Abstract
The term “cerebral small vessel disease” (SVD) describes a range of neuroimaging, pathological and associated clinical features. The latter range from none, to discrete focal neurological symptoms (stroke), to insidious global neurological dysfunction and dementia. The public health burden is considerable. The pathogenesis is largely unknown. Although associated with vascular risk factors, and generally considered to result from an intrinsic cerebral arteriolar occlusive disease, the pathological processes leading to the arteriolar disease, how these result in brain disease, how SVD lesions contribute to neurological or cognitive symptoms and the relationship to risk factors, have been the subject of much speculation. Pathology often reflects end-stage disease making determination of the earliest stages difficult. Neuroimaging provides considerable insights: the small vessels are not easily seen themselves, but the effects of their malfunction on the brain can be tracked on detailed brain imaging. We review the growing evidence for the most likely mechanisms.
Introduction
’Cerebral small vessel disease‘ (SVD) is the term now commonly used to describe a syndrome of clinical, cognitive, neuroimaging and neuropathological findings that are thought to arise from disease affecting the perforating cerebral arterioles, capillaries and venules and the resulting brain damage in the cerebral white and deep grey matter.1 These perforating vessels are essential for maintaining optimal functioning of the brain’s most metabolically active nuclei and complex white matter networks.2
SVD has only recently been recognised for the serious problem that it is. It is very common, causes substantial cognitive,3 psychiatric,4 and physical disabilities5 in older people,6 about a fifth of all strokes7 more than doubles the future risk of stroke,8,9 and contributes to up to 45% of dementias.10 The cost to society is huge. The cause is unknown therefore prevention and treatment, still mostly empirical, are probably suboptimal11,12 or even hazardous.13 Lack of awareness until now may have resulted from i) the large attention given to other stroke mechanisms (i.e. cortical atherothromboembolic and cardioembolic stroke), ii) the cognitive component being overshadowed by Alzheimer’s disease, and iii) most research focusing on individual features of SVD rather than recognising the combined components as one problem.
Why do we know so little about such an important problem? Small vessels are difficult to visualise and investigate in vivo.14 The clinical manifestations are diverse and include sudden onset stroke symptoms or syndromes, recently recognised covert neurological symptoms that include mild, largely ignored, neurological symptoms, signs15 and self-reported cognitive difficulties16 and progressive cognitive decline,6 dementia, depression,4 and physical disabilities.5 Reliance on clinical features and CT scanning to differentiate lacunar from non-lacunar stroke is imprecise and has probably confounded epidemiological and observational studies of risk factor associations.17 Many trials have not differentiated ischaemic stroke subtypes explicitly, potentially overlooking any differences in treatment response between subtypes.18 Death resulting directly from lacunar stroke is rare, so most pathology reflects late stage disease,19 there are few studies of human lacunar stroke pathology,19 and few pathology-imaging correlations.20 Backtracking from a late stage ‘scar’ to the initiating pathology is difficult. Much of SVD is largely clinically silent until late and experimental models are limited by lack of a mechanism to mimic.21,22 Terminology for clinical, imaging23 and pathology24 of SVD is highly varied. For all these reasons, lack of understanding of SVD mechanisms is hardly surprising. Fortunately, standardisation of terminology for imaging features is currently the subject of an international collaboration of experts and due to report in 2013.25 In the meantime, for the purpose of this review, we will use some traditional terms as these were used in the reports that formed the basis for this review.
The pathogenesis of the microvascular and brain abnormality in most SVD is still undetermined and is the focus of this review. We here define SVD as a sporadic intrinsic process affecting small cerebral arterioles, capillaries and sometimes venules. Features of SVD probably develop over many years before becoming clinically evident. The core mechanism underlying SVD-related brain injury is usually assumed to be ischaemia, acting through arteriolar narrowing or occlusion either structural or functional (e.g. vasospasm, impaired autoregulation, or hypotension). However, arteriolar occlusion may be a late stage phenomenon and does not explain the early pathology. Some discussion of specific SVD imaging features will help put the commonest suspected mechanisms in perspective: then we will focus on what we suggest may be a key problem: diffuse cerebrovascular endothelial failure. Specifically, we will summarise evidence suggesting that endothelial damage leads to increased permeability with leakage of material into the vessel wall and perivascular tissue, damage to the vessel wall, inflammation, demyelination, glial scarring, vessel wall thickening and stiffness, impaired autoregulation and at a late stage, luminal narrowing and occlusion, precipitating discrete focal brain parenchymal ischaemia/infarction.
Methods used in this review
We have used systematic reviews where available, searched Medline and Embase extensively for papers on lacunar stroke, for all SVD components in imaging or pathology studies, on the role of reduced blood flow and inflammation, the endothelium, other potential mechanisms, risk factors, in human and animal studies, population-based, cohort studies and clinical trials. The literature search included in our prior systematic reviews extended to the early 1900s. Articles were also identified through searches of the authors’ own files, from conferences, abstract presentations and web sources such as www.strokecentre.org/trials. We included English and non-English language publications where possible. Our focus is on common “sporadic” SVD. We will not discuss any of the rare hereditary forms of SVD (CADASIL, CARASIL, COL4AI, Fabry’s, HERNS) except where these have immediate relevance to sporadic SVD. Nor, for space reasons, will we discuss details of amyloid-associated angiopathy (cerebral amyloid angiopathy) as this has been the subject of recent reviews.26 The final reference list reflects key papers that are most relevant to the broad scope of this review, as space limitations precluded inclusion of many other aspects of potential relevance to pathogenesis of SVD (eg genetic predisposition, or an extensive review of blood pressure).
Recent history of concepts about SVD pathophysiology
Modern concepts concerning aetiology and pathogenesis derive from the seminal post-mortem work of C. Miller Fisher undertaken between 1955 and 1973. His work was based on detailed clinicopathological-vascular post-mortem examinations of 20 patients in whom he studied between one and 50 individual lesions (lacunes, lacunar infarcts, perforating arterioles).27-31 After the introduction of CT scanning in 1973, pathological examination of the brain in patients with lacunar stroke virtually completely ceased.31 Fisher’s pathological studies, mostly conducted long after the original stroke, focused on the lacune (fluid filled cavity) that was thought to represent the originally symptomatic lacunar infarct. The lacune still dominates the field,9 being much more likely to be recognised as responsible for a clinical lacunar stroke than are non cavitated lesions.23
Features of SVD on MR imaging
The main imaging features of SVD, now recognised all to be inter-related, visible on conventional magnetic resonance (MR) imaging (MRI) at 1.5 or 3T, include acute lacunar (or small subcortical) infarcts or haemorrhages, lacunes (fluid-filled cavities thought to reflect old infarcts, many clinically silent),9 white matter hyperintensities (WMH, in which many investigators include small deep grey matter hyperintensities, also mostly clinically covert),32 visible perivascular spaces (PVS),33 microbleeds,34 and brain atrophy (Figure 1).35 Other emerging features detectable at higher field strengths include microinfarcts.36,37 Additional “sub-visible” damage detectable on advanced MRI (e.g. diffusion tensor imaging, DTI, magnetization transfer ratio, MTR) includes altered white matter integrity and disrupted axonal connections,38,39 increased brain water content,40 altered myelination,39 and secondary focal thinning of the cortical grey matter.41 We will briefly describe the imaging and the related clinical components of SVD that are particularly germane to understanding the underlying pathophysiology. We will start with lacunar stroke and acute lacunar infarction because, by causing sudden discrete focal neurological symptoms, it provides a useful ‘alert’ to the presence of SVD and might allow the disease to be caught earlier in its development than in patients presenting with late stage global brain dysfunction.
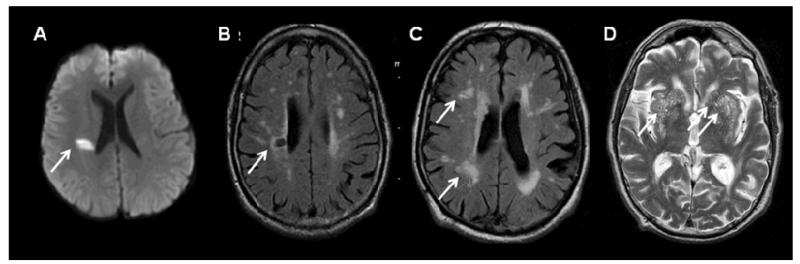
Figure 1.
Key imaging characteristics of features of SVD. A) diffusion-weighted image of an acute small deep (‘lacunar’) infarct (arrow): <2cm diameter, hyperintense on diffusion imaging, FLAIR and T2-weighted imaging, hypointense on T1-weighted imaging. B) Lacune on FLAIR imaging: a CSF-containing cavity, >3mm and <1.5cm diameter, in white or deep grey matter or brainstem, signal characteristics of CSF on other sequences. C) WMH on FLAIR imaging: hyperintense areas on FLAIR and T2 in white and deep grey matter and brainstem, occasionally hypointense on T1 but often not visible, may coalesce when numerous. D) Perivascular spaces on T2-weighted imaging, hyperintense due to containing CSF-like fluid, <3mm diameter, round or linear in white or deep grey matter, visible on T1-weighted imaging when prominent.
Lacunar infarction
The lesion underlying most lacunar strokes42 is an infarct, rounded, ovoid or tubular in shape, less than 20 mm in axial diameter. Tubular lesions seem to be more likely in the basal ganglia/internal capsule, as noted by Fisher31 (Figure 2, Supplementary Figure 1). A small proportion (5%) are due to a small deep haemorrhage. An acute lacunar infarct is of increased signal on diffusion weighted imaging (DWI), reduced signal on apparent diffusion coefficient (ADC) map, and of increased signal on FLuid Attenuated Inversion Recovery (FLAIR), T2-weighted imaging, reduced signal on T1-weighted MRI, and low attenuation on CT scanning, compared to normal grey or white matter. Only about 50% of recent infarcts are visible on CT,43 whereas at least 70% are visible on MR DWI.44 Stroke subtyping on clinical features alone is imperfect, misdiagnosing about 20% of acute lacunar clinical syndromes as cortical stroke and vice versa,17 and will lead to ‘noise’ in studies that do not include DWI. The original size definition was established from pathology which, being late stage, underestimated the size of acute lesions (Figure 3). Imaging shows that acute lesions are usually larger than old lesions, has questioned the maximum permissible sizes of acute lacunar infarcts45 and emphasised the importance of noting the age of the lesion (Figure 3). Acute infarcts generally shrink to either leave a small cavity (lacune) or a small lesion of similar signal characteristics to a WMH or occasionally disappear (Figure 3). Acute lesions larger than 20mm axial diameter are likely to be striatocapsular, i.e. due to middle cerebral artery (MCA) embolism/occlusion or atheroma occluding multiple perforating arterioles.46 However lesions that were quite definitely striatocapsular infarcts when acute can shrink markedly to leave only a small lacunar-like cavity. Thus, it is easy to see why pathology or late stage imaging studies would associate atheromatous or embolic disease with lacunar infarction (Supplementary Figure 2). Probability mapping shows that the main location of acute DWI-proven symptomatic lacunar infarcts is in the primary motor and sensory pathways (note distribution in all images shown),47 explaining why such small lesions present as stroke whereas other SVD lesions accumulate ‘silently’ but otherwise have very similar long term appearances. Fisher also found that location in the internal capsule and not size determined whether the lesion had been symptomatic in life or not.19
Figure 2.
An acute small deep (lacunar) infarct on diffusion imaging (serial axial views from basal ganglia to centrum semiovale, left to right) and T1-weighted imaging (coronal view, right). Note the tubular shape in the coronal plane as the infarct follows the line of a perforating arteriole. A wider range of examples of acute small deep (lacunar) infarcts is shown in Supplementary Figure 1.
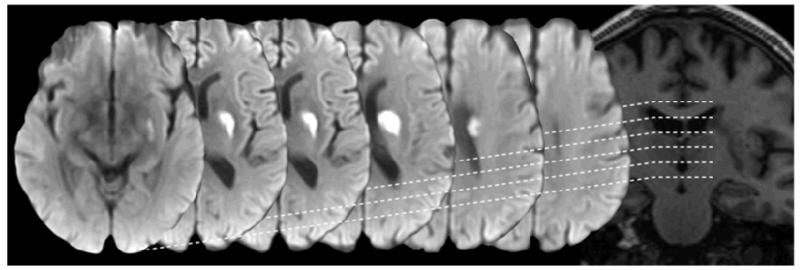
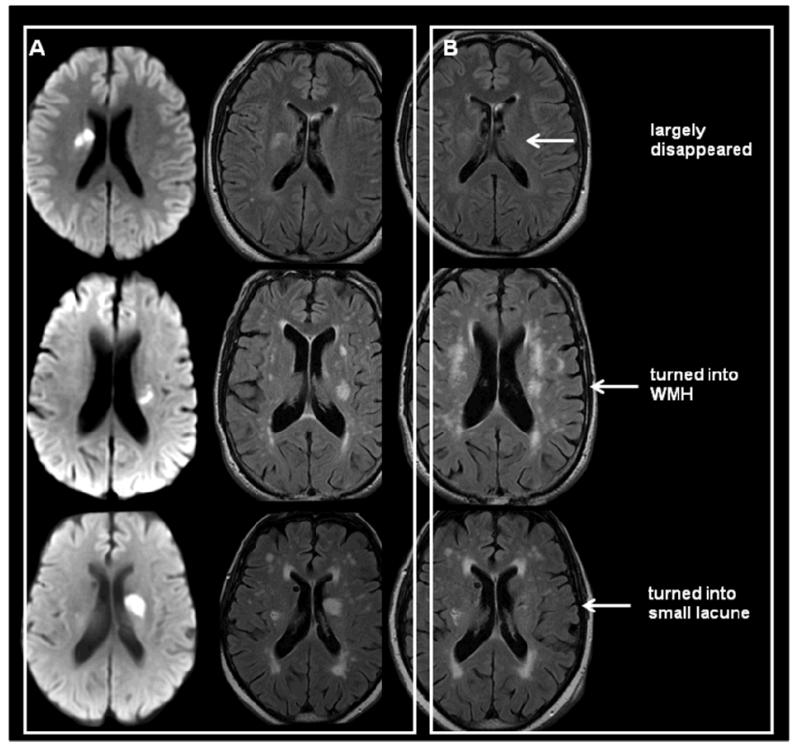
Figure 3.
Illustrating common late sequelae of acute small deep (lacunar) infarcts. Acute stage DWI (left column), FLAIR (middle column) and about one year later FLAIR (right column). These can: disappear (top), look like a WMH indefinitely (middle), or cavitate to create a lacune (bottom).
Lacunes are small cerebrospinal fluid-containing cavities located in the deep grey or white matter, typically larger than 3mm and (most consider) smaller than 15mm in diameter (Figure 1 and 3). Lesions larger than 15mm in some literature are considered as lacunes, but in general the larger the lesion the more likely that the lesion was caused by mechanisms other than SCD (Supplementary Figure 2). Many lacunes were never symptomatic but appear silently in the brain (Supplementary Figure 3 for pathology examples).9 The proportion of definite DWI-confirmed acute lacunar infarcts that progress to lacunes varies from about 28% to 94%48,49 depending on how cavitation is defined and other as yet undetermined factors including duration of follow-up. Whatever the true proportion, not all lesions cavitate: some quite large acute DWI-confirmed lacunar infarcts disappear completely (Figure 3) while others appear long term like a non-cavitated WMH.
White matter hyperintensities (WMH) are rounded areas of decreased attenuation on CT, increased signal on T2-weighted and FLAIR, often decreased on T1-weighted MR imaging with respect to normal brain, but not as attenuated or intense as CSF (Figure 1 and 3). They are distributed in the periventricular and deep white matter of the cerebral hemispheres, in the basal ganglia (i.e. deep grey matter), in the pons and occasionally in other parts of the brainstem and cerebellar white matter. They are almost always symmetrically distributed and are usually numerous in the cerebral hemispheres before appearing in the brainstem. Eventually, when very numerous, they coalesce. It is unclear whether differential periventricular or deep distribution reflects distinct mechanisms or just different disease stages. WMH are more common and more extensive in patients with acute lacunar stroke (vs. other stroke subtypes),32 associated with lacunes,50 perivascular spaces,33 microbleeds34 and brain atrophy.35
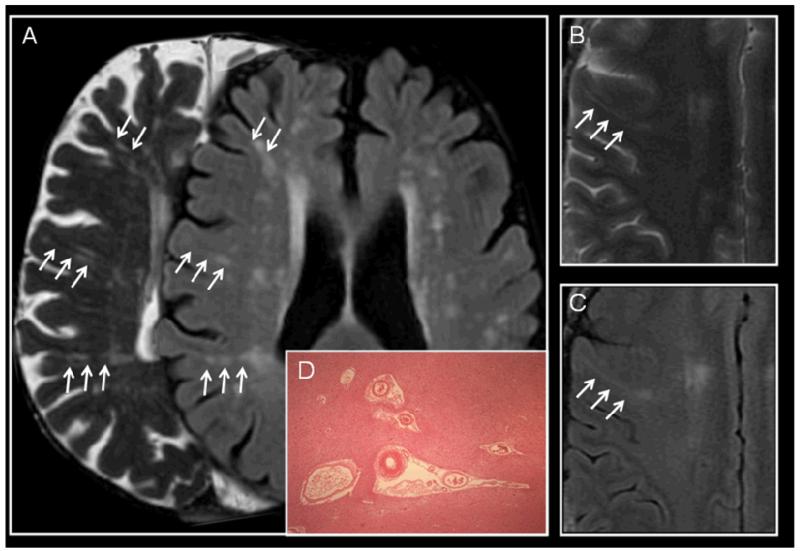
Virchow-Robin, or visible perivascular spaces (PVS) surround the small deep perforating arterioles as they pass through the deep grey and white matter, made visible on T2- or T1-weighted MR by containing increased fluid of similar signal to CSF. On MR imaging, PVS appear round where perpendicular to and linear where parallel to the imaging plane, so typically on axial imaging are round in the basal ganglia and linear in the subcortical white matter of the lateral parts of the temporal, parietal and frontal lobes (Figures 1 and 4). While a few visible PVS may be normal at any age,51 many are not normal.33,52,53 Visible PVS around perforating arterioles, although observed for many years histologically in older people often in association with other SVD features, were often dismissed as an artefact of tissue processing (Figure 4). The relevance of PVS to SVD is illustrated by their presence in larger numbers in association with WMH33,53 and with symptomatic lacunar ischaemic stroke.33,54 An increase in their number also indicates active inflammation, e.g. in multiple sclerosis (where their diameter also increased during active inflammation)55 and in lacunar stroke.56 They are not simply a consequence of global brain atrophy53 as they are frequently seen in patients who have little atrophy, although they might be an alternative manifestation of atrophy.
Figure 4.
Examples of perivascular spaces (PVS) on MRI and histology. (a) 72 year old asymptomatic subject, right, T2-weighted image shows linear PVS in the plane of the image, and on left FLAIR shows WMH around the PVS; (b) 49 year old man with left internal capsule acute small deep infarct (not shown) on T2-weighted imaging shows a perivascular space extending from the periventricular to subcortical tissues and (c) on the corresponding FLAIR image, one WMH running longitudinally around the PVS. (d) PVS on histology (H&E x40) showing parenchymal tissue retraction from around small perforating vessels; these have been dismissed as a processing artefact but are typically seen in ageing brain sections, and often associated with SVD.
Other features of SVD include microbleeds, which are small punctuate areas of hypointensity on T2* or susceptibility-weighted imaging measuring up to 10mm in diameter corresponding to small collections of haemosiderin-laden macrophages around small perforating vessels.20,57 Microbleeds are associated with lacunar stroke and WMH,34,58 and are clearly part of the spectrum of SVD. As the focus of this review is the aetiology of the non-haemorrhagic components of SVD, space precludes detailed consideration of microbleeds. However, we refer the reader to several excellent recent comprehensive reviews of microbleeds59 and cerebral amyloid angiopathy.26
Mechanisms underlying most acute lacunar infarcts, lacunes and WMH
The commonest abnormality described pathologically19,27,30,31 is a diffuse, intrinsic disease of the smaller (40-200μm diameter) arterioles, referred to by Fisher as arteriolosclerosis, lipohyalinosis or fibrinoid necrosis depending on its severity, which he thought largely to result from hypertension. The vessel wall changes include infiltration of plasma components and inflammatory cells into the vessel wall and perivascular tissue with resulting vessel wall and perivascular brain tissue damage (Figure 5). Many of these features were recognised over 100 years ago.60 The mechanisms are largely unknown but this process has been variously attributed to ‘microatheroma’ (ie diffuse deposition of lipid in arteriolar walls, hence ‘lipohyalinosis’), or to be entirely a consequence of hypertension, vasospasm, or more recently to be a consequence of subtle endothelial failure. We will return to consider these mechanisms shortly, but first we will discuss what role, if any, there is for the well established ischaemic stroke mechanisms of embolism, atheroma, and for vascular risk factors, in causing lacunar infarcts and WMH.
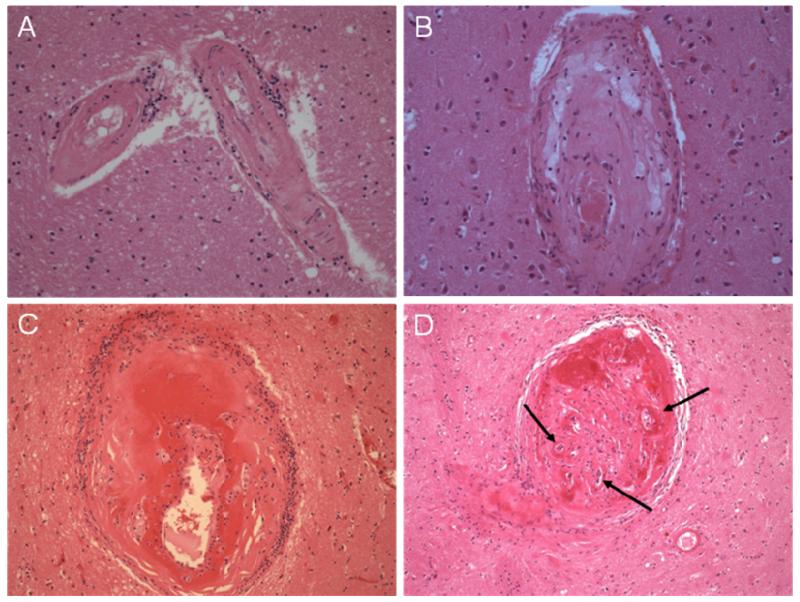
Figure 5.
Histological appearances typical of arterioles affected by SVD pathology, from early arteriolosclerosis through to fibrinoid necrosis (all H&E x200). a) Arterioles where the smooth muscle is being replaced by collagenous tissue and there are small clusters of perivascular inflammatory cells. B) Lipohyalinosis with collagenous thickening of the vessel wall, foamy macrophage deposition and inflammatory cell infiltrate; the residual lumen contains some post mortem thrombus. C) Fibrinoid necrosis with segmental vessel wall destruction and prominent surrounding inflammation; the endothelium is not visible and there is some aneurysmal vessel wall dilatation. d) Severely disrupted arteriole with evidence of previous occlusion and recanalisation, arrows.
Fisher suggested that atherosclerosis and embolism affected the largest perforating arterioles (200-850μm diameter).31 The size of the lacunar infarct was thought to be related to the size of the affected arteriole and, as the larger lesions were thought to be more likely to cause symptoms, it seemed logical that the symptomatic lacunar infarcts would be due to atherosclerosis or embolism in the larger arterioles and the silent ones would be smaller and due to lipohyalinosis or fibrinoid necrosis in the smaller arterioles. However, as suggested above, that conclusion may have resulted from interpreting a small cavity left after a striatocapsular infarct as the sequelae of a lacunar infarct (Supplementary Figure 2).
Emboli
An acute lacunar infarct can be caused by embolism, but no more than 10-15% of lacunar infarcts and few WMH can be traced to emboli across a range of individual cohort studies and meta-analyses.61-63 In experimental models, very few emboli (<6%) injected into the carotid arteries enter the perforating arterioles.21,64 Compared with non-lacunar ischaemic stroke, lacunar stroke is much less likely to be associated with overt embolic sources (atrial fibrillation or proximal ipsilateral carotid stenosis).61,62 Tight internal carotid and intracranial artery stenosis is equally frequent on the contralateral as on the ipsilateral side to an acute lacunar stroke suggesting that the stenosis is unlikely to have caused the infarct.62 Similarly, there is little if any direct association between ipsilateral carotid stenosis and WMH.63 The few acute lacunar infarcts that are due to emboli are more likely to be in the basal ganglia, but in all other respects appear similar to acute lacunar infarcts due to intrinsic arteriolar disease.65
Atheroma of the parent artery or perforating arteriole
Amongst his 20 detailed clinico-pathological examinations, Fisher described 10 lacunar infarcts in the MCA territory of which six were attributed to atheroma in a perforating arteriole, two to lipohyalinosis and two to embolism (because the arteriole appeared normal and the embolus was assumed to have dissolved).31 To put this in perspective, he also described 45/50 lacunar infarcts where the supplying arteriole showed lipohyalinosis or fibrinoid necrosis. Infarct patterns associated with atheromatous MCA stenosis vary between studies, perhaps related to patient selection; many appearing large enough to be striatocapsular rather than true lacunar.66 In the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial to reduce recurrent stroke in patients with intracranial artery stenosis, only 38/347 (11%) were randomised in the trial following a lacunar stroke the rest being cortical territorial; all recurrent strokes in the 11 patients entering WASID with a lacunar index stroke were non-lacunar.67 Thus while MCA stenosis may occasionally lead to lacunar stroke,68,69 most strokes (>90%) in patients with intracranial stenosis are non-lacunar.67 Intracranial stenosis, although apparently common in some ethnic groups, is very uncommon (<1-2%) in Caucasians70 and yet 25% of ischaemic strokes are lacunar in type in Caucasians. A potential association with atheroma has lead to the suggestion that there may be two different subtypes of lacunar stroke,71,72 those due to proximal perforating arteriolar atheroma and those due to lipohyalinosis/arterioloscerosis, the former thought to be more likely if the infarct is larger, in the proximal basal ganglia, associated with progressing symptoms,73 a perfusion abnormality on MRI74 and poor prognosis, and the latter when there are additional features of SVD (WMH, lacunes, e.g. see Supplementary Figure 1).75 Whether or not there are two different subtypes of lacunar stroke, and how reliably they can be differentiated, is at present, unclear. However it may be important to differentiate any due to focal atheroma from non-atheromatous lacunar ischaemic stroke as patients without atheroma may get less long term benefit from anti-atheromatous treatments.13,18,76 We found that lacunar infarct size (</>15mm), shape (ovoid/tubular) or location (basal ganglia/centrum semiovale)65 did not vary with a range of vascular risk factors (e.g. hypertension, hypercholesterolaemia, diabetes), although basal ganglia lesions more often had any proximal embolic source (e.g. carotid stenosis, atrial fibrillation; 13 vs 4%) than centrum semiovale lesions (although note that the absolute proportion, 13%, is still very low). The perforating arteriolar lumen and atheroma in the perforating arteriole wall are difficult to identify on conventional imaging. High field MR (7T) may identify microatheromatous perforating arteriolar plaques45 but the reliability and relevance is yet to be determined.77 However, high definition imaging of perforating arterioles to identify mini atheromatous plaques in the proximal perforating arterioles or around the arteriolar ostium, or follow longitudinal changes in arteriolar patency, will be a powerful tool both to diagnose microatheroma where it is likely to be causative in acute lacunar ischaemic stroke and for research into SVD mechanisms.
‘Traditional’ vascular risk factors
Here, relationships with SVD are still not completely understood. Hypertension, diabetes mellitus, hypercholesterolaemia and smoking are equally common in cortical atherothromboembolic as in lacunar stroke.61 Many patients with SVD are not hypertensive, e.g. amongst 70 consecutive autopsies in patients with pathologically-verified SVD, vascular risk factors were mostly absent.78 Many sporadic cases and the monogenetic variants of SVD occur in normotensive patients. Hypertension is a key risk factor for WMH79-83 but the relationship with blood pressure (BP) is complex. Elevated BP some years previously appears to be more strongly associated with WMH in some studies than concurrent BP, although it is unclear whether diastolic84,85 or systolic82 BP are more important. Both prior and concurrent systolic and diastolic BP predict WMH progression in some81 but not other86 studies. Effective antihypertensive treatment is associated with reduced progression of WMH in observational studies.79,80,83 However in randomised controlled trials, antihypertensive treatment has shown limited,87 or no11 effectiveness in slowing WMH progression. This may be related to the short intervention period or youngish age range of the included patients. Both white matter tract integrity88,89 and WMH burden90-93 are highly heritable. It is possible that the association between SVD and BP is at the genetic locus level rather than being directly causal.94,95 However this finding in a study of SVD-gene associations in families has yet to be replicated. Nonetheless, hypertension is likely to add harm and is at least modifiable. The point is not to devalue the role of BP in SVD, but rather not to overlook other mechanisms. If the apparent WMH-hypertension relationship is partly explained by genetic co-association, then antihypertensive treatment may not be as effective in preventing SVD as is hoped, even if it is very effective in preventing large artery atheromatous disease. On the other hand, risk factors like hypertension and smoking may particularly exacerbate SVD in those with any vulnerability.96 Hence prevention of exacerbating risk factors like hypertension and stopping smoking may be particularly effective if SVD vulnerability can be identified.
In terms of other common vascular risk factors, concurrent smoking also appears to be a key risk factor for WMH and their progression,97 whereas statins did not prevent WMH progression.12 Clopidogrel plus aspirin versus aspirin alone caused an excess of haemorrhage (mostly systemic) and death in patients with lacunar stroke,13,76 possibly because patients with lacunar as opposed to other ischaemic stroke subtypes lacked any large artery atheromatous disease to prevent (eg myocardial infarction, large artery stroke, etc),18 further evidence of the largely non-atheromatous nature of SVD. The role of homocysteine, B12 and folate are complex. While homocysteine appears not to be related to large artery atherothrombotic disease (eg myocardial infarction), there may be an association with SVD.98 There is some evidence of slowing of WMH progression with B12/Folate supplementation in patients with more severe WMH.99 Further data on homocysteine, B12 and folate are needed.
If not ‘traditional’ ischaemic mechanisms, then what?
Some years ago we observed that in the centre of an acute symptomatic lacunar infarct it was sometimes possible to see an abnormal perforating arteriole.14 The arteriolar wall appeared thickened as it passed through the ‘infarct’ not proximal to it (as one might expect with a typical cortical atherothromboembolic infarct), with signal indicating thrombus in the lumen and blood products in and around the arteriole wall. This lead to scrutiny of Fisher’s original descriptions of the microvascular pathology.100 This suggested that a diffuse process that started in the endothelium and consisted of failure of the cerebral arteriolar and capillary endothelium to function effectively as a barrier, could explain the observed arteriolar wall infiltration, thickening and perivascular tissue changes. The concept emerging from these new observations was that the loss of normal endothelial function would result in leakage of plasma fluid components and migration of cells into the vessel wall with disruption of the normal architecture including damaged arteriolar smooth muscle and fibrin deposition (recognised as lipohyalinosis and fibrinoid necrosis, Figure 5).101 The arteriolar wall changes would be patchy and diffuse and result both in vessel lumen dilation and narrowing, as described by Fisher, and stiffened vessels with loss of normal autoregulation, as long suspected in SVD.102,103 In earlier stages, the perivascular oedema, which is toxic to neurons, glia, astrocytes, in fact all brain cells,101,104 would lead to insidious perivascular cumulative tissue damage resulting eventually in the rarefaction and demyelination seen pathologically in WMH on pathology105 and imaging.106 It is interesting that the pathological appearance of non-cavitated lacunar lesions (Supplementary Figure 3b) has long been interpreted as infarction even when the arteriole in the centre of the lesion was not occluded and there was little specific indication of ischaemia. In capillaries, where there is no smooth muscle layer, the endothelial failure and leakage of plasma components into the tissue would result more directly in oedema and tissue damage. In arterioles, the endothelial disruption, vessel wall thickening and luminal distortion would lead eventually to secondary perforating arteriolar thrombosis, luminal occlusion and traditional ’infarction’ (Figure 5). Loss of the normal autoregulatory ability in the thickened, stiffened vessels would contribute further to tissue damage through reduced ability to vasodilate when required, leading to “ischaemic” changes107 superimposed on the endothelial failure.108
Proposed role for early endothelial failure
Since then, much more evidence for this hypothesis as a primary precipitant of sporadic SVD has accumulated. At the capillary level, the endothelium forms a key part of the blood-brain barrier (BBB),109 a phylogenetically important structure for conserving neuronal function present from drosophila to man.110 The BBB is commonly regarded as a nuisance111 because, by impeding the passage of many drugs into the brain, it limits potential therapeutic approaches. This, combined with a paucity of methods for studying its activity in vivo, means that our understanding of the role of cerebrovascular endothelium including the BBB in disease pathogenesis is really only in its infancy.112
The cells of the cerebrovascular endothelium are joined together by tight junctions made up of the occludins and claudins. The BBB is functionally a more complex structure than just the endothelium, encompassing various basement membranes, associated with the perivascular space,113 pericytes,114 the glia limitans and astrocyte end feet.109,115 These components are all inter-related, contribute to the barrier function and are important to consider when trying to understand the aetiopathogenesis of SVD (Figure 6). Autoregulation largely occurs at arteriolar level, but local rapid blood flow responses to neuronal activity also involve capillary responses mediated through pericytes.114,116 In this context, it is worth remembering that there are more glia, endothelial cells and pericytes in the brain than there are neurons, the surface area of the cerebrovascular endothelium approximates that of a tennis court and around 20% of the cardiac output at rest is required to service the metabolic demands of the brain.114 It should therefore be no surprise that minor changes occurring over many years could have profound effects on the cerebrovascular endothelium and in turn on brain function.117 In many regards it is more surprising that the impact is not greater.
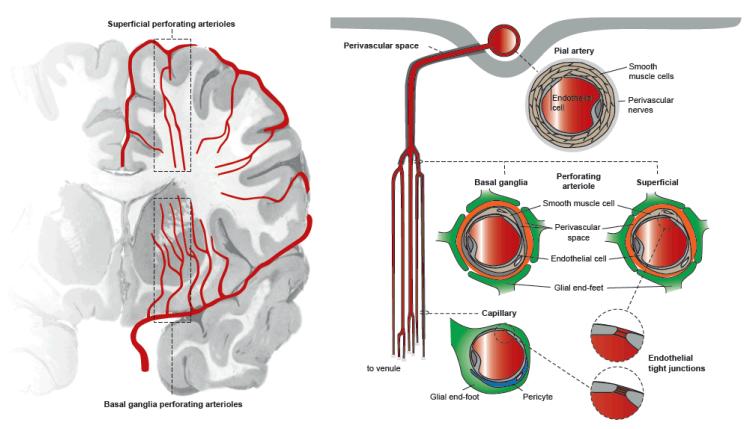
Figure 6.
Schematic diagram of the basal ganglia and superficial perforating arterioles showing key features of the arteriolar and capillary wall. Perforating arterioles show a branching pattern that resembles that of poplar trees rather than oak trees. Arterioles have a smooth muscle layer and are surrounded by perivascular spaces that are delineated by membranes related to pial membranes: there are two layers around basal ganglia arterioles but one layer around superficial perforating arterioles.118,119 The capillary endothelium forms the blood-brain barrier and is closely related via pericytes, microglia, astrocytes and glial cells to neurons; the endothelium continues in the arterioles but at the arteriolar level, the endothelial cell tight junctions are less ‘tight’ than at the capillary level.109 Hence arteriolar walls are less protected from the consequences of endothelial failure than are capillary walls; tissue around the basal ganglia arterioles is more protected from the effects of vascular disease than is tissue around the superficial perforating arterioles. The figure was prepared by Antonia Weingart, Institute for Stroke and Dementia Research, University of Munich.
Two points may help explain how SVD might produce apparently different lesions in different brain and vascular tree locations (Figure 6). First, the endothelial tight junctions are tightest in capillaries where the barrier function is most important, and relatively looser in arteriolar and venular endothelium.109 The hypothetical consequence is that the effects of endothelial failure would be seen earlier in the larger proximal perforating arterioles than in capillaries, leading for example as seen in some patients, to differential development of proximal arteriolar disease and lacunar infarcts in the basal ganglia in advance of more diffuse WMH in the centrum semiovale where tissue is served by smaller arterioles and capillaries. The second related point concerns how the anatomy of the perivascular spaces differs by location, the basal ganglia perforating arterioles having two leptomeningeal layers whereas arterioles entering the deep white matter from the superficial cortex having one leptomeningeal layer.118,119 Perivascular spaces drain interstitial fluid (Figures 4, 6).120 MR imaging demonstrates that basal ganglia and centrum semiovale visible PVS are highly correlated with each other; additionally, basal ganglia PVS are associated with lacunar stroke, while total brain PVS (ie basal ganglia and centrum semiovale combined) are associated with WMH.33,54 The regional differences in PVS anatomy could explain this complex association, with SVD pathology leading to visible PVS as well as perforating arteriolar damage and lacunar infarcts mainly in the basal ganglia, whereas multiple WMH tend to form mainly in the centrum semiovale.
Why should the endothelium fail?
As exemplified by the BBB, the cerebrovascular endothelium becomes increasingly permeable with normal ageing:121 normal subjects aged 70-80 have a near two-fold more permeable BBB compared with younger subjects. Up to age 60, the increase in BBB permeability per decade is probably less marked, suggesting that, as with many ageing-related features, loss of endothelial integrity may start in different people at different ages and progress at different rates, with some evidence of an exponential rather than linear decline in function with advancing age.121 Any process that aggravates endothelial failure or contributes to damage outside the endothelium may also have progressively worse effects with advancing age.122
In addition to advancing normal age, a range of non-specific stimuli, as yet not well understood, affect the cerebrovascular endothelium.117 For example, in experimental models, non specific peripheral pain, e.g. pain to a foot pad in the rat, opens the BBB.123 Amyloid increases BBB permeability,124,125 a possible explanation for the altered BBB permeability detected in AD.121 Inflammation affects the BBB,126 and is a prominent, long-observed although poorly understood component of SVD: inflammatory cell infiltrates were noted pathologically over 110 years ago in the penetrating arterioles and perivascular tissue in SVD,60 and consistently ever since (Figure 5, Supplementary Figure 3);19 plasma inflammatory markers are elevated in lacunar stroke56,127 and also associated genetically with WMH progression and lacunar infarcts.128 However the cause of the inflammation, whether indicating non-specific responses to peripheral systemic stimuli,117 or systemic inflammation, or brain specific,103,109,129 is unknown. High dietary salt intake raises blood pressure and increases stroke risk,130 although the salt-stroke association is non-linear and not wholly accounted for by hypertension.131 High dietary salt exacerbates oxidative stress in salt-sensitive people;132 reduced salt intake not only reduced BP but also reduced oxidative stress and vascular stiffness.132 Salt affects human vascular function by reducing nitric oxide and impairing vasodilatation.133 In an animal model of spontaneous SVD, even modest short term salt exposure exacerbated inflammation, oxidative stress and small vessel pathology and to a lesser but nonetheless measureable extent in control rats without raising BP.134,135 Perivascular spaces drain interstitial fluid from the brain parenchyma120 and dilate visibly on MR in overt inflammatory neurological disorders like MS,55,136 in SVD (Figure 1),56 and associate with increased endothelial permeability in SVD.122 Interestingly,WMH appear around PVS on imaging (Figure 4), suggesting focal failure of interstitial fluid drainage136 with increased focal brain water,39 or some effect of focal inflammatory cell activity as in multiple sclerosis.55 Ischaemia, which might occur secondary to endothelial dysfunction and impaired autoregulation,102 e.g. in stiffened vessels unable to respond to vasodilatory stimuli during exertion, induces matrix metalloproteinase activity which damages the endothelial tight junction proteins claudins and occludins, and could accelerate endothelial damage.137,138 Interstitial fluid drainage along the perivascular spaces may be dependent on ’milking‘ of fluid by pulsation of the adjacent arterioles.139 The ‘milking’ could become less effective as arterioles stiffen e.g. with SVD.139
Changes in BBB permeability also occur in dementia, particularly in vascular dementia versus Alzheimer’s disease or normal age-matched subjects.121 SVD is the commonest cause of vascular dementia140 and SVD commonly occurs with Alzheimer’s disease. Most of these meta-analytic data121 were determined using the CSF/plasma albumin ratio which has limitations. More recently it has been possible to show, using gadolinium contrast-enhanced MR imaging, that the background BBB in white matter remote from any acute infarct is more permeable in patients with lacunar than with large artery cortical stroke (Figure 7),122 in WMH than in normal appearing white matter,141 and in WMH in patients with vascular dementia.142 Interestingly, although the gadolinium signal detected in the brain with these MR techniques is very low, nonetheless it was still possible to demonstrate increasing BBB permeability with increasing age and with increasing numbers of PVS122 consistent with the other evidence for associations between advancing age, SVD and PVS described above. In earlier work, where the subject was lying supine in the MR scanner for more than half an hour, we identified an increasing gradient of gadolinium from the frontal to the occipital white matter suggesting some gravity-dependence of BBB permeability143 and offering some explanation for the tendency for microbleeds to cluster in the occipital lobes in patients with amyloid angiopathy144 (amyloid being another cause of increased BBB permeability125). In a small longitudinal study, greater BBB permeability soon after non-disabling stroke was associated with dependency at three year follow-up, older age, and having more WMH at initial stroke presentation, suggesting that BBB may predate progression of SVD, but larger studies are required.145 Diffusion tensor imaging demonstrates that decreasing white matter integrity in normal appearing white matter (notably changes in mean diffusivity indicating altered water content) correlate with increasing WMH.38,39
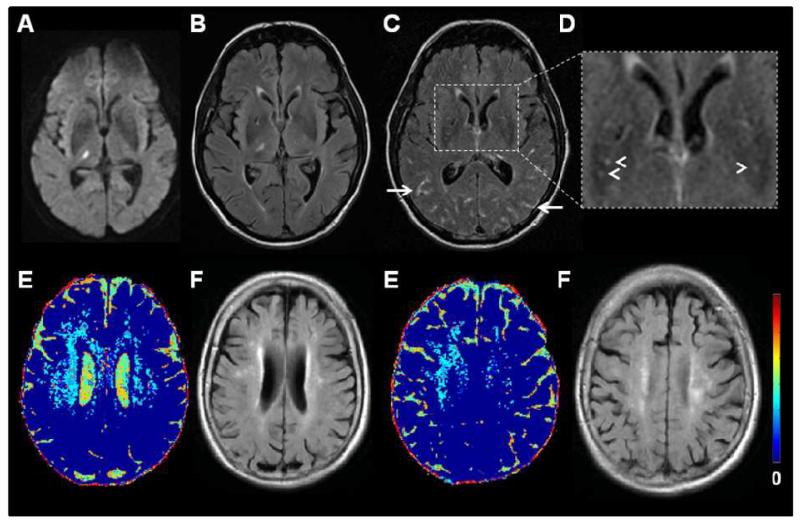
Figure 7.
MR imaging of cerebrovascular endothelial permeability. Top row: 56 year old patient with a right thalamic lacunar infarct: A) DWI, B) FLAIR two days after symptom onset. C) Two months later, FLAIR image after iv. gadolinium (Gd) showing Gd in the perivascular spaces (arrowheads) and sulci (arrows) and (D) inset magnified image of (C). Bottom row: Older patient with left internal capsule lacunar infarct (not shown): E) colour mapping of cerebrovascular permeability following intravenous Gd and F) corresponding FLAIR images showing WMH. Blue indicates low cerebral vascular endothelial permeability, yellow and red indicate increasing permeability. Permeability changes are diffuse. (E) courtesy of Dr Maria Valdes Hernandez.
Evidence for endothelial dysfunction in SVD also comes from CADASIL, a hereditary SVD caused by Notch3 mutations,146 where extensive WMH were associated with increased brain volume on MRI suggesting an increase of brain water content147 and CSF/plasma albumin ratios were increased,148 in keeping with BBB failure. Studies in CADASIL transgenic mice found no evidence for BBB dysfunction,149 but these mice also develop very little brain parenchymal pathology. In contrast, the most relevant experimental model of sporadic SVD, the spontaneously-hypertensive stroke prone rat (SHRSP),21,22,135 has multiple modest endothelial and perivascular tissue defects in early life,21 including impaired endothelial tight junctions, poor myelination, overactive microglia, and tendency to glial scarring detectable in protein expression.134 The SHRSP shows inflammation (again non-specific) and increased BBB permeability early in life,21 predating any rise in BP or development of overt vessel wall or tissue damage.150 Other models of human SVD using quite different mechanisms such as induction of mild carotid stenosis with external carotid coils151 also show evidence of BBB failure early after induction predating brain damage and consistent with the notion that various stimuli might induce SVD through effects on the cerebrovascular endothelium.
Insights from pathology of SVD
In the study of SVD so far, for reasons outlined above, pathology has had a relatively minor although pivotal role. Patients rarely die in the acute phase; the SVD pathology is often observed at post mortem almost as an incidental finding (Figure 5). Rarely, subacute lesions are observed (Supplemental Figure 3) which, on the basis of size and in the absence of any significant relevant clinical dysfunction in life, are assumed to be asymptomatic lacunes (Supplemental Figure 3a). Imaging correlation is rarely available. The need to develop a unified approach to the description of vascular pathology for use by research groups has recently been highlighted,24 and a scoring system has been suggested.152
Parenchymal changes associated with SVD range from PVS (Figure 4) and areas of relatively subtle white matter rarefaction through to cavitated cystic lesions (Supplemental Figure 3). In general, while tissue pathology changes seen at post mortem are frequently interpreted as “ischaemic”, there are changes that also support the endothelial dysfunction concept. While some, particularly larger lacunar lesions with cystic degeneration may be a consequence of end-stage occlusive SVD, as suggested by Fisher, the pathophysiology of PVS and localised white matter changes is less well understood. PVS have been ascribed to the pulsatile effects of hypertensive arterioles153 or changes in interstitial fluid handling,119 amongst others. The latter hypothesis is of particular interest, especially in light of the evolving literature around fluid drainage in the perivascular spaces and development of cerebral amyloid angiopathy.136 Perivascular rarefaction is poorly studied. Damaged endothelium, passage of plasma proteins into the vessel wall and vessel wall damage,154 leakage of fluid,78,101 albumin,108 other plasma proteins, inflammatory cells120,155,156 into the perivascular tissue have long been observed (Figure 5). Loss of normal vascular integrity was the only factor associated with increasing WMH score on MRI amongst several potential components examined neuropathologically, including myelin degradation, microglial activation, vascular density and vascular integrity.155 Additionally BBB was impaired in WMH, and there was increased astrocyte staining with IgG, suggesting BBB failure in deep white matter.155 These observations support the concept that, while pathological changes seen at post mortem are frequently interpreted as “ischaemic”, based on their histological similarities to thromboembolic infarcts, there are changes that also support the altered BBB permeability concept. Increased endothelial expression of endothelial thrombomodulin is seen in arterioles in SVD, possibly indicating a protective mechanism against local thrombosis due to changes in blood flow secondary to altered arteriolar structure.157 The presence of hypoxia-inducible factors in WMH at autopsy in elderly patients may be secondary to impaired autoregulation due to SVD, as vessel walls were thickened without luminal reduction; but capillary endothelial and microglial activation were also observed.107
Other mechanisms suspected from imaging
Falling cerebral blood flow (CBF) has been suggested as a cause of SVD, but why might CBF fall? Earlier, we described the general lack of association between acute lacunar infarcts or WMH and carotid stenosis, suggesting that carotid stenosis is not a common mechanical cause in humans. Relatively few studies have measured CBF in SVD: these have produced conflicting results. CBF and blood flow velocities fall with normal ageing. A reduction in CBF was observed using MR imaging methods in patients with more WMH in some,158 but not other159 studies; both found associations between CBF and atrophy suggesting that declining CBF might occur secondary to tissue loss, rather than that tissue loss and WMH occur secondary to falling CBF. Increasing age and WMH scores were independently associated with lower MCA flow velocities (on average, a 0·2 cms−1 fall in velocity per year increase in age, p=0·045; a 3·75 cms−1 fall in flow velocity per point increase in WMH score, p=0·004). This suggests that increasing brain tissue loss with advancing age and damage due to increasing WMH result in declining blood flow levels as there is less tissue to supply, rather than the other way round.70 Clearly larger longitudinal studies are needed that examine cerebrovascular reactivity and not just resting CBF.160
Recently, jugular venous reflux was suggested as a further potential mechanism for WMH.161 Jugular venous reflux increases with advancing age as do WMH. Elderly subjects with more WMH had more jugular venous reflux detected on Doppler ultrasound not accounted for by hypertension, diabetes, hyperlipidaemia or smoking. However other factors that might be associated both with jugular venous reflux and WMH, such as lung disease, were not evaluated. More studies of vascular dynamics and SVD are required.
Current clinical implications
Until we know more, we should continue clinically with careful management of vascular risk factors, particularly hypertension.140 Multiple combined antiplatelet agents should be used cautiously in patients with pure SVD without other large artery risk factors.18 We should avoid referring to WMH as ‘ischaemic’ – why not just call them ‘WMH’ or ‘ageing-related WMH’ or ‘WMH of presumed vascular origin’ until we understand their cause. We should stop considering the individual components of SVD as different disorders until we have better evidence that they are different; having a few lacunes may have similar impact in terms of cognition or stroke or dementia risk as do having some WMH. Stroke should be subtyped as accurately as possible to avoid confounding due to misdiagnosis of lacunar as cortical stroke and vice versa.
Future research
We need standard terminology and definitions for vascular pathology on neuroimaging with standards for image acquisition, analysis, and reporting to facilitate interpretation and meta-analyses. A unified effort to agree on such standards is currently underway.25 We further need more studies matching detailed pathology with individual SVD lesions on MRI, combined with detailed vascular, cognitive, medication and other health-related data in life. We need better ways of assessing subtle endothelial and BBB function in vivo, preferably using imaging, as that allows localisation of affected tissues that can be followed over time. This should include general and specific markers of endothelial function, such as markers of active and passive transport mechanisms and cerebral blood flow at rest and in response to stimuli. The foregoing work in humans is based on changes in non-specific, largely passive, endothelial function detected by intravenous gadolinium contrast agents and MRI (Figure 7): we need better information about how other aspects of endothelial dysfunction might contribute – how failing vasodilatation might contribute to disease pathogenesis and whether this can be modified by specific drug or lifestyle interventions. Research to determine the relative contributions of largely ischaemic SVD pathologies (arteriolosclerosis) versus largely haemorrhagic SVD pathologies (amyloid angiopathy) are required. Other mechanisms for ageing-related WMH, such as jugular venous reflux or abnormal vascular reactivity, should be tested in replication studies.161 Methods to assess the ’total SVD load‘ on imaging are required to avoid over-reliance on single features as has been the case in most previous studies. Trials and observational studies should aim to subtype (lacunar) stroke according to underlying mechanism (e.g. intrinsic SVD vs. atheroma) while avoiding risk-factor based definitions.61 Where possible, MR DWI soon after stroke should be used to identify the acute infarct. We should be cautious not to assume causation between vascular risk factors and SVD until proven by randomised trials. We need more information on the role of homocysteine, B vitamins and inflammation. Most importantly, we need to test interventions that target suspected mechanisms such as ways to improve endothelial function, to prevent the effects of oedema and inflammation on the brain, to prevent increasing endothelial permeability, to manage platelet activity without increasing risks of haemorrhage. The sooner we start thinking of SVD as a disorder of the endothelium together with the surrounding glia, astrocytes, pericytes and last but not least the neurons, the faster we will progress towards effective prevention and treatment.
Supplementary Material
Acknowledgements
We are grateful to Antonia Weingart, of the Institute for Stroke and Dementia Research, University of Munich for preparing Figure 6. We are grateful to Dr Maria Valdez Hernandez for preparing Figure 7E.
Funding Sources There were no specific funding sources for this work.
Dedication: To C. Miller Fisher, clinical neuroscientist extraordinaire, sadly recently deceased, for providing the original inspiration, insight and clarity of thought about lacunar disease that has informed the present work, and who, despite some misinterpretations of his work by others over the years, did not waver, or mistake subsequences for consequences.
Footnotes
Conflicts of Interest All three authors hold academic grants from government and charitable funding agencies some of which are relevant to this topic. None have industry consultancies or honoraria that are relevant to this subject.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–49. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 3.van der Flier WM, van Straaten EC, Barkhof F, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–20. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–24. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 5.de Laat KF, Tuladhar AM, van Norden AGW, Norris DG, Zwiers MP, de Leeuw F-E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 6.Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:279–82. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudlow CLM, Warlow CP. Comparable studies of the incidence of stroke and its pathological types. Results from an international collaboration. Stroke. 1997;28:491–9. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 8.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeer SE, Longstreth WT, Jr., Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–9. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 10.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) MRI substudy. Stroke. 2012;43:2336–42. doi: 10.1161/STROKEAHA.111.648576. [DOI] [PubMed] [Google Scholar]
- 12.ten Dam VH, van den Heuvel DM, van Buchem MA, et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology. 2005;64:1807–9. doi: 10.1212/01.WNL.0000161844.00797.73. [DOI] [PubMed] [Google Scholar]
- 13.The SPS3 Investigators Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–25. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardlaw JM, Dennis MS, Warlow CP, Sandercock PA. Imaging appearance of the symptomatic perforating artery in patients with lacunar infarction: occlusion or other vascular pathology? Ann Neurol. 2001;50:208–15. doi: 10.1002/ana.1082. [DOI] [PubMed] [Google Scholar]
- 15.Saini M, Ikram K, Hilal S, Qiu A, Venketasubramanian N, Chen C. Silent stroke: not listened to rather than silent. Stroke. 2012;3:3102–4. doi: 10.1161/STROKEAHA.112.666461. [DOI] [PubMed] [Google Scholar]
- 16.Haley AP, Hoth KF, Gunstad J, et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiatry. 2009;17:976–85. doi: 10.1097/JGP.0b013e3181b208ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter G, Doubal F, Jackson C, Sudlow C, Dennis M, Wardlaw J. Associations of clinical stroke misclassification (‘clinical-imaging dissociation’) in acute ischemic stroke. Cerebrovasc Dis. 2010;29:395–402. doi: 10.1159/000286342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacio S, Hart RG, Pearce LA, Benavente OR. Effect of addition of clopidogrel to aspirin on mortality: systematic review of randomized trials. Stroke. 2012;43:2157–62. doi: 10.1161/STROKEAHA.112.656173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey EL, Smith C, Sudlow CLM, Wardlaw JM. Pathology of lacunar ischaemic stroke in humans - a systematic review. Brain Pathol. 2012;22:583–91. doi: 10.1111/j.1750-3639.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–35. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 21.Bailey EL, McCulloch J, Sudlow C, Wardlaw JM. Potential animal models of lacunar stroke: a systematic review. Stroke. 2009;40:e451–e458. doi: 10.1161/STROKEAHA.108.528430. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood Flow Metab. 2008;28:1877–91. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- 23.Potter GM, Marlborough FJ, Wardlaw JM. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke. 2010;42:359–66. doi: 10.1161/STROKEAHA.110.594754. [DOI] [PubMed] [Google Scholar]
- 24.Alafuzoff I, Gelpi E, Al-Sarraj S, et al. The need to unify neuropathological assessments of vascular alterations in the ageing brain: Multicentre survey by the BrainNet Europe consortium. Exp Gerontol. 2012;47:825–33. doi: 10.1016/j.exger.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Lancet Neurology. A united approach to vascular disease and neurodegeneration. Lancet Neurol. 2012;11:293. doi: 10.1016/S1474-4422(12)70050-9. [DOI] [PubMed] [Google Scholar]
- 26.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–80. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. 1965;15:774–84. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 28.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1969;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 29.Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979;36:65. doi: 10.1001/archneur.1979.00500380035003. [DOI] [PubMed] [Google Scholar]
- 30.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 31.Fisher CM. Lacunar infarcts - a review. Cerebrovasc Dis. 1991;1:311–20. [Google Scholar]
- 32.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75:1670–7. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–4. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 34.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 35.Aribisala BS, Valdes Hernandez MC, Royle NA, et al. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur Radiol. 2012 doi: 10.1007/s00330-012-2677-x. DOI 10.1007/s00330-012-2677-x. [DOI] [PubMed] [Google Scholar]
- 36.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–82. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab. 2012;32:425–36. doi: 10.1038/jcbfm.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Sullivan M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke. 2010;41:S154–S158. doi: 10.1161/STROKEAHA.110.595314. [DOI] [PubMed] [Google Scholar]
- 39.Bastin ME, Clayden JD, Pattie A, Gerrish IF, Wardlaw JM, Deary IJ. Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging. 2009;30:125–36. doi: 10.1016/j.neurobiolaging.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 40.MacLullich AMJ, Ferguson KJ, Reid LM, et al. Higher systolic blood pressure is associated with increased water diffusivity in normal-appearing white matter. Stroke. 2009;40:3869–71. doi: 10.1161/STROKEAHA.109.547877. [DOI] [PubMed] [Google Scholar]
- 41.Duering M, Righart R, Csandi E, et al. Incident subcortical infacts induce focal thinning in connected cortical regions. Neurology. 2012;79:2025–2028. doi: 10.1212/WNL.0b013e3182749f39. [DOI] [PubMed] [Google Scholar]
- 42.Donnan GA, Norrving B, Bamford JM, Bogousslavsky J. Subcortical infarction: classification and terminology. Cerebrovasc Dis. 1993;3:248–51. [Google Scholar]
- 43.Wardlaw JM, West TM, Sandercock PA, Lewis SC, Mielke O. Visible infarction on computed tomography is an independent predictor of poor functional outcome after stroke, and not of haemorrhagic transformation. J Neurol Neurosurg Psychiatry. 2003;74:452–8. doi: 10.1136/jnnp.74.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross sectional study. J Neurol Neurosurg Psychiatry. 2011;82:540–2. doi: 10.1136/jnnp.2009.190298. [DOI] [PubMed] [Google Scholar]
- 45.Bang OY, Joo SY, Lee PH, et al. The course of patients with lacunar infarcts and a parent arterial lesion: similarities to large artery vs small artery disease. Arch Neurol. 2004;61:514–9. doi: 10.1001/archneur.61.4.514. [DOI] [PubMed] [Google Scholar]
- 46.Donnan GA, Bladin PF, Berkovic SF, Longley WA, Saling MM. The stroke syndrome of striatocapsular infarction. Brain. 1991;114:51–70. [PubMed] [Google Scholar]
- 47.Valdes-Hernandez MC, Maconick LC, Doubal F, et al. Does the differential distribution of acute lacunar infarcts and white matter lesions in the brain explain why lacunar infarcts but not white matter lesions present with acute symptoms? Cerebrovasc Dis. 2012;33:69. [Google Scholar]
- 48.Potter GM, Doubal FN, Jackson CA, et al. Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke. 2010;41:267–72. doi: 10.1161/STROKEAHA.109.566307. [DOI] [PubMed] [Google Scholar]
- 49.Moreau F, Patel S, Lauzon ML, et al. Cavitation after acute symptomatic lacunar stroke depends on time, location, and MRI sequence. Stroke. 2012;43:1837–42. doi: 10.1161/STROKEAHA.111.647859. [DOI] [PubMed] [Google Scholar]
- 50.Vermeer SE, den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–6. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 51.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48:745–54. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 52.MacLullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–23. doi: 10.1136/jnnp.2003.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–90. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 54.Potter G, Doubal F, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2012 doi: 10.1111/ijs.12054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wuerfel J, Haertle M, Waiczies H, et al. Perivascular spaces - MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332–40. doi: 10.1093/brain/awn171. [DOI] [PubMed] [Google Scholar]
- 56.Rouhl RP, Damoiseaux JG, Lodder J, et al. Vascular inflammation in cerebral small vessel disease. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.04.008. Published Online First: 19 May 2011. doi:10.1016/j.neurobiolaging.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32:528–34. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- 58.Wardlaw JM, Lewis SC, Keir SL, Dennis MS, Shenkin S. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006;37:2633–6. doi: 10.1161/01.STR.0000240513.00579.bf. [DOI] [PubMed] [Google Scholar]
- 59.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrand J. Essai sur l’hémiplégie des vieillards. Les lacunes de desintegration cérébrale. Avec 8 planches. Jules Rousset; Paris: 1902. [Google Scholar]
- 61.Jackson CA, Hutchison A, Dennis MS, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–9. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- 62.Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. Severe ipsilateral carotid stenosis and middle cerebral artery disease in lacunar ischaemic stroke: innocent bystanders? J Neurol. 2002;249:266–71. doi: 10.1007/s004150200003. [DOI] [PubMed] [Google Scholar]
- 63.Potter GM, Doubal FN, Jackson CA, Sudlow CLM, Dennis MS, Wardlaw JM. Lack of association of white matter lesions with ipsilateral carotid artery stenosis. Cerebrovasc Dis. 2012;33:378–84. doi: 10.1159/000336762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macdonald RL, Kowalczuk A, Johns L. Emboli enter penetrating arteries of monkey brain in relation to their size. Stroke. 1995;26:1247–51. doi: 10.1161/01.str.26.7.1247. [DOI] [PubMed] [Google Scholar]
- 65.Del Bene A, Makin SDJ, Doubal FN, Wardlaw JM. Do risk factors for lacunar ischaemic stroke vary with the location or appearance of the lacunar infarct? Cerebrovasc Dis. 2012;33:21. [Google Scholar]
- 66.Bang OY, Yeo SH, Yoon JH, et al. Clinical MRI cutoff points for predicting lacunar stroke may not exist: need for a grading rather than a dichotomizing system. Cerebrovasc Dis. 2007;24:520–9. doi: 10.1159/000110422. [DOI] [PubMed] [Google Scholar]
- 67.Khan A, Kasner SE, Lynn MJ, Chimowitz MI. Risk factors and outcome of patients with symptomatic intracranial stenosis presenting with lacunar stroke. Stroke. 2012;43:1230–3. doi: 10.1161/STROKEAHA.111.641696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adachi T, Kobayashi S, Yamaguchi S, Okada K. MRI findings of small subcortical “lacunar-like” infarction resulting from large vessel disease. J Neurol. 2002;247:280–5. doi: 10.1007/s004150050584. [DOI] [PubMed] [Google Scholar]
- 69.Wong KS, Gao S, Chan YL, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 70.Wardlaw JM, Doubal FN, Eadie E, Chappell F, Shuler K, Cvoro V. Little association between intracranial arterial stenosis and lacunar stroke. Cerebrovasc Dis. 2011;31:12–8. doi: 10.1159/000319773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boiten J, Lodder J, Kessels F. Two clinically distinct lacunar infarct entities? A hypothesis. Stroke. 1993;24:652. doi: 10.1161/01.str.24.5.652. [DOI] [PubMed] [Google Scholar]
- 72.de Jong G, Kessels F, Lodder J. Two types of lacunar infarcts: further arguments from a study on prognosis. Stroke. 2002;33:2072–6. doi: 10.1161/01.str.0000022807.06923.a3. [DOI] [PubMed] [Google Scholar]
- 73.Del Bene A, Palumbo V, Lamassa M, Saia V, Piccardi B, Inzitari D. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke. 2012;7:321–9. doi: 10.1111/j.1747-4949.2012.00789.x. [DOI] [PubMed] [Google Scholar]
- 74.Gerraty RP, Parsons MW, Barber PA, et al. Examing the lacunar hypothesis with diffusion and perfusion magnetic resonance imaging. Stroke. 2002;33:2019–24. doi: 10.1161/01.str.0000020841.74704.5b. [DOI] [PubMed] [Google Scholar]
- 75.Bezerra DC, Sharrett AR, Matsushita K, et al. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78:102–8. doi: 10.1212/WNL.0b013e31823efc42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benavente O, McClure LA, Coffey CS, Hart RG. The Secondary Prevention of Small Subcortical Strokes (SPS3) study: results of the antiplatelet trial. Cerebrovasc Dis. 2012;33:79. [Google Scholar]
- 77.Kang C-K, Park C-A, Park C-W, Lee Y-B, Cho Z-H, Kim Y-B. Lenticulostriate arteries in chronic stroke patients visualised by 7 T magnetic resonance angiography. Int J Stroke. 2010;5:374–80. doi: 10.1111/j.1747-4949.2010.00464.x. [DOI] [PubMed] [Google Scholar]
- 78.Lammie GA, Brannan F, Slattery J, Warlow C. Nonhypertensive cerebral small-vessel disease. An autopsy study. Stroke. 1997;28:2222–9. doi: 10.1161/01.str.28.11.2222. [DOI] [PubMed] [Google Scholar]
- 79.Dufouil C, De Kersaint-Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–6. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 80.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–72. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 81.van Dijk EJ, Breteler MMB, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: Cardiovascular Determinants of Dementia Study. Hypertension. 2004;44:625–30. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 82.Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white-matter disease progression in a biethnic cohort. Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123:266–73. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- 84.Marcus J, Gardener H, Rundek T, et al. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the northern Manhattan study. Stroke. 2011;42:2639–41. doi: 10.1161/STROKEAHA.111.617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X, Pantoni L, Simoni M, et al. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension. 2009;54:57–62. doi: 10.1161/HYPERTENSIONAHA.109.129700. [DOI] [PubMed] [Google Scholar]
- 86.Gouw AA, van der Flier WM, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period. The leukoaraiosis and disability study. Stroke. 2008;39:1414–20. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- 87.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–50. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 88.Chiang MC, Barysheva M, Shattuck DW, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–24. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kohannim O, Jahanshad N, Braskie MN, et al. Predicting white matter integrity from multiple common genetic variants. Neuropsychopharmacology. 2012;37:2012–9. doi: 10.1038/npp.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carmelli D, DeCarli C, Swan GE, et al. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29:1177–81. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- 91.Turner ST, Jack CR, Fornage M, Mosley TH, Boerwinkle E, de Andrade M. Heritability of leukoaraiosis in hypertensive sibships. Hypertension. 2004;43:483–7. doi: 10.1161/01.HYP.0000112303.26158.92. [DOI] [PubMed] [Google Scholar]
- 92.Atwood LD, Wolf PA, Heard-Costa NL, et al. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35:1609–13. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- 93.Kochunov P, Glahn D, Winkler A, et al. Analysis of genetic variability and whole genome linkage of whole-brain, subcortical, and ependymal hyperintense white matter volume. Stroke. 2009;40:3685–90. doi: 10.1161/STROKEAHA.109.565390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kochunov P, Glahn D, Lancaster J, et al. Whole brain and regional hyperintense white matter volume and blood pressure: overlap of genetic loci produced by bivariate, whole-genome linkage analyses. Stroke. 2010;41:2137–42. doi: 10.1161/STROKEAHA.110.590943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kochunov P, Glahn DC, Lancaster J, et al. Blood pressure and cerebral white matter share common genetic factors in Mexican Americans. Hypertension. 2011;57:330–5. doi: 10.1161/HYPERTENSIONAHA.110.162206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt H, Zeginigg M, Wiltgen M, et al. Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease. Brain. 2011;134:3384–9. doi: 10.1093/brain/awr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–9. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 98.Spence JD. Homocysteine-lowering therapy: a role in stroke prevention? Lancet Neurol. 2007;6:830–8. doi: 10.1016/S1474-4422(07)70219-3. [DOI] [PubMed] [Google Scholar]
- 99.Cavalieri M, Schmidt R, Chen C, et al. B Vitamins and MRI-Detected Ischemic Brain Lesions in Patients With Recent Transient Ischemic Attack or Stroke: The VITAmins TO Prevent Stroke (VITATOPS) MRI-Substudy. Stroke. 2012;43:3266–3270. doi: 10.1161/STROKEAHA.112.665703. [DOI] [PubMed] [Google Scholar]
- 100.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–12. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 101.Lammie GA, Brannan F, Wardlaw JM. Incomplete lacunar infarction (type 1b lacunes) Acta Neuropathol. 1998;96:163–71. doi: 10.1007/s004010050877. [DOI] [PubMed] [Google Scholar]
- 102.Stevenson SF, Doubal FN, Shuler K, Wardlaw JM. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke. 2010;41:e434–e442. doi: 10.1161/STROKEAHA.109.569855. [DOI] [PubMed] [Google Scholar]
- 103.Knottnerus ILH, Ten Cate H, Lodder J, Kessels F, van Oostenbrugge RJ. Endothelial dysfunction in lacunar stroke: a systematic review. Cerebrovasc Dis. 2009;27:519–26. doi: 10.1159/000212672. [DOI] [PubMed] [Google Scholar]
- 104.Silberberg DH, Manning MC, Schreiber AD. Tissue culture demyelination by normal human serum. Ann Neurol. 1984;15:575–80. doi: 10.1002/ana.410150610. [DOI] [PubMed] [Google Scholar]
- 105.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 106.Fazekas F, Ropele S, Enzinger C, et al. MTI of white matter hyperintensities. Brain. 2005;128:2926–32. doi: 10.1093/brain/awh567. [DOI] [PubMed] [Google Scholar]
- 107.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–8. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 108.Simpson JE, Wharton SB, Cooper J, et al. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci Lett. 2010;486:246–51. doi: 10.1016/j.neulet.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 109.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;29:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29:3538–50. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cecchelli R, Berezowski V, Lundquist S, et al. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–61. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 112.Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 113.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–52. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 114.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 116.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 117.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 118.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–23. [PMC free article] [PubMed] [Google Scholar]
- 119.Pollock H, Hutchings M, Weller RO, Zhang E-T. Perivascular spaces in the basal gangli of the human brain: their relationship to lacunes. J Anat. 1997;191:337–46. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–44. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 121.Farrall AJ, Wardlaw JM. Blood brain barrier: ageing and microvascular disease - systemic review and meta-analysis. Neurobiol Aging. 2007;30:337–52. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 122.Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 123.McCaffrey G, Seelbach MJ, Staatz WD, et al. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106:2395–409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS One. 2011;6:e23789. doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–6. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–47. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hassan A, Hunt BJ, O’Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 128.Fornage M, Chiang YA, O’Meara ES, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke. 2008;39:1952–9. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 130.Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Dietary sodium and risk of stroke in the Northern Manhattan Study. Stroke. 2012;43:1200–5. doi: 10.1161/STROKEAHA.111.641043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low-sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens. 2009;23:826–35. doi: 10.1038/jhh.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bragulat E, de La Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–8. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- 134.Bailey E, Wardlaw J, Graham D, Dominiczak A, Sudlow C, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5-to 21-week old rats. Neuropathol Appl Neurobiol. 2011;37:711–26. doi: 10.1111/j.1365-2990.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 135.Bailey EL, Smith C, Sudlow CLM, Wardlaw JM. Is the spontaneously hypertensive stroke prone rat a pertinent model of subcortical ischaemic stroke? A systematic review. Int J Stroke. 2011;6:434–44. doi: 10.1111/j.1747-4949.2011.00659.x. [DOI] [PubMed] [Google Scholar]
- 136.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 137.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40:S20–S23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006;238:962–74. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 140.Dichgans M, Zietemann V. Prevention of Vascular Cognitive Impairment. Stroke. 2012 doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]
- 141.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–7. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 142.Taheri S, Gasparovic C, Huisa BN, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–63. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–6. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–34. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 145.Wardlaw JM, Doubal FN, Valdes-Hernandez MC, et al. Blood-brain barrier permeability and long term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2012 doi: 10.1161/STROKEAHA.112.669994. doi: 10.1161/STROKEAHA.112.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 147.Yao M, Jouvent E, Duering M, et al. Extensive white matter hyperintensities may increase brain volume in CADASIL. Stroke. 2012 doi: 10.1161/STROKEAHA.112.664854. in press. [DOI] [PubMed] [Google Scholar]
- 148.Dichgans M, Wick M, Gasser T. Cerebrospinal fluid findings in CADASIL. Neurology. 1999;53:233. doi: 10.1212/wnl.53.1.233. [DOI] [PubMed] [Google Scholar]
- 149.Joutel A, Monet-Lepretre M, Gosele C, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–45. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sironi L, Guerrini U, Tremoli E, et al. Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J Neurosci Res. 2004;78:115–22. doi: 10.1002/jnr.20219. [DOI] [PubMed] [Google Scholar]
- 151.Ueno M, Tomimoto H, Akiguchi I, Wakita H, Sakamoto H. Blood-brain barrier disruption in white matter lesions in a rat model of chronic cerebral hypoperfusion. J Cereb Blood Flow Metab. 2002;22:97–104. doi: 10.1097/00004647-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 152.Smallwood A, Oulhaj A, Joachim C, et al. Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathol Appl Neurobiol. 2012;38:337–43. doi: 10.1111/j.1365-2990.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 153.Hughes W. Origin of lacunes. Lancet. 1965;286:19–21. [Google Scholar]
- 154.Munoz DG. Small vessel disease: neuropathology. Int Psychogeriatr. 2003;15:67–9. doi: 10.1017/S1041610203008986. [DOI] [PubMed] [Google Scholar]
- 155.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–11. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 156.Black S, Gao F, Bilbao J. Understanding white matter disease. Imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 157.Giwa MO, Williams J, Elderfield K, et al. Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology. 2012;78:167–74. doi: 10.1212/WNL.0b013e3182407968. [DOI] [PubMed] [Google Scholar]
- 158.Appelman APA, van der Graaf Y, Vincken KL, et al. Total cerebral blood flow, white matter lesions and brain atrophy: the SMART-MR study. J Cereb Blood Flow Metab. 2008;28:633–9. doi: 10.1038/sj.jcbfm.9600563. [DOI] [PubMed] [Google Scholar]
- 159.ten Dam H, van den Heuvel D, de Craen A, et al. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243:198–203. doi: 10.1148/radiol.2431052111. [DOI] [PubMed] [Google Scholar]
- 160.Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chung CP, Wang PN, Wu YH, et al. More severe white matter changes in the elderly with jugular venous reflux. Ann Neurol. 2011;69:553–9. doi: 10.1002/ana.22276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.