Abstract
Background
The objectives of this study were to determine effects of severe traumatic brain injury (TBI) on cerebrospinal fluid (CSF) concentrations of myelin basic protein (MBP) and to assess relationships between clinical variables and CSF MBP concentrations.
Methods
We measured serial CSF MBP concentrations in children enrolled in a randomized controlled trial evaluating therapeutic hypothermia (TH) after severe pediatric TBI. Control CSF was obtained from children evaluated, but found not to be having CNS infection. Generalized estimating equation models and Wilcoxon Rank-Sum test were used for comparisons of MBP concentrations.
Results
There were 27 TBI cases and 57 controls. Overall mean (±SEM) TBI case MBP concentrations for 5 days after injury were markedly greater than controls (50.49 ± 6.97 vs. 0.11 ± 0.01 ng/ml, p < 0.01). Mean MBP concentrations were lower in TBI patients < 1 year versus >1 year (9.18 ± 1.67 vs. 60.22 ± 8.26 ng/ml, p = 0.03), as well as in cases with abusive head trauma (AHT) versus non-abusive TBI (14.46 ± 3.15 vs. 61.17 ± 8.65 ng/ml, p = 0.03). TH did not affect MBP concentrations.
Conclusions
Mean CSF MBP increases markedly after severe pediatric TBI, but is not affected by TH. Infancy and AHT are associated with low MBP concentrations, suggesting that age-dependent myelination influences MBP concentrations after injury. Given the magnitude of MBP increases, axonal injury likely represents an important therapeutic target in pediatric TBI.
Keywords: Axonal injury, Pediatrics, Secondary injury
Introduction
Axonal injury is a fundamental mechanism in the patho-genesis of severe traumatic brain injury (TBI). Evidence of diffuse axonal injury on brain MRI can portend significant eventual functional morbidity [1, 2]. Axonal injury can mechanistically involve primary traumatic axotomy from direct disruption, secondary axotomy from calcium accumulation and cytoskeletal compaction, and Wallerian degeneration [3, 4]. These processes involve damage to the intact myelin sheath, which encircles long axons traversing brain white matter, and release of sheath constituents. Myelin basic protein (MBP), a constituent of the sheath, is essential for normal myelination and axonal signal conduction, and mediates adhesion between cytoplasmic surfaces of individual myelin layers [5]. Though also present in the peripheral nervous system, MBP exists predominantly in the central nervous system. The process of myelination is age dependent and progresses from infancy into adolescence [6], and degrees of myelinated axonal injury may thus differ in relation to patient age.
Increases in CSF MBP have been reported following an array of neurological insults including TBI in adults and children [7–11]. Mukherjee and colleagues [9], while evaluating the utility of a MBP radioimmunoassay in CSF samples from patients with neurological disease, noted markedly elevated CSF MBP concentrations obtained at random time points in 22 age-unspecified patients with severe TBI compared with controls.
Though investigations of CSF MBP in TBI did not describe associations between concentrations and outcome, serum MBP concentrations in pediatric TBI have been predictive of eventual GOS score [12, 13]. A recently published study of 72 pediatric TBI patients found that serum MBP temporal trajectories with early and large increases in serum MBP were associated with a poor outcome [Glasgow Outcome Scale (GOS) score 3–5] [14]. Given that MBP concentrations in the CSF are not influenced by blood–brain barrier (BBB) permeability as serum concentrations are, MBP directly measured in CSF may demonstrate different concentrations than in the serum following injury, and consequently reflect the temporal course of axonal injury more accurately.
Therapeutic hypothermia (TH) has been proposed as a potential clinical intervention targeted at improving outcome. In several animal models of TBI, TH reduces histological evidence of axonal injury [3, 15]. In particular, calpain proteolysis and neurofilament sidearm compaction [1, 2, 15] are reduced after TH in rat TBI models, suggesting that TH attenuates axonal injury after TBI. Roelfsema and colleagues [3, 4, 16] have also demonstrated that TH reduces demyelination and loss of MBP from injured tissue after a hypoxic-ischemic insult in neonatal sheep. Assessment of MBP in the context of TH after TBI has not been performed previously in humans. Characterization of the effect of TBI on serial CSF MBP concentrations in children would lend insight into the evolution of axonal injury after TBI, effects of therapy such as TH on secondary injury, and whether demographic variables affected measured concentrations. Therefore, we hypothesized that CSF MBP concentrations are elevated after TBI in pediatric patients, and that TH would attenuate that increase. To test this hypothesis, we measured CSF MBP concentrations in a cohort of pediatric TBI patients enrolled in a study of the safety and efficacy of TH following TBI, as well as in a group of controls. We also compared CSF MBP concentrations between groups based on age, gender, etiology of injury (abuse vs. non-abuse), treatment with TH versus normothermia (NT), initial GCS, and 6-month GOS.
Methods
Patients
This study was approved by the Institutional Review Board of the University of Pittsburgh. Children < 18 years old and those who were admitted to Children's Hospital of Pittsburgh of UPMC with TBI between July 1999 and December 2003 were eligible for enrollment in the study. Patients were enrolled in two consecutive Phase II randomized controlled trials of the safety and efficacy of TH in infants and children admitted with severe TBI. The details of the study protocols have been previously reported [5, 17]. All patients either presented with or deteriorated to a GCS score ≤ 8, and were treated with passive continuous CSF drainage via an extraventricular drain (EVD). Patients treated with TH were cooled to 32–33 °C within 24 h of injury. TH was maintained for 48 h, and then patients were gradually re-warmed. Rewarming occurred at a rate of 1 °C every 1–2 h to goal temperature, and was not guided by patient ICP. Patients randomized to NT were maintained at 36.5-37.5 °C for the initial 48-h-study period. Patient management was otherwise in accordance with the published Guidelines For Management Of Severe Pediatric TBI [6, 18]. CSF samples were collected daily during the first 5 days following injury, or until the EVD stopped draining and/or was removed.
Control CSF was obtained from children who underwent lumbar puncture (LP) in the evaluation of suspected CNS infection. All LPs were non-diagnostic for infection based on culture, cell count, and Gram stain. All CSF samples were centrifuged for 10 min at 5,000×g and stored at — 70 °C until analysis.
Clinical data collected in TBI cases included demographic information, whether or not the injury was the result of abusive head trauma (AHT), initial GCS, and 6-month GOS. Only demographic data were collected for controls. Favorable outcome was defined as GOS 1–2 as evaluated in the outpatient setting by a site neuropsychologist or neuropsychology technician under supervision of the neuropsychologist, blinded to therapeutic intervention. The diagnosis of AHT was made with consensus of the CHP Child Protection Team, which is consulted on all cases of possible AHT in the hospital. When the time of injury was not known, it was defined as the latest time the injury could have occurred.
Measurement of MBP Concentrations
CSF MBP concentrations were measured using a commercial enzyme-linked immunosorbent assay kit (International Point of Care, Toronto, ON, Canada). The ELISA was performed according to manufacturer's instructions.
Statistical Analysis
All values are expressed as mean ± standard error of the mean. Generalized estimating equations (GEE) were used for bivariate comparisons of CSF MBP concentrations between groups for the 5 days of observation for assessment of effects of time after injury in hours, age (< 1 vs. ≥1 year old), etiology of injury (AHT vs. no AHT), treatment group (TH vs. NT), admission GCS score, 6-month GOS score, and gender. Wilcoxon Rank-Sum test was used for individual univariate comparisons of peak CSF MBP concentrations between groups on the same day after injury. Categorical demographic data between groups were assessed using Fisher's Exact Test. The decision to dichotomize age in < 1 versus ≥ 1 year of age was based on neuroimaging data indicating the age of 1 year is a transition point into an adult pattern of myelination [6–11, 19]. p < 0.05 was considered statistically significant.
Results
Demographics
A total of 105 samples were collected from 27 TBI patients; 14 patients were randomized to TH, 13 were randomized to NT. There were 57 controls. The characteristics of TBI and controls are described in Table 1. There were no significant clinical or demographic differences between patients and controls with the exception of patients being significantly older (6.86 ± 0.94 vs. 0.73 ± 0.28 years, p <0.01). Ages ranged from 7 weeks to 16 years for the TBI patients, and 8 days to 11 years for the controls. There were 22 TBI patients ≥1 year old, and 6 controls ≥1 year old. The clinical, demographic, and outcome variables of TBI patients treated with TH were not significantly different from those patients treated with NT.
Table 1. Demographic characteristics of TBI cases and controls.
| TBI— hypothermia | TBI— normothermia | Controls | |
|---|---|---|---|
| Number of cases | 14 | 13 | 57 |
| Age (year) | 6.78 ± 1.39 | 6.96 ± 1.30 | 0.73 ± 0.28 |
| Gender (% male) | 71 | 31 | 47 |
| Initial GCS (%) | |||
| 13–15 | 1 (7) | 2 (15) | |
| 9–12 | 2 (14) | 3 (23) | |
| 3–8 | 11 (79) | 8 (62) | |
| 6-month GOS (%) | |||
| 1–2 | 6 (55) | 9 (75) | |
| 3–5 | 5 (45) | 3 (25) | |
| AHT (%) | 3 (21) | 3 (23) | |
AHT abusive head trauma, GOS glasgow outcome scale, GOS 1–2, good outcome; GOS 3–5, bad outcome/death
Mean MBP Concentration in TBI Patients Versus Controls
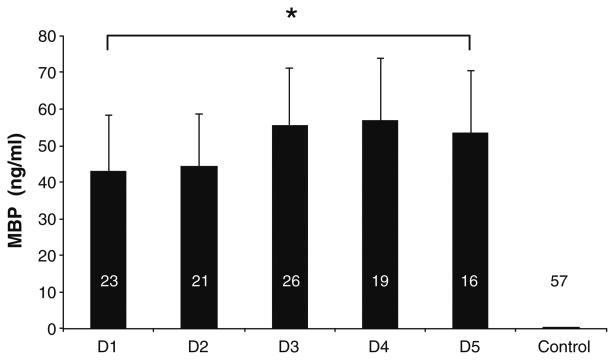
Mean CSF MBP concentration in TBI patients from all 5 days after injury category was significantly and markedly greater than in controls as evaluated by GEE (50.49 ± 6.97 vs. 0.11 ± 0.01 ng/ml, p < 0.01, Fig. 1). Mean CSF MBP concentration was increased in comparison with controls at the first day after injury (43.02 ± 15.34 vs. 0.11 ± 0.01 ng/ml, p < 0.02). The high CSF MBP concentrations seen in the cases were sustained through the 5 days of measurement, and there were no significant differences between mean concentrations on individual days after injury. There was no overlap between MBP concentrations in TBI patients versus controls in any sample.
Fig. 1.
Mean CSF MBP concentrations for all TBI patients, during the first 5 days (D1-5) of admissioncompared with controls. Concentrations for D1-5 and peak for TBI patients were significantly higher than those for controls. Bars Mean value for given day. Error bars indicate standard error of the mean
Effect of Demographic Variables on Mean MBP Concentrations in TBI Patients
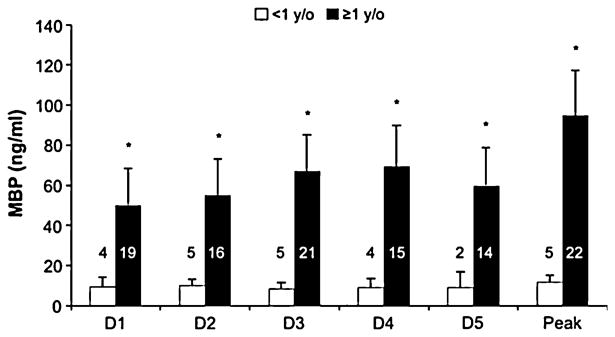
TBI patients ≥ 1 year old had higher mean CSF MBP concentrations (60.22 ± 8.26 vs. 19.18 ± 1.67 ng/ml, p =0.03, Fig. 2). Patients with AHT manifested lower mean CSF MBP concentrations than those with non-abusive TBI (14.46 ± 3.15 vs. 61.17 ± 8.65 ng/ml, p = 0.03). The mean age of AHT patients was 1.06 ± 0.32 years, and significantly younger than patients with non-abusive TBI (8.52 ± 0.19 years, p < 0.01). There was no significant difference in mean CSF MBP concentrations in males versus females for either the TBI patients or controls, but peak CSF MBP concentrations were lower in females than in males (65.48 ± 28.46 vs. 115.88 ± 6.42 ng/ml, p = 0.02). The severity of initial injury was no different between males versus females as reflected in initial GCS (7.56 ± 0.87 vs. 7.55 ± 0.97).
Fig. 2. Mean CSF MBP concentrations for all TBI patients < 1 versus those ≥ 1 year old. Patients < 1 year old had significantly lower mean CSF MBP concentrations.
TH and Mean MBP Concentration
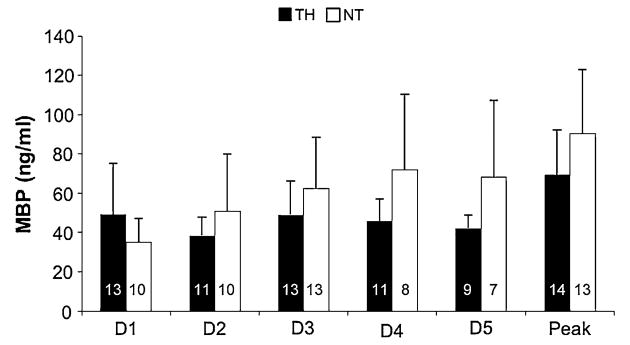
There was no difference in mean or peak CSF MBP concentrations between patients treated with TH versus NT (Mean 45.27 ± 7.48 vs. 56.70 ± 12.44, Peak 69.38 ± 22.76 vs. 90.33 ± 32.37, Fig. 3). No significant differences in CSF MBP concentrations were detected between postinjury days within either the TH or NT subgroups.
Fig. 3. Mean CSF MBP concentrations for all TBI patients treated with TH, versus those maintained at NT. There was not a statistically significant difference in concentrations between the two groups.
GCS and GOS with Mean MBP Concentrations in TBI Patients
Mean CSF MBP concentrations were not different between patients whose GCS was initially > 8 and then declined versus those who presented with GCS ≤8. There was no relationship between mean or peak CSF MBP concentrations and 6-month GOS.
Discussion
This is the first study demonstrating that children with severe TBI manifest marked and sustained increases in mean CSF MBP concentrations compared with controls. This increase occurs immediately after injury and remains sustained for 5 days after injury without any overlap between patients and controls at any time point. This prolonged increase could be consistent with processes affecting myelin sheath breakdown persisting several days following TBI, perhaps because of secondary injury or Wallerian degeneration, both of which are delayed processes [3, 4, 9].
The observation that CSF MBP rises rapidly after TBI, and that it remains sustained, was surprising given prior investigations by members of this group which demonstrated a delayed increase in serum MBP following TBI [12-14]. Our finding of an early rise in CSF MBP may be explained by MBP release into the CSF immediately following injury, followed by progressive MBP leakage across the BBB as secondary injury progresses. A study examining CSF MBP following neonatal HIE by Garcia-Alix and colleagues found no significant difference between CSF MBP concentration at 12 and 72 h in HIE patients, also suggesting that MBP concentration rises more rapidly in CSF than in the serum after injury [7, 14]. Given that ongoing CSF MBP release may imply continuing myelin sheath degradation, CSF MBP may also be a temporally relevant indicator of secondary injury. Further investigation of MBP CSF clearance kinetics in TBI could help resolve the significance of persistently increased MBP concentration in the CSF. Our findings also suggest that serum concentrations of this bio-marker underestimate how rapidly axonal injury occurs after severe TBI.
The magnitude of mean CSF MBP concentration elevation in TBI cases is also noteworthy—concentrations in patients were generally 400–500-fold greater than concentrations in controls (50.49 ± 6.97 vs. 0.11 ± 0.01 ng/ml). Similar concentrations of CSF MBP have been seen in adults with TBI [3, 9, 11, 15] as well as in serum [10].
Effect of Age
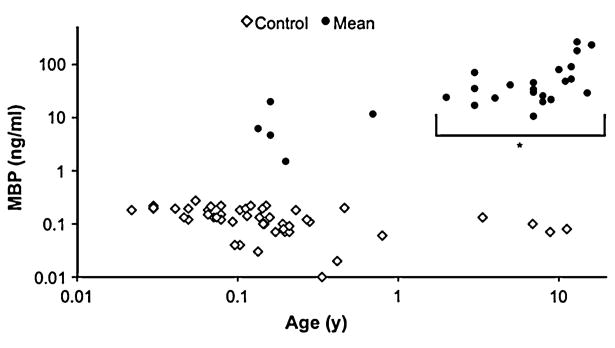
The inverse relationship between MBP concentrations and age in TBI patients was not surprising as there is a lower relative fraction of MBP in the immature brain of infants. This suggests that increases in CSF MBP concentration in infants after TBI may underestimate the severity of injury for a given insult relative to an older child or adult—where myelination is complete. No significant effect of age was seen on CSF MBP in controls (Fig. 4). This is compatible with published CSF MBP data from LP on 79 children and adults found negative for neurological disease, which described only a 1 % increase in MBP per year with age over this broad age range [20]. The 95th percentile concentration for these patients was 1.57 ng/ml, far below concentrations seen in this study's patients, however, and does not explain the exuberant release of MBP in the CSF of these patients after trauma. This association of age and CSF MBP concentration is reflected in our TBI patients in that those < 1 year old manifested lower mean concentrations of CSF MBP than those ≥ 1 year old. Based on results from this and other investigations [20], the effect of age on MBP must be considered when interpreting MBP as a biomarker in pediatric TBI.
Fig. 4.
Scatter plot of mean CSF MBP concentration for each patient (filled circle) andcontrol (open diamond) versus age. Horizontal and vertical scales are logarithmic. There was no significant difference in CSF MBP concentrations between controls <1 versus those ≥1 year old, though TBI patients ≥1 year old demonstrated significantly higher concentrations than those <1 year old
MBP and AHT
Given the importance of diffuse axonal injury in the pathophysiology of AHT [21], we were initially surprised by lower mean CSF MBP concentrations in children with AHT versus non-abusive TBI. However, the mean age of subjects with AHT was significantly lower than the mean age for subjects with non-abusive TBI, and brain maturity likely influenced CSF MBP concentrations measured in AHT patients. The lack of any cases of non-abusive TBI who were <1 year of age in our study population precluded a multivariable analysis to evaluate the influence of age independent of injury mechanism. Ideally, we would be able to compare CSF MBP concentrations in children with AHT to age-matched children with non-abusive TBI, however, the majority of AHT being inflicted on patients <1 year of age is well recognized.
Effect of Gender
In multiple TBI clinical series, females have fared better than males in parameters such as overall outcome [22], memory [23], and oxidative injury [24]. Our finding that female TBI patients demonstrate lower peak (but not mean) CSF MBP concentration than male patients despite similar initial GCS scores may indicate a proclivity toward reduced axonal injury in females. The effects of female sex hormones have been proposed as being neuroprotective [22–24]. Given that the majority of the patients in this study are prepubescent (19 of 27 patients were ≤10 years old), the influence of sex hormones appears less likely, and perhaps other unknown mechanisms contributed to the lower peak MBP concentrations observed in female patients [25].
Effect of TH
Because of the preponderance of animal data demonstrating that TH has a beneficial effect on axonal injury [3, 15, 16], as well as human pediatric data indicating TH after TBI leads to lower CSF concentrations of the cerebral biomarkers alpha synuclein [26], NSE, S100B, and CK-BB [27], it was surprising that patients treated with TH did not have lower mean CSF MBP concentrations compared with patients treated with NT. One possibility for the discontinuity between the experimental and clinical studies in the efficacy of TH on axonal injury is that rewarming in the clinical trial was too rapid. Suehiro and Povlishock [28] demonstrated that traumatic axonal injury is exacerbated by rapid re-warming in experimental TBI. It is not clear what rewarming rate might be potentially deleterious with regard to axonal injury in clinical TBI, however, the patients treated with hypothermia in our study did show rebound intracranial hypertension during rewarming, suggesting that axonal injury could also have been exacerbated [17]. There was no significant difference between CSF MBP concentrations in TH and NT patients on D1 or D2 before rewarming, however. Another possible reason TH was not associated with a difference in MBP concentration could be that the majority of MBP dispersed into the CSF in TBI occurs soon after injury. Persistently elevated concentrations might therefore reflect slow MBP clearance as opposed to ongoing secondary injury. In this paradigm, TH may not have a significant effect on total CSF MBP concentration even if it reduces secondary injury. A third possible explanation for the failure of hypothermia to attenuate MBP concentrations as a reflection of a reduction in axonal injury is that time to onset of cooling and target temperature was much slower in the clinical (hours) versus experimental studies (immediate). This explanation is supported by the fact that MBP concentrations did not differ between groups even during the period where hypothermia was being applied. Further studies of MBP concentrations in the Cool Kids trial, which used a more conservative approach to rewarming, may provide additional insight into this question.
Associations with Admission GCS and Six-Month Outcome
The observation that admission GCS did not correlate with mean CSF MBP is also important. This may suggest a significant contribution of secondary injury to axonal pathology following TBI, and CSF MBP release may reflect damage more from the evolution of TBI than the initial insult. Despite previous work demonstrating an inverse correlation between outcome and peak serum [13, 14] and CSF [10] MBP concentrations, peak CSF MBP concentrations in this study were not associated with 6-month outcome. Previous study has demonstrated that CSF MBP concentration may correlate with outcome post injury. The study of CSF MBP concentrations in neonatal HIE by Garcia-Alix and colleagues [7] found that mean MBP concentration at 12 and 72 h correlated with neuro-cognitive outcome at 1 year of age. Noseworthy and colleagues [10] demonstrated 6-month GOS score correlated with a single peri-operative CSF MBP measurement in adults who underwent surgery within a week of TBI. This study did not demonstrate an association between 6-month GOS score and CSF MBP concentration. The Phase II study of TH of which this investigation was a part did not find an association between favorable and unfavorable GOS.
Limitations
Despite the intrinsic heterogeneity of pediatric TBI presentation and management, this study demonstrated a significant effect of TBI, age, and mechanism on mean CSF MBP concentrations as a biomarker of myelin sheath damage. Limitations in this investigation included significantly younger age of controls compared with TBI patients due to the tendency of patients undergoing LP for occult CNS infection evaluation to be young infants. However, no members of the control population over the age of 1 year old had significantly elevated CSF MBP levels above those less than 1 year. Consequently, the 400–500-fold mean CSF MBP concentration elevation above controls seen in the TBI patients is not likely to be solely because of age-related myelin maturation. It is also reasonable to believe that MBP should not normally be present in the CSF of controls, given that it is primarily a structural protein in myelinated axons. Another factor affecting CSF MBP concentration is that TBI patients in this study all experienced severe injury with GCS ≤8, and milder injury was not reflected. However, EVD placement is very rarely performed on admission in cases with milder injury.
Conclusions
In conclusion, this investigation demonstrates that there is an exuberant and persistent increase in mean CSF MBP concentrations in pediatric patients after severe TBI compared with controls, suggesting an important component of axonal injury in pediatric TBI that is initiated very early after injury. This rise is less pronounced in the young and those who have suffered AHT. A lower peak CSF MBP concentration was also demonstrated after TBI in females.
Acknowledgments
This investigation was supported by Grant Nos.: T32HD040686 (ES, PMK), RO1 NS 38448-01(PDA), and NS30318 (PMK).
Abbreviations
- AHT
Abusive head trauma
- BBB
Blood-brain barrier
- CHP
Children's Hospital of Pittsburgh
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- EVD
Extra-ventricular drain
- GCS
Glasgow coma scale
- GOS
Glasgow outcome scale
- MBP
Myelin basic protein
- NT
Normothermia
- TBI
Traumatic brain injury
- TH
TH Therapeutic hypothermia
Contributor Information
E. Su, Email: esu4u@jhmi.edu, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA.
M. J. Bell, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pediatrics, University of Pittsburgh, Pittsburgh, PA, USA
P. M. Kochanek, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pediatrics, University of Pittsburgh, Pittsburgh, PA, USA
S. R. Wisniewski, Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA, USA
H. Bayır, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pediatrics, University of Pittsburgh, Pittsburgh, PA, USA
R. S. B. Clark, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pediatrics, University of Pittsburgh, Pittsburgh, PA, USA
P. D. Adelson, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA, USA; Barrow Neurological Institute at Phoenix Children’s Hospital and University of Arizona College of Medicine, Phoenix, AZ, USA
E. C. Tyler-Kabara, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA, USA
K. L. Janesko-Feldman, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA
R. P. Berger, Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Pediatrics, University of Pittsburgh, Pittsburgh, PA, USA
References
- 1.Scheid R, Walther K, Guthke T, Preul C, Cramon von DY. Cognitive sequelae of diffuse axonal injury. Arch Neurol. 2006;63(3):418–24. doi: 10.1001/archneur.63.3.418. [DOI] [PubMed] [Google Scholar]
- 2.Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010;113(3):556–63. doi: 10.3171/2009.9.JNS09626. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell WL, Donnelly S, Sun X, Fenton T, Puri N, Graham DI. Axonal cytoskeletal responses to nondisruptive axonal injury and the short-term effects of posttraumatic hypothermia. J Neuro-trauma. 1999;16(12):1225–34. doi: 10.1089/neu.1999.16.1225. [DOI] [PubMed] [Google Scholar]
- 4.Okonkwo DO, Pettus EH, Moroi J, Povlishock JT. Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury. Brain Res. 1998;784(1–2):1–6. doi: 10.1016/s0006-8993(97)01075-5. [DOI] [PubMed] [Google Scholar]
- 5.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63(17):1945–61. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–66. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Alix A, Cabañas F, Pellicer A, Hernanz A, Stiris TA, Quero J. Neuron-specific enolase and myelin basic protein: relationship of cerebrospinal fluid concentrations to the neurologic condition of asphyxiated full-term infants. Pediatrics. 1994;93(2):234–40. [PubMed] [Google Scholar]
- 8.Levin SD, Hoyle NR, Brown JK, Thomas DG. Cerebrospinal fluid myelin basic protein immunoreactivity as an indicator of brain damage in children. Dev Med Child Neurol. 1985;27(6):807–13. doi: 10.1111/j.1469-8749.1985.tb03806.x. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee A, Vogt RF, Linthicum DS. Measurement of myelin basic protein by radioimmunoassay in closed head trauma, multiple sclerosis and other neurological diseases. Clin Biochem. 1985;18(5):304–7. doi: 10.1016/s0009-9120(85)80037-0. [DOI] [PubMed] [Google Scholar]
- 10.Noseworthy TW, Anderson BJ, Noseworthy AF, et al. Cerebro-spinal fluid myelin basic protein as a prognostic marker in patients with head injury. Crit Care Med. 1985;13(9):743–6. doi: 10.1097/00003246-198509000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DG, Palfreyman JW, Ratcliffe JG. Serum-myelin-basic-protein assay in diagnosis and prognosis of patients with head injury. Lancet. 1978;1(8056):113–5. doi: 10.1016/s0140-6736(78)90415-4. [DOI] [PubMed] [Google Scholar]
- 12.Beers SR, Berger RP, Adelson PD. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma. 2007;24(1):97–105. doi: 10.1089/neu.2006.0055. [DOI] [PubMed] [Google Scholar]
- 13.Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma. 2007;24(12):1793–801. doi: 10.1089/neu.2007.0316. [DOI] [PubMed] [Google Scholar]
- 14.Berger RP, Bazaco MC, Wagner AK, Kochanek PM, Fabio A. Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev Neurosci. 2010;32(5–6):396–405. doi: 10.1159/000316803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Büki A, Koizumi H, Povlishock JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol. 1999;159(1):319–28. doi: 10.1006/exnr.1999.7139. [DOI] [PubMed] [Google Scholar]
- 16.Roelfsema V, Bennet L, George S, et al. Window of opportunity of cerebral hypothermia for postischemic white matter injury in the near-term fetal sheep. J Cereb Blood Flow Metab. 2004;24(8):877–86. doi: 10.1097/01.WCB.0000123904.17746.92. [DOI] [PubMed] [Google Scholar]
- 17.Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740–54. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–54. [DOI] [PubMed] [Google Scholar]
- 18.Froelich M, Ni Q, Wess C, Ougorets I, Härtl R. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37(4):1433–41. doi: 10.1097/CCM.0b013e31819c1933. [DOI] [PubMed] [Google Scholar]
- 19.Gao W, Lin W, Chen Y, et al. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol. 2009;30(2):290–6. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Engelen BG, Lamers KJ, Gabreels FJ, Wevers RA, van Geel WJ, Borm GF. Age-related changes of neuron-specific enolase, S-100 protein, and myelin basic protein concentrations in cerebrospinal fluid. Clin Chem. 1992;38(6):813–6. [PubMed] [Google Scholar]
- 21.Shannon P, Smith CR, Deck J, Ang LC, Ho M, Becker L. Axonal injury and the neuropathology of shaken baby syndrome. Acta Neuropathol. 1998;95(6):625–31. doi: 10.1007/s004010050849. [DOI] [PubMed] [Google Scholar]
- 22.Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A. The effect of gender on patients with moderate to severe head injuries. J Trauma. 2009;67(5):950–3. doi: 10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- 23.Donders J, Woodward HR. Gender as a moderator of memory after traumatic brain injury in children. J Head Trauma Rehabil. 2003;18(2):106–15. doi: 10.1097/00001199-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bayır H, Marion DW, Puccio AM, et al. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21(1):1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 25.Du L, Bayır H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279(37):38563–70. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 26.Su E, Bell MJ, Wisniewski SR, et al. α-Synuclein levels are elevated in cerebrospinal fluid following traumatic brain injury in infants and children: the effect of therapeutic hypothermia. Dev Neurosci. 2010;32(5–6):385–95. doi: 10.1159/000321342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Lu G, Shi W, Zheng S. Protective effect of moderate hypothermia on severe traumatic brain injury in children. J Neurotrauma. 2009;26(11):1905–9. doi: 10.1089/neu.2008.0828. [DOI] [PubMed] [Google Scholar]
- 28.Suehiro E, Povlishock JT. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J Neurosurg. 2001;94(3):493–8. doi: 10.3171/jns.2001.94.3.0493. [DOI] [PubMed] [Google Scholar]