Abstract
The feasibility of using a sensitive polymerase chain reaction (PCR) to evaluate malaria vaccines in small group sizes was tested in 102 adult Gambian volunteers who received either the malaria vaccine regimen FP9 ME-TRAP/MVA ME-TRAP or rabies vaccine. All volunteers received the antimalarial drugs primaquine and Lapdap plus artesunate to eliminate malaria parasites. Volunteers in a further group received an additional single treatment with sulfadoxine-pyrimethamine (SP) to prevent new infections. There was substantially lower T-cell immunogenicity than in previous trials with this vaccine regimen and no protection against infection in the malaria vaccine group. Using the primary endpoint of 20 parasites per mL, no difference was found in the prevalence of low-level infections in volunteers who received SP compared with those who did not, indicating that SP did not reduce the incidence of very low-density infection. However, SP markedly reduced the incidence of higher density infections. These findings support the feasibility and potential of this approach to screen pre-erythrocytic vaccines for efficacy against infection in small numbers of vaccinees in endemic areas.
INTRODUCTION
Increased funding for malaria vaccine development, advances in vaccine technology and the sequencing of the Plasmodium falciparum genome have led to an increasing number of candidate malaria vaccines reaching the phase of clinical evaluation. Although pre-erythrocytic vaccines can be tested using sporozoite challenge with P. falciparum in non-immune volunteers,1–3 it is uncertain how well this model will predict vaccine efficacy in populations exposed to natural infection with genetically heterogeneous P. falciparum and provide evidence of efficacy in phase II studies in populations exposed to natural challenge that may be required before proceeding to a large phase III trial. RTS,S/AS02A, the most widely studied malaria vaccine, has consistently protected 30–45% of volunteers challenged experimentally with sporozoites about 2 weeks after final vaccination. Efficacy of this vaccine against natural challenge with heterologous parasite strains in semi-immune adults in the field was 34% using a time-to-patent infection analysis4 and 30% and 35% after 6 and 18 months, respectively, against clinical malaria in Mozambican children.5,6 These results for RTS,S/ASO2A suggest that for this vaccine, efficacy in the field against patent infection is similar to that measured in the experimental challenge model. A randomized trial of DNA-MVA vaccination undertaken in semi-immune adults in The Gambia in 2002 showed no significant efficacy against infection,7 although the same vaccination regimen resulted in significantly delayed time to parasitemia compared with unvaccinated controls in challenge experiments with a heterologous strain in Oxford and fully protected one of eight non-immune volunteers.8
As long as uncertainty remains about the correlation between the results obtained with the experimental sporozoite challenge model and results obtained under conditions of natural challenge, field-efficacy trials will continue to play an essential part in the early evaluation of candidate pre-erythrocytic vaccines. Because the power of a trial to detect efficacy depends on the number of events observed, more sensitive methods of detection would allow smaller sample sizes to be used. In semi-immune populations, most infections are cleared by blood-stage immunity prior to patency. Comparison of estimates of entomological inoculation rates with observed infection rates detected by peripheral blood microscopy in villages near the town of Farafenni, The Gambia, in 2002 suggested that the great majority of infectious bites did not lead to infections detectable by blood film.7 However, many blood-stage infections may have been missed because of the low sensitivity of microscopy, which has a lower limit of detection of ~10–50 parasites per μL.9 Polymerase chain reaction (PCR) assays that increase the sensitivity of detection of blood-stage malaria infections by at least a hundred fold compared with traditional microscopy have recently been developed.1,3,10
Although PCR monitoring is being used increasingly in the context of malaria vaccine volunteer challenge studies in developed countries,11–14 few such studies have been conducted in endemic areas. In endemic areas, many malaria infections in adults fail to reach a parasite density that is high enough to be detected by microscopy, and the sample size and hence the cost of efficacy trials is typically large, requiring hundreds of volunteers per study arm.5,7 If low-density infections could be detected reliably prior to blood-stage clearance, then the sample size needed for the evaluation of pre-erythrocytic vaccines could be reduced. We have, therefore, undertaken a randomized trial using repeated blood sampling with PCR monitoring to evaluate the feasibility of this method for vaccine evaluation and to make a preliminary assessment of the safety, immunogenicity, and efficacy against malaria infection of the FP9 ME-TRAP/MVA ME-TRAP candidate malaria vaccine, which has shown significant efficacy in volunteer challenge studies in the UK11,15 and promising immunogenicity in Gambian adults.16
MATERIALS AND METHODS
Study area and study population
The study took place in nine villages east of Farafenni, The Gambia, from June to October 2004, the time of year when the incidence of malaria in The Gambia is highest. The entomological inoculation rate in the study area was ~10–50 infectious bites per year. Volunteers of age 15–45 years, identified using the demographic surveillance system (DSS), were invited to take part in the study after prior consultations with local civil and religious leaders. Experienced field workers gave detailed explanations of the nature of the trial in English and in their respective local languages, and written consent was obtained. In the case of volunteers of age 15–17 years, written informed consent was also obtained from a parent or guardian. Prior to screening, the age and identity of each volunteer were checked, and a trained member of the study team provided pre-HIV test counseling. Screening involved a thorough physical examination as well as laboratory evaluation. The latter included measurement of a full blood count (FBC), packed cell volume (PCV), plasma creatinine, and alanine amino transferase (ALT) concentrations as well as HIV 1 and 2 screening by ELISA (Capillus HIV1/HIV2 Kit, Trinity Biotech PLC, Ireland). A glucose-6-phosphate dehydrogenase (G6PD) deficiency test (visual colorimetric assay, Sigma Diagnostics, US) was carried out because of the risk of hemolysis when primaquine is given to volunteers who are G6PD deficient. Volunteers were considered eligible if they had no clinically significant disease. Exclusion criteria included a low PCV (< 30%), raised plasma creatinine (> 130 μmol/L), raised ALT concentration (> 42 IU/L), G6PD deficiency, simultaneous participation in another clinical trial, blood transfusion in the month prior to vaccination, previous experimental malaria vaccination, administration of another vaccine within 2 weeks of vaccination, allergy to any previous vaccination or to sulfadoxine-pyrimethamine (SP), a history of splenectomy, and any treatment with immunosuppressive drugs. Eligible volunteers were assigned a unique study number and a photo identity card.
Study vaccines
Details of study vaccines have been described elsewhere.14 The malaria DNA sequence known as ME-TRAP encodes the entire TRAP antigen of the T9/96 strain of P. falciparum and a string of epitopes from six pre-erythrocytic P. falciparum antigens. The sequence is expressed either in fowlpox (FP9 ME-TRAP) or in modified vaccinia virus Ankara (MVA ME-TRAP). The vaccines were manufactured according to good manufacturing practice by Impfstoffwerk Dessau-Tornau (IDT, Rosslau, Germany). FP9 ME-TRAP was administered at a dose of 1 × 108 plaque-forming units (PFU/mL) given as 2 intradermal injections into the skin overlying the right or left deltoid muscle. MVA ME-TRAP was administered at a dose of 1.5 × 108 PFU/mL given as 2 intradermal injections into the skin overlying the deltoid muscle of the non-dominant arm. Rabies vaccine was administered as two 0.1 mL intradermal injections into the skin overlying the deltoid muscle.
Study design
This was a randomized, open, controlled trial that compared the efficacy of the malaria vaccine regimen with a control (rabies) vaccine. Half the volunteers in the rabies vaccine group received a single treatment with sulfadoxine-pyrimethamine (SP) prior to the surveillance period to provide a “positive” control group in whom protection against malaria could be expected for a period of several weeks. P. falciparum remains sensitive to SP in the study area.
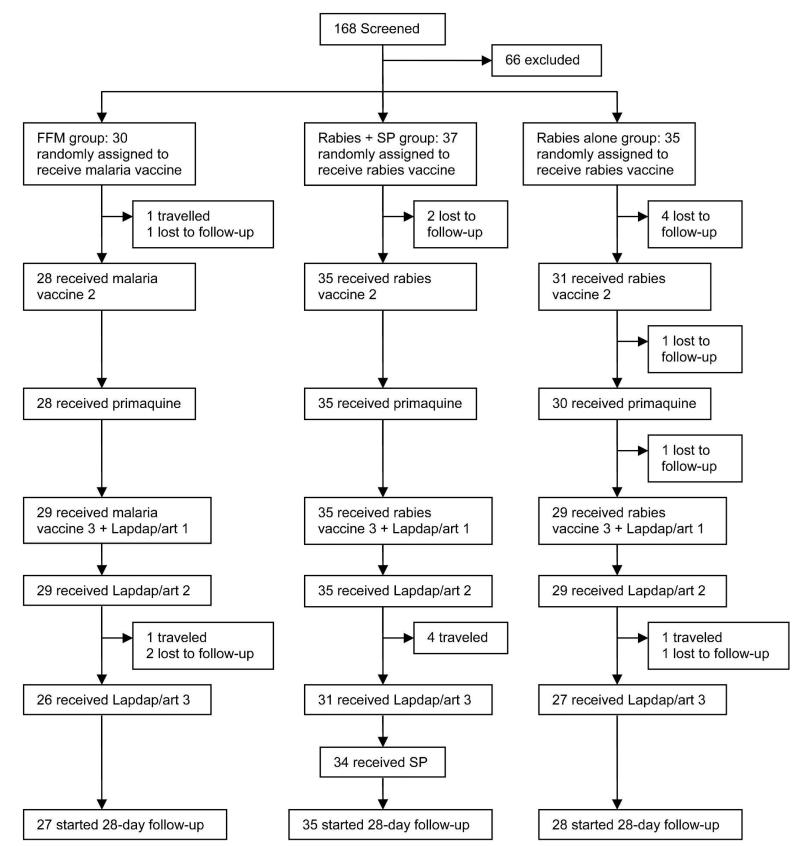
Allowing for a steady rate of drop-out during follow-up amounting to total of 20% of subjects by the end of the trial, the trial had at least 80% power (using 2-sided 5% significance level) to detect a difference in time to infection between vaccine and control groups if the vaccine efficacy was at least 60%, and at least 70% of the control group volunteers developed parasitemia during the trial. A randomization list was generated using a block size of 6; individuals were allocated to treatment groups in a 1:1:1 ratio. Pre-prepared randomization envelopes, numbered 1–120, contained slips with the treatment assignment. On the day on which the first dose of vaccine was due, treatments were assigned according to pre-prepared numbered envelopes. For logistic reasons, 30 volunteers were enrolled in the malaria vaccine group, 37 in the rabies vaccine plus SP group, and 35 in the rabies-alone group (Figure 1). All volunteers were scheduled to receive 3 doses of either malaria or rabies vaccine given 4 weeks apart.
FIGURE 1.
Trial profile.
After vaccination, volunteers were observed for 1 hour to detect any immediate side effects and given a course of anti-pyretic (paracetamol, 500 mg, 3 times a day) to take if required. Field workers made home visits on days 1 and 2 after vaccination, and volunteers were seen by the study physician on days 7 and 28 after each vaccination to record any adverse events on a standard diary card. All volunteers received the antimalarial drug primaquine (30 mg) 7 days before the final vaccination and a 3-day course of Lapdap (2 mg/kg body weight of chlorproguanil and 2.5 mg/kg body weight of dapsone given as standard adult dose) plus artesunate (4 mg/kg body weight divided into 3 doses) commencing on the day of final vaccination to eliminate asexual- and sexual-stage malaria parasites from peripheral blood before surveillance commenced 7 days later. Both dapsone and chlorcycloguanil have half-lives of ~30 hours, so drug levels would have fallen well below inhibitory levels before surveillance started. Clearing existing blood-stage parasites in this manner facilitated the detection of new infections resulting from the bite of an infectious mosquito during the surveillance period. In addition, volunteers in the rabies + SP group received a single treatment of SP when the surveillance period began.
Vaccine efficacy was determined by comparing the incidence of infections in the malaria vaccine and rabies vaccine groups. During the 28-day surveillance period, daily finger-prick blood samples were obtained to provide 0.5 mL of blood for PCR analysis and for preparation of two blood films. Laboratory staff who read blood films or conducted immunoassays and PCR analysis were blind to the group allocation of volunteers until after approval of the analysis plan by the data safety monitoring board (DSMB).
Volunteers could contact a study nurse and a physician at anytime during the course of the study if they had concerns about their health. The study was conducted in accordance with the Declaration of Helsinki principles for the conduct of clinical trials, the International Committee of Harmonization Good Clinical Practices Guidelines, and with the local rules and regulations of the UK Medical Research Council unit in The Gambia and monitored by independent external monitors. An independent DSMB, including a local safety monitor, provided oversight for the trial. The Gambia government/MRC, London School of Hygiene & Tropical Medicine, and the University of Oxford ethics committees approved the study. The trial was registered with ClinicalTrials.gov, a service of the US National Institutes of Health, and allocated the trial number NCT00121823.
Laboratory analysis
Duplicate thick blood films were stained with Giemsa and examined by two microscopists. When discrepancies occurred in the readings, a senior microscopist was available to confirm the presence of parasitemia. Blood samples were collected 1 week after the final vaccination and at the end of the study for estimation of FBC, PCV, ALT, and creatinine. Full blood counts were done using a Medonic CA620 cell analyzer (Medonic, Stockholm, Sweden). ALT and creatinine were measured using a Bio-Merieux visual analyzer (Bio-Merieux, Craponne, France).
Immunogenicity
Ex vivo IFN-γ ELISPOT assays were carried out on days 0, 63, and 150 as described elsewhere.7,11,15,18 The AutoImmun Diagnostika ELISPOT Plate Reader (Strassberg, Germany) was used for automated counting of spots. The results were expressed as the number of spot forming units (SFU) per million peripheral blood mononuclear cells (PBMC). The SFU for a given stimulant was calculated by subtracting the average of the two negative control wells (cells plus culture medium) from the average of the two duplicated stimulant wells (cells plus culture medium plus stimulant). A “positive” response was reported if the SFU were greater than the background and over 50 SFU per million PBMC. Phytohemagglutinin (PHA) was used as a positive control. All peptides were used at a concentration of 25 μg/mL. A single pool contained all ME peptides. Six peptide pools were used to stimulate the cells. These contained 7–10 20-mer peptides overlapping by 10 amino acids and spanning the entire TRAP antigen from the T9/96 strains of P. falciparum (Pool 1 = amino acids 1–110; Pool 2 = 101–210; Pool 3 = 201–310; Pool 4 = 301–395; Pool 5 = 385–495; and Pool 6 = 486–559).
DNA preparation
Finger-prick blood samples for PCR analysis were collected in EDTA Vacutainer tubes. Samples were stored at 4°C for up to 72 hours. The blood volume was recorded, and the samples were flicked to check for blood clots. Large sample volumes were reduced to 0.5 mL after mixing to ensure that whole blood was removed and not plasma alone. Whatman 24-well, double-layer filter plates were used to remove leukocytes from the blood, as described previously, while allowing erythrocytes to pass through.3 Samples under 250 μL were not filtered as it was thought that a low number of parasites might be lost on the filter membrane. Clotted samples were not filtered. Filtered blood was stored at −20°C until DNA extraction. DNA was extracted from the filtered, clotted, or low-volume samples using the QIAamp DNA Mini Blood Kit (Qiagen Ltd., Crawley, UK) with adaptations.3 DNA samples were frozen at −20°C until PCR analysis.
Quantitative real-time PCR
Parasitemia was detected by quantitative real-time PCR using a Rotorgene 3000 machine (Corbett Research, Sydney, Australia) and Qiagen Quantitect SYBR Green I PCR kit, as described elsewhere.3 Samples were analyzed for the presence of the parasite multicopy 18S (small sub-unit) ribosomal RNA genes. Parasite density was quantified using a standard curve of known parasitemia with a limit of sensitivity of 20 parasites/mL.3
Statistical analysis
An analysis plan was approved by the DSMB before analysis of data commenced. Primary analysis of efficacy was according to protocol (limited to subjects who received the full course of vaccination). The time at risk for each volunteer was considered to start 7 days after the third dose of vaccine had been given and to end either on the day of first positive PCR blood sample or, if none of the samples were positive, on the day the last sample was collected. The primary endpoint, set before analysis was undertaken, was time to first parasitemia by PCR with a density ≥ 20 parasites/mL, followed by a second positive result on the next sampling day. This endpoint was the limit of parasite detection by PCR and the most sensitive assay of malaria infection. Secondary analyses were time to first infection with parasite density ≥ 100 or ≥ 1000 parasites/mL and also time to first infection at these densities followed by a second positive result. Vaccine efficacy (VE) was defined as VE = 1 – R, where R is the hazard ratio (malaria vaccine group:rabies group) estimated using the Cox proportional hazards model including all the covariates. A 95% confidence interval for VE was computed. The primary analysis included adjustment for important covariates that were recruitment center, age, bed net use, and the condition of the net. Statistical analysis of the cell-mediated responses induced by vaccination was done using the Wilcoxon signed rank test or the Mann-Whitney U test.
RESULTS
Volunteers
We screened 168 volunteers, of whom 41 were excluded (3 due to low PCV, 28 were G6PD deficient, 2 had a positive HIV test, 6 had clinical abnormalities, and 2 had raised ALT concentrations) and 127 were found to be eligible for the study. Of the eligible volunteers, a total of 102 volunteers were recruited into the study and received the first vaccination. Ninety-three and 94 of the recruited volunteers received the second and third doses of vaccine respectively. Eighty-seven volunteers received all 3 doses (1 volunteer withdrew consent, and 14 others had traveled out of the area at the time of vaccination). Ninety volunteers commenced the 28-day follow-up period (Figure 1). The age range of volunteers 25–45 years was comparable (Table 1). Over the 28-day follow-up period, finger-prick blood samples were obtained each day from an average of 70% of volunteers. Compliance among the study volunteers during the surveillance period was high, with 25 volunteers giving samples on all 28 days (24.5%) and 59 volunteers missing only 5 or fewer time points (57.8%). Eleven vaccinated volunteers gave no samples at all, and the same number gave fewer than 10 samples.
Table 1.
Demographic characteristics of volunteers enrolled in the trial
| Characteristic | Malaria vaccine group (N = 30) n (%) |
Rabies + SP group (N = 37) n (%) |
Rabies group (N = 35) n (%) |
Total (N = 102) |
|---|---|---|---|---|
| Ethnic group | ||||
| Wollof | 4 (13%) | 14 (38%) | 13 (37%) | 31 |
| Mandinka | 21 (70%) | 20 (54%) | 18 (51%) | 59 |
| Fula | 5 (17%) | 3 (8%) | 4 (11%) | 12 |
| Age (years) | ||||
| 15–24 | 16 (53%) | 22 (59%) | 21 (60%) | 59 |
| 25–45 | 14 (47%) | 15 (41%) | 14 (40%) | 43 |
| Bed net | ||||
| Good | 3 (10%) | 1 (3%) | 5 (14%) | 9 |
| Fair | 6 (20%) | 10 (27%) | 13 (37%) | 29 |
| Poor | 10 (33%) | 10 (27%) | 6 (17%) | 26 |
| No bed net | 11 (37%) | 16 (43%) | 11 (31%) | 38 |
Vaccine safety and reactogenicity
All vaccines were safe and well tolerated. All hematological and biochemical parameters remained within normal range for the duration of the study. All solicited adverse events (systemic and local) were assessed as mild or moderate and of short duration. No serious adverse event was recorded. Generally, there was a significantly greater occurrence of solicited adverse events in the malaria vaccine group compared with volunteers in the rabies group (Table 2). There were more episodes of limited arm motion in volunteers after the first dose of the malaria vaccine (23% [7/30]) compared with the control group (1% [1/72], P < 0.001). This side effect was present in 1 of 28 volunteers after the second dose but was not recorded after the third dose of the malaria vaccine. Dry blisters and pain at the site of injection occurred in 53% (16/30) and 50% (15/30) of volunteers after the first dose of the malaria vaccine, but the prevalence of these side effects fell to 24% (7/29) and 21% (6/29), respectively, after the third dose.
Table 2.
Frequency of adverse events after each vaccination
| Adverse effects | FP9 dose 1 | Rabies dose 1 | FP9 dose 2 | Rabies dose 2 | MVA (after 2 doses of FP9) | Rabies dose 3 |
|---|---|---|---|---|---|---|
| Headache | 4 (13%) | 3 (10%) | 2 (8%) | 6 (9%) | 9 (31%) | 7 (11%) |
| (4, 31) | (0, 10) | (1, 24) | (3, 19) | (15, 51) | (5, 21) | |
| Fever | 0 | 0 | 0 | 0 | 0 | 0 |
| (0, 10) | (0, 4) | (0, 10) | (0, 4) | (0, 10) | (0, 5) | |
| Malaise | 2 (6%) | 2 (3%) | 1 (4%) | 0 | 7 (24%) | 11 (19%) |
| (1, 22) | (0, 10) | (0, 18) | (0, 4) | (10, 44) | (9, 29) | |
| Nausea or vomiting | 0 | 2 (3%) | 0 | 1 (2%) | 6 (21%) | 5 (12) |
| (0, 10) | (0, 10) | (0, 10) | (0, 8) | (8, 40) | (3, 17) | |
| Pain | 15 (50%) | 4 (6%) | 13 (46%) | 4 (6%) | 6 (21%) | 1 (2%) |
| (31, 69) | (2, 14) | (28, 66) | (2, 15) | (8, 40) | (0, 8) | |
| Discoloration | 19 (63%) | 19 (26%) | 16 (57%) | 22 (33%) | 8 (28%) | 6 (9%) |
| (44, 80) | (17, 38) | (37, 76) | (22, 46) | (13, 47) | (4, 19) | |
| Itching | 8 (27%) | 5 (7%) | 8 (29%) | 4 (6%) | 2 (7%) | 2 (3%) |
| (12, 46) | (2, 16) | (13, 49) | (2, 15) | (1, 23) | (0, 11) | |
| Induration | 30 (100%) | 25 (35%) | 24 (86%) | 23 (35%) | 24 (83%) | 27 (42%) |
| (90, 100) | (24, 47) | (67, 96) | (24, 48) | (64, 94) | (30, 55) | |
| Blistering | 16 (53%) | 3 (4%) | 7 (25%) | 0 | 7 (24%) | 0 |
| (34, 72) | (1, 12) | (11, 50) | (0, 4) | (10, 44) | (0, 5) | |
| Limited arm motion | 7 (23%) | 1 (1%) | 2 (3%) | 0 | 0 | 0 |
| (10, 42) | (0, 8) | (0, 18) | (0, 4) | (0, 10) | (0, 5) | |
| Total | 30 | 72 | 28 | 66 | 29 | 64 |
Note: Adverse events were assessed 1 hour and 1, 2, 7, and 28 days after each vaccination. Observations were made in 30 volunteers in the malaria vaccine group (FFM), in 37 in the rabies + SP group, and in 35 in the rabies group who received at least one dose of vaccine. Results are presented as n (%) and 95% CI.
Immunogenicity after prime-boost vaccination with FP9 and MVA
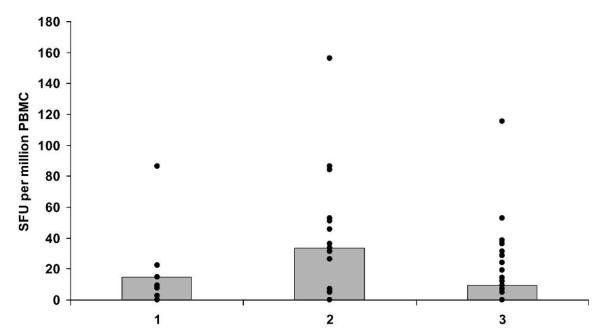
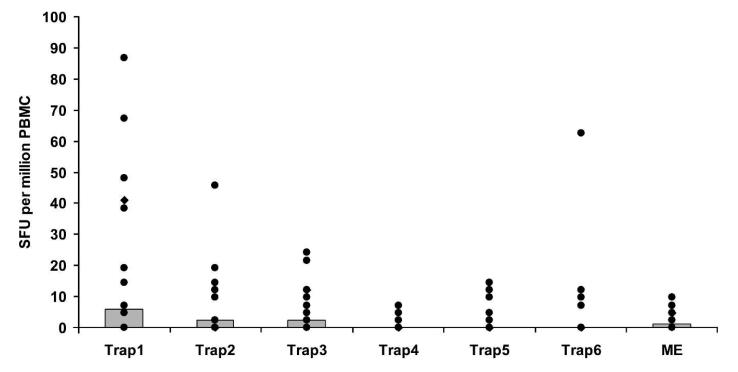
IFN-γ responses to TRAP were measured by ex vivo ELISPOT on freshly isolated PBMC 7 days after the final vaccination in volunteers in the malaria vaccine and rabies groups. Initial samples from a group of 6 randomly selected volunteers were analyzed before vaccination to obtain a baseline reading (median values, Figure 2). One volunteer was excluded from the analysis due to an unacceptably high background level (cells plus culture medium well = 67 SFU per million PBMC). Overall vaccine immunogenicity was unexpectedly low compared with previous studies of this regimen in the UK11,15 and The Gambia.16 Although a significant difference was seen between the malaria vaccine and rabies groups (Wilcoxon signed ranks test: P = 0.044), the responses are ~10-fold lower than in previous studies with this regimen and well below the level associated with protection in volunteer challenge studies.11 Seven of 18 volunteers responded with an ex vivo ELISPOT above the group average. The response to different peptide pools in the ELISPOT assay was measured. TRAP Pool 1 induced the highest response, which was significantly different from Pools 4 and 5 (Wilcoxon signed ranks test: Pool 4, P = 0.006; Pool 5, P = 0.006, Figure 3). Pool 4 contained the least immunogenic peptides, and was significantly less immunogenic than Pools 1, 2, or 3 (Wilcoxon signed ranks test: Pool 1, P = 0.006; Pool 2, P = 0.004; Pool 3, P = 0.002).
FIGURE 2.
Ex vivo IFN-γ ELISPOT responses to P. falciparum TRAP vaccination, 7 days after final vaccination in the malaria vaccine (FFM) and the rabies-vaccinated volunteers. PBMC were stimulated with 6 pools of TRAP peptides containing 7–10 20-mer peptides overlapping by 10 amino acids. Median values (gray boxes) and individual values (black spots) are shown.
FIGURE 3.
Ex vivo IFN-γ ELISPOT responses to P. falciparum TRAP and multi-epitope (ME) peptide pools. Responses are from volunteers in the malaria vaccine (FFM) group 7 days after the final vaccination. Median values (gray boxes) and individual values (black spots) are shown. Significant differences were observed between traps 1 and 2 (P = 0.0058), traps 1 and 5 (P = 0.0058), traps 2 and 4 (P = 0.003), and traps 3 and 4 (P = 0.001). Tests for significance were carried out using the Wilcoxon signed rank test.
Drug treatment
Ninety-three volunteers (91%) received a single dose of primaquine, and 84 (82%) received all 3 doses of Lapdap plus artesunate. Ninety-two percent (34/37) of volunteers allocated to the rabies + SP group received the SP 1 day prior to the start of the 28-day follow-up period. The 3 volunteers who did not receive SP were not included in the PCR analysis.
One volunteer had a parasitemia of ~12,000 parasites/mL on the first day of surveillance and was excluded from further participation in the study and the analysis. Drug treatment was successful in eliminating blood-stage parasites in the remaining volunteers.
PCR analysis
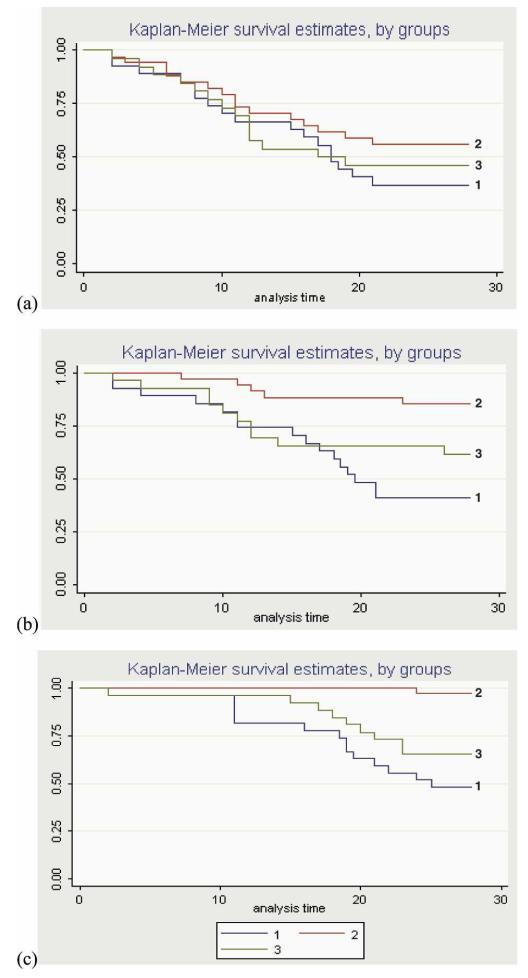
Twenty percent of the total number of blood samples (2033) analyzed were PCR positive. Overall, 71% of the volunteers had one positive PCR result > 20 parasites/mL, and 55% had two positive PCR results above this level. The results of PCR analysis in the study groups at parasite densities of 20, 100, and 1000 parasites/mL are shown in Kaplan-Meier plots (Figure 4a–c). There was no significant difference in time to first infection between the malaria vaccine and the (Figure 4a) rabies groups (adjusted hazard ratios 1.1, 1.75, and 2.3; P = 0.735, 0.184, and 0.056, respectively). Thirty-three volunteers had occasional low-level parasitemia, or “blips,” followed by negative PCR results (maximum, 2,521,353 parasites/mL; minimum, 22 parasites/mL; geometric mean, 152 parasites/mL; median, 87 parasites/mL); these were mainly in the rabies + SP group (malaria vaccine = 7; rabies + SP = 20; rabies = 6). Overall, 28 volunteers remained PCR negative throughout the 28-day follow-up. They were mostly in the rabies-vaccinated control groups (malaria vaccine = 4; rabies + SP = 12; rabies = 11).
FIGURE 4.
Kaplan-Meier survival estimates showing the probability of remaining free of parasitemia during the 28-day follow-up period: estimates were done using thresholds of (a) 20, (b) 100, and (c) 1000 parasites/mL followed by a second positive PCR result. The population used for efficacy analyses included volunteers who received a full course of malaria or rabies vaccines, excluding volunteers who were PCR-positive on the first day of their surveillance (Group 1 = FFM; Group 2 = rabies + SP; Group 3 = rabies alone). This figure appears in color at www.ajtmh.org.
Blood films
Duplicate blood films were prepared at the same time as samples were collected for PCR analysis. Overall 97/2070 (4.7%) blood films were positive for P. falciparum asexual parasites. P. falciparum asexual parasites were detected in 49/399 (12.3%) samples positive at the 20 parasites/mL threshold in the PCR assay, in 48 of 316 positive (15.2%) at the 100 parasites/mL threshold, and in 41 of 178 (43.4%) positive using the 1000 parasites/mL threshold. Thirty-three of the 1634 samples that were negative by PCR were blood-film positive. This represents a 2.0% discrepancy between the PCR and blood-film results. Only 5 cases of clinical malaria were recorded during the follow-up period (1 in the rabies + SP and 4 in the malaria vaccine groups, respectively).
DISCUSSION
This study has demonstrated the feasibility of repeated blood sampling for PCR-based detection of low-level infections, providing a possible approach to the rapid evaluation of pre-erythrocytic malaria vaccines. About half of the Gambian adult men observed over a 28-day period were infected with P. falciparum detected by a sensitive PCR test, although only 28.8% had parasitemia detectable by microscopy.
Participation in this study involved a major commitment from the volunteers who were required to take a combination of drugs to ensure clearance of all asexual and sexual P. falciparum parasites before the surveillance period commenced. The drug combination of Lapdap plus artesunate and primaquine proved effective, as only one volunteer had malaria parasitemia detected by PCR when the surveillance period began. Lapdap and artesunate were chosen to clear asexual parasites, as both are short-acting drugs whose blood concentrations would have fallen below inhibitory concentrations at the commencement of surveillance. In addition to the requirement of taking a mixture of antimalarial drugs, volunteers were also required to provide a daily finger-prick sample for 28 consecutive days. It was uncertain whether this would be acceptable, but > 70% of all possible samples were obtained, and only 21 (20.5%) volunteers withdrew from the study.
Despite the unexpectedly low immunogenicity of the vaccine regimen, we proceeded with the monitoring phase of the trial. As expected, in view of the low immunogenicity, analysis of efficacy using the primary endpoint of 20 parasites/mL showed no evidence for protection against infection in the malaria vaccine group. An opposite effect was suggested when a higher threshold was used, but this was not statistically significant. An unexpected finding was the number of low-parasitemia, short-lived infections detected in volunteers in the rabies + SP group during the first few days of surveillance. It is likely that this was caused by parasites released from liver schizonts before they were eliminated by SP, which is known to clear parasites less rapidly than other drugs, such as the artemisins. Further studies will be required to assess if these observations with SP can be extended to other drugs used for malaria prophylaxis. It is not clear what the potential effects of long-term exposure to low levels of blood stage infections in individuals on long-term drug prophylaxis might be. This population includes not only long-term non-immune residents of malaria endemic countries who take regular prophylaxis but also pregnant women and children receiving intermittent preventive treatment (IPT). Our data suggest that these individuals may be exposed to low-level parasitemia, and it is possible that this could induce some blood-stage immunity over time. Indeed, a recent volunteer challenge study with low doses of blood-stage parasites demonstrated the rapid development of protective immunity to re-infection.19 In addition, a study of IPT in Tanzanian infants using SP also showed that protection lasted for many months longer than the pharmacological effect of SP, suggesting that some protective immunity was acquired while on this prophylaxis.20,21 Our findings suggest a possible explanation for these reports.
We have shown that the use of repeated blood sampling over a short period of time combined with a sensitive PCR assay is a promising approach to the evaluation of malaria vaccines that deserves further study, particularly for preliminary trials in which different doses or vaccine formulations need to be compared. Reduction of the time of follow-up needed for vaccine evaluation to a period of 1 month provides a means of reducing cost and speeding up the evaluation of new vaccines.
Table 3.
Incidence of parasitemia detected by PCR in the two vaccine groups
| Positive defined as | Vaccine | % Positive | Hazard ratio (95% CI) | P value | Hazard ratio adusted for covariates (95% CI)* |
P value |
|---|---|---|---|---|---|---|
| ≥ 20 ppmL | ME-TRAP | 74% (17/23) | 1.14 (0.56, 2.3) | 0.709 | 1.12 (0.53, 2.4) | 0.776 |
| Rabies | 56% (14/25) | 1 | 1 | |||
| ≥ 100 ppmL | ME-TRAP | 70% (16/23) | 1.64 (0.74, 3.6) | 0.219 | 1.75 (0.77, 4.0) | 0.184 |
| Rabies | 42% (10/24) | 1 | 1 | |||
| ≥ 1000 ppmL | ME-TRAP | 81% (17/21) | 2.0 (0.92, 4.4) | 0.082 | 2.3 (0.98, 5.6) | 0.056 |
| Rabies | 42% (10/24) | 1 | 1 |
Adjusted for effects of age, center, bed net use, and ethnic group.
Acknowledgments
The authors thank the field team led by Sheriff Jobe and the volunteers for their patience and understanding; the safety monitor Ousman Nyan; the head of the MRC Farafenni Field Station, Sam Dunyo; members of the Data Safety and Monitoring Board (Diana Lockwood, Richard Hayes, and Automan Gaye); and trial monitors Ceri McKenna and Carol Hall. The contribution of the Malaria Vaccine Initiative at PATH to earlier clinical trials of these vaccines is acknowledged. The Gates Malaria Partnership at the London School of Hygiene and Tropical Medicine, which receives support from the Bill and Melinda Gates Foundation, and the University of Oxford sponsored the study with additional funding from the Wellcome Trust.
Footnotes
Disclosure: AVSH is a co-founder of and shareholder in Oxxon Therapeutics plc, which is developing prime-boost vaccination for therapeutic applications. AVSH is a Wellcome Trust Principal Research Fellow, and EBI was, at the time of the study, a Gates Malaria Partnership Training Fellow. EBI is currently in the employment of the European Malaria Vaccine Initiative.
Contributor Information
EGERUAN B. IMOUKHUEDE, European Malaria Vaccine Initiative, 12 Bell House, Ewen Crescent, Tulse Hill, London, UK, Telephone: +44 (0) 2086748318, Fax: +44 (0) 2032560070, ebimoukhuede@hotmail.co.uk..
LAURA ANDREWS, Wellcome Trust Centre for Human Genetics, University of Oxford, UK, Telephone: +44 (0) 1865 287592..
PAUL MILLIGAN, London School of Hygiene and Tropical Medicine, Keppel Street, London, UK, Telephone: +44 (0) 207927 2126..
TAMARA BERTHOUD, Centre for Clinical Vaccinology & Tropical Medicine, University of Oxford, UK, Telephone: +44 (0) 1865 857444..
KALIFA BOJANG, Medical Research Council Laboratories, Fajara, The Gambia, Telephone: +220 4495442..
DAVIS NWAKANMA, Medical Research Council Laboratories, Fajara, The Gambia, Telephone: +220 4495442..
JAMILA ISMAILI, Medical Research Council Laboratories, Fajara, The Gambia, Telephone: +220 4495442..
CAROLINE BUCKEE, Centre for Clinical Vaccinology & Tropical Medicine, University of Oxford, UK, Telephone: +44 (0) 1865 857444..
FANTA NJIE, Medical Research Council Laboratories, Fajara, The Gambia, Telephone: +220 4495442..
SAIKOU KEITA, Medical Research Council Laboratories, Fajara, The Gambia, Telephone: +220 4495442..
MAIMUNA SOWE, Medical Research Council Laboratories, Fajara, The Gambia, Telephone: +220 4495442..
TRUDIE LANG, Centre for Clinical Vaccinology & Tropical Medicine, University of Oxford, UK, Telephone: +44 (0) 1865 857444..
SARAH C. GILBERT, Wellcome Trust Centre for Human Genetics, University of Oxford, UK, Telephone: +44 (0) 1865 287592.
BRIAN M. GREENWOOD, Gates Malaria Partnership, London School of Hygiene and Tropical Medicine, Keppel Street, London, UK, Telephone: +44 (0) 207 299 407.
ADRIAN V. S. HILL, Wellcome Trust Centre for Human Genetics, University of Oxford, UK, Telephone: +44 (0) 1865 287592.
REFERENCES
- 1.Hermsen CC, Telgt DS, Linders EH, van de Locht LA, Eling WM, Mensink EJ, Sauerwein RW. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol. 2001;118:247–251. doi: 10.1016/s0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 2.Bejon P, Andrews L, Andersen RF, Dunachie S, Webster D, Walther M, Gilbert SC, Peto T, Hill AV. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Dis. 2005;191:619–626. doi: 10.1086/427243. [DOI] [PubMed] [Google Scholar]
- 3.Andrews L, Andersen RF, Webster D, Dunachie S, Walther RM, Bejon P, Hunt-Cooke A, Bergson G, Sanderson F, Hill AV, Gilbert SC. Quantitative real-time polymerase chain reaction for malaria diagnosis and its use in malaria vaccine clinical trials. Am J Trop Med Hyg. 2005;73:191–198. [PubMed] [Google Scholar]
- 4.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WH, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood BM, Hill A, McAdam KP, Tornieporth N, Cohen JD, Doherty T. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 5.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 6.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, Sigauque B, Milman J, Mandomando I, Bassat Q, Guinovart C, Espasa M, Corachan S, Lievens M, Navia MM, Dubois MC, Menendez C, Dubovsky F, Cohen J, Thompson R, Ballou WR. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 7.Moorthy VS, Imoukhuede EB, Milligan P, Bojang K, Keating S, Kaye P, Pinder M, Gilbert SC, Walraven G, Greenwood BM, Hill AS. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 2004;1:e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunachie SJ, Walther M, Epstein JE, Keating S, Barthoud T, Andrews L, Anderson RF, Bejon P, Goonetilleke N, Poulton I, Webster DP, Butcher G, Watkins K, Sinden RE, Levine GL, Richie TL, Schneider J, Kaslow D, Gilbert SC, Carucci DJ, Hill AV. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospodin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006;74:5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood B. The molecular epidemiology of malaria. Trop Med Int Health. 2002;7:1012–1021. doi: 10.1046/j.1365-3156.2002.00980.x. [DOI] [PubMed] [Google Scholar]
- 10.Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB, Jr, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, McConkey SJ, Poulton I, Andrews L, Andersen RF, Bejon P, Butcher G, Sinden R, Skinner MA, Gilbert SC, Hill AV. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci USA. 2005;102:4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, Berthoud T, Andrews L, Andersen RF, Moore A, Gilbert SC, Poulton I, Dubovsky F, Tierney E, Correa S, Huntcooke A, Butcher G, Williams J, Sinden RE, Hill AV. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus Ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect Immun. 2006;74:2706–2716. doi: 10.1128/IAI.74.5.2706-2716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, Maier C, Andrews L, Andersen RF, Gilbert S, Poulton I, Webster D, Dubovsky F, Tierney E, Sarpotdar P, Correa S, Huntcooke A, Butcher G, Williams J, Sinden RE, Thornton GB, Hill AV. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. 2005;23:857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, Andrews L, Bejon P, Poulton I, Butcher G, Watkins K, Sinden RE, Leach A, Moris P, Tornieporth N, Schneider J, Dubovsky F, Tierney E, Williams J, Gray Heppner D, Jr, Gilbert SC, Cohen J, Hill AV. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine. 2006;24:2850–2859. doi: 10.1016/j.vaccine.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AV. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 16.Moorthy VS, Imoukhuede EB, Keating S, Pinder M, Webster D, Skinner MA, Gilbert SC, Walraven G, Hill AVS. Phase 1 evaluation of 3 highly immunogenic prime-boost regimens, including a 12-month reboosting vaccination, for malaria vaccination in Gambian men. J Infect Dis. 2004;189:2213–2219. doi: 10.1086/421118. [DOI] [PubMed] [Google Scholar]
- 17.Webster DP, Dunachie S, McConkey S, Poulton I, Moore AC, Walther M, Laidlaw SM, Peto T, Skinner MA, Gilbert SC, Hill AV. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2006;24:3026–3034. doi: 10.1016/j.vaccine.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, Elliott S, Eisen DP, Weinberg JB, Saul A, Good MF. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 20.Schellenberg D, Menendez C, Kahigwa E, Aponte J, Vidal J, Tanner M, Mshinda H, Alonso P. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet. 2001;357:1471–1477. doi: 10.1016/S0140-6736(00)04643-2. [DOI] [PubMed] [Google Scholar]
- 21.Schellenberg D, Menendez C, Aponte JJ, Kahigwa E, Tanner M, Mshinda H, Alonso P. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet. 2005;365:1481–1483. doi: 10.1016/S0140-6736(05)66418-5. [DOI] [PubMed] [Google Scholar]