Abstract
Long-lived plasma cells (LLPC) that maintain humoral immunity to previously encountered antigens occupy a compartment in the BM. The rules and mechanisms by which cells enter (and leave) this compartment are poorly understood. We have looked at what happens to the LLPC compartment and to plasma cell (PC) lifespan in general, in situations where antigen stimulation and/or inflammation persist. We find that chronic antigen supply causes the generation of short-lived PC in the local lymphoid organ, at the expense of any LLPC production. Furthermore, we find that inflammation caused by infection (mediated via TNFα) causes a dramatic mobilization of LLPC from the BM, with a concomitant reduction in circulating antibody levels against previously immunized antigens. These data are discussed in the context of the capacity of the BM LLPC compartment and competition for entry to it.
Keywords: Plasma cell, cell lifespan, inflammation, antibodies, TNFα
Introduction
Following vaccination or infection, antibody levels persist for many years [1, 2], and this humoral immunity is key to providing protection from re-infection. The main source of these antibodies is long-lived plasma cells (LLPC) that are generated in the secondary lymphoid organs, but reside primarily in the bone marrow (BM) [3-5]. However, during the early phases of T-dependent immune responses many short-lived PCs (SLPC) are also generated. These two types of PC are the result of two distinct waves of differentiation; a first wave occurs following initial interactions between activated, antigen-specific CD4 T and B cells at the border of the T zone [6]. Some B cells become plasmablasts and up-regulate CXCR4, migrating to the red pulp in response to CXCL12 gradients [7]. Here they form the extra-follicular foci of PC [8], that no longer divide and have lost many B cell-associated markers (eg. MHC II, B220, CD19, CD20). Although massive in scale this extra-follicular PC response is transient, as most of these cells die within a few days [9]. Several days later, a second wave of plasmablasts emerges from the germinal centre (GC) reaction. These cells are generally class-switched and secrete high affinity antibody molecules containing V-region mutations. If the GC was initiated by classical immunization, generally, the plasmablasts emerge at a time when the extra-follicular PC response has waned. The GC-derived plasmablasts, therefore, do not settle in the red pulp, but rather respond to CXCL12 [7] in the circulation and migrate to the BM [10-12]. Under other conditions, such as infection, the local CXCL12 production may be increased and prolonged and so GC-derived plasmablasts may remain in the spleen. The GC-derived plasmablasts are also capable of migrating to sites of inflammation guided by CXCR3 (attracted by CXCL9, 10 and 11) [13].
PC are inherently short-lived, surviving for just 2-3 days unsupported, and yet their lifespan can be extended, almost indefinitely, by the provision of survival factors [14]. These include soluble factors (such as APRIL, BAFF, IL-6, TNFα and CXCL12) and membrane receptors (such as CD44, LFA-1:ICAM-1, VLA-4:VCAM-1 [14] and act synergistically to provide a survival niche. BCMA ligands (APRIL and BAFF) seem especially important for long-term survival in the bone marrow [15, 16], as is CD93 [17] and possibly CD28 [18, 19]. These factors are enriched only in the BM under homeostatic conditions, provided by a complex cellular niche; eosinophils [20], megakaryocytes [21] and myeloid cells [22] possibly clustering around VCAM-1+ stromal cells (attracted by CXCL12) where they provide signals to plasmablasts and early PC that also migrate towards CXCL12. Supported by a survival niche, PC endure at this site for many months or even years without division or replenishment from the peripheral B cell pool. Survival niches are also induced in other organs during inflammation, although their characterisation is incomplete [23]. In the secondary lymphoid organs, IL-6 secreting CD11c+CD8α− dendritic cells support plasmablast survival, while APRIL- and BAFF-secreting populations support PC survival in the red pulp and medullary cords [8, 24]. These populations are expanded during inflammation allowing for the temporary survival of large numbers of plasmablasts and PCs. However, under steady-state conditions, relatively few LLPC are supported in the secondary lymphoid organs [9].
In this study we wished to find out how chronic antigen supply and/or inflammation affected the survival and turnover of PC in local lymphoid tissues and in the LLPC compartment of the BM. Both myeloid and B lymphocyte development are known to be influenced by systemic inflammatory mediators [25] and mobilization of BM PC upon antigen re-challenge has been noted previously [26]. This suggests that inflammatory signals might also affect the LLPC compartment. Therefore, we have investigated how competing immune responses under inflammatory or non-inflammatory conditions affect pre-existing BM PC populations and the laying down of new LLPC in the BM. Our findings suggest that, in addition, to antigen-chronicity and inflammation causing the generation of SLPC at the expense of LLPC, inflammation (eg. TNFα) caused a general mobilization of existing LLPC from the BM that may be important to facilitate entry of newly-formed LLPC into the compartment.
Materials & Methods
Mice
C57Bl/6 mice, K/BxN mice [27], MyD88 [28]-, TLR4 [29]- and TRIF [30]-deficient mice were bred and maintained in specific pathogen free conditions at the School of Biological Sciences Animal Facility at the University of Edinburgh. Mice were aged 6-10 weeks at the start of experiments, unless otherwise stated. The MyD88, TLR4 and TRIF-deficient mice were generously supplied by Dr Shizuo Akira, Osaka University, Japan. K/BxN mice were generated by crossing NOD mice with heterozygous T cell receptor transgenic KRN mice [27]; the latter were originally kindly supplied by Drs Diane Mathis and Christophe Benoist (Harvard Medical School, Boston). Development of arthritis was assessed by scoring joint swelling. Non-arthritic littermates were used as controls in all experiments. Experiments were covered by a Project Licence granted by the Home Office under the Animal (Scientific Procedures) Act 1986. This licence was approved locally by the University of Edinburgh Ethical Review Committee.
Immunisations and Infections
For primary immunisations, mice received 100μg alum (Sigma) -precipitated FITC-OVA (Biosearch), NP-KLH or both intraperitoneal (IP) injection together with 2×108 killed Bordetella pertussis (Lee Labs). For secondary immunisations, mice received 100μg NP-KLH or FITC-OVA either in soluble form or alum-precipitated with or without B. pertussis also IP unless otherwise stated. Recombinant mouse TNF-α (R&D Systems) was supplied to mice intravenously (IV) in PBS. Mice received 0.5ug per day for 8 consecutive days. Mice were infected IV with approximately 106 CFU of the Salmonella enterica serovar Typhimurium vaccine strain SL3261. To assess cell division in vivo, 5-Bromo-2′-deoxyuridine (BrdU) (Sigma) was given to the mice in the drinking water at 0.8mg/ml (protected from light and changed every 3 days). For short-term labelling mice were injected IP with 1mg of BrdU. For recombinant cytokine administration, 3 doses of 1μg recombinant mouse TNFα (Biolegend) or 10μg IFN-γ (Biolegend), diluted into PBS, were injected I.V. at 24-hour intervals. Mice were sacrificed 48 hours after the final injection.
FACS staining
Cells were stained with Aqua Live/Dead kit (Invitrogen) before surface staining with anti-CD19-PE-Cy7 (6D5; Biolegend), anti-MHC class II-Pacific blue (M5/114.15.2; Biolegend), anti-CD138-APC or biotin (281-2; BD), anti-CD11b-APC-Cy7 (M1/70; BD), anti-Gr1-AF488 (RB6-8C5, ABD Serotech), anti-F4/80-Pacific Blue (BM8, Biolegend), anti-Siglec-F-PE (E50-2440, BD), anti-CXCR3-Brilliant Violet 421 (CXCR3-173, Biolegend) and anti-CXCR4-APC (2B11/CXCR4, BD) and FcR block (2.4G2, in house) diluted in FACS buffer for 30 minutes at 4°C. Cells were washed in FACS buffer and suspended in FoxP3 fixation/permeabilisation kit (eBioscience) overnight at 4°C prior to intracellular staining. For this cells were stained with goat anti-mouse IgG-PerCP (Jackson ImmunoResearch), goat anti-mouse kappa light chain (FITC or PE), goat anti-mouse IgM (FITC or PE) (both Southern Biotech) or anti-APRIL-PE (A3D8, Biolegend), in permeabilisation buffer (eBioscience), for 30 minutes at 4°C. For antigen-specific intracellular staining, 1μg nitrophenyl(NP)-PE (Biosearch) or FITC-OVA (Biosearch) were used per 1×106 cells in conjunction with IgG or IgM antibodies. For BrdU staining, cells were treated 10μg/ml DNase I (Sigma), diluted in permeabilisation buffer for 45 minutes at 37°C before washing and staining with anti-BrdU-FITC (B44; BD) for 30 minutes at room temperature. Samples were acquired on an LSR II or FACSCANTO (BD) and analysed using FlowJo (Treestar).
Migration Assays
Splenic leukocytes were prepared using Lympholyte (Cedarlane). Washed cells were re-suspended at 1×107/ml (in 0.5% bovine serum albumin (BSA; Sigma) in Iscove’s modified Dulbecco’s Medium (IMDM; Sigma)). Transwell plates (0.5μm pore size, Corning) were coated with mouse fibronectin (Sigma; 10μg/ml in sterile water) for 60 minutes at 37°C following which fibronectin was removed and plates dried for 2 hours at 37°C. 600μl medium with or without recombinant mouse chemokines at 400nM (CXCL9 or CXCL12 from R&D systems) was added to the lower well and 100μl spleen cells to the upper well. Cells were allowed to migrate for 3 hours at 37°C. The contents of the lower well were harvested and re-suspended in 5% FCS IMDM before culturing on ELISPOT plates overnight. For assay of chemokines in spleen or BM ex vivo, cells were mashed or flushed into PBS, spun down and supernatants collected for assay.
ELISPOT
96-well multiscreen plates (Millipore) were treated with 15% ethanol for 1 minute, washed in PBS and coated with NP-BSA or FITC-OVA at 10μg/ml in carbonate/bicarbonate buffer (Sigma) overnight at 4°C. Plates were washed (PBS) and blocked with 10% FCS-PBS for 2 hours and washed. Duplicate in 5-fold cell dilutions were incubated overnight at 37°C before washing with PBS and PBS-0.1% Tween (9 washes). Finally, anti-IgG-alkaline phosphatase (Southern Biotech), was added to each well for 4 hours at room temperature. Plates were developed using Sigmafast (Sigma) and spots were scored under a microscope.
ELISA
96-well Nunc-maxisorp plates (Fischer Scientific) were coated in NP-BSA or FITC-OVA (at 10μg/ml or cytokine/chemokine capture antibodies (at manufacturer’s recommended concentration) and incubated overnight at 4°C. Plates were washed in PBS+0.1% tween, and blocked with PBS+1% BSA. Sera (2-fold dilutions) were incubated for 2-hours, bound antibodies were detected with alkaline phosphatase-labelled anti-IgM, IgG1, IgG2b, IgG2c, IgG3 or total IgG (all Southern Biotech). Titres are the dilution at which samples reached half of the maximal optical density (OD) of the standard for each plate.
Statistics
Throughout the study, Students’ t-test, one-way or two-way ANOVA were used to determine statistical significance. Where required, for one-way ANOVA, the Dunnet post-test or Bonferroni post-test were used. For two-way ANOVA, the Bonferroni post-test was used. Tests were performed on Graphpad Prism software (Graphpad Software Inc., San Diego, CA). P values are represented by asterisks (where * represents p=0.01 to p=0.05, ** represents p=0.001 to p=0.01, *** represents p<0.001 and NS represents not significant (p>0.05)).
Results
Dynamics of accumulation of antigen-specific PC populations in spleen and BM following primary and secondary immunization with NP-KLH
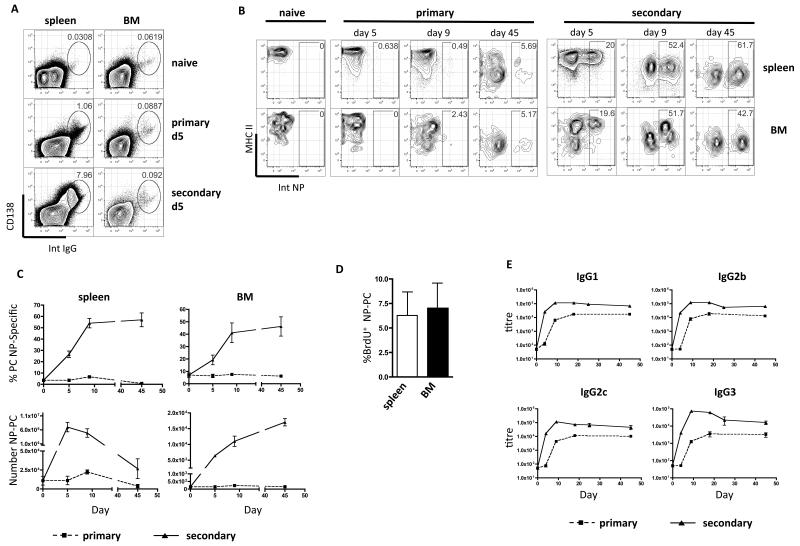
In these studies PC were identified by expression of both CD138 and intracellular immunoglobulin (IgG, kappa light chain) or antigen-binding. Mature PC were distinguished from less mature plasmablasts by their lack of MHC class II expression. In Figure 1A we show that 5 days after primary immunisation using alum-precipitated NP-KLH with killed B. pertussis, large numbers of PC were formed in the spleen (rising from 0.03% to 1% in this time). Few of these splenic PC were NP-specific at day 5 (Fig.1B), and were likely secreting antibody against B. pertussis antigens, or were activated in a polyclonal manner through Toll-like receptor (TLR) stimulation. By day 45 after primary immunization, a significant frequency (5.48% ± 0.16) of NP-specific PC had accumulated in the spleen (Fig.1B and 1C). In the BM, throughout the primary response, the frequency of PC (Fig.1A) or the total number of PC (not shown) changed little. Antigen-specific PC did, however, accumulate in the BM over time, reaching 6.17% (± 0.52) at 45 days (Fig. 1C).
Figure 1. Secondary immunization with soluble NP-KLH generates large numbers of NP-specific LLPC that persist in the BM, but not in the spleen.
(A) PC expressing intra-cellular IgG in spleen and BM after primary immunization with alum-precipitated NP-KLH with killed B. pertussis and boosting with soluble NP-KLH. FACS plots show individual mice that are representative of 24 mice used in 3 experiments. (B) The frequency (%) of NP-specific PC in the spleen and BM at various time points following primary or secondary immunisation, with NP-KLH (plots are representative of data from 15 mice in 3 separate experiments). (C) Summary graphs showing frequencies and numbers of NP-specific IgG PC in the spleen and BM following primary (dashed) and secondary (solid) immunisation with NP-KLH. Each point on graphs is the mean and standard error of data from 5 mice. The experiment was repeated twice with similar results. (D) BrdU incorporation in NP-specific IgG PC in the spleen and BM between day 15 and 24 following secondary immunisation with NP-KLH (bars show the means and standard errors of data from 5 mice per group and is representative of 2 experiments). (E) Serum titers of NP-specific antibody (of indicated class) at various time points following primary or secondary immunization with NP-KLH (points on graph represent 4-5 mice from 1 of at least 6 experiments).
Following secondary immunization (boosting) with soluble NP-KLH, very large numbers of PC were generated in the spleen (7.51% ± 1.15; Fig.1C) and at day 5 post-boost, 21.25%(±1.97) of the PC were NP-specific. The frequency of NP-specific PC increased rapidly to over 50% at day ± 9 and surprisingly was maintained at around 60% of PC at day 45 (Fig. 1B and C), although the total number of PC in the spleen had waned by this point (Fig. 1C, lower left panel). In the BM, secondary immunization caused very little increase in total PC numbers (Fig. 1A and 1C), however, there was a rapid appearance of large numbers of NP-specific PC (with similar kinetics to the spleen) and these were maintained stably until the end of the study at day 45 (Fig. 1B & C). Interestingly, despite the BM being a site where PC of many specificities persist as a form of humoral memory, after boosting with soluble antigen around half of IgG PC in the BM were specific for the single immunising antigen (NP) and formed a stable population for 45 days or longer, (Fig. 1C). A BrdU pulse between 15-24 days following NP-KLH boost showed that very few of the PC became labelled (Fig. 1D) and so the majority of NP-specific PC in both spleen and BM had already become non-dividing LLPC. While the numbers of NP-specific PC rapidly declined in the spleen, they were maintained in the BM for at least 45 days after boosting (Fig. 1C). This was reflected in NP-specific antibody levels in the serum, which peaked by day 9 and declined slightly by day 24 but were thereafter maintained until at least day 45 (Fig. 1E).
Persistent antigenic stimulation causes SLPC production at the expense of LLPC
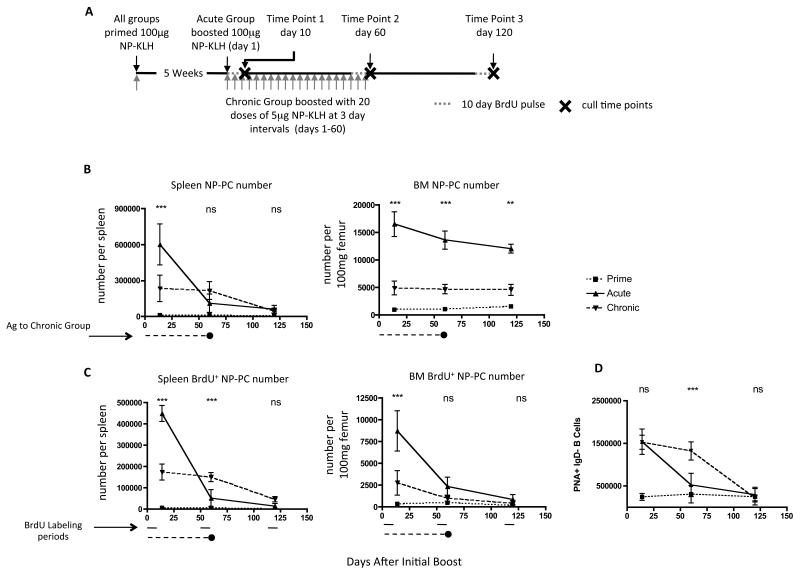
We wished to know how PC turnover and lifespan was affected in situations where immunogens persist (autoimmune disease or chronic infection). In patients with autoimmune disease treated with rituximab to deplete B cells, the titers of autoantibodies fall much more rapidly after B cell depletion than antibodies to vaccine antigens [31]. This has been taken to indicate the autoantibody response is derived from SLPC; in other words continued input from CD20+ B cell precursors is required to sustain an SLPC population. In mice, in a model of SLE, some autoantibodies (eg. anti-dsDNA) are derived from SLPC, while others (against RNA-containing complexes) are the product of LLPC [32]. Also, in the K/BxN model of arthritis the PC making anti-glucose 6-phosphate isomerase (G6PI) antibodies seem to be short-lived [33]. To investigate whether persistent supply of antigen affects PC lifespan, we primed mice with NP-KLH and after 5 weeks boosted them with either a single dose of 100μg soluble NP-KLH (acute) or 20 × 5μg doses of NP-KLH at 3-day intervals (chronic) (Fig. 2A). Providing a single 100μg dose of NP-KLH generated far greater numbers of NP-specific PC in the spleen at d10 and, as before, a proportion of these became established in the BM where they declined only marginally over the course of the 120-day experiment (Fig. 2B). Few new NP-specific PC were generated after the first 10-days, as revealed by 10-day BrdU pulses at days 1-10, 50-60 or 110-120 of the experiment (Fig. 2C). Conversely, continuous supply of 5μg NP-KLH generated lower numbers of NP-specific PC in the spleen (Fig. 2B), but in contrast to the acute immunization, these cells were maintained until day 60. This was due to continued generation of new PCs, as shown by BrdU incorporation (Fig. 2C). In contrast to the spleen, few of these PC became established in the BM; only a third of the numbers seen following acute NP-KLH administration (Fig. 2B). BrdU incorporation data suggests that during the first 10-days, PC generated in the periphery entered the BM, however at later time points these PC either failed to migrate to the BM or were unable to survive (Fig. 2C). The failure to establish LLPC populations in the BM after persistent stimulation was not due to any failure to generate germinal centers; Fig. 2D shows that the numbers of GC B cells in the spleens of the chronic antigen group remained high until 60 days, when antigen administration was stopped. Also there was no discernable difference in the balance of IgM versus IgG isotypes in the anti-NP antibody responses in chronic compared to acute immunizations (data not shown).
Figure 2. Chronic delivery of antigen generates SLPC without establishment of LLPC in the BM.
(A) Schematic of experimental design. All mice were primed with alum-precipitated NP-KLH and B. pertussis. All mice were pulsed with BrdU for 10 days prior to culling at time-points 1, 2 or 3. (B) Numbers of NP-specific IgG PC in the spleen (left) or BM (right) of mice from the groups described above (acute: solid line, chronic: dashed line, no boost: dotted line). Period of chronic antigen administration is indicated below graph. (C) Numbers of BrdU+ NP-specific IgG PC in the spleen and B of BM of groups of mice described above. Numbers of BM cells were adjusted to number per 100mg femur, as the bones increase in size with the age of the mice over the course of the experiment. Points on graphs represent the mean and standard error of data from 5-6 mice. (D) Enumeration of GC B cells (PNA+/IgD−) by flow cytometry at points during or following acute or chronic antigen delivery. Two-way ANOVA with Bonferroni post-test was used to evaluate the significance of observed differences.
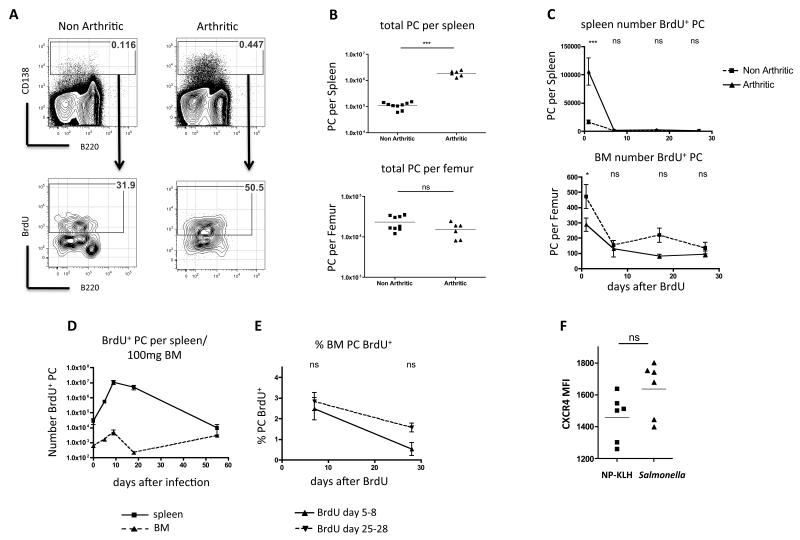
To test whether other chronic inflammatory conditions led to reduced establishment of PC in the BM, we used K/BxN mice that develop arthritis as a result of production of circulating antibody (IgG) to G6PI [27]. At 3 weeks after birth, arthritic mice had increased levels of splenic PC, compared to non-arthritic littermates (Fig. 3A & B). BrdU pulses revealed that these PC had a consistent turnover of around 50% in 3 days (Fig. 3A) and both the percentage (not shown) and especially the number of BrdU+ PC was greater in arthritic compared to non-arthritic mice (Fig. 3C). Despite the uptake of BrdU by many PC in the spleen during a 10-day pulse (50.79% ± 3.06 in arthritic mice), very few BrdU+ PC could be detected in the BM, indeed fewer than in non-arthritic control mice (Fig.3C). This suggested a failure to enter or survive in the BM compartment. To investigate if this phenomenon was also a feature of chronic infection we gave 4-day BrdU pulses to mice at various time points following infection with Salmonella. This revealed an increase in the number of dividing PC in the BM at day 10, this declined by day 18 (Fig. 3D), but increased again at day 55, when anti-Salmonella antibody titers are maximal [34]. To see whether these dividing PC (in BM or spleen) were able to survive as LLPC in the BM, we performed a BrdU pulse-chase experiment: mice were given 4-day BrdU pulses at days 5-8 or 25-28 post-infection, and the survival of labelled PC was determined 7 and 28 days after BrdU. Few BM PC retained BrdU for more than 7 days when pulsed at either time point (Fig. 3E) suggesting that PC entering the BM at early time points in Salmonella infection do not have the capacity to become long-lived.
Figure 3. PC generated in the spleens of arthritic K/BxN mice do not become established as LLPC in the BM.
(A) Representative plots showing CD138+ PC in the spleen of non-arthritic or arthritic litter-mates (top row) and BrdU incorporation in PC following a 3-day pulse (bottom row). Representative of 5 mice from 1 of 5 similar experiments). (B) Summary data of total PC numbers in the spleen (top panel) and BM (per femur) (bottom panel) of non-arthritic and arthritic mice (line indicates median value). (C) Numbers of PC labelled with BrdU in the spleen and BM of non-arthritic or arthritic mice following a 10-day BrdU pulse at day 1, 7, 14 or 28 after BrdU. (D) BrdU-labelled PC in the spleen (solid line) or BM (dashed line) following a 4-day BrdU pulse prior to each indicated time-point after Salmonella infection. (E) Survival of BrdU-labelled PC in the BM of Salmonella infected mice 7 or 28 days after receiving 4-day BrdU pulses at day 5-8 or 25-28 post-infection. Mean and standard error of data from 5 mice per group are shown (representative of 3 experiments). (F) Mean fluorescence intensity of CXCR4 on PC in the spleen of mice boosted with soluble NP-KLH or infected with Salmonella (at day 5). Statistical significance was evaluated using student’s t test (B and F) or two way ANOVA with Bonferroni post-test (C and E).
The lack of LLPC in the BM in these models could be due to failure to migrate there (K/BxN arthritis) or the failure to incorporate into the LLPC pool (Salmonella). The chemokine CXCL12 has a principal role in effecting entry of PC into the BM and so expression of its receptor CXCR4 might indicate reasons for the failure to incorporate in the BM PC compartment. We assessed the expression of CXCR4 on the surface of PC from the spleens of mice 5 days after secondary immunization with soluble NP-KLH (which generates many PC that travel to the BM) and 16 days after infection with Salmonella. In the spleen, expression of CXCR4 was similar (Fig 3F) between the two models, indicating that this did not underlie the failure of PC entry.
Salmonella infection causes depletion of previously established LLPC populations in the BM
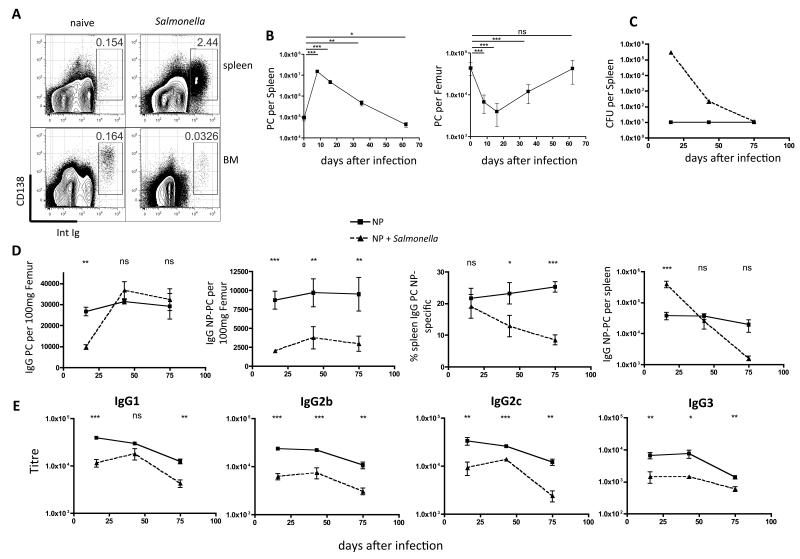
Salmonella infected mice generated large numbers of PC in the spleen, but these failed to persist in the BM (Figs. 3D). Moreover, intriguingly, we also observed a considerable contraction of the BM PC compartment following Salmonella infection (Fig. 4A & B). We, therefore, investigated the effects of Salmonella infection on previously established BM PC populations. To induce a readily detectable NP-specific PC population, mice were primed and boosted with NP-KLH and then rested for 5 weeks before infection with Salmonella. Bacterial loads in the spleen peaked between days 7 and 14 (Fig. 4C). By day 16 post-infection a dramatic depletion of NP-specific PC was seen in the BM (Fig. 4D, panels 1 & 2). Interestingly, at the same time, increased numbers of NP-PC were detected in the spleen (Fig. 4D, panel 4). This is not the result of the generation of new NP-specific PC (no BrdU was incorporated into NP-specific PC after 48 hour pulses on days 6-8 or 14-16), however, it could be due to an influx of NP-binding cells PC from the BM. Splenic NP-specific PC declined over time post-infection (by day 75 post-infection were 10-fold lower than uninfected mice), while, in contrast, they were maintained at stable levels in uninfected controls (Fig. 4D, panel 3). Total PC numbers in the BM recovered to normal between day 25 and 60 post-infection (Fig. 4B), however, the NP-specific PC remained lower than uninfected controls until the end of the analysis at 75 days (Fig. 4D, panels 2 & 3). This indicates that there was no significant re-entry to the BM of PC that were lost during infection (Fig. 4D). Finally, we observed an overall negative effect of Salmonella infection on the NP-specific antibody levels in the serum (Fig. 4E). These data indicate that Salmonella infection causes a disruption of the steady state equilibrium of BM PC populations that affects circulating antibody levels.
Figure 4. Salmonella infection causes loss of LLPC from BM.
(A) Plots showing PC induction in the spleen 16 days after infection with Salmonella, and reduced frequencies of PC in BM (plots representative of groups of 5 mice in experiments done 6 times). (B) Numbers of PC in the spleen and BM at various time points following infection with Salmonella. Points show mean and standard error of 5 mice per group. (C) CFU in the spleens of mice primed and boosted with NP-KLH 5-weeks before infection with Salmonella (dashed line), compared to uninfected controls (solid line). (D) Total IgG (left) or NP-specific IgG PC (left middle) per 100mg femur, or BM NP-specific PC as a percentage of total IgG PC (right middle), or number of NP-specific IgG PC per spleen of mice shown in (C). Numbers of BM cells were adjusted to number per 100mg femur, as the bones increase in size with the age of the mice over the course of the experiment. (E) Titers of NP-specific antibodies of indicated class) in serum of mice from (C). Points on graph are mean and standard errors of data from 5 mice per group from 1 of 2 experiments. Statistical significance was evaluated using one way ANOVA (B) or two way ANOVA with Bonferroni post-test (D and E).
Inflammation not influx of new PC causes the loss of pre-existing LLPC in the BM
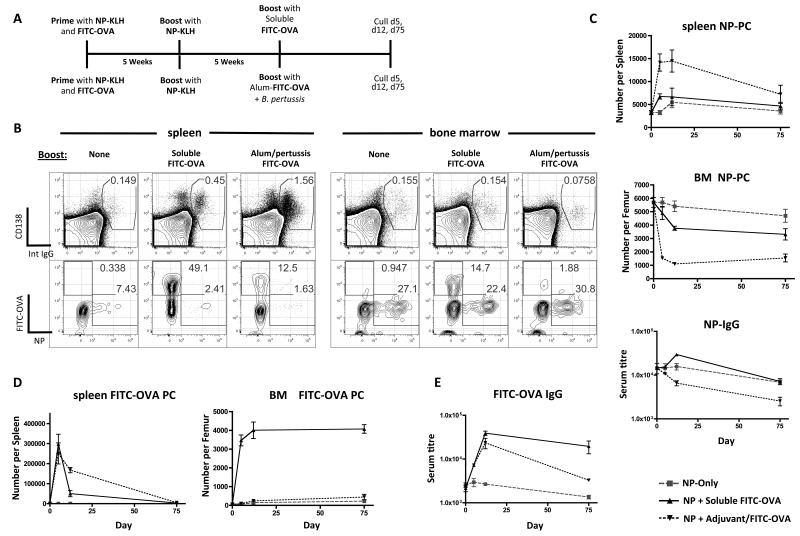
In humans, following booster vaccination with tetanus-toxoid (TT), TT-specific PC can be observed entering the BM from around day 5 and an increased frequency of mature non-TT-specific PC are briefly detected in the blood [26]. The PC in the blood are thought to be LLPC of other specificities that have been “pushed out” of their survival niches by the new influx of TT-specific PC [35]. The mechanism of this competition between new and old BM PC is unknown. The loss of BM PC populations that we saw during Salmonella infection was unlikely to be the result of newly-arrived PC pushing out old ones as there was no early influx of PC and the decrease in numbers was also very pronounced. We hypothesised that the change in BM PC numbers was more likely the result of circulating inflammatory mediators. To test whether simple competition for niches [35] or inflammatory signals were important we developed a model to observe the competition between PC of 2 antibody specificities (NP and FITC) delivered in soluble or inflammatory formulations (Fig. 5A). Mice were primed with both NP-KLH and FITC-OVA. Five weeks later the mice were boosted with soluble NP-KLH to establish NP-specific BM LLPC (see Fig. 1B). A further 5 weeks later, mice were boosted with either soluble FITC-OVA or alum-precipitated FITC-OVA with killed B. pertussis (Fig. 5A). As with Salmonella infection FITC-OVA delivered with adjuvant caused a significant loss of total PC (around 50%) from the BM (Fig. 5B, right hand panel, CD138 vs intracellular IgG stain). In the spleen the opposite was true, in that antigen+adjuvant caused a large expansion of total PC numbers (Fig. 5B, left hand panel). Focusing first on the fate of the pre-existing NP-specific PC, the frequency of NP-specific cells within the BM PC population remained the same following all forms of boosting (Fig. 5B, right hand panel, FITC vs NP plots); however, the numbers of NP-specific PC in the BM fell quite dramatically following the antigen+adjuvant formulation, being reduced to less than 20% of pre-boost numbers (Fig 5C, lower panel; mean of 5.6×103 per femur down to 1×103 at day 10); a much greater proportional depletion than seen in total PC numbers in these mice (mean of 3.2×104 per femur down to 2.1×104 at day 10; data not shown). Soluble FITC-OVA also accelerated the decay of NP-specific PC in the BM (compared to non-boosted controls), but this was not as striking as the effect of FITC-OVA + adjuvant (Fig. 5C). In the spleen we saw a transient increase in NP-specific PC after the antigen+adjuvant boost, which might arise from an influx of PC from the BM or from a non-specific activation/differentiation of NP-specific memory B cells; the study of Benson et al [36] suggests the latter is less likely. Importantly, with regard to serum antibody responses, the antigen+adjuvant caused a noticeable lowering of anti-NP levels over a period of 75 days (Fig. 5C, bottom panel). The adjuvant effect that we describe here is mediated by the killed B. pertussis component, as alum alone has no effect on BM PC numbers (Suppl Fig. S1).
Figure 5. The effect of antigenic competition and adjuvant on the exchange of new for existing PC in the BM.
(A) Schematic of experimental design. All mice were primed with alum-precipitated NP-KLH and FITC-OVA with killed B. pertussis and boosted 5 weeks later with soluble NP-KLH. 5-weeks later mice were boosted with FITC-OVA in different formulations. (B) Representative FACS plots showing total IgG PC staining in spleen and BM at day 5 following boost with FITC-OVA (top row), and NP- or FITC-specificity of IgG PC (bottom row). Data shown is representative of 5 mice per group in this experiment, which was repeated 4 times. (C) Summary graphs showing numbers of NP-specific PC in the spleen and BM of mice immunized as described in (A). (D) Summary graphs showing numbers of FITC-specific PC in the spleen and BM of mice described in (A). (E) FITC- (left) or NP- (right) specific IgG titres in the serum of mice described in (A).
Turning to the generation of new FITC-specific PC following the boost with FITC-OVA: In the spleen both antigen formulations caused a considerable increase in FITC-specific PC numbers (Fig. 5D), although the frequency of FITC-specific cells was much greater after the soluble boost (Fig. 5B). The adjuvant caused a splenomegaly that explains the differences in frequency. In the BM only soluble FITC-OVA caused a major (>20-fold) increase in the number FITC-specific PC. Thus, the adjuvant formulation, although it had made space in the BM compartment, did not allow entry of newly-generated PC into this compartment. The anti-FITC titers were similar in both groups at 30 days post-boost, but by 60 days after boost the antigen+adjuvant group exhibited reduced titers compared to the mice receiving soluble FITC-OVA, reflecting the failure to establish a population of FITC-specific long-lived bone marrow plasma cells (Fig. 5E). Although these data show there is some effect on resident PC of the arrival of new PC into the BM (soluble FITC-OVA caused some loss of NP-specific PC; Fig. 5C), a much more profound influence is exercised by adjuvant- or infection-induced inflammation. Intriguingly, this infection/inflammation driven loss of BM PC does not involve TLRs, as MyD88, TRIF and TLR4 knockout mice all show equivalent or greater degrees of PC depletion after Salmonella infection (Suppl. Fig. 2)
The effect of inflammation on the BM microenvironment
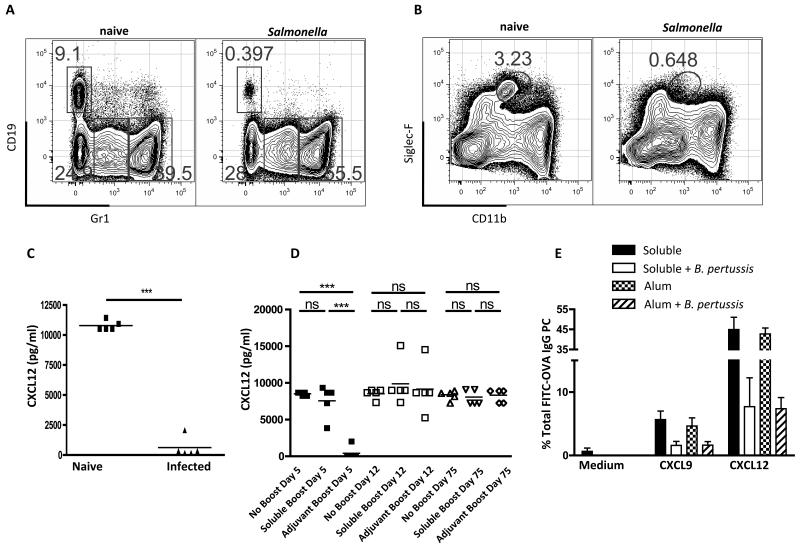
The BM microenvironment is sensitive to systemic inflammation [37], for instance, TNFα (induced by adjuvant) has been observed to cause a significant drop in BM lymphopoiesis [25], accompanied by an expansion of granulocytes and MCP1 causes the mobilization of monocytes from the BM [38]. The former was related to a decrease in CXCL12 production made by resident VCAM-1+ stromal cells [25]. CXCL12 is also the main chemokine to mediate entry of PCs into the BM [7]. We could confirm that that both Salmonella infection (Fig. 6A) and alum-precipitated NP-KLH + B. pertussis (not shown) caused a decrease in B lineage cells in the BM and an increase in granulocytes. In addition, we noted that, at the peak of Salmonella infection, Siglec-F+ eosinophils were much reduced in the marrow (Fig. 6B). Eosinophils have been identified as providing a survival niche for PC in the BM [20]. Furthermore, we found that the BM cells flushed from the bone of infected mice secreted little if any CXCL12 compared to those from non-infected mice (Fig. 6C). We also looked at the CXCL12 production in the BM from mice receiving boost injections of soluble versus alum-precipitated antigen + B. pertussis. At day 5 post-boost with the adjuvant formulation, we could detect no CXCL12 production, compared to normal levels in the marrow from the mice receiving the soluble boost (Fig. 6D). The production of CXCL12 in the marrow returned to normal by 12 days post-boost (Fig. 6D). In the case of Salmonella infection the decrease in CXCL12 correlated with the increase in the circulating levels of a number of inflammatory mediators (Suppl. Fig. S3), including TNFα. IFN-γ (prevalent in the response to Salmonella) has been linked to the induction of CXCR3-expressing PCs that migrate towards CXCL9 [13]; leading to their preferential migration to inflammatory sites rather than the BM. However, the splenic PCs generated following immunization increased both CXCR3 and CXCR4 expression especially when B. pertussis was included in the adjuvant (Suppl. Fig. S3). Importantly, and despite the up-regulation of CXCR3 ad CXCR4, migration of PCs across trans-well membranes towards both CXCL9 and CXCL12 was impaired in mice immunized with B. pertussis as adjuvant (Fig. 6E). It seems likely that the loss of CXCL12 production by BM cells and the reactivity of PCs to this chemokine have important roles to play in the homeostatic control of LLPC populations after infection.
Figure 6. Changes to the BM cellular compartments following Salmonella infection or bacterial adjuvant injection.
(A) Frequencies of CD19+ (B cells) and Gr1+ cells (granulocytes) in the BM of naïve or Salmonella infected mice (at day 16). (B) Frequency of eosinophils (siglec-Fhi / CD11bint) in the bone of naïve or Salmonella infected mice (at day 16). Plots from (A) and (B) are representative of 5 mice from 1 of 5 experiments. (C) CXCL12 levels in the supernatant of BM cultures from mice Salmonella infected (at day 16). (D) CXCL12 levels in the supernatant of BM cultures from mice receiving no boost, soluble FITC-OVA boost, or FITC-OVA + adjuvant boost at day 5, 12 and 60 (all mice primed with alum FITC-OVA + B pertussis). Each point is data from 1 mouse, bars represent the mean, this is representative of 3 other experiments. Migration across transwells towards medium or medium containing CXCL12 (400nM) or CXCL9 (400nM); expressed as the percentage of FITC-OVA IgG PC that migrated across the transwell). Mean and standard errors of ELISA titers from 5 mice per group (experiment run 3 times). Statistical significance was evaluated using student’s t test (C) or one-way ANOVA with Bonferroni post test (D).
TNFα causes the loss of PCs from the BM
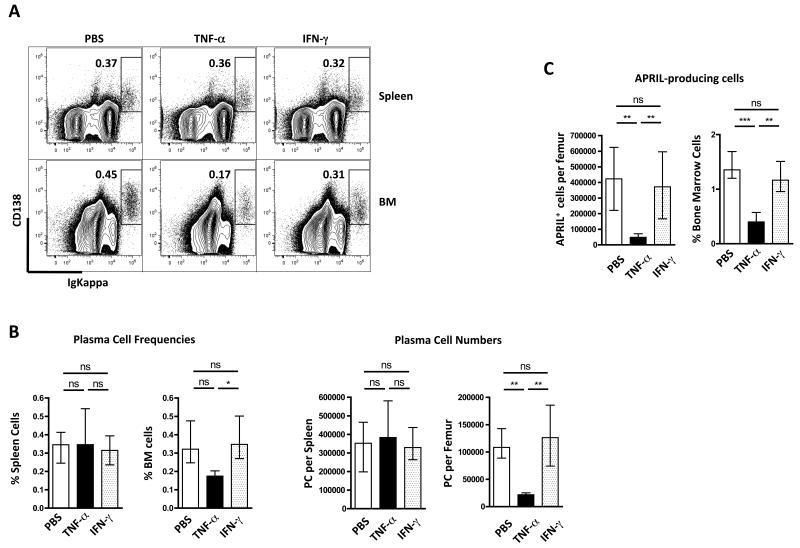
Ueda et al[25] previously reported that TNFα reduced levels of CXCL12 from the BM. To test whether TNFα was responsible for the mobilization of PCs from the BM we injected mice 3 times at 48-hour intervals with 1μg of TNFα. Analysis of the BM PCs 2 days after the last injection showed that the number and frequency of PC was significantly reduced (Figure 7A & B). There was no change in splenic PC populations. Given the role of IFN-γ and downstream mediators in the mobilization of myeloid populations from the marrow[38] and the large increase in IFN-γ in the circulation of infected mice (Suppl. Fig. S3), we also injected this cytokine, however, no change in BM or spleen PC numbers was observed (Fig. 7), despite changes to MHC class II levels on monocyte/macrophages (not shown). On this basis we conclude that TNFα-induced by Salmonella infection or B. pertussis-containing adjuvant is a major cause of PC mobilization from the BM. We also noted that TNFα and Salmonella infection caused a transient loss of cells within the BM that were making the PC survival factor APRIL (Fig 7C).
Figure 7. LLPC and APRIL-producing cells in BM are mobilized by TNFα but not IFN-γ.
(A) Representative FACS plots of PC in the spleen and BM following IV injection of TNFα or IFN-γ (x3 over 72hrs). (B) Summary graphs of PC frequencies (left) or numbers (right) in spleen and BM of TNFα / IFN-γ injected mice. (C) The number and frequency of APRIL-producing cells, identified by intracellular flow cytometric staining, in BM of mice injected with TNFα or IFN-γ. Means and standard errors are shown and this experiment was performed twice. Statistical significance was evaluated using one-way ANOVA with Bonferroni post-test.
Discussion
Following acute immunization the generation of PC in the local lymphoid organ is transient, even following secondary immunization the number of Ag-specific PC wanes significantly beyond 2 weeks (eg. in the spleen, Fig. 1). If antigen is supplied over a longer period the generation of PC is also extended, presumably evoked by continued stimulation of antigen-specific B cells. While in the spleen the persistent antigenic stimulus causes cell turnover (BrdU incorporation) throughout the period of Ag-administration, in the BM newly-divided PC only enter during the first few days following immunization (boosting). There could be several reasons for this. First, the source of new LLPC precursors, the GC, might be impaired in some way, however, this is probably not the case as GC are enhanced in mice given antigen chronically (Fig. 2D). There are two other possibilities that, at present we cannot differentiate; either the BM is receptive to new PC entrants only for a defined period following immunization (both acute and chronic groups exhibit increased numbers of BrdU+ PC in the BM when labelled from day 1-10, but not after this) or the environment of the spleen during chronic Ag administration retains the new PCs locally. The latter may be a reflection of ‘stromal cells’ providing chemotactic and survival factors over a prolonged period as a result of the inflammatory signals. We observed these same phenomena in two other models of chronic stimulation (arthritis and prolonged infection). In the K/BxN arthritis model we found many SLPC were generated in the spleen, but they did not appear in the BM (Fig 3). Following Salmonella infection SLPC were generated early and these did enter the BM but again did not persist as long-lived cells. Some insight may be provided by our experiments comparing the BM incorporation of Ag-specific PC after soluble or adjuvant-associated boosting. After soluble antigen there is no barrier to entry of new PC into the BM compartment, however, inclusion of adjuvant (especially bacterial) inhibits this process. So in the case of the soluble antigen boost the plasmablasts are allowed to enter the BM, while bacterial adjuvant causes retention of these cells in the spleen. More generally, the window for entry of new PC into the BM compartment is controlled by inflammatory signals arising from infection or bacterial adjuvant and as a result of chronic stimulation/inflammation. We (in this work) and others [33] observe that chronic stimulation drives the formation of SLPC; these cells seem incapable of converting into stable LLPC (in the marrow) and so must be sustained locally. This is possible as inflamed tissues contain a number of cell types that can provide survival signals (a “niche”), but only for a short period as the size of this niche is limited and the input of new PC from the chronic activation of B cells is likely to overwhelm the niche capacity (hence cells are pushed out and die). A formal demonstration of this hypothesis remains to be provided. The observation that chronic antigen supply favors SLPC production at the expense of LLPC may have profound consequences for certain vaccination protocols, such as DNA vaccination, whereby small amounts of antigen are administered over a prolonged period.
We noted the dramatic loss of total PC numbers from the BM in the first week following Salmonella infection and supposed that this may underlie the exchange of newly-formed PC with previous occupants. We provide ample evidence that infection- and adjuvant-induced inflammatory signals do cause the mobilization of PC from the marrow and that this efflux does reduce the size of the existing LLPC populations, with concomitant reduction in circulating antibodies derived from these PC populations (see Figs 4 & 5). This is associated with dramatic drop in the amount of CXCL12 produced by BM cells isolated from infected or adjuvant (pertussis) immunized mice (Fig 6), and also with a loss of cells that have previously been shown to provide survival signals for LLPC in the BM, ie. eosinophils (Fig 6) [20]. As a generalised loss of B lineage cells from the BM has been shown following infection [39] and is a feature of TNFα administration [25], we wondered if the mobilization of PC would also be TNFα-dependent. Indeed this was the case and as in previous studies [25] is likely to be the result of decreased CXCL12 production (Fig 6). Thus, injection of TNFα caused rapid loss of PC from the marrow and interestingly this also provoked a noticeable fall in the numbers of cells in the BM that produce APRIL (Fig 7). It seems unlikely that TNFα acts directly on PC to cause their exit from the marrow. Interestingly a number of mediators that affect PC survival (eg. IL-6, stem cell factor, VCAM-1) are raised in the circulation during Salmonella infection (Suppl. Fig. S3). In summary, systemic inflammation, specifically that mediated by TNFα, has quite profound effects on the PC compartment. Our competitive immunization experiments (Fig 5), show that these effects have significant long-term consequences for the antibody response; pre-existing anti-hapten antibody responses drop rapidly when adjuvant-delivered immunization or an infection is introduced.
Contrary to our expectations, the loss of existing BM PC following immunization in association with a bacterial adjuvant did not lead to enhanced entry of new PC into this compartment. Indeed, when B. pertussis was used as adjuvant the incorporation of new FITC-specific PC in the BM was much reduced compared to a boost with soluble or alum-precipitated antigen. This was not due to a decrease in the numbers of FITC-specific PC generated in the spleen; despite lower frequencies after boosting with alum FITC-OVA + B. pertussis compared with soluble FITC-OVA boost (12% vs 49% FITC-specific PC; Fig 5), the number of FITC-specific PC generated was similar (explained by pertussis-induced splenomegaly). A simple explanation could be that the adjuvant-induced inflammatory milieu in the spleen holds the LLPC precursors in the spleen. We conclude that inflammation impacts negatively on the ability of new PC to enter the BM compartment, while plasmablasts can enter without hindrance under non-inflammatory conditions. This has profound consequences for vaccine delivery protocols. Moreover, our data, added to studies on the effect of bystander inflammation on memory B cell survival and differentiation [36] conclude that inflammation is at best a neutral influence on humoral memory but more often causes attrition.
How does this fit with the notion that the LLPC compartment in the BM is limited in size and that entry is competitive [35]? We propose that this compartment in mice is larger than we supposed. Indeed, looking at the numbers of PC (IgM and IgG) accumulating in the BM over time, we observe that compartment increases in size up to 30 weeks of age and possibly beyond (Suppl. Fig S4). In the experiments described the mice were around 25 weeks old at boosting (Fig 5) and may still at this point maintain sufficient space in their PC compartment to sustain large numbers of newly-recruited PC, without any need to clear out pre-existing cells. This would also explain the very high frequencies of Ag-specific PC generated following priming and boosting (with soluble antigen) that persist for more than 45 days (Fig 1). It would be informative to repeat this experiment in mice in which the BM PC numbers had reached a plateau or in mice that were housed in environments where they were exposed to greater microbiological challenge. Once the BM PC compartment is ‘full’, inflammation-driven PC mobilization may become an important mechanism to allow exchange of LLPC specificities. There may be alternative explanations for these observations, for instance, there may be utility in mobilizing PC to the circulation or the spleen that bring benefits related to their (non-specific or cross-reactive) antibody production or to other functions, as cytokine producers [40] or APC [41].
It remains to be demonstrated whether in older mice or in adult humans the LLPC compartment is replete and so entry into it becomes competitive. If this is the case systemic inflammation would have an important role to play in freeing up space in the BM PC compartment for newly-generated cells. However, the importance of this observation is in the consequence that infection/inflammation has on long-lived PC and the circulating antibody levels that they maintain. These data indicate that each time we are infected, such that TNFα (or other mediators) is released, there is a loss of LLPC and a fall in the titers of serum antibodies to antigens previously met. Thus, the protection afforded by antibodies to vaccine antigens and childhood pathogens will almost certainly be degraded by each subsequent (unrelated) infection. Attrition of CD8 memory T cell populations following unrelated viral infections has previously been noted [42, 43]. Although we have not yet looked, we predict that a similar impairment of these antibody titers may be apparent in patients with chronic autoimmune conditions, when severe flares occur. Estimating LLPC lifespan experimentally is generally done with mice in pathogen-free conditions, based on these findings, we feel that in a more normal, pathogen-challenged environment the decay of LLPC populations may be accelerated.
Supplementary Material
Footnotes
Grant support: This work was supported by a Medical Research Council studentship (to TS) and by a Programme Grant from the Wellcome Trust (to DG).
Abbreviations: BM = bone marrow, PC = plasma cell, LLPC = long-lived plasma cell, SLPC = short-lived plasma cell, GC = germinal center, APRIL = A Proliferation Inducing Ligand, BAFF = B cell activating factor, NP = nitrophenyl
References
- 1.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 2.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 4.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 5.Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 6.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 7.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 9.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatto D, Pfister T, Jegerlehner A, Martin SW, Kopf M, Bachmann MF. Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp-1 and XBP-1. J Exp Med. 2005;201:993–1005. doi: 10.1084/jem.20042239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 14.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 17.Chevrier S, Genton C, Kallies A, Karnowski A, Otten LA, Malissen B, Malissen M, Botto M, Corcoran LM, Nutt SL, Acha-Orbea H. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc Natl Acad Sci U S A. 2009;106:3895–3900. doi: 10.1073/pnas.0809736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Njau MN, Kim JH, Chappell CP, Ravindran R, Thomas L, Pulendran B, Jacob J. CD28-B7 interaction modulates short- and long-lived plasma cell function. J Immunol. 2012;189:2758–2767. doi: 10.4049/jimmunol.1102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, Schoenberger SP, Lee KP. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 21.Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, Roth K, Wong DM, Dame C, Tarlinton DM, Schulze H, MacLennan IC, Manz RA. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood. 2010;116:1867–1875. doi: 10.1182/blood-2009-12-259457. [DOI] [PubMed] [Google Scholar]
- 22.Matthes T, Dunand-Sauthier I, Santiago-Raber ML, Krause KH, Donze O, Passweg J, McKee T, Huard B. Production of the plasma-cell survival factor a proliferation-inducing ligand (APRIL) peaks in myeloid precursor cells from human bone marrow. Blood. 2011;118:1838–1844. doi: 10.1182/blood-2011-01-332940. [DOI] [PubMed] [Google Scholar]
- 23.Winter O, Dame C, Jundt F, Hiepe F. Pathogenic long-lived plasma cells and their survival niches in autoimmunity, malignancy, and allergy. J Immunol. 2012;189:5105–5111. doi: 10.4049/jimmunol.1202317. [DOI] [PubMed] [Google Scholar]
- 24.Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan IC. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 25.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 28.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 30.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 31.Ferraro AJ, Drayson MT, Savage CO, MacLennan IC. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 32.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 36.Benson MJ, Elgueta R, Schpero W, Molloy M, Zhang W, Usherwood E, Noelle RJ. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med. 2009;206:2013–2025. doi: 10.1084/jem.20090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulich TR, del Castillo J, Ni RX, Bikhazi N. Hematologic interactions of endotoxin, tumor necrosis factor alpha (TNF alpha), interleukin 1, and adrenal hormones and the hematologic effects of TNF alpha in Corynebacterium parvum-primed rats. J Leukoc Biol. 1989;45:546–557. doi: 10.1002/jlb.45.6.546. [DOI] [PubMed] [Google Scholar]
- 38.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 39.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kuhl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol. 2010;11:1110–1118. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 43.Selin LK, Vergilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.