Abstract
Background
In humans orbital volume increases linearly with absolute latitude. Scaling across mammals between visual system components suggests that these larger orbits should translate into larger eyes and visual cortices in high latitude humans. Larger eyes at high latitudes may be required to maintain adequate visual acuity and enhance visual sensitivity under lower light levels.
Aim
To test the assumption that orbital volume can accurately index eyeball and visual cortex volumes specifically in humans.
Subjects & Methods
Structural Magnetic Resonance Imaging (MRI) techniques are employed to measure eye and orbit (N=88), and brain and visual cortex (N=99) volumes in living humans. Facial dimensions and foramen magnum area (a proxy for body mass) were also measured.
Results
A significant positive linear relationship was found between (i) orbital and eyeball volumes, (ii) eyeball and visual cortex grey matter volumes, (iii) different visual cortical areas, independently of overall brain volume.
Conclusion
In humans the components of the visual system scale from orbit to eye to visual cortex volume independently of overall brain size. These findings indicate that orbit volume can index eye and visual cortex volume in humans, suggesting that larger high latitude orbits do translate into larger visual cortices.
Keywords: Eye socket, Magnetic Resonance Imaging, Brain, Latitude
INTRODUCTION
Human orbital volume increases with absolute latitude, suggesting that larger visual systems are required to maintain adequate visual function (acuity and sensitivity) under the lower light levels found at high latitudes (Pearce and Dunbar, 2012). There is positive scaling between orbit and eyeball size across primates (Kay and Kirk, 2000; Kirk, 2006; Schultz, 1940), as well as between eyeball and visual cortex size across mammals independently of phylogeny and the size of other brain regions (Barton, 2007). This led Pearce & Dunbar (2012) to argue, firstly, that human orbital volume was an accurate index of eyeball volume and secondly, that the progressive enlargement of visual systems towards the poles would include not only larger eyes but also larger visual cortices at higher latitudes.
However, whether orbit size is an accurate proxy for eye and visual cortex size in humans remains an untested assumption. The orbit is comprised of seven bones and the size and morphology of the orbit can be affected by the developmental trajectories of any or all these components and thus by general cranial and facial form (Bron et al., 1997; Whitnall, 1921). This means that orbital dimensions are to some degree related to overall cranial size, for instance orbital depth is linked to skull length (Bron et al., 1997; Chau et al., 2004; Nitek et al., 2009; Waitzman et al., 1992; Whitnall, 1921).
Since the eye fills only one fifth to one third of the adult human orbit (Bron et al., 1997; Schultz, 1940; Whitnall, 1921), it could be supposed that contrary to initial intuition, the size of the obit might not accurately reflect the size of the eye in adult humans. Nonetheless, the eyeball, lacrimal apparatus and oblique muscles are considered to be the main internal factors influencing the form of the orbital cavity (Bron et al., 1997; Whitnall, 1921). For instance, if the eyeball is removed before the orbit is fully formed, the growth of the orbit is checked in both humans and rabbits (Cummings et al., 2012; Hintschich et al., 2001; Sarnat, 1982). This suggests that the adult size of the eye and orbit should be related because orbit size is partly determined by eye growth, at least during early development (Washburn and Detwiler, 1943). Indeed, significant positive relationships were found between eye and orbit dimensions during human development (Tomasik et al., 2005a; Tomasik et al., 2005b). However, recent study of strepsirrhine primates suggests a generic mammalian pattern of early orbit expansion following eye growth in utero succeeded by slowed eye growth but continued orbital growth postnatally (Cummings et al., 2012). This means that in the neonate eye and orbit size may be closely correlated, but due to a higher rate of postnatal orbital growth, this scaling relationship becomes progressively weaker towards adulthood (Schultz, 1940; Washburn and Detwiler, 1943). Indeed, the only previous study to investigate whether the size of the eye, particularly large myopic eyes, leads to correlated orbital volume changes (i.e. whether eye size influences orbit size) failed to find a significant relationship between eye and orbit volume in humans (Chau et al., 2004). However, Chau et al. used a small sample from a single population, which may not be representative.
Across primates adult orbital volume explains ~83% of eyeball volumetric variation (Kay and Kirk, 2000; Schultz, 1940). Several additional factors influencing orbital size have been proposed, including the degree of orbital convergence and cornea size (Kirk, 2006). Orbital convergence also affects downstream visual system component size: both the lateral geniculate nucleus (LGN) and primary visual area (V1) volumes are positively associated with the degree of orbital convergence in primates, although not in other mammals (Heesy et al., 2011). However, it would be worth testing whether the relationship between orbital convergence and LGN/V1 volume is actually mediated by orbital volume as it relates to eyeball size.
Another possible factor is facial morphology, but both phylogenetic and ontogenetic approaches failed to find a significant relationship between brow-ridge size and orbit volume independently of the degree of prognathism (how far the face is tucked under the frontal bone) in primates (Ravosa, 1991a, b). Nonetheless, the direct relationship between orbit size and prognathism across primates requires further study. In contrast, associated growth between orbital and facial dimensions has been reported in human foetuses (Denis et al., 1998). Furthermore, diachronic reductions in facial and cranial size as well as changes in facial shape (reduced prognathism) has been correlated with reduced orbital volume and breadth but increased orbital height in prehistoric East Asians and Western Europeans (Brown and Maeda, 2004; Masters, 2012). This suggests that changes in face shape can influence orbital morphology. In addition, across primates orbit and eye size is associated with body size (although eye size shows a shallower slope) (Kay and Kirk, 2000; Martin, 1990; Schultz, 1940) and the same may be true within humans. However, although orbital morphology is related to facial, neurocranial and basicranial morphology and body size, we propose that orbital size is at least partially associated with eye size.
In comparison with eye-orbit scaling, there is strong evidence for scaling between the optic tract and visual cortex in the healthy human brain. Andrews and colleagues (1997) took physical soft tissue measurements from post-mortem brains and found significant positive linear relationships between the cross-sectional area of the optic tract, LGN volume (both in total and when divided into parvocellular or magnocellular volumes) and V1 volume (identified by the presence of the stria of Gennari). Principal components analysis showed that most of the variance in the size of the visual system apparatus was explained by a single factor, indicating that the size of the optic tract, LGN and V1 co-varied significantly within individuals. In contrast, this factor explained only a very small proportion of variance in total brain mass, suggesting that visual scaling is not driven by brain size. In addition, visual acuity is positively related to V1 volume, suggesting scaling between retinal cone density/numbers and V1 volume in healthy individuals (Duncan and Boynton, 2003).
Further evidence for scaling between peripheral components of the visual system and the visual cortex comes from pathology. For instance, individuals suffering from albinism often have malformed or absent foveae. In V1 stimuli in the central part of the visual field, which are likely to activate the cones of the fovea, are represented in the occipital pole. Compared to healthy controls, subjects with albinism show reduced grey matter volume in the occipital pole, as well as smaller optic chiasms (Bridge et al., 2012; von dem Hagen et al., 2005). Together these results suggest that reduction in the number of foveal photoreceptors directly impacts upon the size of the optic tract and the volume of associated regions of the visual cortex. Moreover, congenital anophthalmic subjects (whose eyes are underdeveloped or absent) show a reduction in V1 grey matter volume but no difference in overall brain volume compared to healthy controls (Bridge et al., 2009). This again suggests scaling between visual system components independent of other brain areas, albeit in a population without “vision”.
This paper aims to test a number of hypotheses relating to scaling within the visual system in a comparatively large global sample of living humans using structural MRI images: (i) orbital volume is associated with eye volume in humans, (ii) eyeball volume is associated with visual cortex volume in humans and (iii) V1 grey matter volume is associated with V2 grey matter volume, and V2 grey matter volume is in turn related to V5 (Middle Temporal) grey matter volume in humans. However, the current sample did not allow us to distinguish between ontogenetic and phylogenetic (ancestral history) effects on visual system size variation, because our subjects may not have been scanned where they grew up. This and other avenues for further research are explored in the Discussion.
METHODS
Magnetic Resonance Image (MRI) scan sample
Data consisted of MRI scans of 100 normal, healthy subjects from the 1000 Functional Connectomes Project (NITRC, Accessed 2011), which provides an online collection of MRI scans from a number of research groups across the world, mainly from USA and Europe. Ten imaging centres were selected to cover as great a range of latitudes as possible (Atlanta (USA), Baltimore (USA), Berlin (Germany), Newark (USA), Orangeburg (New York), Oulu (Finland), Oxford (England), Palo Alto (USA), Queensland (Australia), Taipei (Taiwan)). Ten subjects were randomly selected from each of these samples. Where possible 5 females and 5 males were selected, but for the Palo Alto sample the only 2 male subjects were selected alongside 8 randomly selected females. Demographic information was unavailable for the Taipei sample; so 10 subjects of unknown sex were selected. Demographic information was also unavailable for 3 of the Orangeburg sample.
The Orangeburg sample was not suitable for Osirix measurement (see below) because the skull had been stripped. The OsiriX measurements were thus made on 90 scans, whereas freesurfer was run on the full sample of 100 subjects. However, one subject was removed from the final analyses because this individual appeared as a very low outlier on all brain-associated plots leaving 99 subjects: 43 females (age M=26.70, SD=6.71), 43 males (age M=29.21, SD=7.58) and 13 subjects of unknown sex (unknown age). In the analyses sex was coded as two dummy variables (male and female) so that all available data could be included in the models.
MRI scan methods
The latitude of the 10 imaging centres was obtained using (Gorissen, Accessed July 2008, July 2010).
Freesurfer
The freely-available freesurfer software (Desikan et al., 2006) allows the automatic reconstruction and segmentation of the subcortical and cortical regions of the brain from T1-weighted images. This process involves using identification of white/grey matter and grey/cerebral-spinal fluid (CSF) boundaries and sulcal and gyral patterns in conjunction with generalised “masks” or averaged “template brains” (Desikan et al., 2006; Hinds et al., 2008). The software provides surface area, grey matter thickness and volume for specific areas of the cortex, as well as hemisphere-specific grey and white matter volumes for the cortex and subcortex and the volumes of various subcortical structures such as the amygdala and the hippocampus. These values were extracted for all subjects and converted to an SPSS database.
The same cortical properties can be extracted from visual (V1, V2 and MT), somatosensory (BA1, BA2, BA3a and BA3b) and motor (BA4a and BA4p) Brodmann areas (Fischl et al., 2008; Hinds et al., 2009; Hinds et al., 2008). Of all the visual areas, V1, V2 and V5/MT are the best documented and most accurately identified across primates and within humans (Lyon and Connolly, 2011; Rosa and Tweedale, 2005). Furthermore, identification of the V1/V2 boundary has been shown to closely match when identified anatomically using the stria of Gennari and functionally using visual stimuli (Bridge et al., 2005). In addition, the automated definition of V1 using gyral and sulcal patterns used by freesurfer is in close correspondence with using the stria of Gennari (Hinds et al., 2008).
The ‘gold standard’ for identifying visual areas in human subjects is the use of retinotopic mapping, which relies on each visual area only having a single representation of space to functionally locate more than 10 visual areas (reviewed in Wandell et al., 2007). However, it is not feasible to undertake this type of data acquisition and analysis in large samples. The freesurfer program provides probabilistic maps of areas V1, V2 and V5/MT that are generated from post mortem studies of human brains in which these areas have been identified using cyto- and myeloarchitecture (Eickhoff et al., 2005). The area definitions from the individual subjects are registered using surface-based registration which takes into account the sulcal anatomy and provides an accurate method of combining across subjects (Van Essen, 2004). Furthermore, we have compared these probabilistic anatomical definitions to our own probabilistic maps derived from retinotopic maps in 16 subjects described in Bridge (2011) and found almost total overlap in the area locations. The most accurate definition is V1 using the calcarine sulcus, while V2, being the adjacent area, likewise has considerable consistency across subjects (Fischl et al., 2008; Hinds et al., 2009; Hinds et al., 2008). Previous work also suggests that V5/MT can also be defined anatomically (Dumoulin et al., 2000). While not as good as defining each area individually, this population approach with freesurfer is the best option for large datasets. Moreover, the results presented here in terms of scaling with eye/orbit or absolute latitude are the same whether V1 (the most consistently delineated area: Fischl et al., 2008; Hinds et al., 2009; Hinds et al., 2008) is considered on its own or whether V2 and MT are also included.
Here the sum of V1, V2 and V5/MT are referred to as “total” visual cortex for convenience. Similarly, motor and somatosensory areas were also created from summing the relevant Brodmann areas.
As a control for overall brain size “rest-of-cortex” grey matter volume was calculated by subtracting total visual (V1+V2+MT) grey matter volume from total cortical grey matter volume. Other control volumes were (i) total brain volume, (ii) brain volume minus total visual grey matter volume, (iii) subcortical grey matter volume and (iv) cerebellum volume plus brainstem volume (roughly equivalent to the hindbrain, which is the cerebellum, medulla and pons, used as a control in mammalian analyses: Barton 2007).
OsiriX
As well as differing in imaging resolution, scan acquisition protocol and scanner type, the quality of the scans varied considerably between the source imaging centres. Scans were therefore graded for quality with regard to the ocular (eye/orbit) region: (1) perfect, clear image, (2) complete eye boundary but some blur or slight distortion, (3) incomplete eye boundary, image blurred or distorted, (4) substantial distortion or reconstruction needed in order to delineate the eye in each slice and (5) unmeasurable (for one subject the anterior section of the eye/orbit was cut off in the image). The eye and orbit analyses using the better quality sample (scans graded 1 or 2) yielded similar results to the whole sample but radically improved the fit. The regression parameters lie within the 95% confidence intervals of the whole sample models for the eye/orbit-visual cortex models but not for the eye-orbit models. In order to maximise sample size, we report only the findings of analyses using the whole sample here.
Ninety scans were measured using the freely available Osirix software (Rosset, Accessed 2011), since the skull-stripped Orangeberg subjects were excluded. One additional subject was excluded from this sample because the image was missing the most anterior part of the eye and orbit, so volumes could not be measured (see above). The eye/orbit sample therefore comprised 88 subjects from 9 imaging centres: 41 females (age M=26.29, SD=5.70), 437 males (age M=27.43, SD=5.58) and 10 subjects of unknown sex (unknown age).
As outlined in the Introduction, orbital size is partially influenced by face and body size. We did not have body weight or stature data for these subjects and we therefore followed Pearce & Dunbar (2012) in using foramen magnum (FM) size as a proxy for body mass, as suggested by Radinsky (1967). We measured maximum facial width (sample mean volume M=11.558cm, SD=0.802cm, N=78) and maximum nasal height (sample mean volume M=5.340cm, SD=0.720cm, N=75) in coronal view since these were the easiest face-dimension measures to standardise. We also measured foramen magnum area (sample mean volume M=6.272cm2, SD=1.397cm2, N=88) in axial view (in the first slice in which the cerebellum was no longer visible). We included these body and face size controls as covariates in multiple ordinary least squares (OLS) regressions testing for a relationship between eye and orbit volumes.
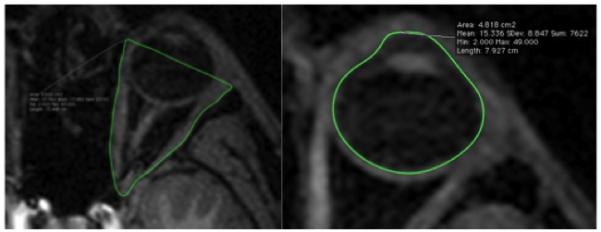
Individual scans were imported into Osirix for measurement of length, area and volume of the right eye/orbit. Regions of interest (Figures 1 & 2) were manually delineated, and lengths, areas and volumes were automatically generated. Maximum axial eye was measured in the axial view to identify possible myopes, since axial ocular length and degree of myopia are strongly linked (Gilmartin, 2004). For each subject linear and areal measurements were taken three times, and volume measurements twice. Means, standard errors (SE) of the means and standard deviations (SD) were calculated as measures of intraobserver error.
Figure 1.
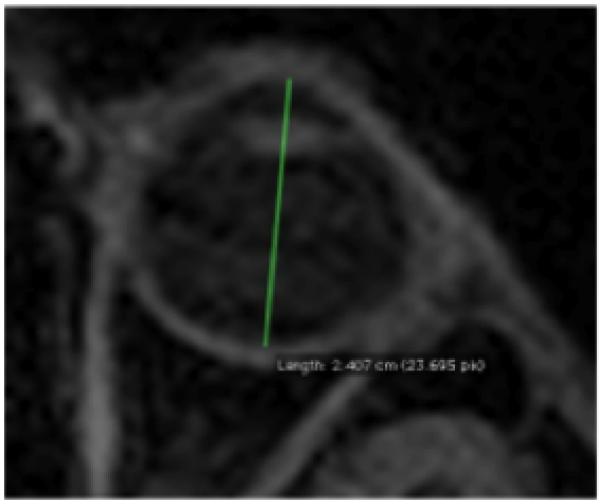
Axial eyeball length taken: maximum length between centre of the cornea and the retina, bifurcating the eye. Due to confidentiality issues with the 1000 Functional Connectomes scans, this scan is not from the current study but serves to illustrate the measurements taken.
Figure 2.
Left – The orbit delineated in a single slice, marked by the nasal cavity on the left and the zygomatic bone on the right. Right – The eyeball delineated in a single slice, with the boundary identified as the edge of the anterior and posterior chambers. The lens is visible in this slice. Due to confidentiality issues with the 1000 Functional Connectomes scans, this scan is not from the current study.
For the linear and area measurements the maximum SEs were <0.1mm/mm2 (SDs <1mm/mm2). For eye volume (sample mean volume M=6.489cm3, SD=0.794cm3, N=88) the maximum SE for the two measurements was 0.64cm3 (SD=0.91cm3) and for orbital volume (sample mean volume M=26.669cm3, SD=2.659cm3, N=88) the SE=3.85cm3 (SD=5.45cm3). The maximum SE for the orbital volume measures was reduced to 1.01cm3 (SD=1.43cm3) when only the higher quality images were considered. Paired-sample t-tests indicated significant correlations between the different measures of orbit and eye volume (r=0.866 and 0.856, respectively, N=88) but whilst no significant difference was found between the means of the two measurement runs for orbital volume, a significant difference was found for eye volume (t87=−2.800, p=0.006), although the mean difference was only −0.132cm3 (SD=0.442cm3).
Analyses
Regression residuals were not significantly different from normal (Kolmogorov-Smirnov one-sample tests) and unless otherwise stated did not show heteroscedasticity. Furthermore, tolerance and VIF statistics did not show excessive collinearity between independent variables in multiple regressions.
RESULTS
Data structure
The data collected are hierarchical, because they were sampled from different imaging centres and therefore subjects sampled from the same centre may not yield independent data-points. The accuracy of results obtained from OLS regression analyses was therefore checked against the output of mixed/multilevel model analyses. However, for all analyses these hierarchical linear models revealed that fitting separate models for each imaging centre (i.e. allowing the slopes and intercepts to vary between groups) did not explain the data significantly better than a single regression model fitted to the whole sample (i.e. ignoring groups). Since the multilevel models did not yield different results, only the OLS analyses are reported here.
Hypothesis 1: Orbital volume is associated with eyeball volume in humans
OLS regression revealed that mean orbital volume was significantly positively associated with mean eyeball volume: t86=4.119, p=8.711×10−5, R2=0.165. This relationship remained significant when an outlier (Figure 3) was excluded from the analysis.
Figure 3.
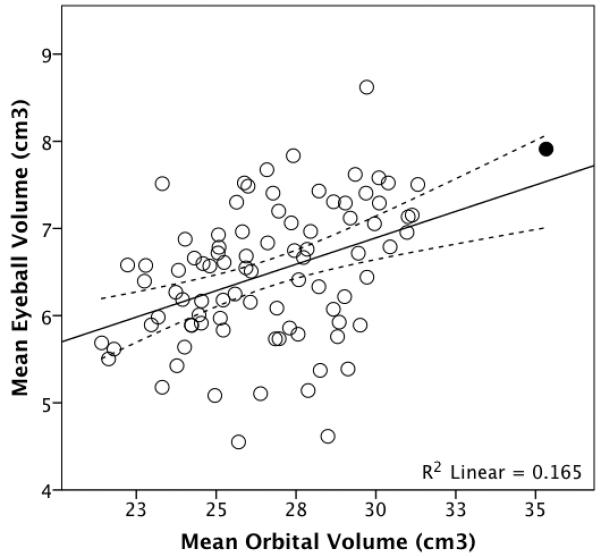
Mean eyeball volume plotted against mean orbital volume for 88 living humans. The dotted lines represent 95% confidence intervals for the regression line. Removing the high outlier (shaded) makes no difference to the analyses.
Orbital volume remained significantly associated with eyeball volume (t85=4.426, p= 2.829×10−5) independently of brain-minus-visual-cortex-grey-matter volume, which itself failed to show a significant partial relationship with eye volume (p=0.125): overall adjusted R2=0.169. Controlling for rest-of-grey-matter (i.e. non-visual) cortical volume, cerebellum plus brainstem or subcortical grey matter volume yielded similar patterns. For subjects for whom the data were available, controlling for FM area and face height and width (proxies for body and face size respectively) made no difference to the results. Similarly, including sex in the model made no difference to the results.
Neither eye nor orbit volume is significantly related to FM area or face height and only orbital volume, but not eye volume, shows significant relationships with face width (t86=2.938, p=0.004, R2=0.103) and brain volume (t86=4.040, p=1.159×10−4, R2=0.159; when two outliers are excluded R2= 0.205).
Hypothesis 2: Eyeball volume is associated with visual cortex grey matter volume in humans
Eyeball - V1
OLS regression revealed that mean eyeball volume was significantly positively associated with total V1 grey matter volume: t86=3.523, p=0.001, R2=0.126. Removing the outlier (Figure 4) did not alter the results. The partial relationship between eyeball volume and V1 grey matter volume remained independently of overall brain size measures (Table I). In the whole sample eyeball volume is related to V1 grey matter volume when rest-of-grey-matter, motor or somatosensory cortex volumes are separately controlled for, whether or not the outlier included. These significant partial relationships between eyeball and V1 grey matter volumes remain when the models also include sex whether or not the outlier is excluded (except for the motor/somatosensory full models when the outlier is removed).
Figure 4.
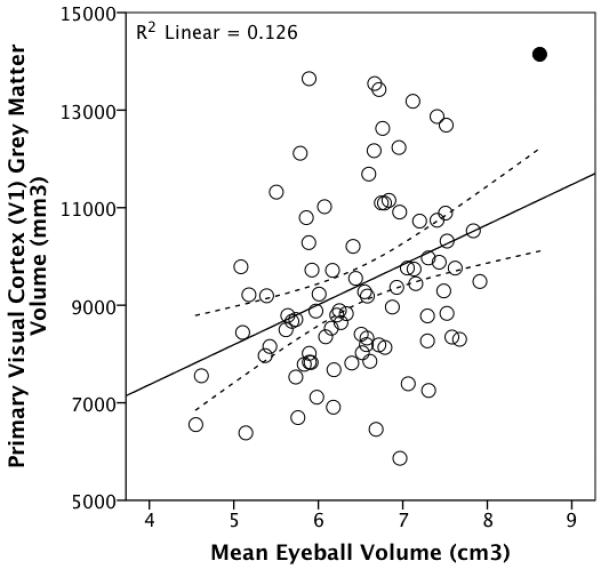
Total V1 grey matter volume plotted against mean eyeball volume for a sample of 88 living humans. The outlier is shaded (see main text). The dotted lines represent 95% confidence intervals for the regression line.
Table I. OLS regression statistics for partial relationship between eyeball volume and V1 grey matter volume, controlling for various measures of overall brain size.
| Eyeball volume predicting V1 volume, separately controlling for: |
OLS Regression Statistics | |||
|---|---|---|---|---|
| t | df | p | Overall adjusted r2 |
|
| Total brain volume | 3.598 | 85 | 0.001 | 0.214 |
| Brain volume minus total visual grey matter volume |
3.607 | 85 | 0.001 | 0.203 |
| Subcortical grey matter volume | 3.680 | 85 | 4.082×10−4 | 0.198 |
| Cerebellum + Brainstem | 3.670 | 85 | 4.230×10−4 | 0.183 |
| Non-visual cortical grey matter volume |
3.316 | 85 | 0.001 | 0.248 |
| Motor cortex grey matter volume | 2.796 | 85 | 0.006 | 0.215 |
| Somatosensory cortex grey matter volume |
2.732 | 85 | 0.008 | 0.252 |
Eyeball – “total” (V1+V2+MT) visual cortex grey matter volume
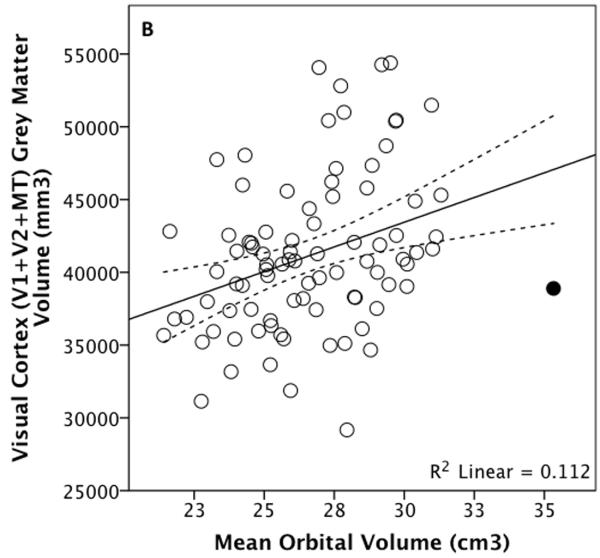
OLS regression analyses revealed a significant positive relationship between eyeball volume and total visual cortex grey matter volume: t86=3.100, p=0.003, R2=0.101, Figure 5. This relationship remains independently of all measures of overall brain size, including non-visual/rest-of grey matter cortical volume as well as independently of motor or somatosensory cortex volumes (Table II). Including sex in the models made no difference.
Figure 5.
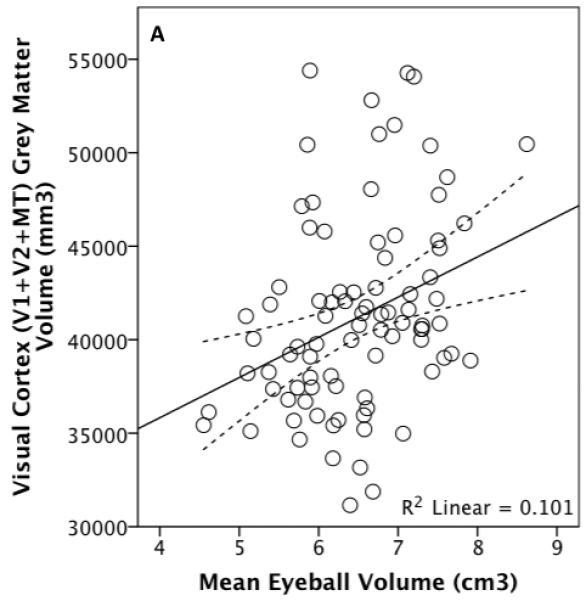
Total visual cortex grey matter volume plotted against eyeball (A) and orbital (B) volume for 88 living humans, with an outlier shaded. The dotted lines represent 95% confidence intervals for the regression line.
Table II. OLS regression statistics for partial relationship between eyeball volume and “total” visual cortex grey matter volume, controlling for various measures of overall brain size.
| Eyeball volume predicting visual cortex grey matter volume, controlling for: |
OLS Regression Statistics for Partial Relationship between Eyeball Volume and “Total” Visual Cortex Grey Matter Volume |
|||
|---|---|---|---|---|
| t | df | p | Overall adjusted r2 |
|
| Total brain volume | 3.353 | 85 | 0.001 | 0.288 |
| Brain volume minus total visual grey matter volume |
3.360 | 85 | 0.001 | 0.269 |
| Subcortical grey matter volume | 3.394 | 85 | 0.001 | 0.225 |
| Cerebellum + Brainstem | 3.338 | 85 | 0.001 | 0.183 |
| Non-visual/rest-of cortical grey matter volume |
2.969 | 85 | 0.004 | 0.370 |
| Motor cortex grey matter volume | 2.127 | 85 | 0.036 | 0.294 |
| Somatosensory cortex grey matter volume | 2.073 | 85 | 0.041 | 0.359 |
Similarly, orbital and total visual cortex volumes are associated (Figure 5) independently of subcortical grey matter (t85=2.531, p=0.013, overall adjusted R2=0.182), motor cortex (t85=2.551, p=0.027, overall adjusted R2=0.298) and cerebellum + brain stem volume (t85=2.713, p=0.008, overall adjusted R2=0.150) whether the outlier is excluded or not. The relationship between orbital volume and total visual grey matter volume does not remain independently of non-visual cortical grey matter volume and is significant independently of non-visual (t84=2.299, p=0.024, overall adjusted R2=0.225) or total brain volume (t84=2.190, p=0.031, overall adjusted R2=0.242) only when the outlier has been removed (Figure 5).
Hypothesis 3: V1 (left and right) grey matter volume is associated with V2 (left and right) grey matter volume, and V2 (left and right) grey matter volume is in turn related to V5/MT (left and right) grey matter volume
Since all the scans provided good image quality for the brain, the within brain scaling models were conducted on the full dataset (N=99). OLS yielded a significant positive relationship between V1 and V2 volumes: Figure 6 (t97=11.468, p<10−36, R2=0.576). Furthermore, V1 remained significantly associated with V2 independently of rest-of-cortex grey matter volume: t96=9.348, p=3.7041×10−15, overall adjusted R2=0.685. This significant partial relationship between V1 and V2 was maintained when heteroscedasticity corrections were performed using the HCREG macro (Hayes and Cai, 2007) (p<0.00001). Similar results are obtained when controlling for the various other measures of overall brain volume (see Methods).
Figure 6.
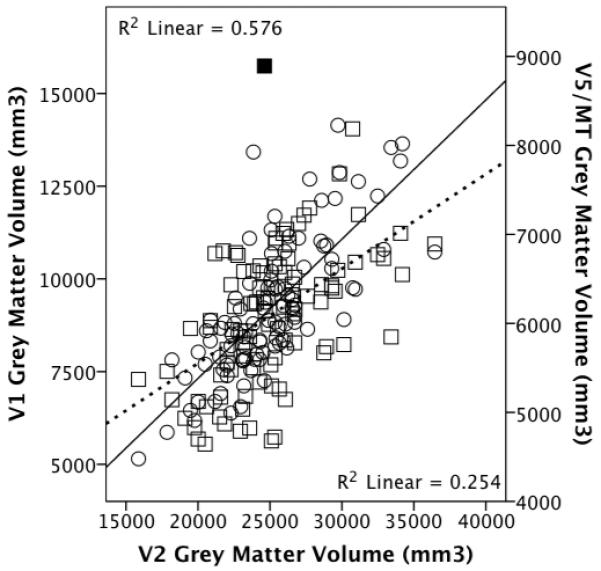
V1 (circles, sold regression line) and V5/MT (squares, dashed line) grey matter volume plotted against V2 grey matter volume (N=99). The outlier is shaded.
OLS regression yielded a significant positive relationship between V2 and V5/MT volumes: Figure 6 (t97=5.742, p<10−36, R2=0.254). Moreover, V2 showed a significant partial relationship with V5/MT independently of rest-of-cortex grey matter volume, which itself did not show a significant partial relationship: t96=3.640, p=4.411×10−4, overall adjusted R2=0.267. When an outlier was removed (Figure 6) both V2 (t95=3.495, p=0.001) and rest-of-cortex grey matter volumes (t95=2.396, p=0.019) were significantly partially associated with V5/MT grey matter volume (adjusted R2=0.322). Similar results are obtained when controlling for the various other measures of overall brain volume.
Furthermore, V1 was significantly positively associated with V2 volume independently of motor (t96=9.511, p=1.672×10−15, adjusted R2=0.660) and somatosensory (t96=8.852, p=4.338×10−14, adjusted R2=0.680) grey matter volumes. In turn, V2 was significantly positively associated with V5/MT volume independently of motor (t96=3.808, p=2.469×10−4, adjusted R2=0.247) and somatosensory (t96=2.823, p=0.006, adjusted R2=0.298) grey matter volumes.
Hypothesis 4: Visual grey matter volume is positively associated with absolute latitude
Since the sample used here are living humans used to artificial lighting, any latitudinal effect on visual system size is likely to be less pronounced than in historical populations studied previously (Pearce and Dunbar, 2012). In addition, the subjects may not have been local to the area where they were scanned, meaning they would have instead the visual system sizes of the latitude of their ancestry or place of birth, for example as might be the case of Australians of European descent.
Bearing these caveats in mind, as a test of the findings of Pearce & Dunbar (2012) regarding a significant positive relationship between orbital volume as an index of visual system size and absolute latitude among human populations, visual cortical grey matter volume was regressed against the absolute latitude of the research centres. OLS regression revealed that total visual cortical grey matter volume was significantly associated with absolute latitude (t96=3.275, p=0. 001) independently of rest-of-cortex grey matter volume (t96=7.701, p<10−36): overall adjusted R2=0.428, Figure 7. This result was replicated when controlling for the alternative measures of overall brain volume and when sex are also included.
Figure 7.
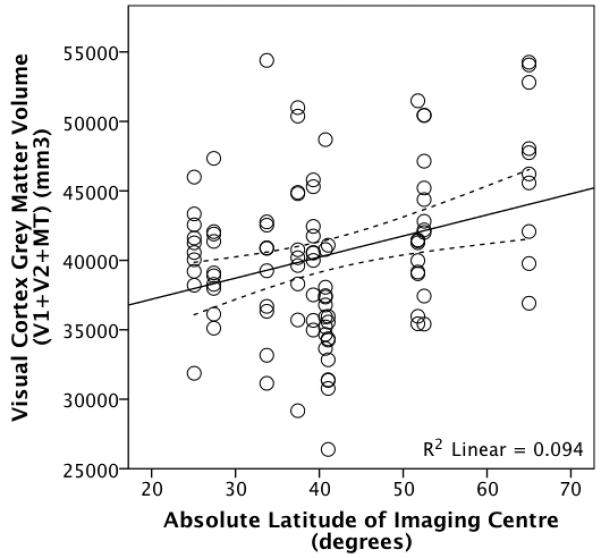
Visual cortex (V1 + V2 + MT) grey matter volume plotted against the absolute latitude of the imaging centres where the scans were taken. The dotted lines represent 95% confidence intervals for the regression line.
In the full sample absolute latitude also shows a positive relationship with eyeball volume (t86=2.045, p=0.044, R2=0.046), which remains independently of non-visual brain volume, brain stem + cerebellum volume and subcortical grey matter volume. In the higher quality subset both orbital and eye volume are positively related to absolute latitude, but not significantly (orbit: t38=0.845, p=0.403; eye: t38=0.797, p=0.430).
Discussion
The results presented here show that contrary to previous reports (Chau et al., 2004), orbital volume is significantly positively associated with eyeball volume within humans, independently of overall brain size, suggesting that on average a larger eye will correspond to a larger orbit.
According to the model human orbital volume explains ~17% of the variance observed in eyeball volume across this human sample (although this rises to ~40% in the higher quality image sample, N=40). Moreover, these results suggest that the eye and orbit scale independently of overall facial and body size. However, this work could be developed by using actual body mass data as well as focusing on the influence of facial shape on orbit size in order to investigate whether these factors impact on eye-orbit scaling during development and in adulthood.
Nonetheless, the strength of the relationship between eyeball and orbital volumes presented here is likely to be an underestimate, for a number of reasons. Firstly, although an updated version of the same software as Chau and colleagues (2004) and the same procedure for measuring orbital volume was used in this study, the MRI scans available were not optimised for orbital and ocular tissue measurements. Since the 1000 Functional Connectome scans were taken in order to study the brain, they were T1-weighted, which allows particularly clear differentiation of white matter and grey matter, whereas T2-weighted images are optimal for delineating the eye (Chau et al., 2004; Singh et al., 2006). Moreover, T1-weighted images show fluid and bones as the same shade (dark/black) meaning that the true extent of the orbit was difficult to ascertain and delineation of the region of interest depended on the external boundary of orbital fat (white), which may not always necessarily follow the orbital walls perfectly.
Secondly, since these scans were not optimised for imaging the eye/orbit region, the quality was variable and resulted in 49 of the available 89 scans requiring “reconstruction” of the eyeball border in some slices in order to measure eyeball volume, reducing measurement accuracy and necessitating removal from the analyses presented here. In combination, these factors likely gave rise to measurement error, thus artificially inflating the unexplained variance in the relationship between orbital and eye volume. Nonetheless, the relationship remained significant. If such error could be eradicated, the relationship would be even stronger.
Another potential reason for artificially inflated error in the model is that unlike in the study reported by Chau et al (2004), visual acuity measures were unavailable. Both myopia and hypermetropia can be due to abnormal axial length of the globe: long in the case of myopia and short in hypermetropia. These conditions may then alter the normal relationship between orbit and eye size for some unidentified subjects, therefore adding to the overall unexplained variance. Taking advice from optometrists at the University of Aston, an axial length of 27mm or above was taken as an indication that the corresponding individual was likely myopic. The three individuals in the full sample who fitted this criterion were not outliers on a plot of orbit versus eyeball volume. However, more detailed information on actual subject visual function, rather than depending on proxies, might reduce unexplained error in future studies into human eyeball-orbit scaling, particularly since corneal curvature is likely to be involved and was not measured here. However, as Chau et al (2004) point out, during childhood the orbital region only continues to grow in breadth, so eyeball enlargement in an axial direction associated with myopia might not have any influence on orbital growth in later development (Chau et al., 2004; Waitzman et al., 1992).
To further test the closeness of the relationship between human orbital and eyeball size, it would be necessary to take one of two directions. The first option would be to run optimised T2-weighted image scans of the eyeball as well as taking CT scans of the same subjects for measurement of the boney orbit. Alternatively, the components of the visual system from orbit to V1 could be measured physically post-mortem. The first option seems preferable in terms of the ease of collecting the measurements, access to larger samples sizes and ethics approval.
The fact that both eyeball and orbital volume remain significantly associated with visual cortex grey matter volume independently of various measures of overall brain size suggests that eyeball volume does explain part of the variance in visual cortex size over and above that explained by allometric scaling to overall brain size. This echoes previous work, which has found that within humans (Andrews et al., 1997) and across mammals (Barton, 2007), the components of the visual system co-vary independently of, respectively, the size of the brain overall and other sensory brain areas. In addition, the lack of a significant relationship between eye and brain volume, which has been reported previously by Todd et al (1940), suggests that eye size is specifically associated with visual cortex size in human adults, even though eye development tends to follow the brain growth trajectory (Scammon and Armstrong, 1925; Todd et al., 1940). However, to strengthen these results, further data collection using T2-weighted images would also allow more accurate measurement of the optic nerve, chiasm and tract to allow a direct link to be made between the orbital and cortical measurements.
Consistent with previous studies (Dougherty et al., 2003; Song et al., 2011), there was a positive significant relationship between V1 and V2 grey matter volumes, independently of rest-of-cortex grey matter. Additionally, there was a significant positive relationship between V2 and V5/MT grey matter volumes, illustrating that the scaling appears to continue through the visual system. As with the V1-V2 relationship, V2 remains significantly associated with V5/MT grey matter volume independently of rest-of-cortex grey matter volume. Furthermore, the V1-V2 and V2-V5/MT associations remain independently of motor and somatosensory area volumes. These findings support scaling between different visual areas in the human brain independently of other cortical systems.
Despite limitations regarding whether subjects were local to the area where they were scanned, as well as the likely diluting effect of widespread artificial lighting, our findings still provide supplementary support for the hypothesis that human visual systems enlarge over progressively higher latitudes (Pearce and Dunbar, 2012). Firstly, significant scaling relationships indicate that larger orbits imply larger eyes at higher latitudes and secondly, a significant positive linear relationship was found between the absolute latitude of the imaging centres and total visual cortex grey matter independently of overall rest-of-cortex grey matter volume (as well as independently of the alternative brain size measures). These results suggest that the positive latitudinal trend in human brain size may be associated with enlarged visual cortices in addition to increasing body mass (Beals et al., 1984; Pearce and Dunbar, 2012).
Although absolute latitude on its own explains only ~9% of the variance in visual cortex grey matter volume (Figure 7), this is probably partly due to the fact that, as mentioned above, the individual subjects measured at each imaging centre were not necessarily local to that area. In other words, subjects may have grown up elsewhere, meaning that the size of their visual systems might reflect their native latitude light levels rather than the ambient light levels associated with the latitude of the imaging centre. In order to test between an ancestral/phylogenetic effect and a developmental effect, data on individual ethnicity and childhood location are necessary. Ocular ontogeny has a high heritability, at least in terms of myopia (Goldschmidt, 2003; Guggenheim et al., 2003), and since eyeball increase would be adaptive in terms of enhanced sensitivity and/or acuity, such global expansion would be positively selected under these conditions. Further research into this is necessary to demonstrate the relative importance of ancestral and childhood ambient light levels in manifested visual system size.
So far the effects of light levels on eye development have been investigated only in relation to myopia, which is generally linked to a large, axially elongated eye (Chau et al., 2004; Masters, 2012; Vera-Diaz et al., 2005). Evidence for the specific effects of light on eyeball size, however, is equivocal. One line of evidence suggests that extended time spent in high light levels, such as during long summer days at higher latitudes, leads to an increase in myopia in humans (Mandel et al., 2008; McMahon et al., 2009) (Vannas et al., 2003). Additionally, some animals (chicks and mice: Cohen et al., 2008; Liu et al., 2004; Zhou et al., 2009) raised under constant exposure to high light levels, also show altered internal ocular dimensions. In contrast, it is also the case that low light levels can affect axial length, in chicks raised in dim light (Cohen et al., 2011), and in humans spending greater time indoors (Rose et al., 2008a; Rose et al., 2008b), perhaps due to insufficient vitamin D (Mutti and Marks, 2011). Moreover, the rate of ocular elongation in child myopes has been reported to be higher in winter than in summer, again suggesting a link between eye growth and lower light levels (Fulk et al., 2002). Overall, it seems that myopic eye enlargement is associated with skewed light/dark cycles and dim light conditions, both of which could be related to latitude in terms of increased day-length seasonality and reduced illuminance. Nonetheless, the impact of light levels on normal visual development requires study.
Once higher resolution data are collected in order to provide baseline equations, the use of orbit size as a proxy for eyeball and visual cortex size may have useful applications for investigating visual system size in fossil hominins. However, as in the case of using orbital volume to associate fossil primates with particular activity periods, caution should be taken when interpreting the results (Kay and Kirk, 2000; Kirk, 2006; Ross and Kirk, 2007).
CONCLUSION
Overall, this paper has demonstrated that in humans orbital volume is significantly positively associated with eyeball volume and that in turn eyeball volume seems to be linked to visual cortex grey matter volume independently of overall brain size. This scaling suggests that the previously identified positive relationship between orbital volume and absolute latitude (Pearce and Dunbar, 2012) indicates not only larger eyes, but also larger visual cortices, in higher latitude humans compared to those living nearer the equator. Direct corroboration of this is also presented here, with the finding that visual grey matter volume is positively associated with absolute latitude independently of overall brain size.
ACKNOWLEDGMENTS
The authors would like to thank Brett Lullo, Sharad Sikka, Maarten Mennes and Mike Milham at the 1000 Functional Connectome Project for access to their scans and Robert Barton for useful comments on this work. We would also like to thank Robin Dunbar for his advice and support. EP is grateful for financial assistance from the Boise Fund, University of Oxford, and a bursary from a European Research Council Advanced grant.
Footnotes
Declaration of interest:
The authors report no conflicts of interest.
REFERENCES
- Andrews TJ, Halpern SD, Purves D. Correlated Size Variations in Human Visual Cortex, Lateral Geniculate Nucleus, and Optic Tract. Journal of Neuroscience. 1997;17:2859–2868. doi: 10.1523/JNEUROSCI.17-08-02859.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA. Evolutionary specialization in mammalian cortical structure. Journal of Evolutionary Biology. 2007;20:1504–1511. doi: 10.1111/j.1420-9101.2007.01330.x. [DOI] [PubMed] [Google Scholar]
- Beals KL, Smith CL, Dodd SM, Angel JL, Armstrong E, Blumenberg B, Girgis FG, Turkel S, Gibson KR, Henneberg M, Roland M, Morimoto I, Sokal RR, Trinkaus E. Brain Size, Cranial Morphology, Climate, and Time Machines. Curr. Anthropol. 1984;25:301–330. [Google Scholar]
- Bridge H. Mapping the visual brain: how and why. Eye. 2011;25:291–296. doi: 10.1038/eye.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Clare S, Jenkinson M, Jezzard P, Parker AJ, Matthews PM. Independent anatomical and functional measures of the V1/V2 boundary in human visual cortex. Journal of Vision. 2005;5:93–102. doi: 10.1167/5.2.1. [DOI] [PubMed] [Google Scholar]
- Bridge H, Cowey A, Raggae N, Watkins K. Imaging studies in congenital anophthalmia reveal preservation of brain architecture in ‘visual’ cortex. Brain. 2009;132:3467–3480. doi: 10.1093/brain/awp279. [DOI] [PubMed] [Google Scholar]
- Bridge H, von dem Hagen EAH, Davies G, Chambers C, Gouws A, Hoffmann M, Morland AB. Cortex. 2012. Changes in brain morphology in albinism reflect reduced visual acuity. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tripathi RC, Tripathi BJ. Wolff’s Anatomy of the Eye & Orbit. 8th Edition Chapman & Hall Medical; London: 1997. [Google Scholar]
- Brown P, Maeda T. Post-Pleistocene diachronic change in East Asian facial skeletons: the size, shape and volume of the orbits. Anthropological Science. 2004;112:29–40. [Google Scholar]
- Chau A, Fung K, Pak K, Yap M. Is eye size related to orbit size in human subjects? Ophthalmic and Physiological Optics. 2004;24:35–40. doi: 10.1046/j.1475-1313.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Avni I, Polat U. Light intensity modulates corneal power and refraction in the chick eye exposed to continuous light. Vision Res. 2008;48:2329–2335. doi: 10.1016/j.visres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under light and dark cycles. Exp. Eye Res. 2011;92:40–46. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cummings JR, Muchlinski MN, Kirk EC, Rehorek SJ, DeLeon VB, Smith TD. Eye Size at Birth in Prosimian Primates: Life History Correlates and Growth Patterns. PLoS ONE. 2012;7:e36097. doi: 10.1371/journal.pone.0036097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis D, Burguière O, Burillon C. A biometric study of the eye, orbit, and face in 205 normal human fetuses. Investigative Ophthalmology & Visual Science. 1998;39:2232–2238. [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. Journal of Vision. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Le Goualher G, Pike GB, Evans AC. A New Anatomical Landmark for Reliable Identification of Human Area V5/MT: a Quantitative Analysis of Sulcal Patterning. Cerebral Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Boynton GM. Cortical Magnification within Human Primary Visual Cortex Correlates with Acuity Thresholds. Neuron. 2003;38:659–671. doi: 10.1016/s0896-6273(03)00265-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JDG, Amunts K. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Human Brain Mapping. 2005;24:206–215. doi: 10.1002/hbm.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BTT, Mohlberg H, Amunts K, Zilles K. Cortical Folding Patterns and Predicting Cytoarchitecture. Cerebral Cortex. 2008;18:1973–1980. doi: 10.1093/cercor/bhm225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DA. Seasonal Variation in Myopia Progression and Ocular Elongation. Optometry & Vision Science. 2002;79:46–51. doi: 10.1097/00006324-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Gilmartin B. Myopia: precedents for research in the twenty-first century. Clin. Exp. Ophthalmol. 2004;32:305–324. doi: 10.1111/j.1442-9071.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E. The mystery of myopia. Acta Ophthalmologica Scandinavica. 2003;81:431–436. doi: 10.1034/j.1600-0420.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- Gorissen P. [Accessed July 2008];2010 Jul; Google Maps Latitude, Longitude Popup [online], available: http://www.gorissen.info/Pierre/maps/googleMapLocation.php?lat=51.618017&lon=2.48291&setLatLon=Set.
- Guggenheim JA, Hill C, Yam T-F. Myopia, genetics, and ambient lighting at night in a UK sample. British Journal of Ophthalmology. 2003;87:580–582. doi: 10.1136/bjo.87.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A, Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: An introduction and software implementation. Behavior Research Methods. 2007;39:709–722. doi: 10.3758/bf03192961. [DOI] [PubMed] [Google Scholar]
- Heesy CP, Kamilar JM, Willms J. Retinogeniculostriate Pathway Components Scale with Orbit Convergence Only in Primates and Not in Other Mammals. Brain, Behavior and Evolution. 2011;77:105–115. doi: 10.1159/000324860. [DOI] [PubMed] [Google Scholar]
- Hinds O, Polimeni JR, Rajendran N, Balasubramanian M, Amunts K, Zilles K, Schwartz EL, Fischl B, Triantafyllou C. Locating the functional and anatomical boundaries of human primary visual cortex. NeuroImage. 2009;46:915–922. doi: 10.1016/j.neuroimage.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana Rosas H, Potthast A, Schwartz EL, Fischl B. Accurate prediction of V1 location from cortical folds in a surface coordinate system. NeuroImage. 2008;39:1585–1599. doi: 10.1016/j.neuroimage.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintschich C, Zonneveld F, Baldeschi L, Bunce C, Koornneef L. Bony orbital development after early enucleation in humans. British Journal of Ophthalmology. 2001;85:205–208. doi: 10.1136/bjo.85.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RF, Kirk EC. Osteological evidence for the evolution of activity pattern and visual acuity in primates. American Journal of Physical Anthropology. 2000;113:235–262. doi: 10.1002/1096-8644(200010)113:2<235::AID-AJPA7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kirk EC. Effects of activity pattern on eye size and orbital aperture size in primates. J. Hum. Evol. 2006;51:159–170. doi: 10.1016/j.jhevol.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Liu J, Pendrak K, Capehart C, Sugimoto R, Schmid GF, Stone RA. Emmetropisation under continuous but non-constant light in chicks. Exp. Eye Res. 2004;79:719–728. doi: 10.1016/j.exer.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Connolly JD. The case for primate V3. Proceedings of the Royal Society B: Biological Sciences. 2011;279:625–633. doi: 10.1098/rspb.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel Y, Grotto I, El-Yaniv R, Belkin M, Israeli E, Polat U, Bartov E. Season of Birth, Natural Light, and Myopia. Ophthalmology. 2008;115:686–692. doi: 10.1016/j.ophtha.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Martin R. Primate origins and evolution. A phylogenetic reconstruction. Chapman & Hall; London: 1990. [Google Scholar]
- Masters MP. Relative size of the eye and orbit: An evolutionary and craniofacial constraint model for examining the etiology and disparate incidence of juvenile-onset myopia in humans. Medical Hypotheses. 2012;78:649–656. doi: 10.1016/j.mehy.2012.02.002. [DOI] [PubMed] [Google Scholar]
- McMahon G, Zayats T, Chen Y-P, Prashar A, Williams C, Guggenheim JA. Season of Birth, Daylight Hours at Birth, and High Myopia. Ophthalmology. 2009;116:468–473. doi: 10.1016/j.ophtha.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Marks AR. Blood Levels of Vitamin D in Teens and Young Adults with Myopia. Optometry & Vision Science. 2011;88:377–382. doi: 10.1097/OPX.0b013e31820b0385. 310.1097/OPX.1090b1013e31820b30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitek S, Wysocki J, Reymond J, Piasecki K. Correlations between selected parameters of the human skull and orbit. Med Sci Monit. 2009;15:BR370–377. [PubMed] [Google Scholar]
- NITRC [Accessed 2011];1000 Functional Connectomes Project [online] accessed at: http://www.nitrc.org/projects/fcon_1000/
- Pearce E, Dunbar R. Latitudinal variation in light levels drives human visual system size. Biology Letters. 2012;8:90–93. doi: 10.1098/rsbl.2011.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radinsky L. Relative Brain Size: A New Measure. Science. 1967;155:836–838. doi: 10.1126/science.155.3764.836. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ. Interspecific perspective on mechanical and nonmechanical models of primate circumorbital morphology. American Journal of Physical Anthropology. 1991a;86:369–396. doi: 10.1002/ajpa.1330860305. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ. Ontogenetic perspective on mechanical and nonmechanical models of primate circumorbital morphology. American Journal of Physical Anthropology. 1991b;85:95–112. doi: 10.1002/ajpa.1330850111. [DOI] [PubMed] [Google Scholar]
- Rosa MGP, Tweedale R. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Philosophical Transactions of the Royal Society B-Biological Sciences. 2005;360:665–691. doi: 10.1098/rstb.2005.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor Activity Reduces the Prevalence of Myopia in Children. Ophthalmology. 2008a;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw S-M. Myopia, Lifestyle, and Schooling in Students of Chinese Ethnicity in Singapore and Sydney. Archives of Ophthalmology. 2008b;126:527–530. doi: 10.1001/archopht.126.4.527. [DOI] [PubMed] [Google Scholar]
- Ross CF, Kirk EC. Evolution of eye size and shape in primates. J. Hum. Evol. 2007;52:294–313. doi: 10.1016/j.jhevol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Rosset A. [Accessed 2011];OsiriX Imaging Software [online] available at: http://www.osirix-viewer.com/
- Sarnat BG. Eye and orbital size in the young and adult. Some postnatal experimental and clinical relationships. Ophthalmologica. 1982;185:74–89. doi: 10.1159/000309228. [DOI] [PubMed] [Google Scholar]
- Scammon RE, Armstrong EL. On the growth of the human eyeball and optic nerve. The Journal of Comparative Neurology. 1925;38:165, 219. [Google Scholar]
- Schultz A. The Size of the Orbit and of the Eye in Primates. American Journal of Physical Anthropology. 1940;26:389–408. [Google Scholar]
- Singh KD, Logan NS, Gilmartin B. Three-Dimensional Modeling of the Human Eye Based on Magnetic Resonance Imaging. Investigative Ophthalmology & Visual Science. 2006;47:2272–2279. doi: 10.1167/iovs.05-0856. [DOI] [PubMed] [Google Scholar]
- Song C, Schwarzkopf DS, Kanai R, Rees G. Reciprocal Anatomical Relationship between Primary Sensory and Prefrontal Cortices in the Human Brain. Journal of Neuroscience. 2011;31:9472–9480. doi: 10.1523/JNEUROSCI.0308-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TW, Beecher h., Williams GH, Todd AW. The weight and growth of the human eyeball. Human Biology. 1940;12:1–20. [Google Scholar]
- Tomasik E, Czepita D, Zejmo M, Czerwinski F. Development of the human eyeball and orbit during fetal life. Ann Acad Med Stetin. 2005a;51:37–40. [PubMed] [Google Scholar]
- Tomasik E, Czepita D, Zejmo M, Czerwinski F. Fetal ocular and orbital development in humans. Durham Anthropology Journal. 2005b;12:2–3. [Google Scholar]
- Van Essen DC. Surface-based approaches to spatial localization and registration in primate cerebral cortex. NeuroImage. 2004;23(Supplement 1):S97–S107. doi: 10.1016/j.neuroimage.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Vannas AE, Ying GS, Stone RA, Maguire MG, Jormanainen V, Tervo T. Myopia and natural lighting extremes: risk factors in Finnish army conscripts. Acta Ophthalmologica Scandinavica. 2003;81:588–595. doi: 10.1046/j.1395-3907.2003.0151.x. [DOI] [PubMed] [Google Scholar]
- Vera-Diaz FA, McGraw PV, Strang NC, Whitaker D. A Psychophysical Investigation of Ocular Expansion in Human Eyes. Investigative Ophthalmology & Visual Science. 2005;46:758–763. doi: 10.1167/iovs.04-0127. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EAH, Houston GC, Hoffmann MB, Jeffery G, Morland AB. Retinal abnormalities in human albinism translate into a reduction of grey matter in the occipital cortex. European Journal of Neuroscience. 2005;22:2475–2480. doi: 10.1111/j.1460-9568.2005.04433.x. [DOI] [PubMed] [Google Scholar]
- Waitzman AA, Posnick JC, Armstrong DC, Pron GE. Craniofacial Skeletal Measurements Based on Computed Tomography: Part II. Normal Values and Growth Trends. The Cleft Palate-Craniofacial Journal. 1992;29:118–128. doi: 10.1597/1545-1569_1992_029_0118_csmboc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual Field Maps in Human Cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Washburn SL, Detwiler SB. An experiment bearing on the problems of physical anthropology. American Journal of Physical Anthropology. 1943;1:171–190. [Google Scholar]
- Whitnall SE. The Anatomy of the Human Orbit and Accessory Organs of Vision. The Oxford Medical Publications, Henry Frowde and Hodder & Stoughton; London: 1921. [Google Scholar]
- Zhou XT, An JH, Wu XM, Lu RX, Huang QZ, Xie RZ, Jiang LQ, Qu J. Relative Axial Myopia Induced by Prolonged Light Exposure in C57BL/6 Mice. Photochem. Photobiol. 2009;86:131–137. doi: 10.1111/j.1751-1097.2009.00637.x. [DOI] [PubMed] [Google Scholar]