Abstract
Decreasing the incidence of chronic rejection and reducing the need for life-long immunosuppression remain important goals in clinical transplantation. In this article, we will review how regulatory T cells (Treg) came to be recognized as an attractive way to prevent or treat allograft rejection, the ways in which Treg can be manipulated or expanded in vivo, and the potential of in vitro expanded/generated Treg for cellular therapy. We will describe the first regulatory T cell therapies that have been or are in the process of being conducted in the clinic as well as the safety concerns of such therapies and how outcomes may be measured.
Keywords: transplantation, T regulatory cells, cellular therapy, drug minimization, translational medicine
1. Introduction
One of the characteristics that defines the mammalian adaptive immune system is the rapid proliferation and expansion of T and B cells following antigen exposure, but in the past two decades it has also become clear that the immune system has evolved multiple peripheral mechanisms for controlling these responses. Growing evidence indicates that it should be possible to engage these inherent regulatory pathways to suppress immune responses to alloantigens following transplantation. Ways to specifically prevent immunity to foreign cells and tissues would offer a new way to minimize reliance on non-specific immunosuppression and could ultimately allow patients to be completely withdrawn from drug-based immunosuppression.
Many different types of T cells with regulatory activity have been described including: CD8+ T cells [1-3]; CD4−CD8− double negative T cells [4, 5]; NK T cells [6]; and γδ T cells [7], but these are all less well studied than their CD4+ T cell counterparts. In this review we will focus on the potential for clinical application of CD4+ T regulatory cells characterized by high and stable expression of CD25 and FOXP3 in the context of organ transplantation. CD25+FOXP3+ T regulatory cells (hereafter Treg) can arise via two distinct developmental pathways. First, so-called “naturally-occurring” or nTreg, arise directly in the thymus, and are thought to primarily function to regulate autoimmunity. Second, when conventional CD4+ T cells encounter their antigen in a tolerogenic environment, e.g. when presented by immature dendritic cells (DCs), or with immunosuppressive cytokines, they differentiate into “adaptive” or aTreg. Establishment of long-term tolerance by nTregs is thought to depend on their ability to stimulate de novo differentiation of aTreg [8]. Despite distinct developmental origins, both nTregs and aTregs rely on continuous expression of FOXP3 for their suppressive function. It is difficult to distinguish nTreg from aTreg, because both are defined as FOXP3+ cells, but recent data suggest that nTregs may be identified by high expression of another transcription factor, Helios [9].
The importance of Treg to the normal immune system came from two recent studies where a transgenic approach examined whether selective depletion of nTreg in otherwise normal mice might replicate some of the characteristics of profound autoimmunity seen in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) patients. Mapping studies had shown that such patients have a point mutation in the gene encoding the transcription factor FOXP3 [10, 11] and a functional Treg deficit in vitro [12]. DREG mice were constructed in which the diptheria toxin receptor gene was inserted into the Foxp3 locus such that administration of the toxin leads to a conditional depletion of Foxp3+ nTreg. Selective Treg depletion led to profound autoimmunity in neonates [13] and lethal autoimmune disease in adults [14] demonstrating that active regulation mediated by nTreg plays an indispensable role in normal immune homeostasis. The implication that nTreg play such an important role in controlling immune responses in mice and in humans gives grounds for cautious optimism that it should be possible to harness the potential of Treg to control rejection in clinical transplantation.
2. Regulatory T cells in vivo: An historical perspective
Although much of our current understanding of immune regulation has come from autoimmunity models, it is important to recognize that transplantation provided some of the earliest evidence for Treg function in vivo. Almost 30 years ago in a rat heart transplant model, Hall et al. showed in the MHC mismatched PVG to DA strain combination that a two-week course of cyclosporine (CyA) led to indefinite allograft survival without further therapy. Importantly, when harvested 100 days post transplant and tested in adoptive transfer models, T cells from these animals had the capacity to prevent rejection mediated by normal effector cells [15]. These data provided a clear indication that long-term allograft survival independent of long-term immunosuppression (operational tolerance) involved T cells with the ability to regulate naïve alloreactive T cells. Subsequently, Hall and colleagues demonstrated that regulation was associated with CD4+ T cells [16, 17] and were the first to suggest that CD25 is a useful Treg marker [18]. Similar data were obtained in a rat renal allograft model where operational tolerance was induced by donor-specific blood transfusion [19, 20].
To determine whether cells isolated on the basis of CD25 expression could be used therapeutically in the transplant setting, Hara et al. reconstituted immunodeficient CBA mice with naïve CBA effector T cells with or without CD4+CD25+ T cells isolated from CBA mice bearing fully allogeneic B10 heart allografts. The reconstituted mice were then transplanted with test B10 skin grafts. Mice reconstituted with effector T cells alone rejected their skin grafts acutely but, in stark contrast, co-transfer of CD4+CD25+ T cells from tolerant animals led to indefinite skin graft survival in 80% of recipients [21]. Strikingly, when used at equivalent cell doses, naïve CD4+CD25+ T cells were unable to control rejection suggesting that exposure to alloantigen in a tolerogenic environment either enhances nTreg function and/or generates a population of induced Treg.
Whilst the observation that long-term tolerant mice contain populations of alloantigen reactive CD25+ Treg was important, these experiments were unable to distinguish between Treg that were generated by the induction strategy itself and those that arose simply by the presence of the accepted allograft. In terms of developing potential clinical approaches, a much more important question is whether induction strategies that ultimately lead to long-term operational tolerance can drive Treg development independently of the graft itself. The presence of sufficient numbers of donor-reactive Treg pre-transplant might offer immediate active regulation perhaps allowing early drug-minimization. In a fully mismatched mouse transplant model, pre-treatment of H2k CBA mice with H2b donor alloantigen (donor specific transfusion, DST) under the cover of a non-depleting anti-CD4 antibody 28 days before transplant leads to the indefinite survival of donor-specific H2b hearts without further therapy [22]. Importantly, when CD4+CD25+ T cells were isolated from mice 28 days after pre-treatment but without transplant, these cells prevented test skin graft rejection in a sensitive adoptive transfer model [23]. Critically, protection was not seen with similar populations isolated from naïve, anti-CD4-only or DST-only mice demonstrating that tolerance mediated by CD25+ Treg can be indeed induced in vivo prior to transplant.
Although a significant body of work has demonstrated that Tregs can control alloreactive responses, most experiments involved adoptive transfer of cells into immunodeficient recipients where allograft rejection is driven by relatively small numbers of effector T cells - typically of the order of 105 per mouse. In terms of translational medicine, a much more relevant question is what role do Tregs play in an intact immune system? In transplantation Treg-specific inactivation was used to show that in the anti-CD4/DST tolerance induction model described above, the survival of primary heart allografts in normal, lymphoreplete recipients is also unequivocally dependent on aTreg driven by the tolerance induction protocol [24]. These data suggest that it should indeed be possible to boost the function of Tregs in non-lymphopenic transplant patients.
3. How might Treg be exploited for therapeutic benefit?
3.1 In vivo induction of Treg
The current success of clinical transplantation depends on immunosuppression and, as in rodent models [15, 25, 26], it may be possible to tailor immunosuppression to promote the generation and/or expansion of donor-reactive Treg. Attempts to identify the emergence of Treg in such circumstances are essential but a number of factors make such identification far from trivial. Firstly, although FOXP3 and CD25 have been and continue to be useful for the identification of Tregs, in humans neither marker is unique to Tregs, and both can be up-regulated on activated non-regulatory populations [27-31]. Thus, accurate identification of Treg is problematic and historical data that did not take this possibility into account must be viewed with caution. Secondly, it is quite likely that different immunosuppressive drugs will be more or less permissive for Treg development/function. For example, some studies have found circumstantial evidence that calcineurin inhibitors (CNI) have a negative impact on regulation whilst rapamycin may preserve or enhance Treg development and or function. Thirdly, the heterogeneity of donor-recipient populations and the use of many different of induction and maintenance immunosuppressive regimens will make it challenging to identify protocols that promote Treg function. Fourthly, even if a given transplant protocol were to induce functional human Treg, identifying the in vivo contribution of such cells against a background of very effective immunosuppression will not be straightforward.
Despite the above limitations, four specific strategies of immunotherapy have been identified that may be permissive for Treg development and function in a transplant setting. These are: 1. anti-CD3 antibody; 2. anti-thymocyte globulin; 3. anti-CD52 antibody; and 4. mTOR inhibitors.
3.1.1 Anti-CD3 antibody
The early observation that T cells are essential for rejection led to the development of anti-T cell reagents including anti-CD3 antibodies which have a long history in transplantation. These agents were used initially as an anti-rejection therapy [32, 33] but evidence began to emerge that they could also induce tolerance in transplant [34, 35] and autoimmunity models [36]. Although anti-CD3 antibodies provide an initial period of global immunosuppression due to T cell receptor (TCR) modulation and enhanced effector T cell apoptosis, in the longer term a state of self-tolerance develops which involves the expansion of TGF-β–producing aTreg [37, 38]. Anti-CD3 antibodies have been used in Phase I/II trials in recent onset diabetic patients and appear to delay the requirement for exogenous insulin [39]. Importantly, the most benefit was in patients with the highest residual β-cell mass and the least advanced autoimmunity. Thus anti-CD3 antibody therapy could be useful in the transplant setting since the problem of pre-existing activated effector cells and organ damage is less of a problem compared to patients with advanced autoimmunity.
3.1.2 Anti-thymocyte globulin
Like anti-CD3 antibodies, anti-thymocyte globulins (ATG) have been used for many years in transplantation, principally as induction agents. Due to their polyclonal nature, these agents have multiple modes of action including T cell depletion, TCR modulation and induction of effector T cell apoptosis. However, as with CD3 antibodies, ATG may also promote an overall shift in T cell responses, due partly to a relative resistance of Treg to ablation and to the fact that following non-Treg depletion, re-populating T cells may default along a Treg pathway. Evidence for both these possibilities has been obtained in the mouse [40] and humans [41, 42]. Although ATG treatment of human T cells does not induce the differentiation of aTreg in vitro [43], this finding does not exclude the possibility that ATG may increase the numbers of donor-reactive aTreg in vivo.
3.1.3 Anti-CD52 antibody
The antibody that became the commercial humanised anti-CD52 reagent Alemtuzumab began life in Cambridge in the early 1980s and an IgG2b variant was selected for further development because of its ability to kill T cells and other leukocytes with high efficiency [44]. The antibody is licensed for use in chronic lymphocytic leukaemia and has been used in multiple sclerosis patients and in kidney transplant recipients. Alemtuzumab causes a rapid and profound depletion of most leukocyte sub-sets including T cells but, as with anti-CD3 and ATG, it is possible that T cell re-population after Alemtuzumab induction therapy could result in a shift in the Treg/non-Treg balance such that there is a functional enrichment of Treg. In a study of 29 kidney transplant recipients, Bloom et al. showed that induction therapy with Alemtuzumab (day 0, +1; total dose, 40mg) was associated with a significant increase in the proportion of CD25+FOXP3+ T cells:- pre-transplant:- mean 3.5% of total CD4+ T cells cf. mean 12% at 6 months post-transplant, [45]. Numbers remained similar at 12 months post-transplant but then declined almost to pre-transplant levels by 24 months. Although it is tempting to speculate that these results indicate an Alemtuzumab-dependent induction of regulatory T cells, such conclusions are complicated by two factors. Firstly, in addition to receiving steroids, the patients in this study also received maintenance sirolimus therapy, which in vitro at least has been shown to favour Treg survival [46]. Secondly, in man (rather unlike the mouse), FOXP3 is up-regulated on activated non-regulatory T cells. Indeed, a previous report demonstrated that treatment of kidney transplant recipients with Alemtuzumab resulted in an enrichment of cells with a memory-like phenotype [47] and since memory is frequently associated with activation, it is possible that the increase in the proportion of CD25+FOXP3+ T cells seen in the later study [45] reflects transient T cell activation and not the expansion of bona fide Treg. One obvious possibility is that global T cell depletion results in the expansion/enrichment of both Treg and activated memory cells but that the former is masked by the latter. If this is the case, it might be possible to consider a two-step procedure in which global depletion is followed by a second depletion to target memory rather than regulatory T cells. A potential candidate for this approach is anti-CD2 antibody which is reported to have some selectivity for memory cells [48] and has been shown to result in Treg enrichment when used as part of a conditioning regimen for haematopoietic stem cell transplantation [49, 50]. Although it is clear that much remains to be learned about the best way in which to manipulate the immune system using global depletion, the possibility that such approach could promote the emergence of donor-reactive Treg means that for many groups this will continue to be an important area of study
3.1.4 mTOR inhibitors
Tregs have a functionally-essential reduction in the activity of the phosphatidyl inositol 3′ kinase (PI3K) pathway [51] and accordingly are resistant to the anti-proliferate effects of inhibitors of various kinases in this pathway. Evidence that rapamycin, which inhibits the mTORC2 complex in the PI3K pathway, promotes the selection and/or preferential survival of mouse [46] and human nTreg [52-54] has led to the idea that mammalian target of rapamycin (mTOR)_inhibitor-based immunosuppressive regimens could promote the development of donor-reactive Treg in vivo. Several groups are currently investigating this possibility in clinical transplantation trials. One study found that whereas calcineurin inhibitors (CNIs) reduce the number of circulating Treg in transplant patients, rapamycin preserves Treg numbers [55]. Whether this ‘Treg preservation’ is due to preferential selection or de novo generation is not clear.
A problem with designing trials of novel immunosuppressive regimens is the choice of the relevant control or comparator population. For example in a study of 21 renal transplant recipients, patients were induced with Alemtuzumab then treated with cyclosporine (CyA) plus low dose mycophenolate mofetil (MMF) or rapamycin plus low dose MMF to compare the impact of CyA versus rapamycin on Treg [56]. In both groups, leukopenia was followed by a gradual recovery in Treg frequency but, while Treg recovered only to pre-transplant levels in the CyA group, in the rapamycin patients there was a 3.6-fold increase in CD25+ cells 12 months post-transplant. These data suggest that Treg recovery depends on calcineurin-dependent IL-2 production but it is difficult to conclude that rapamycin promotes the preferential selection of Treg since the effect could simply be due to the lack of CyA rather than the presence of rapamycin [56, 57]. In a unique study in which the effect of rapamycin on Treg number and function was evaluated in pre-transplant diabetic patients, Monti et al. showed that whilst rapamycin had little effect on the number of circulating Treg, the regulatory potency of these cells in vitro was restored to that seen in normal untreated individuals [58]. This is an important observation because unlike other investigations, the nature of the study allowed the effects of rapamycin to be examined independently from the confounding effects of other immunosuppressive agents. Overall, the circumstantial data obtained thus far are consistent with the idea that, in vivo, rapamycin can contribute to the preservation or generation of functional Treg.
The effect of mTOR inhibition in animal models is generally clearer and rapamycin has been shown to preserve or promote Treg function in graft versus host disease (GVHD) models [59]. Furthermore, in an elegant series of experiments, Gao et al. provided persuasive evidence for rapamycin-mediated generation of Treg from non-Treg precursors [60]. In these experiments, CD4+GFP− cells (non-Treg) from Foxp3GFP reporter mice carrying a congenic marker were adoptively transferred into MHC compatible recipients and then treated with PBS control, CyA or rapamycin. Four days later spleen cells were harvested from these mice and gating on the congenic marker allowed Foxp3(GFP) expression to be examined only within the input population. In these experiments, an appearance of Foxp3+ cells in the relevant gate would indicate a non-Treg to aTreg conversion. Compared with PBS-treated controls, administration of rapamycin resulted in a 10-fold increase in the number of GFP+ cells recovered (2.3×104, cf 2.4×105, PBS and rapamycin respectively). Importantly, in mice treated with CyA, the average number of GFP+ cells recovered was only 50% of that in the PBS group supporting the observation [56] that Treg expansion in vivo relies on calcineurin-dependent IL-2 production. The above data were obtained by adoptive transfer of non-Treg without antigen challenge, but important additional experiments showed that rapamycin also promoted Treg conversion when given as peri-transplant immunosuppression in an allogeneic skin graft model. It is not clear if Gao et al. had the opportunity to ask whether concomitant CyA administration can override the rapamycin effect, but this would be a fascinating line of enquiry highly relevant to current clinical practice.
3.2 Treg as cellular therapy
Compelling data from animal models combined with tentative clinical observations suggest that Treg may emerge in transplant patients as a result of successful engraftment but the difficulty of identifying donor-reactive Treg and influencing their development means that in vivo generation may depend more on serendipity than on deliberate design. These factors indicate that the most likely route for the controlled use of Treg in clinical transplantation will be the administration of in vitro expanded or generated cells delivered as a cellular therapy.
The use of Treg as a cellular therapy has a number of theoretical advantages compared with attempts to generate such populations in vivo. These include: Treg could be produced under defined and reproducible conditions; function according to defined criteria could be validated before use; Treg could be delivered in defined numbers and at a specified time relative to transplant; and cryo-preservation would allow Treg therapy to be ‘topped-up’ if required post-transplant. At present, there are three main strategies being pursued to develop Treg-cell based therapies for transplantation: 1) the use of nTreg, either freshly isolated or expanded in vitro; 2) the generation of aTreg, generated by donor antigen stimulation in vitro; and 3) the generation of Treg by ectopic gene expression.
3.2.1 Naturally occurring Treg
The small number of nTreg accessible in the peripheral circulation means that for cellular therapy, it will be almost certainly be necessary to use a polyclonal stimulus to expand nTreg in vitro. Thus, the first challenge is deciding the basis on which Treg should be isolated to maintain pure populations after in vitro expansion. Purity is an issue because of the potential out-growth of non-Treg that could contribute to rejection or cause autoimmunity. In the absence of a Treg-specific cell surface marker, two different combinations of markers appear to be promising for Treg isolation. The first combination seeks to isolate CD4+CD25hi nTreg but with the addition of an antibody to select for CD45RA+ cells and so eliminate antigen experienced or memory T cells [61]. The second combination also uses CD4 and CD25 but includes an antibody to CD127 (IL-7Rα) on the basis that in human nTreg, there is a reciprocal expression of CD127 and FOXP3 and thus CD127 provides a sort-able surrogate maker for FOXP3+ nTreg [62, 63].
Notably, isolation of CD25+CD45RA+ (naive) T cells yields Treg with a greater suppressive capacity than total CD25hi cells [61]. The reason for this became clear when Miyara et al. examined subpopulations of human FOXP3+ T cells and discovered that whereas CD25+CD45RA−FOXP3hi cells are highly suppressive, CD25+CD45RA−FOXP3lo cells are not suppressive in vitro, contain many IFN-γ and IL-2 producing cells, and also Th17 precursors [64]. Furthermore, after three weeks of in vitro expansion, CD127− Treg became methylated at the Treg-specific demethylated region (TSDR) while CD45RA+ expanded Treg remained demethylated [65], and the CD127− Treg that lost FOXP3 expression were CD45RA− demonstrating that naïve Treg may represent the most stable population for expansion [65, 66]. Naïve Treg have additional benefits of having the greatest expansion potential [66] and of expressing the homing receptors CD62L and CCR7 even after in vitro expansion which may be beneficial for cellular therapy to target the cells to lymphoid organs [61]. One drawback especially in an ageing population, is that numbers of naïve Treg decline in peripheral blood with age [67, 68], hence, isolation based on CD127 expression may still be a practical approach.
Work continues to identify additional markers that may give purer or more potent Treg, or provide more straight-forward isolation procedures. In addition to selection criteria based on CD45RA or CD127, there have been studies investigating the utility of CD121a/CD121b and TGF-β/LAP [69] CD39 [70-72], and glycoprotein-A repetitions predominant (GARP) [73-75] as new Treg markers. However, all of these proteins are only expressed on activated Treg so they would only be useful for re-purifying in vitro expanded Treg if contamination with effector T cells was suspected. Markers have also been identified that separate Treg into different functional subsets. For example, human ICOS+FOXP3+ cells produce IL-10 and TGF-β whilst ICOS−FOXP3+ cells only produce TGF-β [76]. Thus depending on the type of immune response to be suppressed, it may be useful to isolate subsets of nTregs which have specific mechanisms of action.
In view of their low abundance much work has gone into developing in vitro methods to expand nTreg. Methods employed to stimulate Treg include anti-CD3/anti-CD28 coated beads as well as cell-based artificial antigen presenting cells expressing co-stimulation molecules and/or Fc receptors. In addition to stimulus through the TCR, Treg require CD28 co-stimulation [77-80] and exogenous IL-2 [81, 82]. Adding rapamycin to the culture has been shown to preserve Treg purity and allow selective Treg expansion [46, 53, 54, 80, 83]. Notably, as discussed above, rapamycin-expanded Treg retain suppressive capacity in vitro and when tested in a GVHD model were more effective than nTreg expanded under conventional conditions [80]. However, rapamycin also significantly inhibits the proliferation of Treg; thus, addition of this compound for only a portion of the expansion period may be optimal [66, 80]. Importantly, expansion outcomes among individuals are heterogeneous [66], potentially affecting the number or purity of Treg that can be obtained from a given individual. Indeed, in a recently reported clinical study, target doses of expanded nTreg were not achieved for all patients [84]. Improvements in expansion methods that remain GMP compliant should allow Treg therapy to be applicable for a wider range of patients.
Whilst it has been essential to use in vitro suppression assays to determine whether expanded nTreg retain regulatory function, a much more critical matter is whether these cells can regulate alloreactivity in vivo. Fortunately, in the past few years, several humanised-mouse models have been described in which immunodeficient mice are reconstituted with components of the human immune system [85, 86]. In a unique study, Nadig et al. reconstituted immunodeficient BALB/c Rag−/− common γ chain−/− mice with human PBMC with or without expanded nTreg isolated from the same cell donor, then transplanted these mice with segments of human left internal mammary artery side branches as interposition grafts in the descending aorta [87]. Grafts were harvested at day 30 and examined for intimal hyperplasia, one of the hallmarks of immune-mediated vascular damage. Mice transplanted without cellular reconstitution showed no signs of intimal proliferation, whereas mice reconstituted with allogeneic PBMC showed extensive vasculopathy which in some cases resulted in almost complete intimal occlusion. However, co-transfer of expanded nTreg isolated on the basis either of CD4 and CD25 or CD4, CD25 and low expression of CD127, had a striking impact on vasculopathy with some vessels being entirely free from occlusion. On the basis of their ability to prevent vasculopathy in this model, cells sorted on the basis of CD127 appear to be some five times more effective on per cell basis, [87] an observation that could have important implications for the design of future clinical studies (see below). This approach has recently been extended to a human skin graft model in BALB/c Rag−/− common γ chain−/− mice reconstituted with allogeneic human PBMC and as in the vessel model, in vitro expanded CD4+CD25+CD127lo nTreg have been show to be powerful regulators of allograft rejection emphasising their clinical potential [88].
3.2.2 Adaptive Treg
Broadly speaking, the CD4+ T cell compartment of rodents and man contains approximately 5-10% of FOXP3+ nTreg and, as discussed earlier, these clearly play an essential role in normal immune homeostasis [10-12, 14, 89-91]. However, as discussed above, in humans it is difficult to isolate pure populations of nTreg and their low expansion potential continues to limit clinical application. Many groups have thus pursued the development of strategies to induce aTreg in vitro. GVHD models allowed the unequivocal demonstration that stimulation of donor CD4+ T cells with recipient strain APC in the presence of TGF-β and IL-2 resulted in a population of Treg that could prevent lethal GVHD in vivo [92]. Subsequently, two groups made the important observation that Foxp3+ cells with suppressive function in vitro and in vivo could be generated from CD4+CD25− precursors [93, 94]. The ability to generate Treg from non-Treg precursors could have important consequences for eventual Treg cell therapy because CD4+ conventional T cells are 10-20-fold more abundant than pre-existing Treg and thus provide a larger pool of potential Treg precursors.
The potential of adaptive Tregs has been extended from GVHD models to models of organ and tissue transplantation and several groups have shown that in vitro generated aTreg can control allograft rejection. For example, Feng et al. developed a novel in vitro protocol in which recipient strain CD4+ T cells are stimulated for 14 days with donor APC in the presence of IFN-γ [95]. Without further purification, the resulting cells prevent donor-specific skin and vessel allograft rejection mediated by CD25− effector T cells in a sensitive adoptive transfer model [96, 97]. Importantly, T cell stimulation in the presence of IFN-γ enriches for alloreactive Foxp3+ Treg by inducing preferential apoptosis in responding non-Treg, promoting the expansion of responding nTreg and driving the conversion of non-Treg precursors [95]. While these results are encouraging, the aTreg cells in these experiments are required to control only a relatively small population of effector cells. An essential next step is to determine whether logistically feasible numbers of aTreg can influence graft outcome in normal mice with an intact T cell repertoire. Whilst it is perhaps no surprise that alone, even 2×106 IFN-γ conditioned Treg have no impact on the rejection of primary heart allografts, when combined with sub-optimal co-stimulation blockade, as few as 5×105 cells delivered one day before transplant can extend heart graft survival beyond that in controls given co-stimulation blockade alone (Chan and Bushell, preliminary results).
Encouraging primary heart allograft results have also been reported following administration of recipient T cells stimulated in vitro with donor APC in the presence of TGF-β and IL-2 [98]. Delivery of 1×107 in vitro generated Treg on days −1 and +5 resulted in a median graft survival of approximately 50 days with ~30% of animals accepting their grafts long-term. Most remarkable is the fact that these transplants were performed in a fully mismatched strain combination (B6, H2b to DBA/2, H-2d) and no additional immunosuppression was given. Although it is not clear which cell in the injected population is responsible for the regulation described, if it is assumed that regulation is dependent on CD4+CD25+ cells (shown to be ~3×106 per 1×107 cells), then scaling these numbers up to an 80kg human would appear to indicate that a dose of 1.2×1010 might be required. Of course, fewer Treg might be required if delivered with Treg-permissive immunosuppression although this was not explored in this study. A caveat is that there is currently no robust way to generate aTregs in vitro in humans [28], thus testing of this approach in the clinic will await further basic research.
On the other hand, protocols to generate human aTregs characterized by high expression of IL-10, but not FOXP3, are well established. These so-called Tr1 cells arise when conventional T cells encounter their antigen in the presence of IL-10 [99] and mediate antigen-specific suppression via an IL-10-dependent mechanism. In mouse models, Tr1 cells can suppress islet allograft rejection [100] as well as GVHD [101], leading to the development of clinically-applicable protocols to generate alloantigen-specific Tr1 cells for cellular therapy [102]. A trial is ongoing to test whether delivery of Tr1 cells can control GVHD without affecting responses to other antigens.
3.2.3 Generation of Treg by ectopic gene expression
A genetic deficiency in FOXP3 results in profound autoimmunity in Scurfy mice [103] and IPEX patients [10, 11] and it is now known that expression of the FOXP3 transcription factor is essential to maintain functional nTregs. Indeed, Foxp3 over-expression either in vivo in transgenic mice [104] or in vitro using viral transduction [105-107] confers regulatory activity on previously non-regulatory T cells. Similarly over-expression of FOXP3 in human T cells using a vector system which ensures continuous high expression levels allow efficient generation of Tregs from conventional T cells in vitro [108]. Human T cells expressing an inducible form of FOXP3 only acquire regulatory capacity after FOXP3 is expressed for 7-12 days, illustrating the importance of long term and high expression of FOXP3 to achieve functional reprogramming of human T cells [109]. A major benefit of this approach is that a relatively large number of T cells could be isolated and reprogrammed into Treg, overcoming the challenge of limiting cell numbers. Furthermore, over-expression of FOXP3 can also reprogram memory T cells into Tregs, allowing for generation of antigen-specific Treg. The generation of Treg by ectopic expression of FOXP3 thus represents an exciting though challenging prospect in clinical transplantation. In the living-donor transplant setting it would theoretically be possible to stimulate recipient T cells with donor APC and then transduce the responding T cell population with FOXP3 for subsequent delivery as a “personalized” Treg cellular therapy.
Another approach involving a combined gene and cell therapy approach is to introduce alloantigen-specific TCRs into Tregs. Tsang et al. [110] used a mouse model to generate Tregs with both indirect and direct specificity for donor alloantigens. Recipient (B6, H2b) CD25+ T cells were repeatedly stimulated with donor (BALB/c, H2d) to generate Treg with direct alloreactivity, then transduced these cells with the TCR recognising for Kd presented by IAb . The resulting cells were then delivered to B6 recipients as a cellular therapy and assessed for their ability to influence the rejection of fully mismatched BALB/c heart allografts. When combined with sub-therapeutic anti-CD8 antibody plus rapamycin, cells with only direct specificity led to ~50% heart allograft survival at 100 days. In contrast, cells with both direct and indirect specificity led to 100% graft survival in the same strain combination. This study provided important ‘proof of concept’ data showing firstly that transduction of recipient cells with a TCR of known allospecificity can generate functional Treg and, secondly, that Treg with the capacity to recognise alloantigen via the indirect pathway should not be ignored as protocols are developed for clinical testing. Although these data are striking, it should be noted that the doses of cells used in these mice were not insignificant (1×107 on days −1, +7, 14 and 21). When scaled up from a ~25g mouse to an 80kg human, this equates to a total dose approximately 1.2×1011 transduced Treg. This number is put into a logistical and possible safety context by the fact that an average 80kg person has approximately 4×108 circulating CD4+FOXP3+ nTreg and perhaps an equivalent number resident in peripheral lymphoid tissues. Thus, such an approach, though technologically elegant would face enormous practical and licensing issues if it were to be considered for clinical transplantation.
3.3 The current clinical experience
The ‘first-in-man’ study of expanded nTreg to be used as a cellular therapy reported the results of two patients who developed GVHD following bone marrow transplantation [111]. One patient developed chronic GVHD (POD 137) and received triple therapy (prednisolone, tacrolimus and MMF) for two years post-transplant but complications of the immunosuppression prompted the administration of CD4+CD25+ flow-sorted, expanded nTreg from the bone marrow donor at a dose of 1×105/kg. This allowed MMF withdrawal and a reduction in steroids without overt disease recurrence. The second patient was diagnosed with acute GVHD on day 29 post transplant which was refractory to treatment with steroids, tacrolimus, MMF and ATG. This patient received expanded donor nTreg at a total dose of 3×106/kg over three infusions (day+75, +82 and +93) and, although there was a temporary clinical improvement after the first infusion, the patient deteriorated and died of multi-organ failure on day 112.
In late 2010, a much larger Phase I/II study was reported in which 23 patients who received double umbilical cord blood stem cell transplantation were enrolled in a dose escalation Treg trial [84]. In each case, CD4+CD25+ T cells were isolated from a third party unit of cord blood partially matched with the recipient. The study design called for delivery of defined doses on day +1 post-transplant, with some patients receiving a second dose on day +15. Doses ranged from 1×105/kg to 30×105/kg. The rates of GVHD and infectious complications were compared with those from 108 historical controls. Importantly, the study reported no increase in fungal, bacterial or viral infections compared with the control group and although the primary endpoints were safety and feasibility, the authors did report a slight reduction in grade II-IV GVHD in the trial group. It seems highly likely that as various groups begin planning trials of nTreg in bone marrow and solid organ transplantation, this study will provide an essential reference point for cell doses and cell purity for both study teams and the licensing authorities.
Another study has recently been reported in which expanded donor CD4+CD25+ nTreg were administered to patients who underwent HLA-haploidentical hematopoietic stem cell transplantation [112]. Donor conventional T cells (Tconv) were given four days post-transplant with CD34+ cells in a dose escalation study:- 4 patients received 2 × 106 Treg/kg plus 0.5 × 106 Tconv/kg, the next 17 patients received 2 × 106 Treg/kg plus 1 × 106 Tconv/kg, and the next 5 patients received 4 × 106 Treg/kg plus 2 × 106 Tconv/kg. One goal of this study was to deliver Tconv cells in addition to the CD34+ cells to enhance immune reconstitution and function in the recipient without causing GVHD. Importantly, no GVHD prophylaxis was given. Twenty-six of 28 patients achieved full donor-type engraftment, and of those, no patients developed chronic GVHD at the time of reporting (3.6 to 21.4 months post transplantation). However, two of the 26 patients developed ≥ grade II acute GVHD, but these received the highest dose of Tconv cells emphasising that Treg-mediated control is clearly a dose-dependent phenomenon. The study reported an enhancement of immune cell recovery, and an improved immunity to pathogens as judged by in vitro assays. Furthermore, there was no association with an increased risk of leukemia relapse, indicating that the graft-versus-leukemia response was likely intact. It should be noted that in terms of patient survival, the results of this study appear to be disappointing in that 13/26 patients in the study died, particularly from sepsis, viral or fungal infection. However, some of these patients had fungal infections prior to transplant and importantly, no fatal infections occurred after the first two months post transplant indicating a restoration of protective immunity without negative effects of Treg therapy.
In Europe, the European Union has recently funded a multi-centre Phase I/II study to evaluate various types of immunomodulatory cells in living-donor kidney transplantation. The ‘ONE Study’ involves groups from Regensburg, Berlin, London, Oxford, Milano and Nantes and will develop protocols for the use of expanded recipient nTreg (Regensburg, Berlin, London, Oxford), recipient Tr1 cells (Milano), donor regulatory macrophages, Mregs (Regensburg) and donor tolerogenic DC (Nantes). Critical to the study design is that all centres will use a common immunosuppressive protocol (part of the ‘ONE’ concept), which will be closely based on the recently published Symphony study [113]. As with the stem cell trials described above [84, 112], the primary endpoints will be safety and feasibility but therapeutic benefit will be examined through immunological monitoring using lessons learned from the European Unionfunded RISET and Indices of Tolerance initiatives and from similar studies sponsored by the Immune Tolerance Network [114, 115]. Each centre will enrol 20 patients in the control arm to receive Symphony-based immunosuppression and 10 patients who will receive the same immunosuppressive regimen (but without anti-CD25 induction) plus cell therapy. Providing the relevant licensing and ethical approval is obtained, the study design calls for the control patients to be transplanted no later than 2013 with the cell therapy groups transplanted in 2014 thus allowing a follow-up period of at least 12 months. The intention with the nTreg group is that recipient nTreg will be isolated, expanded, assessed phenotypically and functionally, then cryopreserved for delivery at day −1 relative to the time of transplant. Although many details will be subject to modification by the appropriate regulatory and ethical bodies based on the bone marrow transplant experience described above, it is anticipated that the study will begin with doses of the order of 3×106 expanded nTreg/kg.
3.4 Safety concerns of Treg cellular therapy
3.4.1 Global immune suppression
One of the main goals of using Treg as a cellular therapy is to decrease the requirement for life-long global immunosuppression which increases the risk of infection and cancer. While Treg have been shown to suppress graft rejection in multiple studies, whether or not they are globally immunosuppressive in the context of cellular therapy has not been extensively studied. However, promising results from transfer of Treg to treat or prevent GVHD show that Treg can suppress GVHD while still maintaining the critical graft versus leukemia effect [116-118]. Indeed, it has been demonstrated that Treg not only prevent GVHD but also enhance immune reconstitution after bone marrow plus T cell transplant in mice by preventing GVHD-induced damage of the thymus and secondary lymphoid organs, thus allowing protection against lethal cytomegalovirus infection [119].
There have been fewer investigations of global immune suppression when Treg are used as a cellular therapy in solid organ transplantation. In one study, when antigen-specific Treg are induced in vivo to prevent cardiac allograft rejection in a mouse model, they do not prevent antiflu responses after challenged with influenza 7 days post-transplant [120]. The same is true when splenocytes from these in vivo tolerised mice are transplanted to naïve mice which receive allografts and virus challenge. Although these data are encouraging, much more work is required to determine whether the potential benefits of expanded nTreg, aTreg, or FOXP3-transduced cells can be realised in solid organ transplantation without compromising protective immunity.
Limited safety data have been obtained from initial clinical trials. In the phase I clinical trial by Brunstein et al. described above, where nTreg expanded from umbilical cord blood were infused into patients who had undergone double umbilical cord blood transplantation, results indicate that while Treg confer enhanced protection from acute GVHD, they do not increase the incidence of opportunistic infection nor disease relapse [84]. In the clinical trial by Di Ianni et al., freshly isolated donor Treg were infused 4 days prior to haploidentical hematopoietic stem cell transplant, and no GVHD prophylaxis was given [112]. Infused Treg did not inhibit immune reconstitution, and of 26 patients, no CMV-related deaths were reported, an improvement over 40% of non-leukemic deaths caused by CMV that had previously been reported by this group. Furthermore, seven patients were vaccinated against influenza 3-14 months post-transplant, and five acquired protective antibody titres. These studies provide a basis to move on to larger trials that will shed further light on whether polyclonal and/or adaptive Treg result in global immunosuppression causing relapse or infection.
3.4.2 Non-pure populations and plasticity of Treg
Further safety concerns of using Treg as a cellular therapy are the lack of pure populations, where contaminating Tconv cells could cause harm. Even if extremely pure populations can be obtained, there is great debate over the stability of Treg. In inflammatory environments, Treg may lose their suppressive phenotype [121-127], but other groups claim that nTreg are a stable lineage, even in the inflammatory conditions of infection and autoimmunity [128]. One factor to consider is that different populations of Treg may be more stable than others. In particular, aTreg tend to be more highly methylated at the TSDR while nTreg are demethylated in this region, suggesting that aTreg have less stable FOXP3 expression and therefore less functional stability than nTreg [129]. Another emerging idea is that the role of Treg may not be limited to suppression of immune responses since a novel role for mouse Treg as helper cells in some environments has been identified [130-134]. Better understanding of factors that cause Treg to lose or gain suppressive capacity will be required to predict how Treg will behave as cellular therapy for transplantation.
3.4.3 Addressing safety concerns
One way to address inevitable safety concerns would be to engineer Treg to express an inducible suicide gene such that these cells can be removed if they become pathogenic [135, 136]. One example of such a strategy would be to generate Treg populations that express a cell fate control gene, such as HSV-thymidine kinase which has been expressed in genetically-engineered conventional T cells delivered in the context of stem cell transplantation so that they can be eliminated by gancyclovir in vivo should they cause GVHD [137]. An example of more advanced cell fate control gene is an enhanced mutant of thymidylate kinase (TK), an enzyme that phosphorylates 3′-azido-3′-deoxythymidine (AZT), converting it into a toxic form. Administration of AZT could efficiently eliminate TK-expressing transferred cells that have become pathogenic or cells that have become malignant as a result of gene integration [135]. Nonetheless, further advances in gene therapy would be required for this approach to move forward and licensing issues are likely to be less than straightforward. However, it should be recognised that the transplant community is well accustomed to the use of agents such as Alemtuzumab, Basiliximab and ATG for induction therapy. If adverse events were detected that resulted from Treg delivery, there could also be the option to use any one of these antibody preparations to disable and/or deplete the injected population. None of these is Treg specific but the fact that their transient use does not appear to lead to long-term immunodeficiency suggests that if some form of rescue strategy is required by the regulatory authorities, these agents should be acceptable.
3.5 Monitoring outcomes
How outcomes are measured will be a critical aspect of clinical Treg cellular therapy studies. As discussed above, initial trials of Treg therapy will see an introduction of Treg into established ‘gold-standard’ immunosuppressive regimens, but by definition, these regimens give good graft outcomes in their own right. Therefore, identifying an additive effect of Treg therapy will be a challenge. Although the most robust confirmation of graft rejection is currently via a biopsy, it does not follow that the same approach can detect reduced alloreactivity. Thus clinical parameters combined with in vitro assays to measure alloantigen-specific effector T cell function, such as the IFN-γ ELISPOT, will be required to identify therapeutic benefit. In addition, simple phenotypic analyses of circulating Tregs may not give clear results because FOXP3 does not exclusively identify Tregs in humans [27-31] and the presence of Treg in the graft rather than the periphery may be a better indicator of outcome [57, 138].
The most promising methods of monitoring alloantigen-specific tolerance are molecular diagnostics including genetics, epigenetics, transcriptomics, proteomics, and metabolomics (reviewed in [139] and in this issue). Biomarkers of operationally tolerant kidney and liver transplant patients have been identified [140, 141]. Furthermore, investigations using the CDR3-length distribution assay suggest that the TCR repertoire might be a good predictor of graft outcome as investigations suggest the majority of kidney transplant patients with chronic rejection have an accumulation of olio or monoclonal Vbeta expansions while operationally tolerant recipients have a TCR repertoire like that of healthy individuals [142]. The best indicators of rejection will probably come from a combination of monitoring techniques. Once the initial series of Phase I/II trials have been completed it will be necessary to conduct large, multi-centre trials powered sufficiently to identify a reduced incidence of rejection. Success in defining good ways to measure tolerance would set the scene for subsequent trials in which accelerated drug minimization was the principal aim.
3. 6 Impact of concurrent immunosuppression
One of many significant unknowns in the design of Treg-based cell therapy is whether their function will be compromised by immunosuppression. Although there are tentative data indicating that CNIs can attenuate Treg function in vivo [26], definitive data are lacking. Whilst it is easily possible to examine the effect of specific agents on Treg function in vitro, extrapolation of results to the in vivo setting is problematic because of the difficulty in identifying true dose comparisons. Furthermore, any attempt to use in vivo models to ask whether immunosuppressive agents block Treg function and result in normal rejection responses are immediately confounded by the fact that the drug therapy will block the rejection responses themselves. Whilst attempts to develop relevant animal models will continue, the most relevant and most direct information regarding the effect of conventional immunosuppression on Treg function will probably be inferred from the initial clinical trials.
4. Concluding remarks
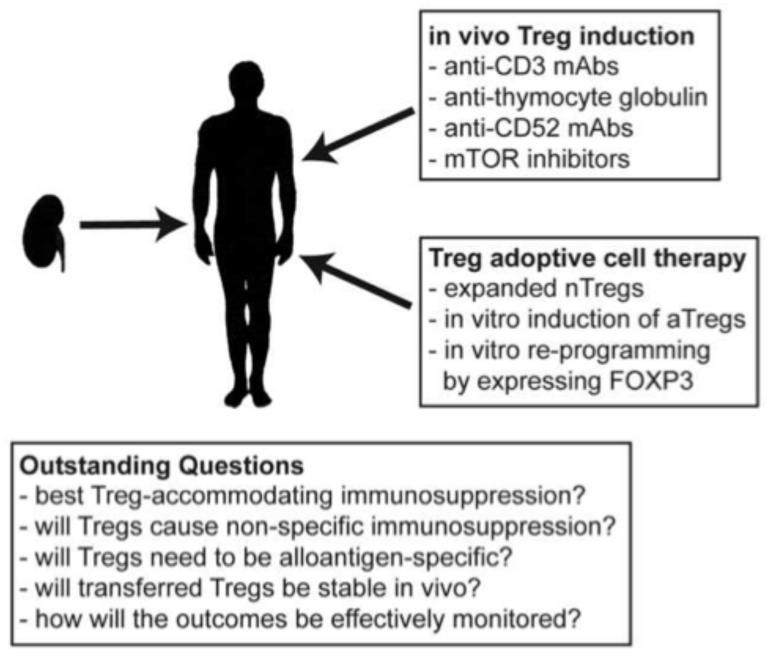
The first trials of Treg cellular therapy in clinical haematopoietic stem cell transplantation have been promising and provide a basis for future trials in solid organ transplantation. The ability to cryo-preserve expanded recipient Treg allows for administration at specified times relative to the transplant and a growing understanding of the various options for Treg therapy should allow consensus protocols to be established. Although there are still many questions to be answered (Figure 1) there is great hope that large scale trials powered to identify clinical benefit will show that Treg-based therapies can accelerate drug minimization or perhaps cessation of immunosuppressive medication.
Figure 1. Approaches and outstanding questions for Treg cell therapy in organ transplantation.
Animal models have illustrated the potential of many different methods to enhance the numbers and/or function of Tregs in the context of solid organ transplantation. Although there are still many outstanding questions that must be answered to optimise this approach, the results from ongoing and planned clinical trials will be critical to illustrate whether this therapy can reduce the dependence on pharmacological immunosuppression.
Acknowledgements
The authors own work is supported by grant from the Canadian Institutes for Health Research (to MKL), the Roche Organ Transplant Foundation (to MKL), and The Wellcome Trust, the Medical Research Council, British Heart Foundation and European Union Framework 7 awards (To AB and KJW). ANM holds a Canada Vanier Scholarship, a MSFHR Junior Graduate Studentship, and a CIHR Transplantation Training Program award. MKL holds a Canada Research Chair in Transplantation.
Abbreviations
- APC
antigen presenting cell
- ATG
anti thymocyte globulin
- aTreg
adaptive T regulatory cell
- AZT
3′-azido-3′-deoxythymidine
- CNI
calcineurin inhibitor
- CyA
cyclosporin A
- DC
dendiric cell
- DST
donor specific transfusion
- GVHD
graft versus host disease
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
- MMF
mycophenolate mofetil
- nTreg
natural T regulatory cell
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidyl inositol 3′ kinase
- Treg
T regulatory cell
- TCR
T cell receptor
- Tconv
T conventional
- TK
thymidiylate kinase
- TSDR
Treg specific demethylation region
References
- [1].Reibke R, Garbi N, Ganss R, Hämmerling GJ, Arnold B, Oelert T. CD8+ regulatory T cells generated by neonatal recognition of peripheral self-antigen. Proc Natl Acad Sci. 2006;103:15142–15147. doi: 10.1073/pnas.0602622103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T Cell (Treg) Subsets Return in Patients with Refractory Lupus following Stem Cell Transplantation, and TGF-β-Producing CD8 + Treg Cells Are Associated with Immunological Remission of Lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li XL, Ménoret S, Bezie S, Caron L, Chabannes D, Hill M, et al. Mechanism and Localization of CD8 Regulatory T Cells in a Heart Transplant Model of Tolerance. J Immunol. 2010;185:823–833. doi: 10.4049/jimmunol.1000120. [DOI] [PubMed] [Google Scholar]
- [4].Zhang Z-X, Yang L, Young KJ, Du Temple B, Zhang L. Identification of a previously unkown antigen specific regulatory T cell and its mechanism of suppression. Nature Medicine. 2000;6:782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- [5].Ford McIntyre MS, Young KJ, Gao J, Joe B, Zhang L. Cutting Edge: In Vivo Trogocytosis as a Mechanism of Double Negative Regulatory T Cell-Mediated Antigen-Specific Suppression. J Immunol. 2008;181:2271–2275. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- [6].Monteiro M, Almeida CF, Caridade M, Ribot JC, Duarte J, Agua-Doce A, et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J Immunol. 2010;185:2157–2163. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]
- [7].Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- [8].Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bennett CL, Christie JD, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- [11].Kobayashi I, Shiari R, Yamada M, Kawamura N, Okano M, Yara A, et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX) J Med Genet. 2001;38:874–876. doi: 10.1136/jmg.38.12.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 2006;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- [15].Hall BM, Jelbart ME, Dorsch SE. Suppressor T cells in rats with prolonged cardiac allograft survival after treatment with cyclosporin. Transplantation. 1984;37:595–600. doi: 10.1097/00007890-198406000-00014. [DOI] [PubMed] [Google Scholar]
- [16].Hall BM, Jelbart ME, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. Mediation of specific suppression by T helper/inducer cells J Exp Med. 1985;162:1683–1694. doi: 10.1084/jem.162.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hall BM, Gurley KE, Pearce NW, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporin. II. Sequential changes in alloreactivity of T cell subsets. Transplantation. 1989;47:1030–1033. doi: 10.1097/00007890-198906000-00022. [DOI] [PubMed] [Google Scholar]
- [18].Hall B, Pearce N, Gurley K, Dorsch S. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III: Further characterization of the CD4+ suppressor cell and its mechanism of action. J Exp Med. 1990;171:141–157. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Quigley RL, Wood KJ, Morris PJ. Transfusion induces blood donor-specific suppressor cells. J Immunol. 1989;142:463–470. [PubMed] [Google Scholar]
- [20].Quigley RL, Wood KJ, Morris PJ. Mediation of antigen induced suppression of renal allograft rejection by a CD4 (W3/25+) T cell. Transplantation. 1989;47:684–688. doi: 10.1097/00007890-198904000-00022. [DOI] [PubMed] [Google Scholar]
- [21].Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell A, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- [22].Saitovitch D, Bushell A, Mabbs DW, Morris PJ, Wood KJ. Kinetics of induction of transplantation tolerance with a nondepleting anti-CD4 monoclonal antibody and donor specific transfusion before transplantation: A critical period of time is required for development of immunological unresponsiveness. Transplantation. 1996;61:1642–1647. doi: 10.1097/00007890-199606150-00016. [DOI] [PubMed] [Google Scholar]
- [23].Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+ CD4+ regulatory T cells prevent graft rejection: CTLA-4 and IL-10 dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- [24].Bushell A, Wood KJ. GITR Ligation Blocks Allograft Protection by Induced CD25+CD4+ Regulatory T Cells without Enhancing Effector T-Cell Function. Am J Trans. 2007;7:759–768. doi: 10.1111/j.1600-6143.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- [25].Qin S, Cobbold SP, Pope H, Elliot J, Kiossis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- [26].Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- [27].Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- [28].Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- [30].Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- [32].Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, et al. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535–539. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- [33].Nooij FJ, Jonker M. The effect of skin allograft survival of a monoclonal antibody specific for a polymorphic CD3-like cell surface molecule in rhesus monkeys. Eur J Immunol. 1987;17:1089–1093. doi: 10.1002/eji.1830170803. [DOI] [PubMed] [Google Scholar]
- [34].Nicolls MR, Aversa GG, Pearce NW, Spinelli A, Berger MF, Gurley KE, et al. Induction of long-term specific tolerance to allografts in rats by therapy with an anti-CD3-like monoclonal antibody. Transplantation. 1993;55:459–468. doi: 10.1097/00007890-199303000-00001. [DOI] [PubMed] [Google Scholar]
- [35].Knechtle SJ, et al. FN18-CRM9 immunotoxin promotes tolerance in primate renal allografts. Transplantation. 1997;63:1–6. doi: 10.1097/00007890-199701150-00002. [DOI] [PubMed] [Google Scholar]
- [36].Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chatenoud L. CD3-specific antibody induced active tolerance: From bench to bedside. Nature Reviews Immunology. 2003;3:123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- [38].Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the tratment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- [39].Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- [40].Minamimura K, Gao W, Maki T. CD4+ Regulatory T Cells Are Spared from Deletion by Antilymphocyte Serum, a Polyclonal anti-T cell antibody. J Immunol. 2006;176:4125–4132. doi: 10.4049/jimmunol.176.7.4125. [DOI] [PubMed] [Google Scholar]
- [41].Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10:2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sewgobind VD, Kho MM, van der Laan LJ, Hendrikx TK, van Dam T, Tilanus HW, et al. The effect of rabbit anti-thymocyte globulin induction therapy on regulatory T cells in kidney transplant patients. Nephrol Dial Transplant. 2009;24:1635–1644. doi: 10.1093/ndt/gfn778. [DOI] [PubMed] [Google Scholar]
- [43].Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4+ T cells in vitro is associated with T-cell activation and not the induction of FOXP3+ T regulatory cells. Blood. 2009;114:5003–5006. doi: 10.1182/blood-2009-04-214437. [DOI] [PubMed] [Google Scholar]
- [44].Waldmann H. A Personal History of the CAMPATH-1H Antibody. Med Oncology. 2002;19:S3–S9. doi: 10.1385/mo:19:2s:s03. [DOI] [PubMed] [Google Scholar]
- [45].Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, et al. CD4+CD25+FOXP3+ Regulatory T Cells Increase De Novo in Kidney Transplant Patients After Immunodepletion with Campath-1H. Am J Transplant. 2008;8:793–802. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- [46].Battaglia M, Stabilini A, Roncarolo M-G. Rapamycin selectively expands CD4+CD25+Foxp3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- [47].Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- [48].Adkins D, Ratanatharathorn V, Yang H, White B. Safety profile and clinical outcomes in a phase I, placebo-controlled study of siplizumab in acute graft-versus-host disease. Transplantation. 2009;88:198–202. doi: 10.1097/TP.0b013e3181abfbf7. [DOI] [PubMed] [Google Scholar]
- [49].Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR, et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp Hematol. 2007;35:1140–1152. doi: 10.1016/j.exphem.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of Donor-Specific Tolerance in Recipients of Haploidentical Combined Bone Marrow/Kidney Transplantation. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03566.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- [52].Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, et al. Rapamycin-Mediated Enrichment of T Cells with Regulatory Activity in Stimulated CD4+ T Cell Cultures Is Not Due to the Selective Expansion of Naturally Occurring Regulatory T Cells but to the Induction of Regulatory Functions in Conventional CD4+ T Cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- [53].Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- [54].Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective Survival of Naturally Occurring Human CD4+CD25+Foxp3+ Regulatory T Cells Cultured with Rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- [55].San Segundo D, Carlos Ruiz J, Izquierdo M, Ferna’ndez-Fresnedo G, Go’mez-Alamillo G, Merino R, et al. Calcineurin Inhibitors, but not Rapamycin, Reduce Percentages of CD41 CD251 FOXP31 Regulatory T Cells in Renal Transplant Recipients. Transplantation. 2006;82:550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- [56].Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T Cells and T Cell Depletion: Role of Immunosuppressive Drugs. J Am Soc Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- [57].Bestard O, Cruzado JM, Mestre M, Calde’s A, Bas J, Carrera M, et al. Achieving Donor-Specific Hyporesponsiveness Is Associated with FOXP3+ Regulatory T Cell Recruitment in Human Renal Allograft Infiltrates. J Immunol. 2007;179:4901–4909. doi: 10.4049/jimmunol.179.7.4901. [DOI] [PubMed] [Google Scholar]
- [58].Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, et al. Rapamycin Monotherapy in Patients With Type 1 Diabetes Modifies CD4+CD25+FOXP3+ Regulatory T-Cells. Diabetes. 2008;57:2341–2347. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gao W, Lua Y, B. EE, Oukka M, Kuchroo VJ, Strom TB. Contrasting Effects of Cyclosporine and Rapamycin in De Novo Generation of Alloantigen-Specific Regulatory T Cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- [62].Liu W, Putnam AL, Xu-yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- [65].Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- [66].Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- [68].Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- [72].Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- [73].Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009;39:3315–3322. doi: 10.1002/eji.200939684. [DOI] [PubMed] [Google Scholar]
- [75].Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- [78].Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- [80].Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- [82].Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- [83].Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol. 2008;180:5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2010 doi: 10.1182/blood-2010-07-293795. pre-published online Oct 15th 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Macchiarini F, Manz MG, Palucka AK, Shultzabcd LD. Humanized mice are we there yet? J Exp Med. 2005;202:1307–1311. doi: 10.1084/jem.20051547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- [87].Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Schiopu A, et al. In vivo prevention of transplant arteriosclerosis by ex vivo expanded human regulatory T cells. Nat Med. 2010;16:809–814. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood KJ. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90:1321–1327. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Powrie F, Correa-Olivera R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- [91].Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- [92].Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- [93].Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex-vivo of TGF-b-producing regulatory T cells from CD4+ CD25− precursors. J Immunol Methods. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- [94].Chen W, Jin W, Hardegan N, Lei K, Li L, Marinos N, et al. Conversion of peripheral CD4+ CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-b induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Feng G, Gao W, Strom TB, Oukka M, Francis R, Wood KJ, et al. Exogenous IFN-g ex-vivo shapes the alloreactive T cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol. 2008;38:2512–2527. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Feng G, Wood KJ, Bushell A. Interferon-g Conditioning Ex Vivo Generates CD25+CD62L+Foxp3+ Regulatory T Cells That Prevent Allograft Rejection: Potential Avenues for Cellular Therapy. Transplantation. 2008;86:578–589. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- [97].Warnecke G, Feng G, Goto R, Nadig SN, Francis R, Wood KJ, et al. CD4+ Regulatory T Cells Generated in Vitro with IFN-g and Allogeneic APC Inhibit Transplant Arteriosclerosis. Am J Path. 2010;177:464–472. doi: 10.2353/ajpath.2010.090292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zheng SG, Meng L, Wang JH, Watanabe M, Barr ML, Cramer DV, et al. Transfer of regulatory cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int Immunol. 2006;18:279–289. doi: 10.1093/intimm/dxh368. [DOI] [PubMed] [Google Scholar]
- [99].Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- [100].Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. [PubMed] [Google Scholar]
- [101].Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- [102].Bacchetta R, Gregori S, Serafini G, Sartirana C, Schulz U, Zino E, et al. Molecular and functional characterization of allogantigen-specific anergic T cells suitable for cell therapy. Haematologica. 2010;95:2134–2143. doi: 10.3324/haematol.2010.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko S-Y, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genetics. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- [104].Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- [105].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcripition factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- [106].Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- [107].Chai JG, Xue SA, Coe D, Addey C, Bartok I, Scott D, et al. Regulatory T cells, derived from naive CD4+CD25− T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation. 2005;79:1310–1316. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- [108].Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- [109].Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol. 2008;38:3282–3289. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- [110].Tsang J, Tanriver Y, Jiang S, Xue S, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2009;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Trzonkowsi P, Bieniasewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD2+CD127− Tregulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [112].Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GvHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011 doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- [113].Ekberg H, Bernasconi C, Tedesco-Silva H, V’ıtko S, Hugo C, Demirbas A, et al. Calcineurin Inhibitor Minimization in the Symphony Study: Observational Results 3 Years after Transplantation. Am J Transplant. 2009;9:1876–1885. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- [114].Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- [117].Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9:243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- [119].Nguyen VH, Shashidhar S, Chang DS, Ho L, Kambham N, Bachmann M, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–953. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bushell A, Jones E, Gallimore A, Wood K. The generation of CD25+ CD4+ regulatory T cells that prevent allograft rejection does not compromise immunity to a viral pathogen. J Immunol. 2005;174:3290–3297. doi: 10.4049/jimmunol.174.6.3290. [DOI] [PubMed] [Google Scholar]
- [121].Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li L, Kim J, Boussiotis VA. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol. 2010;185:4148–4153. doi: 10.4049/jimmunol.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- [130].Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- [132].Vendetti S, Davidson TS, Veglia F, Riccomi A, Negri DR, Lindstedt R, et al. Polyclonal Treg cells enhance the activity of a mucosal adjuvant. Immunol Cell Biol. 2010;88:698–706. doi: 10.1038/icb.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vokaer B, Van Rompaey N, Lemaitre PH, Lhomme F, Kubjak C, Benghiat FS, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 2010;185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- [134].Wang Y, Souabni A, Flavell RA, Wan YY. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J Immunol. 2010;185:5983–5992. doi: 10.4049/jimmunol.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sato T, Neschadim A, Konrad M, Fowler DH, Lavie A, Medin JA. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol Ther. 2007;15:962–970. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- [136].Guillot-Delost M, Cherai M, Hamel Y, Rosenzwajg M, Baillou C, Simonin G, et al. Clinical-grade preparation of human natural regulatory T-cells encoding the thymidine kinase suicide gene as a safety gene. J Gene Med. 2008;10:834–846. doi: 10.1002/jgm.1220. [DOI] [PubMed] [Google Scholar]
- [137].Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- [138].Demirkiran A, Baan CC, Kok A, Metselaar HJ, Tilanus HW, van der Laan LJ. Intrahepatic detection of FOXP3 gene expression after liver transplantation using minimally invasive aspiration biopsy. Transplantation. 2007;83:819–823. doi: 10.1097/01.tp.0000258597.97468.88. [DOI] [PubMed] [Google Scholar]
- [139].Naesens M, Sarwal MM. Molecular diagnostics in transplantation. Nat Rev Nephrol. 2010;6:614–628. doi: 10.1038/nrneph.2010.113. [DOI] [PubMed] [Google Scholar]
- [140].Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A. 2007;104:15448–15453. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Martinez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–2857. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Miqueu P, Degauque N, Guillet M, Giral M, Ruiz C, Pallier A, et al. Analysis of the peripheral T-cell repertoire in kidney transplant patients. Eur J Immunol. 2010;40:3280–3290. doi: 10.1002/eji.201040301. [DOI] [PubMed] [Google Scholar]