Abstract
Amyloid β (Aβ) and tau protein are both implicated in memory impairment, mild cognitive impairment (MCI), and early Alzheimer's disease (AD), but whether and how they interact is unknown. Consequently, we asked whether tau protein is required for the robust phenomenon of Aβ-induced impairment of hippocampal long-term potentiation (LTP), a widely accepted cellular model of memory. We used wild-type mice and mice with a genetic knock-out of tau protein and recorded field potentials in an acute slice preparation. We demonstrate that the absence of tau protein prevents Aβ-induced impairment of LTP. Moreover, we show that Aβ increases tau phosphorylation and that a specific inhibitor of the tau kinase glycogen synthase kinase 3 blocks the increased tau phosphorylation induced by Aβ and prevents Aβ-induced impairment of LTP in wild-type mice. Together, these findings show that tau protein is required for Aβ to impair synaptic plasticity in the hippocampus and suggest that the Aβ-induced impairment of LTP is mediated by tau phosphorylation. We conclude that preventing the interaction between Aβ and tau could be a promising strategy for treating cognitive impairment in MCI and early AD.
Introduction
Amyloid β (Aβ) and tau protein both have well established roles in Alzheimer's disease (AD), forming the two hallmark pathologies visible in postmortem AD brains as amyloid plaques and neurofibrillary tangles (NFTs), respectively (Hardy and Selkoe, 2002; Small and Duff, 2008). Aβ plays a central role in disease pathogenesis, being implicated in the synaptic dysfunction that is considered a major cellular mechanism underlying the cognitive deficits in patients with mild cognitive impairment (MCI) and early AD (Selkoe, 2002). Plaque formation is preceded by elevated levels of soluble, low-N oligomers of Aβ, and evidence suggests it is these that are responsible for synaptic dysfunction (Lue et al., 1999; Hardy and Selkoe, 2002). Acute exposure to synthetic or naturally secreted Aβ has been shown to impair hippocampal long-term potentiation (LTP) (Cullen et al., 1997; Lambert et al., 1998; Walsh et al., 2002; Wang et al., 2004; Townsend et al., 2006; Wei et al., 2010), a widely accepted cellular model of learning and memory (Bliss and Collingridge, 1993).
Tau protein may also play a crucial role in the cognitive decline in MCI and early AD. In the medial temporal lobe, the first area to be affected in AD, NFTs are elevated and their numbers in the hippocampal CA1 correlate with memory decline (Markesbery et al., 2006). Nevertheless, it has been suggested that tau intermediates are more important for the cognitive decline than the tangles themselves (Santacruz et al., 2005).
Thus, Aβ and tau are both fundamental in the early stages of AD, but whether and how these two proteins interact is not yet established. Evidence for an important interaction includes the protection of tau-depleted neurons from Aβ-induced neurodegeneration in culture (Rapoport et al., 2002). Moreover, the cognitive deficits that are seen in human amyloid precursor protein (hAPP)-overexpressing mice (Hsiao et al., 1996) are absent in hAPP-overexpressing mice with a genetic knock-out of tau protein (Roberson et al., 2007). Therefore, to further investigate a possible interaction between Aβ and tau and so help elucidate the mechanism underlying the cognitive decline in MCI and early AD, we asked whether tau is required for the Aβ-mediated impairment of LTP in the hippocampus, one of the earliest regions affected in AD (Braak and Braak, 1991). For this, we used both wild-type mice and mice with a genetic knock-out of tau protein (Tau−/− mice) (Dawson et al., 2001).
To investigate a possible molecular pathway for this interaction, we tested whether glycogen synthase kinase 3 (GSK-3) is involved in the Aβ-induced impairment of LTP. GSK-3 is a serine/threonine kinase known to phosphorylate tau protein (Ishiguro et al., 1993) and to play a role in both synaptic plasticity (Hooper et al., 2007) and Aβ-induced and tau-mediated neurodegeneration (Tackenberg and Brandt, 2009). If GSK-3 is involved in an interaction between Aβ and tau, inhibition of GSK-3 could be a promising therapeutic strategy for MCI and early AD.
Materials and Methods
Mice.
Animal care and experimental procedures were conducted in accordance with UK Home Office regulations under the Animals (Scientific Procedures) Act of 1986. Animals had access to food and water ad libitum. Holding facilities were maintained at a temperature of ∼22°C, humidity of 60–70%, and with a 12 h light/dark cycle. We used 4- to 6-month-old Tau−/− mice on a C57BL/6J background (Dawson et al., 2001) and age-matched C57BL/6J mice purchased from Charles River Laboratories. All mice were housed in the same animal facility under the same conditions for at least 2 weeks before experiments commenced. Mice of both sexes were used for the rodent Aβ experiment; for all other experiments only males were used.
Slice preparation.
Parasagittal hippocampal slices (400 μm) were prepared after decapitation under deep isoflurane-induced anesthesia. After dissection in ice-cold artificial CSF (ACSF) containing (in mm) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 25 NaHCO3, 10 glucose, pH 7.2–7.4, bubbled with carbogen gas (95% O2, 5% CO2), slices were maintained at room temperature (22−25°C) in a submerged-style holding chamber for at least 1 h and then incubated in drug/control solutions. For recording, slices were transferred to an interface-style recording chamber maintained at 33–35°C and superfused with ACSF at a rate of 2 ml/min, and recording started at least 15 min after the slices were transferred.
Pharmacology.
Rodent Aβ1–42 (rAβ1–42) was dissolved in ACSF to a concentration of 10 μm and frozen in aliquots. Single aliquots were defrosted and sonicated for 11 min before final dilution to 500 nm for slice incubation. Human Aβ1–42 (hAβ1–42) was freshly prepared on the day of the experiment. It was initially dissolved in ACSF to a concentration of 5 μm. Aliquots were then sonicated for 11 min before final dilution to 220 nm in ACSF.
Hippocampal slices were incubated in a submerged-style holding chamber in ACSF with or without Aβ1–42 or Aβ42–1 for 1–3 h before recording. Perfusion with a half-concentration of the drug continued after slices were transferred to the interface chamber.
AR-A014418 was dissolved in DMSO at 100 mm concentration and stored in frozen aliquots. For slice incubation, single aliquots of AR-A014418 were defrosted and diluted to a final concentration of 1 μm in ACSF. Slices were incubated for 30–45 min in AR-A014418 before the addition of Aβ1–42 or control ACSF.
To block GABAA receptor-mediated inhibition in some experiments, gabazine (SR 95531) was bath applied at 100 nm concentration to slices from which the CA3 had been removed.
hAβ1–42, hAβ42–1, rAβ1–42, and gabazine were purchased from Tocris Bioscience; AR-A014418 was obtained from Sigma Aldrich.
Electrophysiological protocols.
Extracellular field recordings from CA1 were made with an Axoclamp-2A amplifier in bridge mode, and data were acquired with an ITC-16 A/D board (Instrutech) using Igor Pro software (WaveMetrics). Borosilicate glass recording electrodes were filled with ACSF. Recording and stimulation electrodes were positioned in the stratum radiatum of CA1. Synaptic efficacy was monitored by stimulating the Schaffer collaterals at 0.2 Hz (50 μs, 20–60 μA) with a monopolar tungsten electrode connected to a stimulus isolator unit (ISO-Flex, A.M.P.I.). Stimulation strength was set to elicit a field EPSP (fEPSP) of half-maximal amplitude. fEPSP slopes were monitored for a baseline period of at least 15 min. If synaptic transmission was stable (< 15% change in fEPSP slopes over 15 min), a single high-frequency stimulus train was delivered (100 Hz for 1 s). To measure paired-pulse ratio both before and after LTP induction, two 50 μs pulses with an interpulse interval of 40 ms were given at a low stimulation strength.
Protein extraction and Western blot analysis.
Slices were prepared as described above from wild-type and Tau−/− mice and incubated in ACSF with or without 220 nm hAβ1–42 for 2 h. A subset of slices was preincubated in 1 μm AR-A014418 for 30 min. The hippocampus was dissected from each slice and immediately frozen at −80°C. Total protein was extracted by homogenizing hippocampal slices in radio-immunoprecipitation assay buffer (Sigma Aldrich) supplemented with phosphatase inhibitors (PhosSTOP, Roche) and protease inhibitors (Complete Mini Protease Inhibitor Cocktail, Roche), followed by brief microcentrifugation. Protein concentrations were determined using the Bradford method. Two micrograms of total protein were separated on 3–8% Tris acetate polyacrylamide gels (Invitrogen) and transferred to a polyvinylidene fluoride membrane. Membranes were blocked in phosphate buffered saline containing 0.1% Tween 20 and 10% milk and probed with primary antibody. Membranes were probed initially with an anti-phospho-tau primary antibody (AT8, 1:1000; Thermo Scientific) before stripping in Restore buffer (Pierce) and reprobing with a primary antibody to detect total tau (Tau5, 1:1000; Fitzgerald). Primary antibody binding was detected using an anti-mouse HRP-conjugated secondary antibody (Bio-Rad) and ECL Plus reagent (GE Healthcare) apposed to photographic film (CL-Xposure film, Thermo Scientific). Films were digitized and optical densities were determined using ImageJ (v1.43u; National Institutes of Health). To control for between-membrane variation, all samples from an individual mouse were run on a single gel, and optical densities were expressed as ratios of phospho/total tau normalized to the respective ACSF-only condition. Values presented are the mean of duplicate Western blots.
Data analysis.
Changes in synaptic efficacy were estimated using the mean fEPSP slopes (measured from the middle third of the rising slope of the fEPSP) 30–45 min after high-frequency stimulation normalized to the mean fEPSP slope during the last 5 min of baseline recording. Paired-pulse ratio was expressed as the mean ratio of the amplitude of the second fEPSP to the amplitude of the first fEPSP (average of five paired pulses). Data were analyzed using Igor Pro, Matlab, and SPSS and are given as mean ± SEM. Statistical significance was assessed using the Student's t test or one-way ANOVA, followed by post hoc analysis with Bonferroni corrections when applicable. Unless otherwise stated, numbers (N) refer to the number of slices obtained from at least six animals. Experiments in Figure 1 were performed blind such that the experimenter was not aware of genotype. In a subset (approximately half) of experiments in Figure 2, the experimenter was unaware of drug and genotype. As the average and variability of blind and nonblind experiments were similar, these data were pooled.
Figure 1.
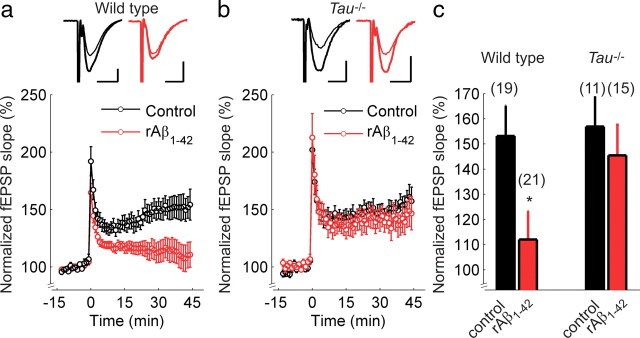
Rodent Aβ1–42 does not reduce LTP in slices of Tau−/− mice. a, b, Hippocampal Schaffer collateral-CA1 LTP in wild-type (a) and Tau−/− mice (b) in control ACSF (black) or after incubation with rAβ1–42 (red). The insets show superimposed example traces before and 40 min after high-frequency stimulation for each condition. Scale bars: 5 ms, 200 μV. c, Summary of results 40–45 min after high-frequency stimulation. Error bars are SEM; *p < 0.05. The numbers of slices are shown in parentheses.
Figure 2.
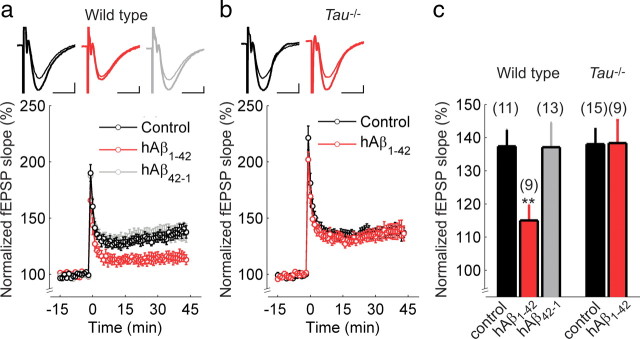
Human Aβ1–42 does not reduce LTP in slices of Tau−/− mice. a, b, Hippocampal Schaffer collateral-CA1 LTP in wild-type (a) and Tau−/− mice (b) in control ACSF (black), or after incubation with hAβ1–42 (red) or the control peptide hAβ42–1 (gray). The insets show superimposed example traces before and 40 min after high-frequency stimulation for each condition. Scale bars: 5 ms, 200 μV. c, Summary of results 40–45 min after high-frequency stimulation. Error bars are SEM; **p < 0.01. The numbers of slices are shown in parentheses.
Results
LTP in Aβ1–42-exposed slices from Tau−/− mice
First, we wanted to confirm that Aβ1–42 inhibits LTP in hippocampal slices from wild-type mice. We monitored fEPSPs evoked by extracellular stimulation of the Schaffer collateral pathway and induced LTP using high-frequency stimulation (100 Hz for 1 s). In slices pretreated with 500 nm rAβ1–42 for 1–3 h, LTP was almost completely blocked (control: 153 ± 12%, N = 19; rAβ1–42: 112 ± 11%, N = 21; t test, p < 0.05) (Fig. 1a,c). Similarly, hAβ1–42 also strongly impaired LTP (control: 137 ± 5%, N = 11; hAβ1–42: 115 ± 5%, N = 9; t test, p < 0.01) (Fig. 2a,c), consistent with previous reports (Walsh et al., 2002; Wang et al., 2004; Townsend et al., 2006). This was a specific effect of hAβ1–42, because slices pretreated with a control peptide containing the hAβ peptide sequence in reverse order (hAβ42–1) showed a synaptic potentiation (137 ± 7%, N = 13) (Fig. 2a,c) equivalent to that in control slices (t test, p = 0.49). These results demonstrate that, in wild-type mice, acute application of Aβ1–42 impairs one or more of the cellular mechanisms necessary for LTP.
To investigate a possible interaction between Aβ and tau, we tested the effect of Aβ1–42 on LTP in Tau−/− mice (Dawson et al., 2001). Slices incubated in control solution showed normal levels of LTP and, remarkably, slices preincubated in rAβ1–42 showed LTP of similar magnitude (control: 157 ± 11%, N = 11; rAβ1–42: 145 ± 12%, N = 15; t test, p = 0.51) (Fig. 1b,c). Equivalent results were obtained using hAβ1–42 (control: 138 ± 5%, N = 15; hAβ1–42: 138 ± 7%, N = 9; t test, p = 0.48) (Fig. 2b,c). Univariate ANOVA revealed a main effect of treatment, as well as a genotype–treatment interaction for both rodent and human Aβ1–42 (rAβ1–42: F(1,65) = 3.23, p < 0.05; hAβ1–42: F(1,40) = 5.03, p < 0.05), resulting from a significant effect of Aβ in wild-type slices.
To investigate whether this result could be caused by different basic synaptic properties between wild-type and Tau−/− mice, we measured the synaptic input–output relationship and paired-pulse ratio (PPR). We found no difference in synaptic input–output relationships between wild-type and Tau−/− mice, indicating equivalent basal synaptic transmission (supplemental Fig. 1a, available at www.jneurosci.org as supplemental material). Moreover, we observed no difference in the PPR (supplemental Fig. 1b) between wild-type and Tau−/− mice [wild-type PPR before LTP: 1.67 ± 0.05, PPR after LTP: 1.68 ± 0.10, N = 12; Tau−/− PPR before LTP: 1.63 ± 0.11, PPR after LTP: 1.72 ± 0.06, N = 10; repeated measures ANOVA revealed no effect of genotype (between-subjects factor F(1,17) = 0.323; p = 0.58) on PP value before or after LTP induction (within-subjects factor F(1,17) = 0.55; p = 0.47] (supplemental Figure 1). There was also no significant difference in post-tetanic potentiation (PTP) between wild-type and Tau−/− mice (wild-type PTP: 193 ± 9%, N = 28 mice, Tau−/− PTP: 211 ± 10%, N = 18 mice, t test p = 0.18) (Fig. 1a,b, 2a,b). To confirm that the LTP results were not caused by differences in GABAergic inhibition, experiments were repeated with equivalent results in the presence of 100 nm gabazine in slices with the CA3 removed (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Altogether, these results suggest that differences in basic synaptic properties are unlikely to account for the lack of Aβ1–42-induced impairment of LTP in Tau−/− mice.
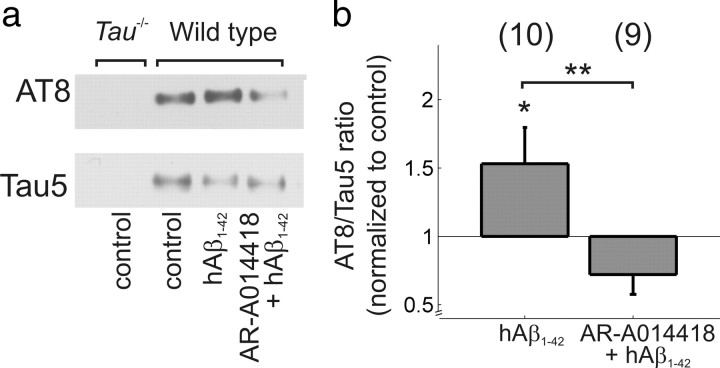
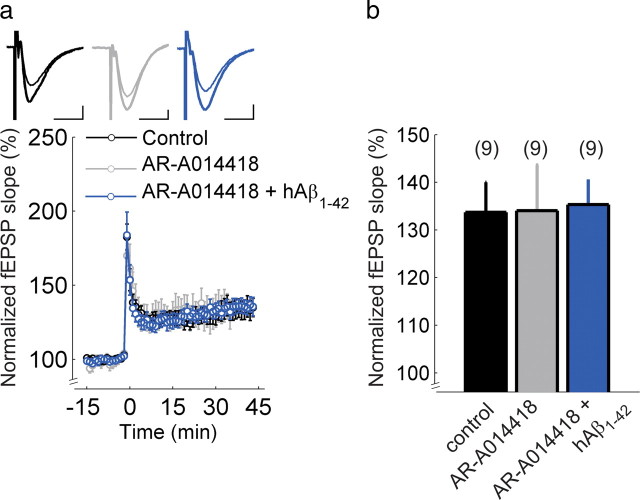
Given that NFTs comprising hyperphosphorylated tau are a prominent AD pathology alongside Aβ plaques, we asked whether the Aβ1–42-induced impairment of LTP is associated with tau phosphorylation and requires activity of GSK-3, a serine/threonine kinase known to phosphorylate tau protein (Ishiguro et al., 1993). We used AR-A014418, which is a highly specific GSK-3 inhibitor that does not significantly inhibit closely related kinases such as cdk2 or cdk5 (Bhat et al., 2003). First, to test whether hAβ1–42 increases tau phosphorylation and AR-A014418 inhibits tau phosphorylation under our experimental conditions, we used Western blot analysis of hippocampal tissue from slices incubated in ACSF with or without 220 nm hAβ1–42. In a subset of experiments, slices were preincubated in 1 μm AR-A014418 to inhibit GSK-3 activity. A significant effect of treatment on the ratio of phosphorylated tau to total tau was found (ANOVA, F(2,22) = 3.99 p < 0.05) (Fig. 3). Incubation with hAβ1–42 significantly increased tau phosphorylation in hippocampal tissue compared with control slices (AT8/Tau5 ratio = 1.53 ± 0.26, N = 10, one-sample t test, p < 0.05), and the phosphorylation ratio in slices preincubated with AR-A014418 was significantly lower than those incubated with hAβ1–42 alone (AT8/Tau5 ratio = 0.72 ± 0.14, N = 9, two-sample t test, p < 0.01). Slices exposed to 1 μm AR-A014418 alone showed tau phosphorylation equivalent to that observed in control conditions (AT8/Tau5 ratio = 0.96 ± 0.16, N = 4). Next, we tested whether this concentration of AR-A014418 could block the hAβ1–42-induced impairment of LTP. When hippocampal slices from wild-type mice were pre-exposed to 1 μm AR-A014418 for 30 min before incubation with hAβ1–42, we observed LTP of a magnitude equivalent to that in control slices (control: 134 ± 6%, N = 9, hAβ1–42: 135 ± 5%, N = 9, t test p = 0.58) (Fig. 4a,b) and in slices exposed to AR-A014418 alone (134 ± 6%, N = 9, t test p = 0.51, compared with control) (Fig. 4a,b). This recovery of LTP was attributable to AR-A014418, since LTP was not observed in slices pre-exposed to vehicle (0.01% DMSO) for 30 min before incubation with hAβ1–42 (supplemental Fig. 3a,b), and vehicle alone did not affect LTP (supplemental Fig. 3a,b). The finding that AR-A014418 completely blocked the hAβ1–42-induced, augmented tau phosphorylation, as well as impairment of LTP, suggests that activation of GSK-3 and its phosphorylation of tau are required for the effect of Aβ1–42 on synaptic plasticity.
Figure 3.
A specific inhibitor of GSK-3 reduces Aβ1–42-induced phosphorylation of tau. a, Example Western blot showing immunoreactivity to Tau5 (total Tau) and AT8 (phosphorylated Tau). b, Pooled data for AT8/Tau5 ratios normalized to immunoreactivity observed in control conditions in each mouse; *p < 0.05; **p < 0.01. The numbers of mice are shown in parentheses.
Figure 4.
The specific GSK-3 inhibitor AR-A014418 prevents impairment of LTP by human Aβ1–42 in wild-type slices. a, Hippocampal Schaffer collateral-CA1 LTP in wild-type mice in control ACSF (black) or after incubation with AR-A014418 alone (gray) or AR-A014418 followed by incubation with hAβ1–42 (blue). The insets show superimposed example traces before and 35 min after high-frequency stimulation for each condition. Scale bars: 5 ms, 200 μV. b, Summary of results 30–35 min after high-frequency stimulation. Error bars are SEM. The numbers of slices are shown in parentheses.
Discussion
Two principal findings emerge from this work. First, in Tau−/− mice, neither the rodent nor human version of Aβ1–42 impaired hippocampal LTP, in contrast to the robust impairment of LTP they caused in slices from wild-type mice. Second, a specific inhibitor of the tau kinase GSK-3 prevented both the augmented tau phosphorylation and the impairment of LTP that were otherwise seen following Aβ1–42 treatment in wild-type mice.
Because cognitive decline in early AD is associated with pathology in the medial temporal lobe (Braak and Braak, 1991), we investigated LTP at the hippocampal CA3-CA1 synapse, a widely accepted cellular model for learning and memory. Our confirmation that Aβ1–42 impaired LTP in slices from wild-type mice is in agreement with a wealth of previous reports (Cullen et al., 1997; Lambert et al., 1998; Chen et al., 2002; Walsh et al., 2002; Wang et al., 2004; Townsend et al., 2006) showing that soluble Aβ oligomers have an inhibitory effect on LTP. It also suggests that, under our experimental conditions, Aβ assembled into the oligomeric complexes thought to be the synaptotoxic form of the peptide. We show that the magnitude of LTP is not reduced in slices from Tau−/− mice exposed to either human or rodent Aβ1–42. This suggests that tau protein is one element required for the synaptotoxic effects of Aβ1–42, consistent with the finding that tau-depleted neurons do not degenerate in the presence of Aβ (Rapoport et al., 2002). The similar synaptic input–output relationships and unchanged paired-pulse ratios with LTP in wild-type and Tau−/− mice that we observed suggests that this result is not caused by different presynaptic properties in Tau−/− mice, but that the absence of tau uncouples Aβ from its downstream effects that impair the postsynaptic mechanisms required for LTP. In support of this postsynaptic interpretation, a dendritic role for tau in mediating Aβ toxicity was recently reported (Ittner et al., 2010). Nevertheless, this does not exclude a contribution of presynaptic mechanisms; for example, Aβ-induced deficits in axonal transport are prevented in Tau−/− neurons (Vossel et al., 2010).
It is a priority to establish which molecular pathways underlie the Aβ1–42-induced impairment of LTP. We found that the specific GSK-3 inhibitor AR-A014418 can prevent impairment of LTP by hAβ1–42 in wild-type slices. This suggests that GSK-3, via its subsequent phosphorylation of tau (Ishiguro et al., 1993), is a key component in the pathway by which Aβ exerts its pathogenic downstream effects on LTP, similar to Aβ-mediated neurodegeneration (Tackenberg and Brandt, 2009). In support of this, we demonstrate that inhibition of GSK-3 prevents the augmented tau phosphorylation seen following incubation in Aβ alone. However, while our results show that Aβ-induced impairment of LTP is associated with increased levels of tau phosphorylation and that a specific inhibitor of GSK-3 blocks both increased phosphorylation and impairment of LTP, they do not provide conclusive evidence that phosphorylated tau protein mediates this impairment. The results also do not exclude the involvement of other kinases previously implicated in the Aβ-induced impairment of LTP, including c-Jun N-terminal kinase, cdk5, p38 mitogen-activated protein kinase (Wang et al., 2004), and protein kinase A (Vitolo et al., 2002). Nevertheless, the complete recovery of LTP magnitude in the presence of AR-A014418 is striking.
Previous studies have shown a correlation between behavior and synaptic plasticity, with both learning and LTP being impaired in the same animal model of AD (Chapman et al., 1999; Stéphan et al., 2001). Combined with the behavioral findings of Roberson et al. (2007), the present data are the first to link LTP and behavior through tau protein. This both strengthens the link between synaptic plasticity and memory and suggests a mechanism underlying the cognitive dysfunction in early AD. Nevertheless, our data were obtained with acute application of synthetic Aβ peptides, whereas the lack of cognitive deficits was found in hAPP-overexpressing and tau knock-out mice (Roberson et al., 2007); there may be subtle differences between chronic in vivo and acute in vitro exposure. For example, 1–2 d APP overexpression or Aβ exposure can reduce spine density, whereas 1 h exposure to Aβ peptides alters the structural plasticity of individual spines (Wei et al., 2010).
Overall, the findings presented here indicate that the absence of tau prevents the synaptic dysfunction induced by Aβ1–42, and support previous suggestions that tau acts downstream of Aβ (Hardy and Selkoe, 2002), although parallel pathways remain possible (Small and Duff, 2008). These results, using acute exposure to soluble Aβ1–42, may be relevant to early AD, when synaptic dysfunction is present but before extensive plaque formation has occurred. However, later in the disease process the absence of functional tau protein may render the brain more vulnerable to Aβ (Dawson et al., 2010). Nevertheless, the molecular cascades being uncovered suggest that GSK-3 might be a potential drug target for treating synaptic dysfunction and, hence, improving cognitive function during early AD.
Footnotes
This work was supported by the Wellcome Trust and an equipment grant from the Alzheimer's Research Trust (funded by Doris Field Charitable Trust). O.A.S. holds a Wellcome Trust OXION studentship. E.M.T. is a Royal Society Research Fellow. F.D. held a Wellcome Trust Studentship in Neuroscience. R.W.-M. held a Wellcome Trust Research Career Development Fellowship, and the work was supported in part by CurePSP. M.V.-C. holds a Wellcome Trust OXION Training Fellowship. O.A.S., J.R.L., J.D., C.E.J.A., E.M.T. and M.V.-C. conducted experiments; H.N.D, M.P.V. and F.D. generated and maintained the Tau−/− mouse strain; O.A.S., J.R.L. and M.V.-C. analyzed the data. R.W.-M., O.P. and M.V.-C. designed the experiments. O.A.S., O.P. and M.V.-C. wrote the manuscript. All authors discussed the project and approved the final version of the manuscript.
References
- Bhat R, Xue Y, Berg S, Hellberg S, Ormö M, Nilsson Y, Radesäter AC, Jerning E, Markgren PO, Borgegård T, Nylöf M, Giménez-Cassina A, Hernández F, Lucas JJ, Díaz-Nido J, Avila J. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278:45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by β-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer's disease. Neuroscience. 2010;169:516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, Uchida T, Imahori K. Glycogen synthase kinase 3-β is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325:167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Path. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery MR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Small SA, Duff K. Linking Aβ and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stéphan A, Laroche S, Davis S. Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackenberg C, Brandt R. Divergent pathways mediate spine alterations and cell death induced by amyloid-β, wild-type tau, and R406W tau. J Neurosci. 2009;29:14439–14450. doi: 10.1523/JNEUROSCI.3590-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Aβ-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid β from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]