Abstract
Background
Individuals with ulcerative colitis (UC) are at increased risk for colorectal cancer. The standard method of surveillance for neoplasia in UC by colonoscopy is invasive and can miss flat lesions. We sought to identify a gene expression signature in non-dysplastic mucosa without active inflammation that could serve as a marker for remote neoplastic lesions.
Methods
Gene expression was analyzed by cDNA microarray in 5 normal controls, 4 UC patients without dysplasia, and 11 UC patients harboring remote neoplasia. Common gene ontology pathways of significantly differentially expressed genes were identified. Expression of genes which were progressively and significantly up-regulated from controls, to UC without neoplasia, to UC with remote neoplasia were evaluated by real time PCR. Several gene products were also examined by immunohistochemistry.
Results
468 genes were significantly up-regulated and 541 genes were significantly down-regulated in UC patints with neoplasia compared to UC patients without neoplasia. Nine genes (ACSL1, BIRC3, CLC, CREM, ELTD1, FGG, S100A9, THBD, and TPD52L1) were progressively and significantly up-regulated from controls to non-dysplastic UC to UC with neoplasia. Immunostaining of proteins revealed increased expression of S100A9 and REG1α in UC-associated cancer and in non-dysplastic tissue from UC patients harboring remote neoplasia, compared to UC patients without neoplasia and controls.
Conclusions
Gene expression changes occurring as a field effect in the distal colon of patients with chronic UC identify patients harboring remote neoplastic lesions. These markers may lead to a more accurate and less invasive method of detection of neoplasia in patients with inflammatory bowel disease.
Keywords: Inflammatory bowel disease, ulcerative colitis, dysplasia, colorectal cancer, gene expression
Introduction
Patients with ulcerative colitis (UC) are at increased risk for colorectal cancer (CRC), although mechanisms of carcinogenesis remain poorly understood (1–3). The risk of inflammatory bowel disease (IBD)-associated colon cancer is related to the extent, duration, and severity of inflammation (4–6). With chronic inflammation, the epithelium undergoes a transformation from injured and regenerating yet non-dysplastic tissue to progressively neoplastic epithelium, ranging from low-grade dysplasia (LGD), to high-grade dysplasia (HGD), and eventually to adenocarcinoma (3, 7). Molecular and genetic changes accompany this histologic progression (3, 8–11).
Previous reports have also identified molecular abnormalities in normal-appearing, non-dysplastic mucosa from patients with UC who harbor a remote dysplastic lesion (11–15). Aneuploidy, chromosomal alterations, p53 mutations, and p53 loss of heterozygosity appear in large areas of normal appearing mucosa adjacent to areas with cancer (12–14). Furthermore, chromosomal instability can be seen in normal appearing mucosa prior to the development of dysplasia (15).
Earlier microarray studies have demonstrated changes in gene expression from mucosa in patients with UC compared to normal controls and patients with Crohn’s disease (16, 17). Although one study identified gene expression changes in UC tissue remote from neoplastic lesions compared to UC without dysplasia, the study did not report on inflammatory activity, which is known to alter gene expression (18). No study has addressed gene expression changes in quiescent UC at risk for colon cancer. We hypothesized that gene expression profiles in random, normal appearing UC mucosa might provide a ‘genetic signature’ to distinguish colons harboring dysplasia from non-dysplastic colons. Identification of gene expression changes could provide valuable insights into mechanisms driving cancer development in IBD and potentially identify biomarkers for IBD patients harboring neoplastic lesions.
Materials and Methods
Patients
The study was approved by the institutional review board at the University of Chicago. Informed consent was obtained from patients with UC undergoing surveillance colonoscopy, colonoscopy for follow up of a previous diagnosis of dysplasia, or surgical colectomy with a diagnosis of dysplasia. Microarray data was obtained from colonic mucosal biopsies from normal controls, UC patients without dyspasia, and non-dysplastic mucosa from UC patients harboring a remote neoplastic lesion. Patients with UC were included if they had a previous clinical diagnosis of UC confirmed by an expert GI pathologist, a disease duration > 7 years, and an extent of disease >20 cm proximal to the anal verge. Background information was collected on the age of the patient, duration and extent of disease, history of primary sclerosing cholangitis (PSC), and a family history of colon cancer in a first degree relative.
Histology Review
Original surgical pathology was reviewed for neoplasia by two expert GI pathologists (JH/AN). All rectosigmoid samples submitted to pathology were reviewed from the same date as the tissue biopsies obtained for the study. On histological review, all patients had IBD with quiescent disease (no active inflammation) or with minimal inflammatory activity in the rectosigmoid colon. No patient had a neoplastic lesion at the site where study biopsies were obtained.
Sample Collection
During endoscopic procedures, 4 mucosal biopsies were obtained 10–20cm proximal to the anal verge and placed in RNAlater®. After 24–48 hours at 4° C, the biopsies were then stored at −80° C until RNA extraction was performed. Specimens obtained at the time of surgery were acquired through biopsy of tissue from the rectosigmoid colon immediately following removal of the colon during colectomy. Surgical specimens were placed in RNAlater® without delay and transferred to 4° C storage for 24–48 hours and then stored at −80° C until RNA extraction was performed.
RNA Extraction
RNA was extracted using the RNeasy® kit (Quiagen, Hilden, Germany) that included a one-column DNase step according to the manufacturer’s specifications. RNA purity and concentration were determined using the Agilent nanodrop ND1000 spectrophotometer and RNA integrity assessed by Agilent 2100 N (Agilent Technologies, Palo Alto, CA). All samples used in the analysis had a RNA integrity number (RIN) >7.
Microarray Analysis
Microarray assays were carried out on Affymetrix Human U133p2 platform at the Vanderbilt Functional Genomics Facility following Affymetrix protocols (Affymetrix, Santa Clara, CA, USA). The automated hybridization, washing, staining, and scanning of the arrays was completed on the Affymetrix Gene Titan instrument. The quality check and data normalization were performed using R/Bioconductor package "affy" with "rma" approach (19). The microarray .cel files and normalized data were deposited in NCBI GEO repository with accession number GSE37283.
Gene filtering and sample classification were performed using dChip software (http://biosun1.harvard.edu/complab/dchip/) with Pearson's correlation matrix and cetroid-linkage method. Probe sets were filtered by the criteria of standard deviation >0.7 and < 2, and probe set intensity > 5 in at least 50% samples, resulting in 2450 probe sets demonstrating sufficient variation across individual samples. Unsupervised hierarchical clustering of samples was performed using these probe sets to determine whether the clinical conditions could be distinguished by global gene expression pattern.
To identify differentially expressed genes, two-group comparison was performed using Significance Analysis of Microarrays (SAM) software using fold change > 2.0 and false discovery rate (FDR) < 5%. Differentially expressed genes between UC and UCN were further parsed into clusters with distinct expression pattern across the three clinical conditions in the sequence of normal, UC, and UCN using Self-Organizing-Map implemented in GeneCluster2 software (www.broadinstitute.org/cancer/software/genecluster2/gc2.html) (20). Functional enrichment analysis of the identified differentially expressed genes or SOM clusters was performed using Onto-Express software (21). The criteria of significant Gene Ontology biological processes was set at gene number ≥ 4 and FDR ≤5%.
Quantitative Real Time PCR
cDNAs were prepared using 100 ng of total RNA, gene-specific primers, and SuperScript III Platinum Two-Step qRT-PCR (Applied Biosystems, Branchburg, NJ, USA). SYBR Green quantitative real time reverse transcription-polymerase chain reaction (qPCR) was performed with the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Primer sets for THBD, ACSL1, BIRC3, ELTD1, TPD52L1, FGG, CREM, S100A9, CLC, and REG1α were synthesized by Invitrogen (Carlsbad, CA, USA) (Supplemental Data: Table 1). The PCR thermal profile was 50°C for 2 min, 95°C for 2 min, and 40 cycles at: 95°C for 3 s followed by 60°C for 30 s in a Roche LightCycler 480 II (Indianapolis, IN, USA).
For qPCR reactions, statistical analyses were performed with SAS (Cary, NC, USA). Gene expression levels were compared using the comparative CT method (ΔCT) and normalized to β-actin (22). Significance between individual groups and across the three groups (normal, UC, and UC with remote neoplasia) was calculated using a mixed effect analysis of variance (23).
Immunohistochemistry
Sigmoid colon mucosa was compared between normal controls, UC patients without neoplasia, and nondysplastic tissue from UC patients with a remote neoplatic lesion. Tissue from UC patients with a remote neoplastic lesion were from a subgroup of the same patients used in the microarray and pcr analysis. Five micron sections from formalin-fixed, paraffin-embedded tissue were cut and mounted on Vectabond-coated Superfrost Plus slides. A tissue microarray was also prepared with punch biopsies from 27 formalin-fixed paraffin embedded UC-associated cancers. Following deparaffinization, the slides were hydrated in a graded series of ethanol washes. Antigen retrieval was accomplished by heating in 0.01mol/L citrate buffer (ph 6; CTNNB1) in a steamer for 20 minutes. To block endogenous perioxidases, the slides were incubated in methanol/H2O2 (3%) protected from light. Protein block (Dako) was used to inhibit non-specific staining. The sections were incubated with the primary antibody overnight at 4 degrees [1:50 dilution for S100A9 (Abnova, H00006280-M01); 1:800 for fibrinogen (Dako, A0080), 1:50 for clusterin (Santa Cruz Biotechnology, SC-6420), 1:100 for annexin A3 (Abcam, AB33068), and 1:200 for REG1α (Laboratory Medicine, Czech Republic, RD181078100)]. The slides were incubated with a 1:200 dilution of secondary antibody (Dako) for 35 minutes and stained with DAB solution. The slides were counterstained with hematoxylin and bluing reagent. For negative controls, primary antibodies were omitted. Control sections showed no specific staining. For S100A9, semiquantitative intensity scores were independently assigned by 3 investigators blinded to tissue diagnosis using the immunoreactive score described by Remmele and Stegner (24). When there was disagreement among reviewers, the slides were re-reviewed and a consensus was reached. For the tissue array with S100A9, positive staining was reported as stromal, glandular, or both. REG1α positivity was reported as the percent positive staining crypts by three blinded investigators and averaged. Comparisons were made using one-way analysis of variance model (ANOVA), and residuals were checked for normality. P values for the pairwise comparisons were Bonferroni-adjusted. For the REG1α tissue array, positive staining of tumor glandular cells was characterized as none, focal weak, diffuse weak, focal strong, and diffuse strong. Because the data were not normally distributed, the overall difference between three groups was assessed using the Kruskal-Wallis test, and pairwise group comparisons were done using the Wilcoxon ranksum test. Analyses were performed using Stata v12 (College Station, TX).
Results
Patients
Microarray results were obtained in 24 patients. Of these patients, five were normal controls, four had quiescent UC, and 15 had UC with neoplasia. After removal of two patients with primary sclerosing cholangitis (PSC) and two patients because of poor RNA quality by normalization, 11 patients with remote neoplastic lesions were included in the analysis. Of the patients with neoplasia, four had low-grade dysplasia (LGD), three had high-grade dysplasia (HGD), and four had adenocarcinomas. Two neoplastic lesions were located in the ascending colon, three in the transverse colon, one in the descending colon, three in the sigmoid colon, and two in the rectum.
The mean age of the patients analyzed was 46 years in the control group, 48.3 years in patients with quiescent ulcerative colitis, and 46.9 years in patients with UC associated neoplasia. The median disease duration was 13.5 years in UC patients with neoplasia and 20 years in UC patients without neoplasia. All patients included in the analysis were male. Ten of the 11 patients with UC-associated neoplasia had pancolitis. Of the four UC patients without dysplasia, two had extensive colitis and two had left-sided colitis (Table 1).
Table 1.
Baseline Patient Characteristics
| Normal | UC | UC with neoplasia |
|
|---|---|---|---|
| N | 5 | 4 | 11 |
| Sex (M/F) | 5/0 | 4/0 | 11/0 |
| Race (AA/C/A/U)* | 2/3/0/0 | 0/3/0/1 | 1/6/1/3 |
| Age (Mean) | 46 | 48.3 | 46.9 |
| Disease Duration (Median) | X | 20 | 13.5 |
| Left sided colitis | X | 2 | 1 |
| Pancolitis | X | 2 | 10 |
| Dysplasia Histology (LGD/HGD/Adenocarcinoma) | X | X | 4/3/4 |
| Medications | |||
| Mesalamine | 4 | 8 | |
| Immunomodulators | 2 | 3 | |
| Anti-TNF | 0 | 1 | |
AA=African American; C= Caucasian; A= Asian; U= Unknown
Microarray
In UC patients without neoplasia (UC) compared to normal controls, 324 genes were significantly up-regulated and 17 genes were significantly down-regulated. In UC patients with neoplasia (UCN) compared to normal controls, 1667 genes were significantly up-regulated and 508 genes were significantly down-regulated. In UCN compared to UC, 488 genes were significantly up-regulated and 541 genes were significantly down-regulated (Table 2) (The 25 genes with greatest up-regulation and down-regulation between groups are presented in Supplementary Data: Table 2-4).
Table 2.
Prioritization of differentially expressed genes underlying disease progression.
| Two Group Comparison |
FDR% | Fold Change |
Up-Regulated | Down-Regulated |
|---|---|---|---|---|
| UC vs. Normal | 5.2 | 2.0 | 324 | 17 |
| UCN vs. Normal | 2.5 | 2.0 | 1667 | 508 |
| UCN vs. UC | 5.0 | 2.0 | 468 | 541 |
Note. Gene filtering criteria and number of significantly up- or down-regulated gene loci indentified by SAM.
Nine genes (ACSL1, BIRC3, CLC, CREM, ELTD1, FGG, S100A9, THBD, and TPD52L1) were progressively and significantly up-regulated from healthy controls to quiescent UC without neoplasia to UC patients harboring remote neoplasia (Table 3). No genes were progressively down-regulated across these patient groups. For the purpose of this pilot study, we focused on progressively up-regulated genes for further validation by real time PCR and immunostaining.
Table 3.
Real time PCR results for genes which were progressively and significantly upregulated between normal controls to UC to UCN.
| UC vs. Normal | UCN vs. Normal | UCN vs. UC | |||||
|---|---|---|---|---|---|---|---|
| Symbol | Gene Name | Fold Change | P value | Fold Change | P value | Fold Change | P value |
| ACSL1 | Acyl-CoA synthetase long-chain family member 1 | 1.4 (0.9–2.0) | 0.1 | 2.1 (1.5–2.9) | <0.0001 | 1.5 (1.1–2.2) | 0.02 |
| BIRC3 | Baculoviral IAP repeat-containing 3 | 1.3 (1.0–1.8) | 0.08 | 6.0 (4.6–7.8) | <0.0001 | 4.6 (3.4–6.1) | <0.0001 |
| CLC | Charcot-Leyden crystal protein | 1.5 (1.0–2.3) | 0.3 | 1.7 (1.2–2.5) | 0.008 | 1.1 (0.8–1.7) | 0.6 |
| CREM | cAMP responsive element modulator | 2.5 (1.6–2.8) | <0.0001 | 4.5 (3.1–6.5) | <0.0001 | 1.8 (1.2–2.7) | 0.004 |
| ELTD1 | EGF, latrophilin and seven transmembrane domain containing 1 | 0.2 (0.2–0.3) | <0.0001 | 2.5 (1.8–3.4) | <0.0001 | 10.2(7.2–14.4) | <0.0001 |
| FGG | Fibrinogen gamma chain | 1.9 (1.2–3.0) | 0.007 | 7.2(4.8–10.9) | <0.0001 | 3.8 (2.4–5.8) | <0.0001 |
| S100A9 | S100 calcium binding protein A9 | 10.3(6.3–16.7) | <0.0001 | 13.2(8.7–20.2) | <0.0001 | 1.3 (0.8–2.0) | 0.3 |
| THBD | Thrombomodulin | 2.4 (1.5–3.7) | 0.0004 | 7.2 (4.8–10.7) | <0.0001 | 3.0 (2.0–4.7) | <0.0001 |
| TPD52L1 | Tumor protein D52-like 1 | 5.5 (3.6–8.2) | 0.004 | 10.9(7.6–15.7) | <0.0001 | 2.0(1.4–3.0) | 0.0006 |
Note. Results are expressed as fold change (95% confidence intervals) and p value between the groups indicated.
Real Time PCR
Changes in nine genes that progressively and significantly increased in expression from normal controls to UC patients without neoplasia to UC patients with neoplasia in microarray analysis were tested by real time PCR. Real time PCR was performed on five normal controls, four patients with UC without dysplasia, and seven patients with UC with neoplasia remote from the biopsy site from the same patient samples used in the microarray analysis. The highest expression levels in all nine genes analyzed were in UC patients with neoplasia. Furthermore, all genes displayed significant expression differences between UC with neoplasia and normal controls. In comparing UC without neoplasia to normal controls, five genes were significantly up-regulated (CREM, S100A9, THBD, FGG, TPD52L1) and one gene was significantly down-regulated (ELTD1). Compared to UC without neoplasia, seven of the nine genes displayed significantly increased expression in UC patients with neoplasia (ACSL1, BIRC3, CREM, ELTD1, FGG, THBD, and TPD52L1) (Table 3).
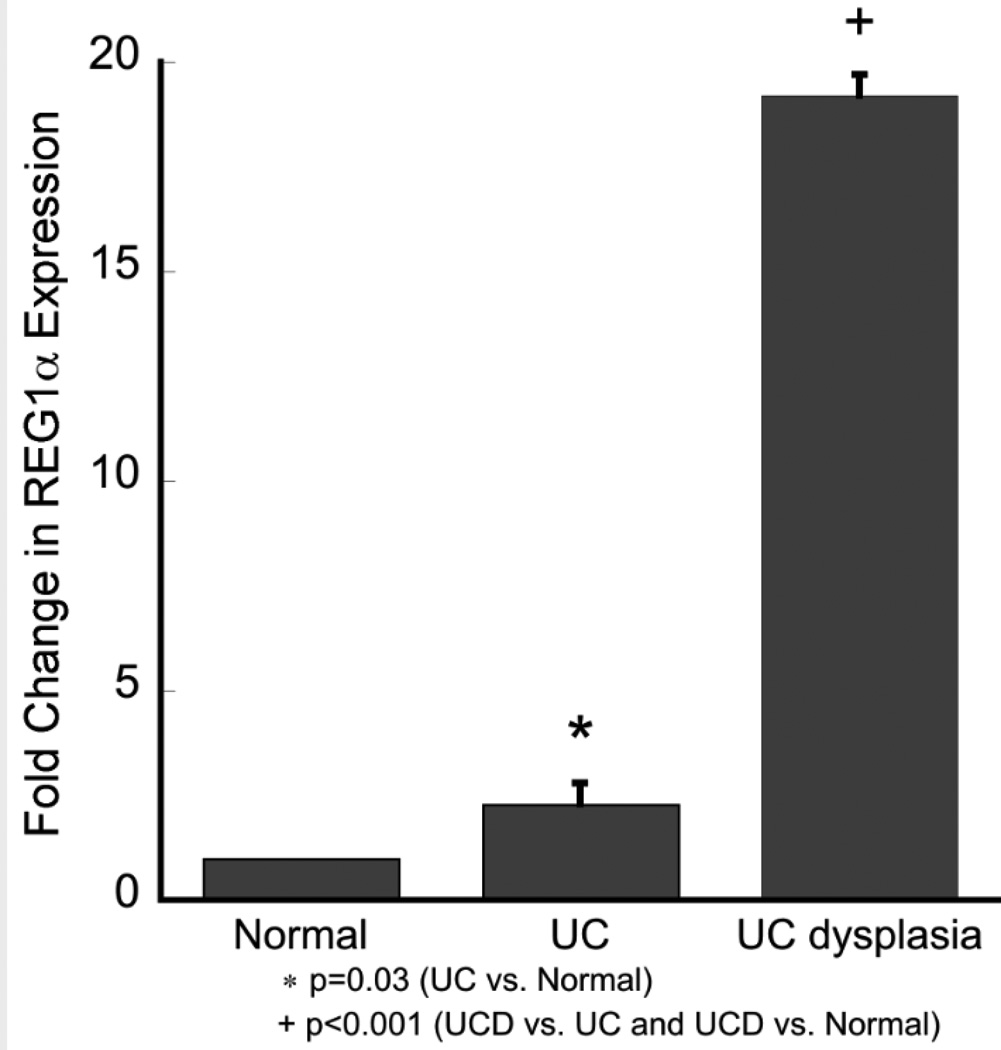
We also performed real time PCR analyses for REG1α as this gene had the greatest differential expression between UC without dysplasia and UC tissue harboring remote neoplasia in the microarray. The real time PCR results confirmed the microarray results with highest expression in UC patients harboring remote neoplasia. As shown in Figure 3, REG1α was up-regulated 2.3 (1.1–4.9)-fold in UC patients (p=0.03) and 19.2 (10.0–36.8)-fold in patients harboring remote neoplasia (p<0.001) compared to normal controls. Compared to UC patients without dysplasia, UC patients harboring remote neoplasia had 8.2 (4.1–16.5)-fold upregulation of REG1α (p<0.001) (Figure 2).
Figure 3.
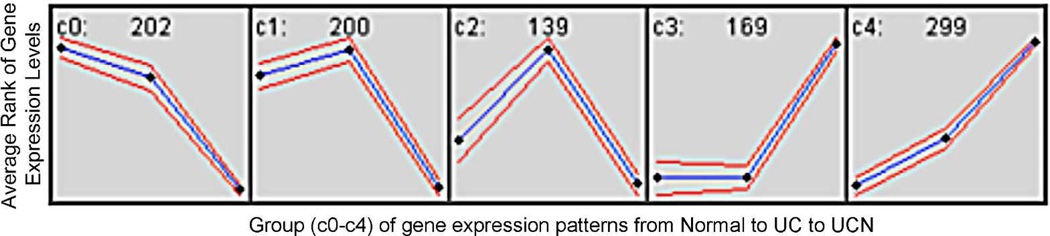
Genes clustered into 5 groups (C0-C4) based on expression patterns between Normal, UC, and UC harboring remote neoplasia. The number of genes in each group is listed at the top of each box. The blue line represents the expression pattern with standard deviation of the mean in red. The black boxes represent the average rank of gene expression levels by clinical condition (Normal – left, UC – middle, UCN – right).
Figure 2.
REG1α expression by real time PCR. Fold change in expression in UC patients without dysplasia and UC patients harboring remote neoplasia is compared to normal controls. Error bars are model based standard error estimates.
Biological processes enriched with differentially expressed genes
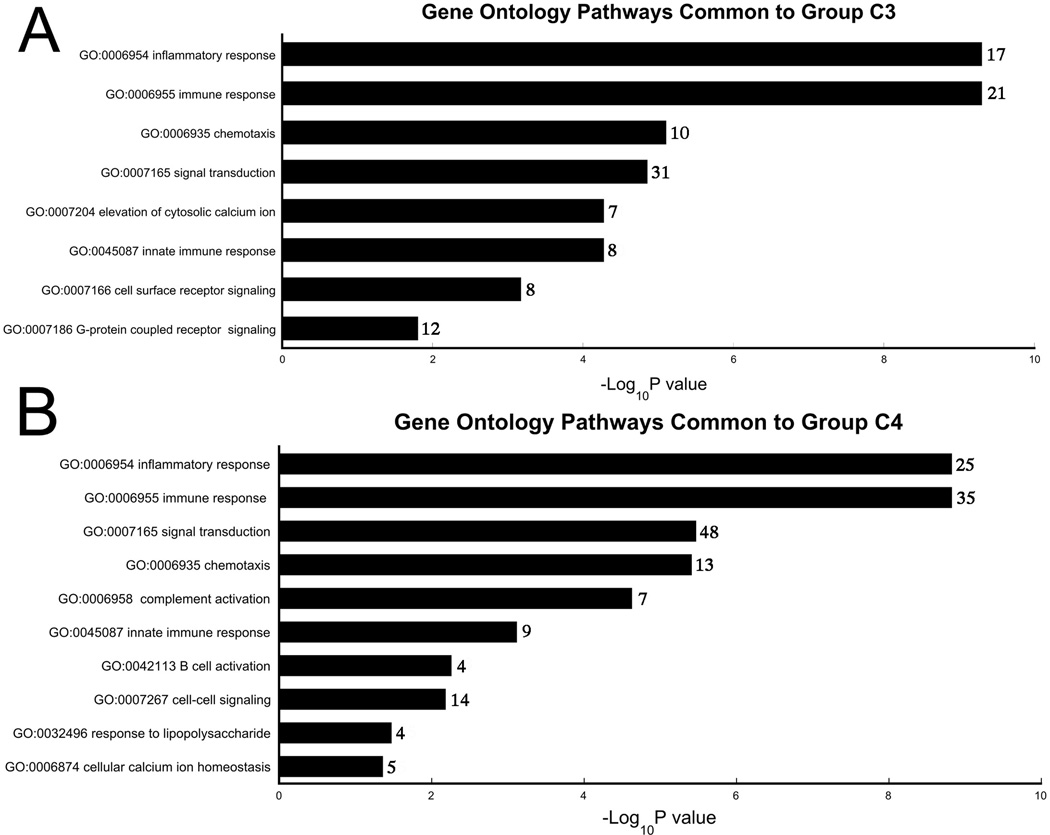
In order to cluster differentially expressed genes by functional classification, we created 5 groups based on expression patterns from normal controls to UC without neoplasia to UC with neoplasia (C0-C4; Figure 3). We focused on groups C3 and C4 as genes clustered into functional ontology classifications in these groups with less random distribution than C0, C1, and C2. One hundred sixty-nine genes had expression patterns consistent with C3. These genes belonged to pathways of immune response, innate immune response, chemotaxis, inflammatory response, G-protein coupled receptor signaling, elevation of cytosolic calcium ion concentration, and signal transduction (Figure 4). There were 299 genes in group C4 with progressive upregulation from normal controls, to UC without dysplasia, to UC harboring remote neoplasia. These genes belong to pathways of cellular calcium ion homeostasis, complement activation, cell-cell signaling, B-cell activation, innate immune response, signal transduction, response to lipopolysaccharide, inflammatory response, chemotaxis, and immune response (Figure 4).
Figure 4.
Gene Ontology Pathways Common to Groups C3 (A) and C4 (B). Gene ontology categories were identified by Ontoexpress software; only biological processes with ≥4 genes and a p-value <0.05 are displayed. The numbers to the right of the column represent the number of unique genes in each pathway.
Immunohistochemistry
By immunohistochemistry, we examined several proteins whose transcripts were significantly up-regulated in UC with remote neoplasia suggesting these proteins could be potentially useful clinical biomarkers. Protein expression examined by immunohistochemistry included: annexin A3, clusterin, fibrinogen gamma (FGG), REG1α, and S100A9. Because of the diffuse stromal staining of annexin A3, clusterin, and FGG, the antibodies were not suitable to demonstrate clinically relevant differences between groups (data not shown).
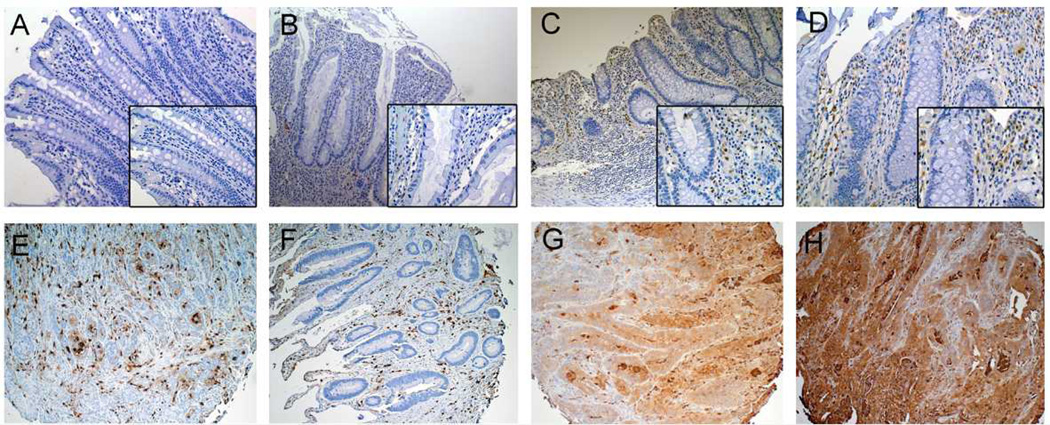
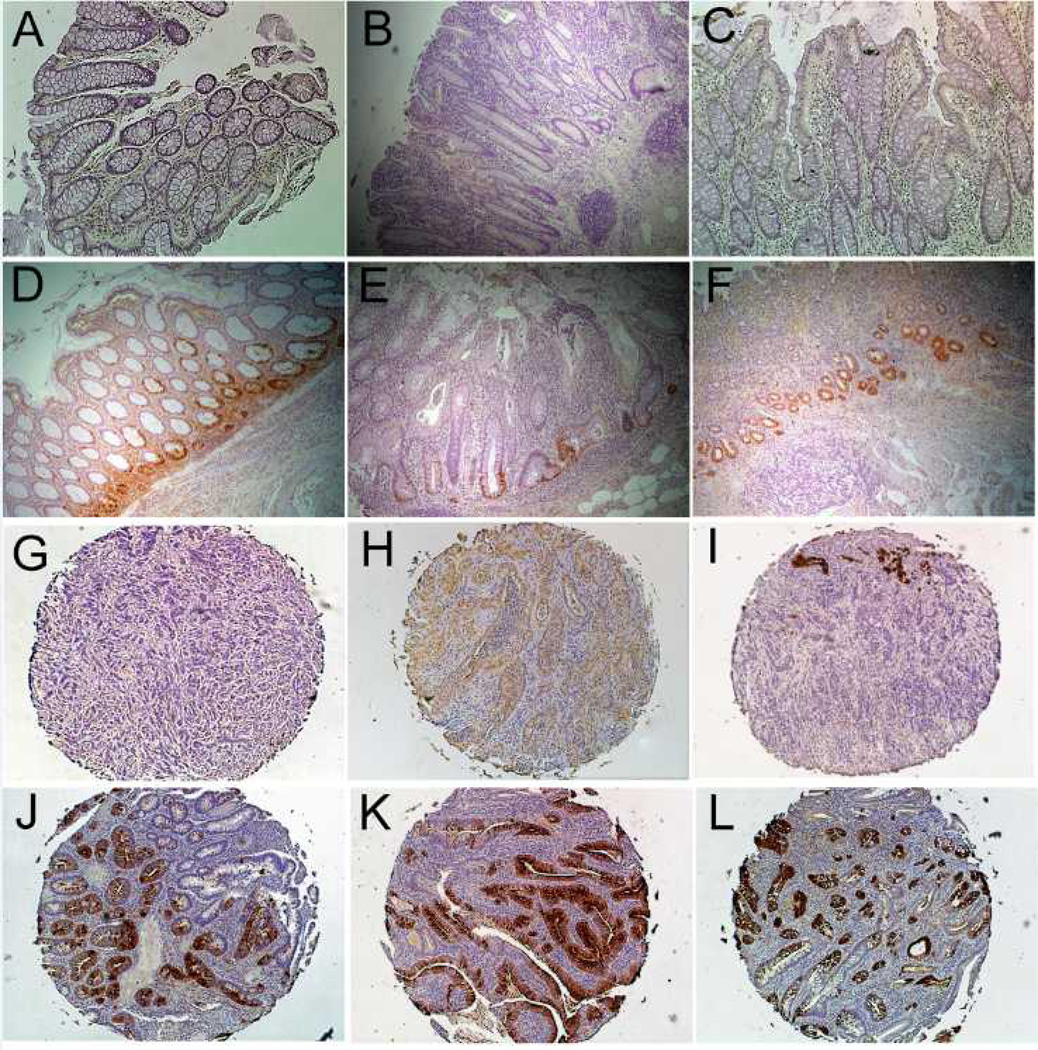
S100A9 immunostaining was performed on nondysplastic mucosa from the sigmoid colons of normal controls (n=6), UC patients without dysplasia (n=6), and UC patients harboring a remote neoplastic lesion (n=9), UC associated dysplasia (n=5), as well as on a tissue array of UC-associated colon cancer tissues (n=27). S100A9 antibodies stained epithelial stroma in all samples. Compared to controls (average score 2.7 (95%CI: −0.04 to 5.4)), S100A9 was increased to 4.8 (95%CI: 2.1 to 7.5) in UC without dysplasia (p=0.80), to 8.9 (95%CI: 6.7 to 11.1) in non-dysplastic tissue from UC patients harboring remote neoplasia (p=0.002), and to 10.2 (95%CI: 7.2 to 13.2) in UC-associated cancer (p<0.001). Compared to quiescent non-dysplastic UC, increases in S100A9 expression were significant in UC-associated dysplasia (p=0.03) and nearly reached significance in non-dysplastic tissue in UC patients harboring remote neoplasia (p=0.06). All tumors displayed stromal staining for S100A9 and 17/27 tumors also showed staining in epithelial cells (Figure 5).
Figure 5.
S100A9 Immunohistochemistry. Representative images from the sigmoid colon in (A) Normal colon (20x) (B) Quiescent UC (20x) and (C 10x, D 20x) non-dysplastic mucosa in a UC patient with a remote dysplastic lesion. Note increased stromal staining in (C, D) compared to (A, B). (E–H) IBD-cancer tissue array: Representative images from 2 sections with positive stromal staining (E, F) and 2 sections with glandular staining (G, H). Larger images are 20x magnification. Insets are at 40x magnification.
REG1α immunostaining was performed on nondysplastic mucosa from the sigmoid colons of normal controls (n=3), UC patients without dysplasia (n=5), and UC patients harboring a remote neoplastic lesion (n=6), and on tissue array of UC-associated colon cancer tissues (n=27). Staining differences were statistically significant between controls, UC, and UC with remote dysplasia patients (p=0.05, Kruskal-Wallis test). The proportion of positive crypts was increased in UC patients harboring remote neoplasia compared to normal controls (p=0.04) but not in normal controls compared to UC patients without dysplasia (p=0.12). In control patients, no staining was identified by immunohisochemistry. UC patients harboring remote neoplasia had higher increase in crypt cell staining compared to UC patients without dysplasia (median percent positive staining 0.97% vs. 22.9%; p=0.09). In a tissue microarray of 27 UC-associated cancers, 25/27 cancers had positive epithelial staining. Of these, nine had focal weak glandular positivity, two had diffuse weak positivity, eight had focal strong positivity, and six had diffuse strong positivity (Figure 6).
Figure 6.
REG1α Immunohistochemistry. (A) Normal colon, (B, C) UC without dysplasia, and (D–F) UC harboring a remote dysplastic lesion. In patients with UC harboring remote dysplasia, there is increased crypt staining, primarily in base of crypts. (G–L) IBD-cancer tissue array (G) A cancer with no positive staining, (H) Cancer with diffuse weak positive staining (I) A cancer with focal strong positive staining (J–L) Cancers with diffuse strong glandular positivity. Images A–G at 10x magnification. Images G–L at 5x magnification.
Discussion
In this cross-sectional analysis, we demonstrated that UC patients harboring a dysplastic lesion display differential gene expression in remote quiescent non-dysplastic mucosa compared to UC patients without dysplasia and normal controls. Gene ontology analysis indicated that differentially expressed genes are involved in pathways of innate immune response and Toll receptor signaling supporting the hypothesis that neoplastic lesions in long standing UC likely result from activation of tumor promoting pathways secondary to longstanding inflammation. In agreement with previous studies demonstrating molecular changes occurring in nondysplastic mucosa of IBD patients remote from a dysplastic lesion, these findings provide further evidence that the entire colonic mucosa is at risk for neoplastic transformation in longstanding ulcerative colitis.
For the purpose of this study, we validated genes by real time PCR and focused on genes which were progressively up-regulated from normal to neoplastic mucosa as these genes may contribute causally to neoplastic transformation from normal mucosa to regenerating, non dysplastic tissue, to mucosa at high risk for neoplasia. We confirmed by real time PCR that all of the progressively up-regulated genes displayed higher expression in UC patients with neoplasia compared to UC patients without neoplasia and normal controls with varying levels of significance. Of these nine genes, BIRC3, THBD, and S100A9, have been reported to be dysregulated in with colonic carcinogenesis in previous studies (25–29). Only two of the genes, BIRC3 and S100A9 have previously been associated with IBD without neoplastic changes (17, 30). Although FGG and S100A9 are known to be up-regulated in colitis-associated cancer in an animal model, none of the genes with up-regulation confirmed by real time PCR have been described before in IBD-associated neoplasia (29, 31).
Although there is limited data implicating these progressively up-regulated genes in IBD-carcinogenesis, the function of the majority these genes has been described in previous studies. ACSL1 and ELTD1 are regulators of signal transduction. ACSL1 is an enzyme that converts long-chain fatty acids into fatty acyl-CoA esters (32). ELTD1 is a member of the epidermal growth factor-transmembrane 7 subfamily of receptors and is known to be up-regulated in endothelial cells of the microvasculature. Members of this class are involved in the innate and acquired immune response, although there is no data in IBD or CRC with ELTD1 (33, 34). CREM is a transcription factor that binds to the cyclic AMP (cAMP) response element and down regulates cAMP induced transcription (35, 36). BIRC3 inhibits apoptosis by binding to TNF receptor associated factors. Up-regulation of BIRC3 has previously been described in IBD and sporadic colon cancer (25, 30). Fibrinogen gamma is the gene that encodes for the gamma chain of fibrinogen. Fibrinogen appears to act as an oncogene as assessed in experimental loss of function mutant mice (31). Previous studies have demonstrated that fibrinogen is synthesized by colon tumors and elevated serum levels are associated with colon cancer and may be of prognostic significance (37, 38). TPD52L1 inhibits MAP3K4 induced apoptosis. It is known to be up-regulated in breast cancer, although it has not been previously described in association with colon cancer (39, 40). Thrombomodulin, THBD, is a membrane receptor that binds thrombin resulting in activation of protein C. Although there is no data on THBD in colon cancer, it is up-regulated in other cancers, including melanoma (41). CLC is the gene that encodes for Charcot-Leyden crystal protein. This protein is secreted by eosinophils and overexpressed in acute myeloid leukemia. Although it increases in allergic inflammation, it has not previously been described in association with IBD (42)
By immunohistochemistry, we examined several proteins which we found were associated with progressively up-regulated gene expression in mucosa from normal controls to UC without neoplasia to UC with neoplasia. In addition to S100A9, we also investigated proteins for fibrinogen gamma (FGG), clusterin, and annexin A3. Because of the diffuse stromal staining of these additional proteins, the antibodies were not suitable to demonstrate clinically relevant differences between groups. In future experiments, we plan to confirm these findings by in situ hybridization. We did demonstrate that S100A9 is increased in the colonic stroma of UC patients harboring remote dysplasia as well as in the stroma and tumor glands of IBD-associated cancer.
We also examined REG1α transcript and protein expression. REG1α is a growth factor involved in regeneration of the epithelium. Previous studies have shown that REG1α has mitogenic and/or antiapoptotic properties in both gastric cancer and UC-associated cancer (43, 44). Two previous analyses have demonstrated increased epithelial staining for REG1α in UC-associated dysplasia and in colon cancer (44, 45). In our microarray studies, REG1α expression was 35-fold up-regulated in mucosa from patient with UC harboring remote neoplasia compared to mucosa from patients with quiescent UC without dysplasia. We confirmed strong protein expression of REG1α in UC-associated neoplastic tissues. Although REG1α expression was increased by PCR in non-dysplastic UC tissue compared to normal controls, it was not significantly increased by immunohistochemistry as demonstrated in previous studies (44). This observation likely reflects the fact that we did not include mucosa with significant inflammation, as inflammation is known to increase REG1α expression (44). It bears emphasis that this is the first study to show increased expression of REG1α in non-dysplastic tissue from UC patients harboring remote dysplasia.
A previous study by Watanabe et al has examined gene expression changes occurring in non-dysplastic mucosa in UC patients harboring remote dysplasia (18). That study identified 40 genes that discriminated between UC patients harboring remote neoplasia and UC patients without neoplasia. Although there were similarities in the results of several key genes and gene ontology pathways between the two studies, we were able to identify many genes which were differentially expressed in nondysplastic mucosa in patients harboring remote dysplasia which have not been previously described. The discrepancy in our findings can be explained at least in part by several key differences between the two studies. In contrast to the study by Watanabe et al, we selected patients without active inflammation, as inflammatory activity alone may account for many changes in gene expression. Furthermore, we were able to demonstrate differences in gene expression not only for patients harboring UC-CRC, but also for patients with LGD and HGD. Lastly, we validated several key genes by real time PCR and immunohistochemistry; qPCR is more sensitive for quantitative measurement, and protein measurement is important as these molecules mediate many biologic processes in carcinogenesis. Although there were not significant differences in disease-specific patient characteristics between the two studies, there was variation in gender and ethnicity between the two study populations; the impact of these differences in gene expression is not known.
This study has several limitations. As this study was a cross-sectional analysis, we cannot conclude that the identified RNA expression changes and protein markers are predictive of future risk of developing a dysplastic lesion. Furthermore, we were not able to assess the impact of tumor location, grade of dysplasia, or impact of medical therapy on gene expression because of the small sample size. Although we were able to control for endoscopic and histologic inflammation between study groups, several genes which were up-regulated are involved in inflammatory pathways. This finding may indicate that ongoing inflammatory activity at a molecular level is more common in patients with a dysplastic lesion. As such, the ability of these genes and proteins to act as clinical markers of dysplasia will need to be validated in a larger cohort of patients which includes controls with active inflammation. The mode of sample collection may also have impacted gene expression differences. Samples from the majority of UC patients with dysplasia were collected at the time of surgery while most of the UC patients without dysplasia and normal controls were collected by colonoscopic biopsy. Although these methods both yield high quality RNA, the depth and size of the biopsy specimens may have varied between the groups, potentially altering gene expression levels. Because of this potential confounding, we validated several proteins of interest through immunohistochemistry. However, a future larger prospective study that controls for method of tissue collection will be needed to further validate these findings for use as predictive biomarkers.
In conclusion, this is the first study to identify gene expression changes occurring as a field effect in UC patients without active inflammation who harbor a remote dysplastic lesion. Patients with UC who harbor a dysplastic lesion display differential gene expression in normal appearing mucosa compared to UC patients without dysplasia. This includes genes that regulate signal transduction, immune function, inflammation, proliferation, and apoptosis. Further characterization of these genes might elucidate pathways of carcinogenesis in IBD. These findings may also lead to the development of more accurate, less invasive markers of dysplasia in those at increased risk.
Supplementary Material
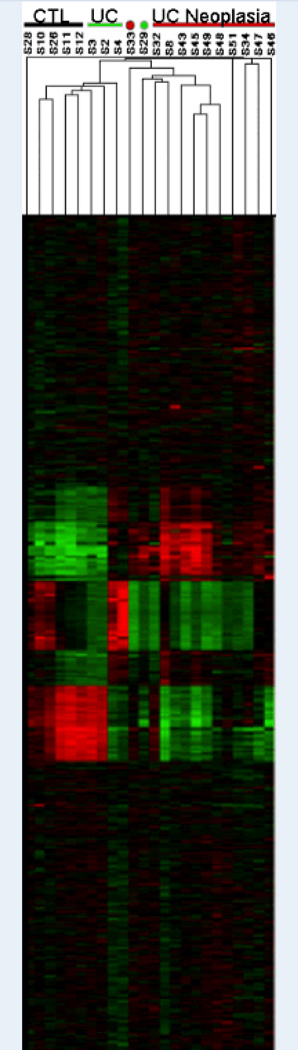
Figure 1.
Heat map with genome wide analysis of gene expression. Using two-way unsupervised hierarchical clustering, patients clustered into phenotypic groups with the exception of one UC patient without dysplasia (S29) who had a gene expression profile more characteristic of those harboring remote neoplasia.
Acknowledgments
Support: Financial support: International Organization for the Study of Inflammatory Bowel Disease (M.B., G.C.), the Crohn’s and Colitis Foundation of America (J.P.), and National Institutes of Health Grant K08DK090152 (J.P.).
References
- 1.Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. quiz 1340-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin DT. The changing face of colorectal cancer in inflammatory bowel disease: progress at last! Gastroenterology. 2006;130:1350–1352. doi: 10.1053/j.gastro.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 7.van der Woude CJ, Kleibeuker JH, Jansen PL, et al. Chronic inflammation, apoptosis and (pre-)malignant lesions in the gastro-intestinal tract. Apoptosis. 2004;9:123–130. doi: 10.1023/B:APPT.0000018794.26438.22. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svec J, Musilkova J, Bryndova J, et al. Enhanced expression of proproliferative and antiapoptotic genes in ulcerative colitis-associated neoplasia. Inflamm Bowel Dis. 2010;16:1127–1137. doi: 10.1002/ibd.21178. [DOI] [PubMed] [Google Scholar]
- 10.Willenbucher RF, Aust DE, Chang CG, et al. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol. 1999;154:1825–1830. doi: 10.1016/S0002-9440(10)65438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burmer GC, Rabinovitch PS, Haggitt RC, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 12.Brentnall TA, Crispin DA, Rabinovitch PS, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 13.Rubin CE, Haggitt RC, Burmer GC, et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611–1620. doi: 10.1016/0016-5085(92)91185-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Rabinovitch PS, Crispin DA, et al. DNA fingerprinting abnormalities can distinguish ulcerative colitis patients with dysplasia and cancer from those who are dysplasia/cancer-free. Am J Pathol. 2003;162:665–672. doi: 10.1016/S0002-9440(10)63860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinovitch PS, Dziadon S, Brentnall TA, et al. Pancolonic chromosomal instability precedes dysplasia and cancer in ulcerative colitis. Cancer Res. 1999;59:5148–5153. [PubMed] [Google Scholar]
- 16.Okahara S, Arimura Y, Yabana T, et al. Inflammatory gene signature in ulcerative colitis with cDNA macroarray analysis. Aliment Pharmacol Ther. 2005;21:1091–1097. doi: 10.1111/j.1365-2036.2005.02443.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn's disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet. 2001;10:445–456. doi: 10.1093/hmg/10.5.445. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Kobunai T, Toda E, et al. Gene expression signature and the prediction of ulcerative colitis-associated colorectal cancer by DNA microarray. Clin Cancer Res. 2007;13:415–420. doi: 10.1158/1078-0432.CCR-06-0753. [DOI] [PubMed] [Google Scholar]
- 19.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 20.Tamayo P, Slonim D, Mesirov J, et al. Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. Proc Natl Acad Sci U S A. 1999;96:2907–2912. doi: 10.1073/pnas.96.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draghici S, Khatri P, Bhavsar P, et al. Onto-Tools, the toolkit of the modern biologist: Onto-Express, Onto-Compare, Onto-Design and Onto-Translate. Nucleic Acids Res. 2003;31:3775–3781. doi: 10.1093/nar/gkg624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Yuan JS, Reed A, Chen F, et al. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 25.Endo T, Abe S, Seidlar HB, et al. Expression of IAP family proteins in colon cancers from patients with different age groups. Cancer Immunol Immunother. 2004;53:770–776. doi: 10.1007/s00262-004-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pucci S, Bonanno E, Sesti F, et al. Clusterin in stool: a new biomarker for colon cancer screening? Am J Gastroenterol. 2009;104:2807–2815. doi: 10.1038/ajg.2009.412. [DOI] [PubMed] [Google Scholar]
- 27.Marshall KW, Mohr S, Khettabi FE, et al. A blood-based biomarker panel for stratifying current risk for colorectal cancer. Int J Cancer. 2010;126:1177–1186. doi: 10.1002/ijc.24910. [DOI] [PubMed] [Google Scholar]
- 28.Hanly AM, Redmond M, Winter DC, et al. Thrombomodulin expression in colorectal carcinoma is protective and correlates with survival. Br J Cancer. 2006;94:1320–1325. doi: 10.1038/sj.bjc.6603098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turovskaya O, Foell D, Sinha P, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–2043. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidelin JB, Vainer B, Andresen L, et al. Upregulation of cIAP2 in regenerating colonocytes in ulcerative colitis. Virchows Arch. 2007;451:1031–1038. doi: 10.1007/s00428-007-0517-1. [DOI] [PubMed] [Google Scholar]
- 31.Steinbrecher KA, Horowitz NA, Blevins EA, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–2643. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mashek DG, Bornfeldt KE, Coleman RA, et al. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J Lipid Res. 2004;45:1958–1961. doi: 10.1194/jlr.E400002-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Wallgard E, Larsson E, He L, et al. Identification of a core set of 58 gene transcripts with broad and specific expression in the microvasculature. Arterioscler Thromb Vasc Biol. 2008;28:1469–1476. doi: 10.1161/ATVBAHA.108.165738. [DOI] [PubMed] [Google Scholar]
- 34.Yona S, Lin HH, Stacey M. Immunity and adhesion-GPCRs. Adv Exp Med Biol. 2010;706:121–127. doi: 10.1007/978-1-4419-7913-1_10. [DOI] [PubMed] [Google Scholar]
- 35.Foulkes NS, Laoide BM, Schlotter F, et al. Transcriptional antagonist cAMP-responsive element modulator (CREM) down-regulates c-fos cAMP-induced expression. Proc Natl Acad Sci U S A. 1991;88:5448–5452. doi: 10.1073/pnas.88.12.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foulkes NS, Borrelli E, Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991;64:739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- 37.Shi HJ, Stubbs R, Hood K. Characterization of de novo synthesized proteins released from human colorectal tumour explants. Electrophoresis. 2009;30:2442–2453. doi: 10.1002/elps.200800767. [DOI] [PubMed] [Google Scholar]
- 38.Grande M, Milito G, Attina GM, et al. Evaluation of clinical, laboratory and morphologic prognostic factors in colon cancer. World J Surg Oncol. 2008;6:98. doi: 10.1186/1477-7819-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrne JA, Mattei MG, Basset P. Definition of the tumor protein D52 (TPD52) gene family through cloning of D52 homologues in human (hD53) and mouse (mD52) Genomics. 1996;35:523–532. doi: 10.1006/geno.1996.0393. [DOI] [PubMed] [Google Scholar]
- 40.Abba MC, Sun H, Hawkins KA, et al. Breast cancer molecular signatures as determined by SAGE: correlation with lymph node status. Mol Cancer Res. 2007;5:881–890. doi: 10.1158/1541-7786.MCR-07-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Wit NJ, Rijntjes J, Diepstra JH, et al. Analysis of differential gene expression in human melanocytic tumour lesions by custom made oligonucleotide arrays. Br J Cancer. 2005;92:2249–2261. doi: 10.1038/sj.bjc.6602612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benson M, Langston MA, Adner M, et al. A network-based analysis of the late-phase reaction of the skin. J Allergy Clin Immunol. 2006;118:220–225. doi: 10.1016/j.jaci.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Sekikawa A, Fukui H, Fujii S, et al. REG Ialpha protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology. 2005;128:642–653. doi: 10.1053/j.gastro.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 44.Sekikawa A, Fukui H, Fujii S, et al. Possible role of REG Ialpha protein in ulcerative colitis and colitic cancer. Gut. 2005;54:1437–1444. doi: 10.1136/gut.2004.053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka H, Fukui H, Fujii S, et al. Immunohistochemical analysis of REG Ialpha expression in ulcerative colitis-associated neoplastic lesions. Digestion. 2011;83:204–209. doi: 10.1159/000321808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.