, Falahatpisheh MH, Nanez A. From genomics to mechanistic insight: a global perspective on molecular deficits induced by environmental agents. Environ Mol Mutagen. 2007;48(5):395–399. doi: 10.1002/em.20310. [DOI] [PubMed] [Google Scholar]
- Rappolee DA. Impact of transient stress and stress enzymes on development. Dev Biol. 2007;304(1):1–8. doi: 10.1016/j.ydbio.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera FP. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26(3):373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rosevear SK, Holt DW, Lee TD, Ford WC, Wardle PG, Hull MG. Smoking and decreased fertilisation rates in vitro. Lancet. 1992;340(8829):1195–1196. doi: 10.1016/0140-6736(92)92895-m. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5(8):1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404(6773):95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- Sahgal N, Canham LN, Konno T, Wolfe MW, Soares MJ. Modulation of trophoblast stem cell and giant cell phenotypes: analyses using the Rcho-1 cell model. Differentiation. 2005;73(9-10):452–462. doi: 10.1111/j.1432-0436.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- Salafia C, Shiverick K. Cigarette smoking and pregnancy II: vascular effects. Placenta. 1999;20(4):273–279. doi: 10.1053/plac.1998.0378. [DOI] [PubMed] [Google Scholar]
- Sanyal MK, Li YL. Differential metabolism of benzo[alpha]pyrene in vitro by human placental tissues exposed to active maternal cigarette smoke. Birth Defects Res B Dev Reprod Toxicol. 2007;80(1):49–56. doi: 10.1002/bdrb.20102. [DOI] [PubMed] [Google Scholar]
- Sanyal MK, Li YL, Belanger K. Metabolism of polynuclear aromatic hydrocarbon in human term placenta influenced by cigarette smoke exposure. Reprod Toxicol. 1994;8(5):411–418. doi: 10.1016/0890-6238(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Sanyal MK, Mercan D, Belanger K, Santella RM. DNA adducts in human placenta exposed to ambient environment and passive cigarette smoke during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79(4):289–294. doi: 10.1002/bdra.20346. [DOI] [PubMed] [Google Scholar]
- Shamsuddin AK, Gan R. Immunocytochemical localization of benzo(a)pyrene-DNA adducts in human tissue. Hum Pathol. 1988;19(3):309–315. doi: 10.1016/s0046-8177(88)80524-0. [DOI] [PubMed] [Google Scholar]
- Shiverick KT, Salafia C. Cigarette smoking and pregnancy I: ovarian, uterine and placental effects. Placenta. 1999;20(4):265–272. doi: 10.1053/plac.1998.0377. [DOI] [PubMed] [Google Scholar]
- Sohn SH, Kim KN, Kim IK, Lee EI, Ryu JJ, Kim MK. Effects of tobacco compounds on gene expression in fetal lung fibroblasts. Environ Toxicol. 2008;23(4):423–434. doi: 10.1002/tox.20335. [DOI] [PubMed] [Google Scholar]
- Solhaug A, Ovrebo S, Mollerup S, Lag M, Schwarze PE, Nesnow S, Holme JA. Role of cell signaling in B[a]P-induced apoptosis: characterization of unspecific effects of cell signaling inhibitors and apoptotic effects of B[a]P metabolites. Chem Biol Interact. 2005;151(2):101–119. doi: 10.1016/j.cbi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Solhaug A, Refsnes M, Holme JA. Role of cell signalling involved in induction of apoptosis by benzo[a]pyrene and cyclopenta[c,d]pyrene in Hepa1c1c7 cells. J Cell Biochem. 2004;93(6):1143–1154. doi: 10.1002/jcb.20251. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132(9):2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Svinarich DM, Bitonti OM, Araneda H, Romero R, Gonik B. Induction and postranslational expression of G-CSF and RANTES in a first trimester trophoblast cell line by lipopolysaccharide. Am J Reprod Immunol. 1996;36(5):256–259. doi: 10.1111/j.1600-0897.1996.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Toft DJ, Linzer DI. Identification of three prolactin-related hormones as markers of invasive trophoblasts in the rat. Biol Reprod. 2000;63(2):519–525. doi: 10.1095/biolreprod63.2.519. [DOI] [PubMed] [Google Scholar]
- Vahdaninia M, Tavafian SS, Montazeri A. Correlates of low birth weight in term pregnancies: a retrospective study from Iran. BMC Pregnancy Childbirth. 2008;8:12. doi: 10.1186/1471-2393-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Cantfort J, Gielen JE. Ontogenetic variation in rat liver, lung and kidney monooxygenase induction by low doses of benzo(A)pyrene and cigarette-smoke condensate. Br J Cancer. 1981;44(6):902–910. doi: 10.1038/bjc.1981.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M, Straube S, Fusch C, Heineck G, Olbertz D, Schneider KT. The shortening of the duration of pregnancy due to smoking and associated costs for perinatal health care in Germany. Z Geburtshilfe Neonatol. 2007;211(5):204–210. doi: 10.1055/s-2007-981326. [DOI] [PubMed] [Google Scholar]
- Wang H, Ikeda S, Kanno S, Guang LM, Ohnishi M, Sasaki M, Kobayashi T, Tamura S. Activation of c-Jun amino-terminal kinase is required for retinoic acid-induced neural differentiation of P19 embryonal carcinoma cells. FEBS Lett. 2001;503(1):91–96. doi: 10.1016/s0014-5793(01)02699-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83(Suppl 1):1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Wang YC, Xie Y, Wygle DL, Shen HH, Puscheck EE, Rappolee DA. A major effect of simulated microgravity on several stages of preimplantation mouse development is lethality associated with elevated phosphorylated SAPK/JNK expression. Reprod Sci. 2009;16:947–959. doi: 10.1177/1933719109337544. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12(1):14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Ain R, Ohboshi S, Soares MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J Endocrinol. 2003a;179(3):335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Shao LJ, Ain R, Dai G, Soares MJ. The mouse prolactin gene family locus. Endocrinology. 2003b;144(1):313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- Wills RA, Coory MD. Effect of smoking among Indigenous and non-Indigenous mothers on preterm birth and full-term low birthweight. Med J Aust. 2008;189(9):490–494. doi: 10.5694/j.1326-5377.2008.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Xie Y, Abdallah M, Awonuga N, Puscheck E, Rappolee D. Benzo(a)pyrene causes AMPK-dependent ID2 loss in placental trophoblast stem cells. Mol Reprod Devt. 2009 doi: 10.1002/mrd.21178. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE, Rappolee DA. Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev. 2008;75(4):689–697. doi: 10.1002/mrd.20787. [DOI] [PubMed] [Google Scholar]
- Xie Y, Puscheck EE, Rappolee DA. Effects of SAPK/JNK inhibitors on preimplantation mouse embryo development are influenced greatly by the amount of stress induced by the media. Mol Hum Reprod. 2006a;12(4):217–224. doi: 10.1093/molehr/gal021. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007a;74(10):1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod. 2006b;75(1):45–55. doi: 10.1095/biolreprod.105.049791. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007b;13(7):473–481. doi: 10.1093/molehr/gam027. [DOI] [PubMed] [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, Depamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134(21):3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogren L, Endo H, Soares MJ, Talamantes F. Co-localization of placental lactogen-I, placental lactogen-II, and proliferin in the mouse placenta at midpregnancy. Biol Reprod. 1994;51(6):1188–1192. doi: 10.1095/biolreprod51.6.1188. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3(1):69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. Placental 11 beta-hydroxysteroid dehydrogenase: barrier to maternal glucocorticoids. Rev Reprod. 1997;2(3):129–132. doi: 10.1530/ror.0.0020129. [DOI] [PubMed] [Google Scholar]
- Yu YM, Han PL, Lee JK. JNK pathway is required for retinoic acid-induced neurite outgrowth of human neuroblastoma, SH-SY5Y. Neuroreport. 2003;14(7):941–945. doi: 10.1097/01.wnr.0000074341.81633.b8. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Xiao Q, Zeng L, Hu Y, Xu Q. TLR4 expression in mouse embryonic stem cells and in stem cell-derived vascular cells is regulated by epigenetic modifications. Biochem Biophys Res Commun. 2006;347(1):89–99. doi: 10.1016/j.bbrc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Zhang L, Connor EE, Chegini N, Shiverick KT. Modulation by benzo[a]pyrene of epidermal growth factor receptors, cell proliferation, and secretion of human chorionic gonadotropin in human placental cell lines. Biochem Pharmacol. 1995;50(8):1171–1180. doi: 10.1016/0006-2952(95)00253-v. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shiverick KT. Benzo(a)pyrene, but not 2,3,7,8-tetrachlorodibenzo-p-dioxin, alters cell proliferation and c-myc and growth factor expression in human placental choriocarcinoma JEG-3 cells. Biochem Biophys Res Commun. 1997;231(1):117–120. doi: 10.1006/bbrc.1997.6053. [DOI] [PubMed] [Google Scholar]
- Zhong W, Xie Y, Proteasa S, Abdallah M, Awonuga A, Slater JA, Rappolee DA. Cellular stress induces placental lactogen-1 in trophoblast stem cell dependent on SAPK/JNK and AMPK. Biol Reprod. 2009 in press. [Google Scholar]
- Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14(6):534–547. doi: 10.1177/1933719107307182. [DOI] [PubMed] [Google Scholar]