Abstract
Sativex is a cannabis-plant extract delivering nearly 1:1 Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) by oromucosal spray. It has been suggested that CBD attenuates THC-induced tachycardia, anxiety, and euphoria. In this study, pharmacodynamic effects were compared over 10.5 h in nine cannabis smokers randomly assigned to receive placebo, 5 and 15 mg oral synthetic THC, and low (5.4 mg THC, 5.0 mg CBD) and high (16.2 mg THC, 15.0 mg CBD) doses of Sativex. At therapeutic doses, no substantial CBD-induced modulation of THC's effects was evident. Oral THC and Sativex produced similar, clinically insignificant increases in heart rate, anxiety, and “good drug effects” with no serious adverse events. Oral and oromucosal THC have slower absorption, lower rate of THC delivery to the brain, and fewer associated adverse events as compared with smoked cannabis. These results indicate that Sativex has a pharmacodynamic safety profile comparable to that of oral THC at low, therapeutic doses.
Δ9-Tetrahydrocannabinol (THC) is approved in the United States as the formulation Marinol for treatment of chemotherapy-associated nausea and vomiting, and anorexia. THC is an analgesic and muscle relaxant1 that reduces muscle spasticity.2 Acute psychological responses to THC include euphoria, relaxation, sedation, anxiety, paranoia, dysphoria, psychosis, and depression.3 Another phytocannabinoid, cannabidiol (CBD), was evaluated for efficacy in treating psychosis,4 dystonic movement disorders,5 Huntington's disease,6 and epilepsy7.
THC is extensively oxidized in the liver to equipotent 11-hydroxy-THC (11-OH-THC) and inactive 11-nor-9-carboxy-THC. After cannabis is smoked, 11-OH-THC concentrations are generally <10% of the THC concentrations, but after oral administration, the concentrations of 11-OH-THC and THC are approximately equal. Therefore circulating plasma 11-OH-THC contributes to THC's subjective effects.
THC binds reversibly to membrane receptors and elicits physiological and behavioral effects. CB1-cannabinoid receptors are primarily located on presynaptic nerve terminals8 and bind endogenous or exogenous cannabinoids, modulating synaptic transmission. CB1-receptors are highly expressed in the regions of the brain that control coordination, movement, learning, memory, and cognitive function.9 Peripheral CB1-receptors are located in reproductive, endocrine, gastrointestinal, and vascular systems.8 CBD is a weak agonist of CB1-receptors and may decrease nociception by binding to transient receptor potential vanilloid10 and other receptors that have not yet been described.
Earlier research has indicated that CBD may attenuate THC-induced tachycardia,11 euphoria, and anxiety,12 but the interaction mechanism remains unclear. Sativex is a standardized medication containing 2.5 mg/actuation CBD and 2.7 mg/ actuation THC. Sativex is approved by Health Canada for treating neuropathic pain associated with multiple sclerosis and pain refractory to opiates in patients with cancer. In June 2010, Sativex was approved in the United Kingdom for multiple sclerosis–associated spasticity. Sativex is an investigational drug in the United States, currently in phase III clinical trials as an adjunct to opiates for cancer pain. To our knowledge, no study has directly compared the pharmacodynamics of oral THC with that of Sativex and explored the relationships between pharmacodynamic effects and cannabinoid pharmacokinetics in the same volunteers. The objectives of this study were to establish physiological and subjective drug effect profiles for oral THC and oromucosal Sativex, to determine whether CBD attenuates THC's effects, and to evaluate relationships between plasma cannabinoid concentrations and physiological and subjective measures.
Results
Twenty-two subjects provided written informed consent to participate in the study. Seven were discharged prior to drug administration for various reasons: loss of contact, refusal to provide venous access, claustrophobia during mock functional magnetic resonance imaging (fMRI), and a positive pregnancy test. Six others were discharged after at least one drug administration. Of these, two could not be contacted, two experienced panic attacks in the fMRI scanner, one had inadequate venous access, and one had orthostatic hypotension, dizziness, and nausea after the fMRI session. Additional adverse events in those who completed the study were two episodes of transient light-headedness in one participant, each episode occurring upon standing after the fMRI scan, and headache and neck pain during the fMRI scan in another participant. All the adverse events were nonserious and expected.
Nine cannabis smokers completed five drug administration sessions (Table 1). Baseline THC concentrations varied on entry and ranged from “not detected” to 6.5 μg/l. No association was observed between self-reported use and baseline plasma THC concentrations. The mean baseline THC concentrations were 1.6, 2.3, 1.8, 2.5, and 2.3 ng/ml after placebo, 5 mg oral THC, 15 mg oral THC, low-dose Sativex and high-dose Sativex, respectively. No statistically significant differences in results were observed between sessions.
Table 1. Participant demographic, self-reported drug use histories, and baseline Δ9-tetrahydrocannabinol (THC) concentration ranges.
| Subject | Gender | Age | Race | Weight (kg) | Height (cm) | Average cannabis use | Duration of longest use (years) | Age of 1st use | Other drugs (in last year) | Baseline THC (μg/l) range |
|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 19 | C | 70 | 175 | 1×/month | 2 | 15 | T, A | ND |
| B | F | 25 | C | 66 | 173 | 3×/week | 5 | 16 | T, A, Coc, H | ND–1.6 |
| C | M | 22 | AA | 95 | 178 | 2×/month | 1 | 21 | A | 1.2–2.3 |
| D | M | 28 | AA | 64 | 168 | 1×/week | 9 | 16 | T, A | 0.5–0.6 |
| E | M | 27 | AA | 73 | 178 | 2×/month | 7 | 12 | T, A | ND |
| F | M | 43 | AA | 109 | 196 | 30×/week | 10 | 16 | — | 3.4–6.5 |
| G | F | 20 | AA | 69 | 145 | 2×/week | 2 | 13 | T, A | 3.9–5.3 |
| H | F | 21 | C | 64 | 165 | 5-6×/week | 2 | 17 | T, A, H | 1.6–3.9 |
| I | M | 23 | AA | 64 | 157 | 9×/week | 5 | 14 | A | 3.2–5.3 |
A, alcohol; AA, African-American; C, Caucasian; Coc, cocaine; F, female; H, hallucinogen (psilocybin mushrooms); M, male; ND, not detected; T, tobacco.
Plasma pharmacokinetics
Peak plasma concentration (Cmax) and time to Cmax (Tmax) values for THC and 11-OH-THC after the four active dosing conditions are presented in Table 2. There were no significant differences in Cmax values of THC and 11-OH-THC for similar doses and in the Tmax values across doses.
Table 2. Plasma Δ9-tetrahydrocannabinol (THC) and 11-hydroxy-THC (11-OH-THC) concentration maximum (Cmax) and time to Cmax (Tmax) following 5 and 15 mg oral THC and low (5.4 mg THC + 5 mg cannabidiol (CBD)) and high (16.2 mg THC + 15 mg CBD)- dose oromucosal sativex.
| Dose | Mean ± SE | Median | Range | |
|---|---|---|---|---|
| THC Cmax | 5 mg oral THC | 4.7 ± 0.9 | 4.6 | 1.4–10.4 |
| 15 mg oral THC | 14.3 ± 2.7 | 11.2 | 3.3–28.5 | |
| Low Sativex | 5.1 ± 1.0 | 5.1 | 1.2–9.6 | |
| High Sativex | 15.3 ± 3.4 | 14.5 | 3.2–38.2 | |
| THC Tmax | 5 mg oral THC | 3.2 ± 0.3 | 3.1 | 1.5–4.5 |
| 15 mg oral THC | 3.4 ± 0.5 | 3.4 | 1.2–5.5 | |
| Low Sativex | 3.3 ± 0.3 | 3.5 | 1.2–4.5 | |
| High Sativex | 4.0 ± 0.5 | 4.5 | 1.2–5.6 | |
| 11-OH- THC Cmax | 5 mg oral THC | 3.0 ± 0.4 | 2.6 | 1.8–5.9 |
| 15 mg oral THC | 11.1 ± 2.0 | 9.3 | 3.6–19.5 | |
| Low Sativex | 4.2 ± 0.7 | 3.7 | 2.1–7.5 | |
| High Sativex | 8.4 ± 1.2 | 7.6 | 3.8–13.7 | |
| 11-OH- THC Tmax | 5 mg oral THC | 3.3 ± 0.4 | 3.3 | 1.5–5.6 |
| 15 mg oral THC | 3.4 ± 0.4 | 3.6 | 1.0–5.5 | |
| Low Sativex | 3.6 ± 0.6 | 3.3 | 1.0–7.5 | |
| High Sativex | 3.9 ± 0.5 | 3.7 | 1.2–5.6 |
Physiological effects
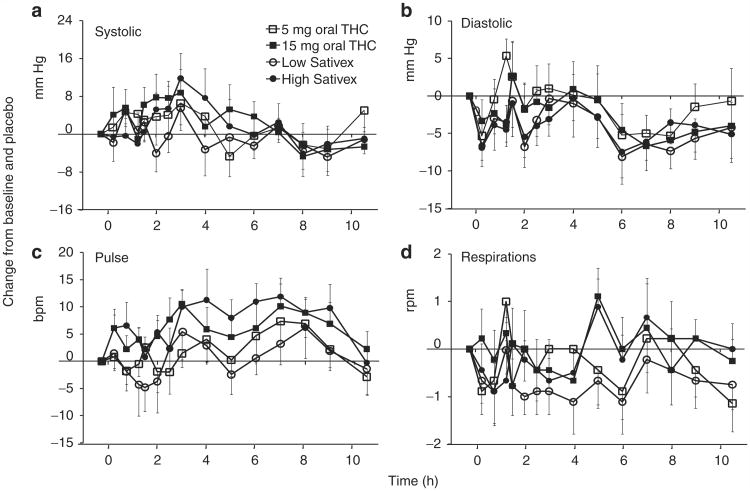
Although systolic blood pressure (SBP) tended to increase (Figure 1a), peaking at ~3 h, there were no significant SBP effects following any of the treatments. Participant B had the largest SBP change from baseline (35.6%, from 101 to 137 mm Hg) occurring at 1.5 h after 5 mg oral THC.
Figure 1.
Mean ± SE (n = 9) changes in (a) systolic blood pressure, (b) diastolic blood pressure, (c) pulse rate, and (d) respiration rate relative to baseline and placebo after 5 and 15 mg oral Δ9-tetrahydrocannabinol (THC) and low-dose (5.4 mg THC + 5 mg cannabidiol (CBD)) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex.
Diastolic blood pressure (DBP) decreased between 4 and 8 h (Figure 1b) after dosing. The values returned to baseline levels by 10.5 h only in the group that received 5 mg oral THC. Relative to the placebo group, DBP was significantly lower in the group that received 15 mg oral THC (avgdiff = 2.71 ± 1.01, P = 0.007) and also in the groups that received low-dose Sativex (avgdiff = 4.52 ± 1.00, P < 0.001) and high-dose Sativex (avgdiff = 4.27 ± 1.01, P < 0.001). Low-dose Sativex also significantly decreased DBP as compared to 5 mg oral THC (avgdiff = 3.05 ± 1.01, P = 0.003). The mean maximum decreases in DBP values were 14.4 ± 2.4 mm Hg at 5.3 ± 0.9 h and 15.1 ± 2.3 mm Hg at 5.4 ± 1.2 h after 5 and 15 mg oral THC, respectively, and 17.7 ± 2.1 mm Hg at 5.4 ± 1.0 h and 16.7 ± 1.6 mm Hg at 4.4 ± 1.0 h after low- and high-dose Sativex, respectively. Participant C had the greatest decrease in DBP from baseline (31.4%, from 70 to 48 mm Hg) at 8 h after 5 mg oral THC.
Heart rate generally increased after all active treatments (Figure 1c), returning to baseline by 10.5 h for all doses. Both 15 mg oral THC (avgdiff = 5.86 ± 1.09, P < 0.001) and high-dose Sativex (avgdiff = 6.93 ± 1.09, P <0.001) significantly increased heart rate as compared to placebo. The higher dose of oral THC resulted in a significantly elevated heart rate than did the lower dose (avgdiff = 4.40 ± 1.10, P < 0.001); similarly, the higher dose of Sativex resulted in a significantly higher heart rate than did the lower dose of Sativex (avgdiff = 6.60 ± 1.10, P < 0.001). The mean maximum heart rate increases were 15.3 ± 3.0 bpm at 6.3 ± 0.6 h and 17.7 ± 3.7 bpm at 5.2 ± 0.9 h for the 5- and 15-mg oral doses of THC, respectively, and 15.7 ± 3.2 bpm at 5.0 ± 1.2 h and 22.9 ± 4.3 bpm at 4.8 ± 0.9 h for the low and high doses of Sativex, respectively. The largest heart rate increase from baseline, from 69 to 98 bpm, occurred in participant A, who experienced this 42.0% increase at 6 h after high-dose Sativex. Only two participants experienced transient tachycardic episodes (>100 bpm); the longest-lasting episode was experienced by participant B (102–125 bpm) from 3 to 5 h after high-dose Sativex. The participants were asymptomatic, and the observed changes were deemed clinically insignificant. The respiration rates (Figure 1d) varied between 12 and 24 breaths per min, with mean adjusted differences fewer than 1 breath per min.
Subjective effect scales
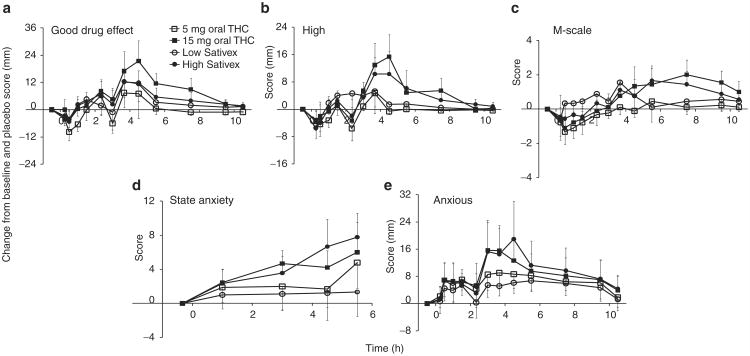
The “good drug effect” peaked at 3.7 h after each of the doses, except with 15 mg oral THC, for which Tmax was 4.5 h (Figure 2a). Visual analog scale (VAS) scores for “good drug effects” returned to baseline by 10.5 h. Post hoc comparisons revealed significantly greater “good drug effects” after 15 mg oral THC (avgdiff = 6.19 ± 1.64, P < 0.001) and high-dose Sativex (avgdiff = 3.79 ± 1.65, P = 0.022) as compared to placebo. Low-dose Sativex was associated with significantly higher (avgdiff = 3.33 ± 1.65, P = 0.044) “good drug effects” than 5 mg oral THC. Four participants had changes ≥40% after low-dose Sativex and three after 5 mg oral THC. “Good drug effects” were also greater (avgdiff = 6.82 ± 1.65, P < 0.001) after 15 mg vs. 5 mg oral THC. There were no significant differences in this regard between the results after low- and high-dose Sativex. The durations to mean maximum “good drug effects” were 2.9 ± 0.7 and 3.9 ± 0.7 h after 5 and 15 mg oral THC, respectively, and 3.5 ± 0.7 and 3.0 ± 0.6 h after low- and high-dose Sativex, respectively. Four of the participants who received low-dose cannabinoid, and three of those who received high-dose cannabinoid, did not report “good drug effects” >5% above baseline values.
Figure 2.
Mean ± SE (n = 9) changes in (a) “good drug effect,” (b) “high,” (c) marijuana (M)-scale, (d) state anxiety, and (e) “anxious” relative to baseline and placebo after 5 and 15 mg oral Δ9-tetrahydrocannabinol (THC) and low-dose (5.4 mg THC + 5 mg cannabidiol (CBD)) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex. “Good drug effect,” “high,” and “anxious” scores were based on a maximum score of 100, M-scale on a maximum of 12, and state anxiety on a maximum of 80 points.
The mean VAS for “high” peaked at 3.7 h after each of the drug treatments, except after 15 mg oral THC, for which this occurred at 4.5 h (Figure 2b). The “high” returned to baseline values by 7.5 and 10.5 h after low and high doses, respectively. The subjective “high” was significantly greater after the 15 mg oral THC as compared to placebo (avgdiff = 2.94 ± 1.25, P = 0.019) and to 5 mg oral THC (avgdiff = 3.82 ± 1.26, P = 0.003). Neither of the Sativex doses produced significant subjective “high” feelings. The mean maximum “high” occurred 3.1 ± 0.8 and 3.4 ± 0.6 h after 5 and 15 mg oral THC, respectively, and 3.5 ± 0.7 and 3.0 ± 0.6 h after low- and high-dose Sativex, respectively. Participant H reported the greatest increase (57%) 3.7 h after 15 mg oral THC. Six participants reported “high” responses <5% above baseline after 5 mg oral THC, three after 15 mg oral THC, and four after each of the Sativex doses.
Marijuana scale (M-scale) scores were highest 3.7 h after low-dose Sativex, 5.5 h after 5 mg oral THC and high-dose Sativex and 7.5 h after 15 mg oral THC (Figure 2c). Baseline M-scale scores ranged between 0 and 3. M-scale scores after each of the active treatments were significantly greater than those after placebo (P < 0.036), except in the case of 5 mg oral THC. As compared to 5 mg oral THC, both low-dose Sativex (avgdiff = 0.83 ± 0.21, P < 0.001) and 15 mg oral THC (avgdiff = 0.78 ± 0.21, P < 0.001) resulted in significantly higher M-scale scores. The mean maximum M-scale increases were 1.0 ± 0.4 and 3.2 ± 0.8 after 5 and 15 mg oral THC, respectively, and 2.1 ± 0.7 and 1.9 ± 0.8 after low- and high-dose Sativex, respectively. The greatest individual M-score increase (8 points) was observed in participant B, 4.5 h after high-dose Sativex. Participant I was the only subject who did not report a positive change in M-scale scores after either of the high-dose formulations.
The participants reported feeling maximally “stimulated” 7.5 h after 5 mg oral THC, 3.7 h after 15 mg oral THC, and 3 h after each of the Sativex doses, returning to near baseline by 10.5 h. Significantly more “stimulation” was reported after the 15 mg dose of oral THC as compared to placebo (avgdiff = 3.22 ± 1.37, P = 0.020), 5 mg oral THC (avgdiff = 3.39 ± 1.38, P = 0.015), and high-dose Sativex (avgdiff = 4.43 ± 1.38, P = 0.002). Low-dose Sativex was significantly more “stimulating” than placebo (avgdiff = 2.95 ± 1.37, P = 0.032), 5 mg oral THC (avgdiff = 3.12 ± 1.38, P = 0.024), and high-dose Sativex (avgdiff = 4.16 ± 1.38, P = 0.003). Participant E had the greatest “stimulation” change (52%) 4.5 h after 15 mg oral THC, similar to his increase in subjective “high” (45%) and “good drug effects” (48%) at that time. Participants C, G, and I reported no “stimulation” changes >5% after either of the high doses. No significant differences were observed between the treatments for VAS “stoned, sedated, and depressed” or for any Likert scale. The subjective effects after placebo are shown in Supplementary Figure S1 online.
State–trait anxiety inventories and VAS “anxiety”
Mean trait anxiety scores were 27.7 ± 0.8, with no significant differences across treatments. Mean state anxiety varied from 25.7 ± 2.1 to 30.4 ± 2.1 across sessions. State anxiety steadily increased to a maximum at 5.5 h after each of the cannabinoid doses (Figure 2d). Relative to placebo treatment, state anxiety scores were significantly higher, after high-dose Sativex (avgdiff = 3.81 ± 1.11, P = 0.008), 15 mg oral THC (avgdiff = 3.52 ± 1.02, P = 0.001), and 5 mg oral THC (avgdiff = 2.05 ± 1.02, P = 0.046).
Participants were most “anxious” (per VAS) at 3.7 h after the 5 mg oral THC, at 3.0 h after 15 mg oral THC, at 5.5 h after low-dose Sativex, and at 4.5 h after high-dose Sativex (Figure 2e). All the active drug treatments induced significantly more “anxiety” as compared to placebo (avgdiff > 4.22 ± 1.27, P ≤ 0.001). State anxiety and VAS “anxiety” were significantly higher after high-dose Sativex than after low-dose Sativex (avgdiff > 3.29 ± 1.11, P ≤ 0.004). The largest increases in VAS “anxiety” (89%) and state anxiety (20 out of 80 points) occurred 4.5 h after high-dose Sativex in participants B and H, respectively.
Correlations between physiological and subjective effects and plasma cannabinoid concentrations
After 15 mg oral THC, the concentration of THC in plasma was observed to have weak, but statistically significant, positive correlations with SBP and DBP (r ≤ 0.23), “good drug effect,” and “high” (r ≤ 0.35). Similarly, after high-dose Sativex, positive correlations (r ≤ 0.32) were also observed between plasma THC concentrations and “anxious,” “good drug effect,” “high,” “stimulated,” and M-scale scores.
Discussion
In ancient China, cannabis was used for surgical analgesia;13 in Western medicine in the nineteenth century, it was used to treat muscle cramps, convulsions, migraines, inflammation, pain, asthma, and neuralgia.13,14 With cannabis later being designated as a Schedule I drug,14 smoked cannabis is at the center of an ethical debate in the medical community. In 1999, the Institute of Medicine recognized the therapeutic potential of cannabis and requested research on alternative cannabinoid delivery systems other than smoking, owing to its known toxicity.15 Alternative routes of administration should deliver a consistent quantity of drug, preferably with diminished central nervous system side effects as compared to smoked cannabis, and without delivering potential carcinogens. The first approved cannabinoid medication was oral, synthetic THC, an appetite enhancer for AIDS wasting disease and an antiemetic during cancer chemotherapy.16 Sativex, a cannabis-plant extract, is delivered through the oromucosal route, offering advantages for patients with difficulty swallowing due to nausea and vomiting. In addition, cannabinoids offer therapeutic benefit in chronic conditions such as muscle spasticity,17 urinary incontinence,18 increased intraocular pressure19 and pain associated with neuropathic conditions,20,21 rheumatoid arthritis,22 and cancer23.
Investigators claimed that CBD was capable of modulating the undesirable effects of THC,11,12 and also of acting on additional cannabinoid receptors24 as well as on other known10 and unknown receptors, to produce desirable therapeutic results. These data show the effects of two Sativex doses (one dose equal to two times the initial titration dose in two actuations and the other a standard single dose in six actuations) and matched 5-and 15-mg oral THC doses.
Tachycardia is the most sensitive and robust cardiovascular response to THC.25–28 Statistically significant heart rate increases relative to placebo treatment were observed after high-dose oral THC and high-dose Sativex; consistent with other findings, CBD did not ameliorate THC-induced heart rate escalation.12,29,30 While no significant alteration in SBP occurred between treatments, significant diastolic hypotension was observed after almost every active dose. In our study, statistically significant cardiovascular responses were of low magnitude and considered clinically insignificant. This is in contrast to the tachycardia produced by smoked cannabis, characterized by mean peak increases of 46.0 ± 18.6 and 55.8 ± 22.2 bpm after 1.75% and 3.55% THC, respectively, which are twofold greater increases than we observed28.
Some earlier studies reported significant attenuation in THC intoxication when CBD was administered in CBD:THC ratios of 1:1 and 2:1 by the oral route11 and 4:1 by the smoked route,30 while others reported no change with a 2:1 ratio administered orally.29 In this study, CBD did not decrease “good drug effects” significantly, but may have attenuated “high” feelings. Statistical differences were determined across the entire time course; significantly greater “highs” were reported, relative to placebo, after the 15-mg oral THC, but not after high-dose Sativex. However, the effects of 15 mg THC and high-dose Sativex did not differ significantly when compared directly. M-scale score differences were significantly greater after low-dose Sativex as compared to 5 mg oral THC, consistent with the increase in “good drug effects.” Contrary to other studies reporting significant decreases in M-scale scores and anxiety associated with the coadministration of CBD and THC in the ratio 2:1,11 we found no reduction in these effects after either of the Sativex doses. The greatest change in the VAS “anxious” score occurred 4.5 h after high-dose Sativex in participant B, corresponding to her maximum M-score increase at 4.5 h and tachycardia at 3–5 h after the dose. It is possible that increases in heart rate, good drug effect, and high—all of which occurred simultaneously—contributed to the increase in anxiety. Increases in anxiety could be of concern in a clinical setting; however, the magnitude of changes in subjective anxiety scores was low. Low therapeutic doses of cannabinoids were perceived as “stimulating,” possibly because of the combination of increases in heart rate, “good drug effects,” and “high.”
Cannabis smokers may be less responsive than drug-naive patients to subtle subjective effects after low oral or oromucosal cannabinoid doses. However, there was no rank order relationship between magnitude of effect produced by THC and frequency of cannabis use. After receiving oral THC and Sativex, many of the participants reported no changes >5% above baseline and placebo on subjective scales. VAS for “stoned,” “sedated,” and “depressed” were low after each of the cannabinoid doses. These low VAS scores were probably due to the oral route of administration, which provides a low rate of drug delivery to the brain as compared to smoked cannabis. Also, Likert scales were similar after each of the treatments, including after placebo, indicating that this instrument may not be sensitive to small THC-induced alterations. Another potential limitation was that VAS descriptors may have been interpreted differently by participants. For instance, “depressed” could be interpreted as either “sedated” or a “depressive” symptom. The small sample size and multiple comparisons limited the statistical power. Another limitation was the inability to test effectively for drug condition × sequence interaction effects because of the limited number of subjects and the large number of drug conditions. We minimized the influence of such effects by dose randomization. Also, data were normalized by correcting for placebo in addition to correcting for baseline, thereby improving accuracy in determining CBD and THC effects. An additional limitation was the inability to evaluate improvement in symptoms in a relevant patient population.
Subjective responses typically reached mean maxima within 1 h of plasma THC and 11-OH-THC Tmax after each of the treatments. However, linear correlations between plasma THC concentrations and physiological and subjective effects were weak. This is consistent with other reports of the absence of strong, statistically significant linear relationships between plasma THC and intoxication scores26,31–33.
Sativex facilitates self-titration, with most patients reporting pain relief at doses lower than those producing intoxication.34 Current recommendations for Sativex suggest a maximum daily dose of 12 sprays (30 mg CBD + 32.4 mg THC) over a 24-h period.35 Future investigations should evaluate higher CBD:THC ratios and higher Sativex doses, which may demonstrate greater THC effect modulation.
At these low therapeutic cannabinoid doses, physiological and subjective effects were not robust, and no modulation of THC's effects by CBD was apparent, with the potential exception of subjective “high.” The absence of clinically significant increases in cardiovascular parameters and euphoria, typical of smoked cannabis, is an advantage in the therapeutic use of Sativex. As compared to smoked cannabis, oral or oromucosal THC administration is characterized by slower THC absorption and less rapid as well as decreased THC delivery to the brain, thereby delaying the onset and magnitude of effects and resulting in fewer adverse events. No strong cardiovascular or intoxication effects or serious adverse events were observed, indicating that Sativex has a pharmacodynamic safety profile comparable to those of 5- and 15-mg oral doses of THC.
Methods
Participant screening
Cannabis smokers, 18–45 years of age, were recruited through print, radio, and television advertisements to participate in this double-blind, double-dummy, within-subject, institutional review board–approved protocol. The participants were restricted to those who had smoked cannabis at least once, but less often than daily, within the 3 months prior to participation. Screening included drug use history, physical examination, 12-lead electrocardiogram, and laboratory tests to detect clinically significant illnesses. SBP, DBP, and heart rate were required to be ≤140 mm Hg, ≤90 mm Hg, and ≤100 bpm, respectively, while seated, after resting for 5 min. Blood donation >500 ml within 30 days prior to the scheduled administration of the study drug precluded enrollment in the study. Individuals with a history of psychosis or current DSM-IV axis I disorder (other than caffeine or nicotine dependence and simple phobia), and those who had earlier experienced significant adverse events associated with cannabis intoxication were excluded. An estimated intelligence quotient ≥85 per the Wechsler Abbreviated Scale of Intelligence and <24 on the Attention Deficit Hyperactivity Disorder Screening Rating subscales were required. Additionally, participants were excluded if they were allergic to sesame seed oil (dronabinol ingredient), propylene glycol, ethanol, or peppermint oil (Sativex ingredients). Pregnant or nursing female volunteers were excluded, and participants were encouraged to use birth control for at least 3 months after completion of the study.
Study design
The subjects provided their written informed consent to participate in the study. They resided at the research unit under continuous medical surveillance for at least 10 h prior to dosing to ensure that there was no acute intoxication. Female participants were required to have a negative urine pregnancy test prior to each treatment session. A standard breakfast was provided ∼1 h before each drug administration. The participants were trained on neurocognitive tasks and screened for claustrophobia in a mock fMRI scanner prior to session 1. At 90 min after each dose, the participants underwent neurocognitive testing by means of fMRI.
Each of the participants received two capsules and six sprays in each of five sessions. Placebo THC capsules contained only lactose, while placebo Sativex contained propylene glycol, ethanol, and peppermint oil. The participants took two capsules with water; subsequently the study physician administered Sativex and/or Sativex placebo actuations sublingually and buccally, within 1–2 min. Five different treatments were administered in random order: (i) one 5 mg oral THC capsule and one placebo THC capsule and six Sativex placebo actuations (administered from two containers); (ii) one dose of 15 mg oral THC (one 5 mg and one 10 mg oral THC capsule) and six Sativex placebo actuations (administered from two containers); (iii) two placebo oral THC capsules; and two Sativex actuations (low-dose 5.4 mg THC and 5.0 mg CBD) and four Sativex placebo actuations (administered from two containers), and (iv) two placebo oral THC capsules and six Sativex actuations (high-dose 16.2 mg THC and 15.0 mg CBD, administered from two containers) and (v) two placebo oral THC capsules and six Sativex placebo actuations (administered from two containers). There were at least 5 days between sessions.
Physiological monitoring
With the subject seated, measurements of blood pressure, heart rate, and respiration were taken at baseline (−0.3 h or −20 min) and at 0.3, 0.8, 1.3, 1.5, 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.5 h after the dose.
Subjective tests
Subjective effects scales consisted of seven 100-mm VASs, seven five-point Likert scale items, and the 12-item M-scale from the Addiction Research Center Inventory.36 Subjective-effects scales were administered prior to (−0.5 h) and at 0.3, 0.5, 1.0, 1.5, 2.3, 3.0, 3.7, 4.5, 5.5, 7.5, 9.5, and 10.5 h after drug administration. The 100-mm VASs were anchored with “not at all” and “most ever” and included “good drug effect,” “high,” “stoned,” “stimulated,” “sedated,” “anxious” and “depressed.” Likert Scales included: “difficulty concentrating,” “altered sense of time,” “slowed or slurred speech,” “body feels sluggish or heavy,” “feel hungry,” “feel thirsty,” and “shakiness/tremulousness.” Participants’ responses were scored as none–1, slight–2, mild–3, moderate–4, and severe–5. The M-scale consisted of 12 true-or-false questions relating to cannabis intoxication. Each true response was given one point, for a maximum of 12 points.
The Spielberger State–Trait Anxiety Inventory (Mind Garden, Menlo Park, CA) is a method of assessing anxiety. The state anxiety scale evaluates the degree of anxiety the person feels at that moment, whereas trait anxiety assesses the baseline anxiety level. Participants were administered the trait anxiety inventory prior to each dosing session (−0.3 h). The state anxiety test was administered prior to dosing (−0.3 h) and at 1.0, 3.0, 4.5, and 5.5 h after the dose. The participants answered 20 state questions with “not at all,” “somewhat,” “moderately so,” or “very much so,” and 20 trait questions with “almost never,” “sometimes,” “often,” or “almost always.” Each question was scored from 1 to 4 using the scoring key provided by Mind Garden. Potential state and trait scores ranged from 20 (minimum) to 80 (maximum), with high scores indicating increased anxiety or stress37.
Plasma specimen collection and analysis
Whole blood was collected through peripheral venous catheters into 7-ml tubes containing sodium heparin. The samples were stored on ice and then centrifuged within 2 h to separate plasma. Plasma was frozen in cryotubes at −20 °C until analysis.
Concentrations of THC and 11-OH-THC in plasma were quantified using a validated two-dimensional gas chromatography mass spectrometry (2D-GCMS) method.38 Briefly, 1.0 ml of plasma was precipitated with 3 ml cold acetonitrile, the supernatants were decanted, and solid phase extracted. Derivatized analytes were analyzed by 2D-GCMS in selected ion-monitoring mode. Low calibration curves were from 0.25 to 25 ng/ml for THC and 0.125–25 ng/ml for 11-OH-THC. Analytical method intra-run, inter-run, and total imprecision were ≤9.5%, and recovery/bias was ≤9.2%.
Statistical methods
In order to estimate physiological and subjective differences between drug conditions, changes relative to baseline values and relative to the placebo session were calculated after the administration of active drug treatments. Mixed models for repeated measures analysis of variance fit the data: changes in response measures relative to baseline and placebo were calculated as (drug condition + time + time × drug condition), where time denotes the data collection time point. Post hoc comparisons were carried out on the results of all active drug treatments with placebo and 5 to 15 mg oral THC, 5 mg oral THC with low-dose Sativex, 15 mg oral THC with high-dose Sativex, and low-dose Sativex with high-dose Sativex. SAS PROC MIXED was used for estimating mean differences (avgdiff ± standard errors) among placebo and the various active drug conditions, averaging across the post-baseline time points. In addition, time × treatment interactions were tested to ascertain whether the differences among the various treatment results varied significantly over time. Mean maximum responses were assessed by determining the maximum change from baseline and placebo for each participant and calculating the mean response and mean time at which maximum response occurred. To examine whether the administered drug had an effect on Likert scales, we computed changes in parameters relative to baseline and placebo in each drug condition and performed a Mantel–Haenszel χ-test stratified by participant to test for differences over time in mean change scores. With only nine subjects, and five drug conditions assigned in random order, it was impossible to test effectively for the existence of drug condition × sequence interaction effects.
The association between changes in plasma cannabinoid concentrations and concurrent changes in pharmacodynamic effects was determined. Because plasma collection and physiological measurements did not occur at exactly the same time points, corresponding plasma THC concentrations were evaluated if they were collected within 30 min of the parameter; otherwise, if a time difference >30 min occurred, plasma THC concentrations before and after the time point were averaged. Within each active drug condition, the Spearman rank correlation coefficient was calculated between changes relative to baseline THC concentrations and changes relative to physiological and subjective baseline and placebo data. The mean correlation across participants within each active drug condition was calculated. Correlation estimates from individuals were normalized using Fisher’s z-transformation, z(r) = ½loge[(1+r)/(1−r)], and a one-sample t-test was performed on transformed correlation scores to test whether mean correlations were significantly different from zero.
Supplementary Material
Acknowledgments
The authors acknowledge G.W. Pharma for generously providing active Sativex and placebo, Geoffrey Guy and colin Stott for technical assistance, and Susan Baskin, Kathleen Demuth, Janeen Nichels, and John Etter for clinical research assistance. This research was supported by the Intramural Research Program of the National Institute on Drug abuse, National Institutes of health.
Footnotes
Supplementary Material is linked to the online version of the paper at http://www.nature.com/cpt.
Conflict of Interest: The authors declared no conflict of interest.
References
- 1.Pertwee RG. Pharmacological and therapeutic targets for delta-9-tetrahydrocannabinol and cannabidiol. Euphytica. 2004;140:73–82. [Google Scholar]
- 2.Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW. Delta-9-Thc in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse. 1987;7:39–50. doi: 10.1300/j251v07n01_04. [DOI] [PubMed] [Google Scholar]
- 3.Johns A. Psychiatric effects of cannabis. Br J Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- 4.Zuardi AW, Morais SL, Guimarães FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- 5.Consroe P, Sandyk R, Snider SR. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci. 1986;30:277–282. doi: 10.3109/00207458608985678. [DOI] [PubMed] [Google Scholar]
- 6.Sandyk R, Consroe P, Stern LZ, Snider SR. Effects of cannabidiol in huntington’s disease. Neurology. 1986;36:342. [Google Scholar]
- 7.Cunha JM, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol. 2005;40:2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- 9.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9 - tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- 12.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 13.Mechoulam R, Hanu L. The cannabinoids: an overview. Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion, in multiple sclerosis and in neuroprotection. Pain Res Manag. 2001;6:67–73. doi: 10.1155/2001/183057. [DOI] [PubMed] [Google Scholar]
- 14.Guy GW, Whittle BA, Robson PJ. The Medicinal Uses of Cannabis and Cannabinoids. Pharmaceutical Press; London: 2004. [Google Scholar]
- 15.Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- 16.Wright S. Cannabinoid-based medicines for neurological disorders–clinical evidence. Mol Neurobiol. 2007;36:129–136. doi: 10.1007/s12035-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 17.Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006;12:639–645. doi: 10.1177/1352458505070618. [DOI] [PubMed] [Google Scholar]
- 18.Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler. 2004;10:425–433. doi: 10.1191/1352458504ms1063oa. [DOI] [PubMed] [Google Scholar]
- 19.Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma. 2006;15:349–353. doi: 10.1097/01.ijg.0000212260.04488.60. [DOI] [PubMed] [Google Scholar]
- 20.Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010;33:128–130. doi: 10.2337/dc09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50–52. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Reyes M, Lipton MA, Timmons MC, Wall ME, Brine DR, Davis KH. Pharmacology of orally administered delta-9-tetrahydrocannabinol. Clin Pharmacol Ther. 1973;14:48–55. doi: 10.1002/cpt197314148. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 27.Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- 28.Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther. 1992;52:31–41. doi: 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- 29.Hollister LE, Gillespie H. Interactions in man of delta-9-tetrahydrocannabinol II Cannabinol and cannabidiol. Clin Pharmacol Ther. 1975;18:80–83. doi: 10.1002/cpt197518180. [DOI] [PubMed] [Google Scholar]
- 30.Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta-9-tetrahydrocannabinol effects. Clin Pharmacol Ther. 1976;19:300–309. doi: 10.1002/cpt1976193300. [DOI] [PubMed] [Google Scholar]
- 31.Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol. 1981;21:171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 32.Guy GW, Robson PJ. A phase I, double-blind, three-way crossover study to assess the pharmacokinetic profile of cannabis based medicine extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers (GWPK0215) J Cannabis Ther. 2004;3:121–52. [Google Scholar]
- 33.Galanter M, Wyatt RJ, Lemberger L, Weingartner H, Vaughan TB, Roth WT. Effects of humans of delta-9-tetrahydrocannabinol administered by smoking. Science. 1972;176:934–6. doi: 10.1126/science.176.4037.934. [DOI] [PubMed] [Google Scholar]
- 34.Russo E. The Solution to the Medicinal cannabis Problem. In: Schatman ME, editor. Ethical Issues in Chronic Pain Management. Taylor & Francis; Boca Raton, FL: 2006. pp. 165–194. [Google Scholar]
- 35.Pharmaceuticals GW. Summary of Product characteristics: Sativex. 2010 [Google Scholar]
- 36.Haertzen CA. An overview of Addiction Research Center Inventory Scales (ARCI): An Appendix and Manual of Scales, DHEW Publication No (ADM) US Government Printing Office; Washington, DC: 1974. [Google Scholar]
- 37.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory for Adults. Consulting Psychologists Press; Palo Alto, CA: 1983. pp. 1–76. [Google Scholar]
- 38.Karschner EL, Barnes AJ, Lowe RH, Scheidweiler KB, Huestis MA. Validation of a two-dimensional gas chromatography mass spectrometry method for the simultaneous quantification of cannabidiol, Delta(9)-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC in plasma. Anal Bioanal Chem. 2010;397:603–611. doi: 10.1007/s00216-010-3599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.