Abstract
Nitric oxide (⋅NO) was originally identified as an innate cytotoxin. However, in tumors it can enhance resistance to chemotherapy and exacerbate cancer progression. Our previous studies indicated that ⋅NO/⋅NO-derived species react with etoposide (VP-16) in vitro and form products that show significantly reduced activity toward HL60 cells and lipopolysaccharide (LPS)-induced macrophages. Here, we further confirm the hypothesis that ÷NO generation contributes to VP-16 resistance by examining interactions of ⋅NO with VP-16 in inducible nitric-oxide synthase (iNOS)–expressing human melanoma A375 cells. Inhibition of iNOS catalysis by N6-(1-iminoethyl)-l-lysine dihydrochloride (L-NIL) in human melanoma A375 cells reversed VP-16 resistance, leading to increased DNA damage and apoptosis. Furthermore, we found that coculturing A375 melanoma cells with LPS-induced macrophage RAW cells also significantly reduced VP-16 cytotoxicity and DNA damage in A375 cells. We also examined the interactions of ⋅NO with another topoisomerase active drug, Adriamycin, in A375 cells. In contrast, to VP-16, ⋅NO caused no significant modulation of cytotoxicity or Adriamycin-dependent apoptosis, suggesting that ⋅NO does not interact with Adriamycin. Our studies support the hypothesis that ⋅NO oxidative chemistry can detoxify VP-16 through direct nitrogen oxide radical attack. Our results provide insights into the pharmacology and anticancer mechanisms of VP-16 that may ultimately contribute to increased resistance, treatment failure, and induction of secondary leukemia in VP-16–treated patients.
Introduction
Topoisomerases constitute an important class of nuclear enzymes responsible for maintaining the topology and function of DNA. Inhibition and/or interference with topoisomerase functions lead to cell death. A large number of clinically active anticancer drugs (e.g., etoposide, adriamycin, and camptothecin) interact with and poison topoisomerases, causing significant DNA damage and cell death. Etoposide (VP-16), a topoisomerase II (topo II) poison, is active against lung and testicular cancers and lymphoma (Henwood and Brogden, 1990). Adriamycin (Adr), also a Topo II poison (Liu, 1989), is active against a wide range of cancers, including leukemias, bladder, breast, lung, and multiple myelomas (Sinha, 1995).
VP-16 is rapidly metabolized by cytochrome P-450 (P450) and peroxidases to o-dihydroxy and o-quinone derivatives of VP-16 (Haim et al., 1986, 1987a,b; VanMaanen et al., 1987; Kalyanaraman et al., 1989; Kagan et al., 2001), and the formation of these metabolites requires the generation of a VP-16 radical (VP-16⋅) formed from the oxidation of the 4′-OH group of VP-16 (Haim et al., 1987a,b). VP-16 metabolites bind to cellular components (Haim et al., 1986, 1987b) and cause DNA strand cleavage through a Topo II–mediated mechanism (Sinha et al., 1990; Gantchev and Hunting, 1998). VP-16⋅ causes oxidative stress both in vitro and in vivo by oxidizing glutathione and forming the glutathione thiol radical (Katki et al., 1987). In addition, VP-16-o-quinone has been reported to form glutathione adducts in HL60 cells (Fan et al., 2006).
Adriamycin has been reported to intercalate into DNA and binds covalently to DNA and proteins via generation of reactive species through reductive bioactivation (Sinha, 1980; Sinha et al., 1984). Reductive activation also generates free radicals via the formation of semiquinone radical and its reaction with molecular oxygen (Kalyanaraman et al., 1980; Myers et al., 1987; Sinha et al., 1987). Although the covalent binding to DNA and formation of free radicals are implicated in the mechanisms of Adr cytotoxicity (Myers et al., 1987; Sinha et al., 1987) and cardiotoxicity (Doroshow 1983; Rajagopalan et al., 1988), poisoning of Topo II is considered to play an important role in tumor cell death.
⋅NO and/or its products (⋅NO2, ONOOH) have been shown to play important roles in cancer biology, including the innate immune response, neovascularization, cancer metastasis, and cell death (Jenkins et al., 1995; Wink et al., 1998; Xu et al., 2002; Chen et al., 2008). Exposure to ⋅NO (via NO donors) modifies activities of certain anticancer drugs, including Adr (Cook et al., 1997; Wink et al., 1997; Evig et al., 2004). ⋅NO and/or its products react with a wide variety of substrates, including amines, thiols, and phenols (Janzen et al., 1993; Cudic and Ducrocq, 2000; Yenes and Messeguer 1999). ⋅NO-derived species rapidly react with phenols to form phenoxyl radicals, quinones, and nitrated phenols. The formation of nitrotyrosine in cellular proteins has been associated with modifications of protein activities (Curtis et al., 1996; Chatterjee et al., 2009). Furthermore, ⋅NO has been reported to react with the tyrosyl radical of ribonucleotide reductase, resulting in the inhibition of DNA synthesis and ultimately cell death (Lepoivre et al., 1994).
VP-16 contains a phenolic OH group in the 4′-position, and both the cytotoxicity and the binding of VP-16 to Topo II depend on the presence of this moiety (Loike and Horwitz, 1976; Long et al., 1984; Sinha et al., 1990). We have recently shown that the phenolic OH of VP-16 reacts with ⋅NO-derived species to generate VP-16⋅, o-quinone, and other products, thereby modulating the cytotoxicity of VP-16 toward cancer cells (Sinha et al., 2013). In this report, we have further examined the interactions of VP-16 and Adr with endogenously formed ⋅NO via iNOS catalysis in A375 human melanoma cells. Our results support the conclusion that the reaction of ⋅NO with VP-16 diminishes its cytotoxic activity toward cancer cells. In contrast, endogenously formed ⋅NO in A375 cells had no significant effects on Adr cytotoxicity, indicating that Adr did not interact with ⋅NO or ⋅NO-derived species. This work extends our in vitro work to the cellular systems and confirms the same processes observed for VP-16 in vitro also operates in tumor cells.
Materials and Methods
VP-16 and Adriamycin were the gift of the Drug Synthesis and Chemistry Branch, Development Therapeutic Program of the National Cancer Institute, National Institutes of Health. Human topoisomerases and SDS/KCl precipitation assay kits were obtained from Topogen (Columbus, OH). The nitric-oxide synthase (inducible, iNOS) inhibitor N6-(1-iminoethyl)-l-lysine dihydrochloride (L-NIL) was obtained from Cayman Chemicals (Ann Arbor, MI). Caspase-3 activity was measured by an Abcam assay kit (Cambridge, MA). A primary antibody for the analysis of iNOS was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A nitric oxide assay kit was purchased from Thermo Fisher Scientific (Waltham, MA).
Cytotoxicity Studies.
A375 melanoma cell lines and macrophage RAW 264.7 cells [American Type Culture Collection (ATCC), Manassas, VA] were grown in phenol red–free RPMI 1640 media supplemented with 10% fetal bovine serum and antibiotics. A375 cells and RAW 264.7 were used for 15 passages, after which the cells were discarded and a new cell culture was started from fresh-frozen stock.
The intracellular reaction between VP-16 and endogenous ⋅NO was examined in A375 cells by preincubating cells either in the presence or absence of an iNOS inhibitor (L-NIL) for 4 hours before the addition of VP-16. A375 cells (2000/well) were plated in phenol red–free medium and cultured for 96 hours. Dimethylsulfoxide was included as the vehicle control, and the cytotoxicity was measured by the microculture tetrazolium toxicity (MTT) assay, as described elsewhere (Alley et al., 1988).
Coculture experiments for cytotoxicity with A375 cells and induced RAW cells were performed in six-well plates separated by 0.4-μM membrane filters (Costar, Corning, NY). Macrophage RAW cells were induced as described previously elsewhere (Sinha et al., 2013). For the cytotoxicity studies, 200,000–250,000/well of A375 cells were plated in 3 ml of complete medium into a six-well plate (in duplicates) and were allowed to attach for 4–6 hours. Membranes were inserted, and various concentrations of VP-16 were added onto the top of membranes containing cells induced with lipopolysaccharide (LPS) (1 × 106) and incubated for 48 hours. Membranes and RAW cells were removed, A375 cells were washed once with ice-cold phosphate-buffered saline (PBS) and trypsinized, and the cytotoxicity was determined by counting the cells. Nitrite concentrations were determined using the Greiss reagent, as described previously elsewhere (Sinha et al., 2013).
Western Blot Assay.
Samples (10 μg of total protein) were electrophoresed under reducing conditions through 4%–12% Bis-Tris NuPage acrylamide gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins were transferred onto a nitrocellulose membrane and probed with anti-iNOS and anti-β actin antibodies. An Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE) was used to acquire images. Rabbit polyclonal anti-iNOS antibody was used to quantitate iNOS proteins in samples using a standard enzyme-linked immunosorbent assay (ELISA) (Ranguelova et al., 2008).
Caspase-3 Assay.
Caspase-3 activity in the A375 cell lysate was measured by a colorimetric assay kit (Abcam) using the manufacturer’s instructions. In brief, 1 million cells were treated with VP-16 (25 and 50 μM) in the presence or absence of L-NIL (250–400 μM) for 18 hours. Cells were collected by centrifugation and lysed with the lysis buffer (Abcam) by incubating for 10 minutes on ice. Cells were centrifuged at 10,000g for 5 minutes, supernatant was collected, and the protein concentration was determined by the BCA assay. Equal amounts of the protein (200–400 μg) were incubated with 10 mM DTT (dithiothreitol) containing the reaction buffer, followed by the addition of caspase-3 substrate (DEVD-Pna, 4 mM), and the reaction mixture was incubated at 37°C for 2–4 hours. The sample was analyzed for absorbance at 405 nm in a multiwell plate reader, and results were expressed as substrate utilization per milligram protein.
SDS-KCl Precipitation Assay.
The formation of covalent Topo II/DNA complexes with VP-16 in the presence or absence of L-NIL was quantitated by the SDS-KCl precipitation assay, as described elsewhere (Liu et al., 1983). In brief, DNA of A375 cells growing in the logarithmic phase (2 × 106 /ml) was labeled with [methyl-3H]thymidine (10 μci, 2 Ci/mmol; PerkinElmer Life and Analytical Sciences, Waltham, MA) for 18–24 hours. Cells were collected and washed twice with the medium, diluted in fresh medium, and seeded into a six-well plate at a density of 2 × 105 cells/ml. L-NIL was preincubated with the cells for 18 hours during the labeling phase. VP-16, dissolved in dimethylsulfoxide, was added and incubated for 1 hour. Cells were washed with PBS (2 times) and lysed with 1 ml of prewarmed lysis solution (Topogen). After lysis and shearing of DNA, DNA–Topo II–VP-16 complexes were precipitated with KCl. The precipitate was collected by centrifugation and washed extensively (4 times) with the washing solution (Topogen) according to the manufacturer’s instructions. The radioactivity was counted in a scintillation counter after adding 5 ml of scintillation fluid.
The effects of endogenously generated ⋅NO from iNOS of LPS-induced macrophage RAW cells on SDS-KCl precipitate formation in A375 cells were investigated as described in the cytotoxicity studies, except that the drug exposure was for 90 minutes and the samples were processed for the SDS-KCl precipitation assay as described previously. In some experiments, VP-16 (10 μM) was preincubated with LPS-induced RAW cells (0.5 × 106 cells/ml, 30 minutes), the mixtures were added to six-well plates fitted with membrane filters (on top), and they were allowed to interact with the 3H-labeled A375 cells (in bottom, 200–250,000 cells/well) for 90 minutes. The medium was removed, and the cells were washed once with PBS; A375 cells were lysed with prewarmed lysis buffer, and the samples were processed as described previously.
Metabolism of VP-16 in A375 Cells.
The metabolic studies with VP-16 in A375 cells in the presence and absence of L-NIL were performed similarly to those described previously elsewhere (Haim et al., 1987a,b). In brief, 1 × 106/ml of cells in 5 ml of phenol red-free media were seeded in a 100 × 15 mm Petri dish (Falcon; Becton Dickinson, Franklin Lakes, NJ) and were allowed to attach for 4 hours. L-NIL (400 μM) was added to the cells and incubated for 18 hours before VP-16 (100 μM) was added at various time points. At the end of the incubation, the cells were washed with ice-cold PBS (5 ml), gently scraped in 5 ml of ice-cold PBS, and collected by centrifugation (2000g, 5 minutes). The cells were suspended in 1 ml of ice-cold PBS, lysed with sonication (4°C), and the resulting mixture extracted with chloroform (4 × 1 ml). The combined organic layers were removed under argon, and the residue was dissolved in 200 μl of methanol and analyzed by high-performance liquid chromatography (HPLC) as described previously elsewhere (Haim et al., 1987a,b), except that a C18 column was used and MeOH-water (60:40) was the mobile phase. Under these conditions, VP-16 and VP-16-o-quinone had retention times of 2.1 minutes and 2.7 minutes, respectively.
Results
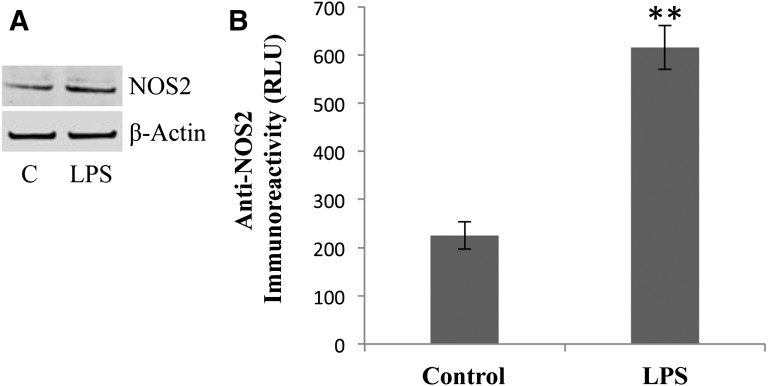
Using purified Topo II enzyme, we had previously reported that products formed from reactions of ⋅NO and/or ⋅NO-derived species with VP-16 were significantly less active than the parent drug in inducing DNA cleavage and were not cytotoxic to HL60 cells (Sinha et al., 2013). In this report, we examined whether endogenous formation of ⋅NO catalyzed by iNOS in cancer cells could affect the cytotoxicity of VP-16 and Adr. To assess this, we used a human melanoma A375 tumor cell line, which has been shown to express iNOS and to produce levels of ⋅NO that are relatively low (Tang and Grimm, 2004; Chanvorachote et al., 2006; Sikora et al., 2010). The presence of iNOS was confirmed in A375 cells with a primary antibody for iNOS (Fig. 1A), and our results are similar to those described previously elsewhere (Sikora et al., 2010). Furthermore, treatment of A375 cells with 10 ng/ml LPS for 18 hours significantly induced this protein, further confirming the presence of the inducible form of NOS in A375 cells (Fig. 1B). It is interesting to note that under these experimental conditions the formation of ⋅NO/NO2 was not detected by the Griess reaction (data not shown). This observation is similar to those previously published by Sikora et al., (2010) and Chin and Deen (2010), as the amount of NO synthesis in A375 cells is well below (100–150 nM) the detection limits of the Griess reaction.
Fig. 1.
(A) The Western blots for iNOS and effects of LPS (10 ng/ml) in A375 cells. (B) Quantitation of the iNOS protein in A375 cells using rabbit polyclonal anti-iNOS antibody in the standard ELISA assay as described in Materials and Methods. For the Western blots, A375 cells were seeded at 1 × 106 in a six-well plate. LPS (10 ng/ml) was added and exposed for 14 to 16 hours. Cells were collected, lysed, and examined for iNOS protein by Western blots. For the Western blots, 10 μg of proteins were loaded, and actin was used to assess equal loading of the proteins. Data are the mean of three separate experiments performed in duplicate. **P < 0.005 compared with the untreated control.
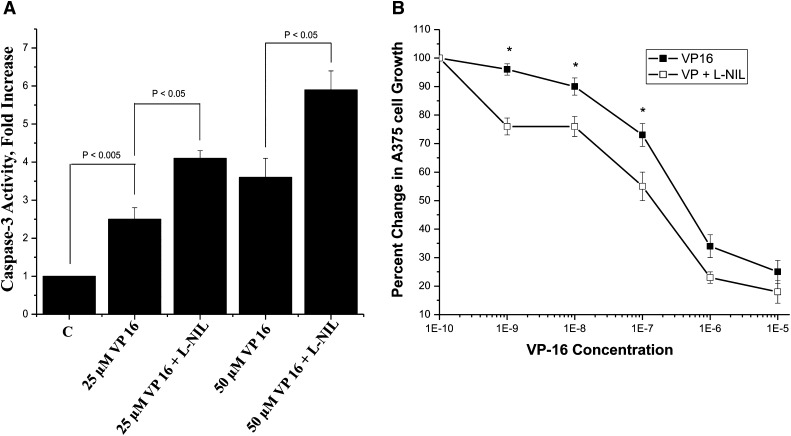
Examination of caspase-3 activity, a marker for apoptosis, in A375 cells showed that VP-16 alone (25 and 50 μM) induced 2.5- and 3.5-fold increases in caspase-3 activity, respectively, after 18 hours (Fig. 2A). Inhibition of iNOS with L-NIL (400 μM) further enhanced caspase-3 activity 4.5- and 6.0-fold in the presence of 25 μM and 50 μM VP-16, respectively. Consistent with these data, application of L-NIL (400 μM) significantly enhanced VP-16 cytotoxicity in A375 cells (2.5–3.5-fold; Fig. 2B). The relative IC50 (concentration required to inhibit cell growth by 50%) is presented in Table 1. Under these conditions, L-NIL alone had no significant effect on caspase-3 activity or A375 cell viability.
Fig. 2.
(A) The caspase-3 assay as an indicator of VP-16 apoptosis in the presence or absence of iNOS inhibitors was performed according to the manufacturer’s protocol. L-NIL (400 μM) was dissolved in the medium and added to A375 cells (1 × 106/well) in six-well plates and incubated for 4 hours before adding VP-16. The cells were incubated for another 18 hours before collecting cells by centrifugation. Data are the mean of three separate experiments performed in duplicate. *P < 0.05, n = 3, Student’s t test. (B) Cytotoxicity studies with VP-16 in human A375 cells in the presence or absence of L-NIL (400 μM) were performed using the MTT assays described in Materials and Methods. Cytotoxicity data are the mean of four separate experiments performed in triplicate. *P < 0.05, n = 4, against concentration-matched VP-16 alone, Student’s t test.
TABLE 1.
Cytotoxicity of VP-16 in human A375 melanoma cells under various treatment conditions
Cytotoxicity studies were performed as described in Materials and Methods and also detailed in their respective figures. Data are the mean ± S.D. of three to four separate experiments.
| Conditions | IC50 |
|---|---|
| M | |
| VP-16 | 2.5 ± 0.5 × 10−7 |
| VP-16 + L-NIL | 8.5 ± 0.5 × 10−8a |
| VP-16 | 2.5 ± 0.5 × 10−7 |
| VP-16 + LPS | 9.0 ± 0.8 × 10−7a |
| VP-16 + LPS + L-NIL | 2.0 ± 0.5 × 10−7a |
| VP-16 | 5.2 ± 0.5 × 10−7 |
| VP-16 + induced RAW cells | 8.5 ± 0.70 × 10−6b |
P < 0.05, Student’s t test.
P < .005, Student’s t test.
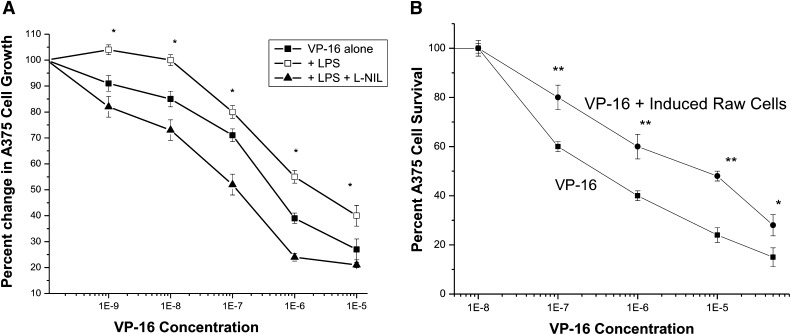
Because LPS significantly induced iNOS (Fig. 1), we examined the cytotoxicity of VP-16 after induction of A375 cells with LPS. Consistent with the increase in the expression of iNOS, VP-16 cytotoxicity was further decreased in the induced A375 cells (Fig. 3A; Table 1). More interestingly, the presence of L-NIL significantly sensitized A375 cells to VP-16 (Fig. 3A; Table 1), which suggests that increased ⋅NO formation from iNOS was responsible for the decrease in VP-16 cytotoxicity in A375 cells. To further examine the roles of exogenously generated ⋅NO on VP-16 cytotoxicity in A375 cells, we performed coculture studies with LPS-induced macrophage RAW cells. As shown in Fig. 3B and Table 1, exogenously formed ⋅NO from iNOS in RAW cells further decreased VP-16 cytotoxicity (>15-fold), suggesting that ⋅NO generated from the induced RAW cells reacted with VP-16 and formed noncytotoxic species. Additionally, the data presented in Table 2 show that considerable amounts of ⋅NO were generated from the induced RAW cells, and the presence of VP-16 (in coculture cytotoxicity studies) significantly decreased ⋅NO formation, indicating a reaction between VP-16 and ⋅NO. Furthermore, there were no significant differences in nitrite formation between the top and bottom layers (compartments) of the six-well plates, suggesting free diffusion of ⋅NO across the membrane. In contrast, the cytotoxicity of VP-16 was not significantly affected (data not shown) in the presence of noninduced RAW cells, indicating that ⋅NO/⋅NO-species generated in the induced RAW cells from iNOS catalysis were responsible for this decrease in VP-16 cytotoxicity.
Fig. 3.
(A) Cytotoxicity of VP-16 in A375 cells after induction with LPS (10 ng/ml 16 hours) and effects of L-NIL (400 µM) on VP-16 cytotoxicity using the MTT assay as described in Fig. 2. Data are the mean of three separate experiments performed in triplicate. *P < 0.05, n = 3, against concentration-matched VP-16 alone and VP-16 + L-NIL, Student’s-t test. (B) Cytotoxicity of VP-16 in A375 cells in the presence of LPS-induced RAW cells (1 × 106 cells/incubation). Coculture studies were conducted as described in Materials and Methods. Data are the mean of three separate experiments performed in duplicate. *P < 0.05 and **P < 0.005, n = 3, against concentration-matched VP-16 alone, Student’s t test.
TABLE 2.
Relative nitrite concentration during coculture studies for cytotoxicity with VP-16 in A375 cells in the presence of LPS-induced RAW cells
Nitrite concentrations were determined using Greiss Reagent by removing samples (100 µl; 4 hours) from the top and bottom layers (separated by 0.4 μM membrane filters; Costar, Corning, NY) in six-well plates. A375 cells were seeded in the bottom layer and the LPS-induced RAW cells were seeded on the top. The concentration of nitrite is expressed as µM/106 RAW cells. Data are the mean of three separate experiments.
| Conditions | Top | Bottom |
|---|---|---|
| Control, Untreated | 52.43 ± 1.0 | 52.32 ± 0.8 |
| + 50 µM VP-16 | 25.35 ± 2.1a | 24.7 ± 2.4a |
| + 10 µM VP-16 | 31.7 ± 1.6a | 25.3 ± 1.0a |
| + 1 µM VP-16 | 37.5 ± 1.0a | 31.8 ± 1.0a |
| + 0.1 µM VP-16 | 36.5 ± 1.0b | 36.7 ± 1.0b |
P < 0.005, compared with untreated controls, Student’s t test.
P < 0.05, compared with untreated controls, Student’s t test.
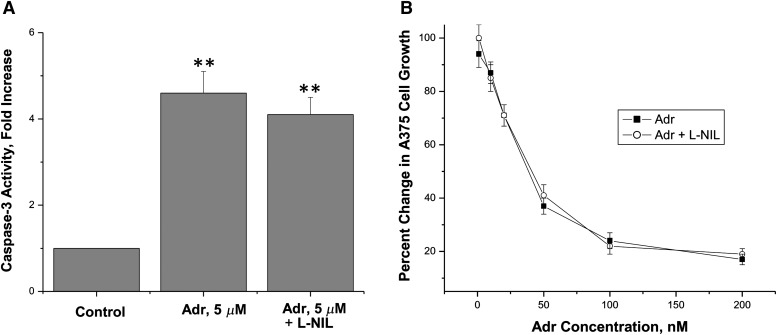
Adriamycin is a Topo II–active drug that induces protein-associated DNA damage leading to cell death, and thus it is similar to VP-16 in its mode of action. We examined the effects of endogenously generated ⋅NO in Adr-induced apoptosis and cytotoxicity in A375 cells. Under similar conditions, the presence of L-NIL had no significant effects on caspase-3 activity induced by Adr (Fig. 4). Furthermore, the presence of L-NIL had no effects on the cytotoxicity of this drug (Fig. 4) in A375 cells. These observations would indicate that Adr does not react with ⋅NO/⋅NO-related species.
Fig. 4.
Caspase-3 activity as an indicator of apoptosis (A) and cytotoxicity (B) induced by Adriamycin, respectively, in A375 cells. These studies were performed as described in Fig. 2. Data are the mean of three separate experiments performed in triplicate for the cytotoxicity and in duplicate for the caspase-3 activity. **P < 0.005, n = 3 compared with control, Student’s t test.
Effects of L-NIL on VP-16–Induced Cleavable Complex in A375 Cells.
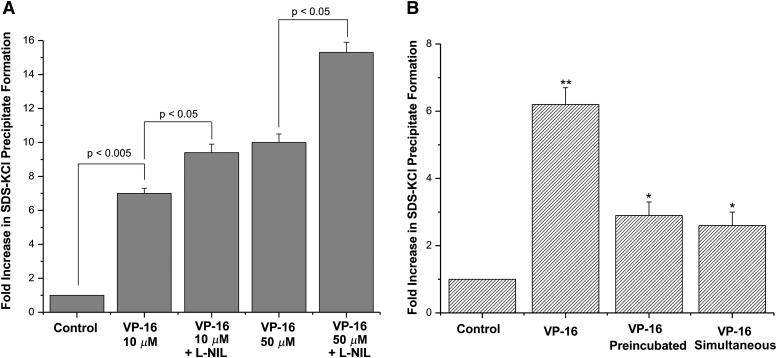
To determine whether iNOS catalysis influences the formation of a VP-16/Topo II complex, we evaluated the formation of the cleavage complex by VP-16 in the presence of L-NIL using an SDS/KCl precipitation assay (Liu et al., 1983). As expected, treatment of A375 cells with VP-16 (10 μM) significantly increased (6- to 7-fold) the formation of the SDS/KCl precipitate (Fig. 5) over the untreated controls. The presence of L-NIL further increased this complex formation (10-fold), equivalent to the effects of VP-16 alone at 5 times the concentration (50 μM; Fig. 5). This result suggests that inhibition of ⋅NO formation in A375 cells by L-NIL increased the DNA damage induced by VP-16 in a Topo II–dependent manner.
Fig. 5.
(A) Measurement of the VP-16 cleavable complex formation. The SDS-KCl precipitation assay with VP-16 in the presence of L-NIL was performed as described in Materials and Methods. L-NIL (400 μM) was incubated with the cells for 18 hours during the labeling phase before VP-16 was added for 1 hour. * P < 0.05, n = 3 compared with controls and 10 μM VP-16 treatment and 50 μM + L-NIL treatment compared with 50 μM VP-16 treatment alone. (B) Coculture experiments for determination of SDS-KCl precipitate formation were performed with LPS-induced (1 μg/ml for 14–16 hours) RAW cells as described in Materials and Methods. **P < 0.005, n = 3, compared with control and VP-16 treated simultaneously or preincubated; *P < 0.05, n = 3 compared with control), Student’s t test.
To further confirm that ⋅NO or ⋅NO-derived species were responsible for this decrease in VP-16–mediated Topo II–dependent DNA damage, we performed coculture experiments with induced macrophage RAW cells. Coincubation of A375 cells with induced RAW cells significantly decreased VP-16–dependent SDS-KCl precipitate formation (Fig. 5B). Similar effects were noted when the coincubations were performed either simultaneously or with preincubated VP-16 (Fig. 5B). Taken together, these observations strongly suggest that ⋅NO and associated nitrogen oxides can react with VP-16, thereby making it less effective at inducing apoptosis and DNA damage and resulting in increased VP-16 resistance toward cancer cells.
Metabolism of VP-16 in A375 Melanoma Cells.
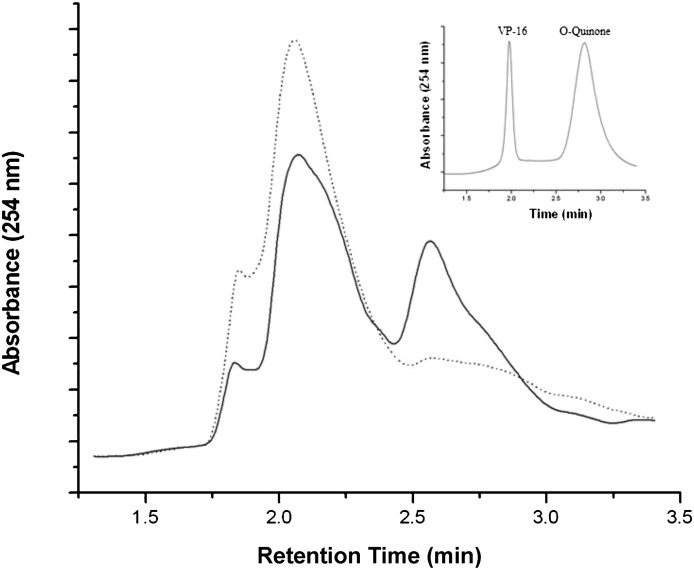
To examine the effects of endogenously produced ⋅NO on VP-16 metabolism in A375 cells, VP-16 was incubated with A375 cells for various times in the presence or absence of L-NIL. The HPLC analysis (Fig. 6) clearly indicated that VP-16 is rapidly metabolized in A375 cells, and a significant amount of VP-16-o-quinone was formed within 15 minutes of incubation. The presence of L-NIL completely inhibited the quinone formation, indicating that ⋅NO and other nitric oxide species formed from iNOS in A375 cells were responsible for the o-quinone formation.
Fig. 6.
HPLC analysis of the cellular metabolism of VP-16 (100 μM) in the presence or absence of L-NIL (400 μM). A375 cells were incubated with L-NIL for 18 hours before VP-16 was added and incubated for 15 minutes afterward. Metabolites were isolated as described in Materials and Methods and analyzed by HPLC. The solid line represents cells treated with VP-16 only, and the dashed line represents cells treated with both VP-16 and L-NIL. Insert: The HPLC analysis of products formed in vitro during reactions of ⋅NO gas with VP-16 at a low ratio in chloroform, as described elsewhere (Sinha et al., 2013).
Discussion
The impact of NOS activity on tumor biology is diverse and dependent on a myriad of host factors (Ambs and Glynn, 2011). Likewise, ⋅NO and its nitrogen oxide products can affect cancer therapy either positively or negatively (Hickok and Thomas, 2010). The present study extends our work in vitro to tumor cells and shows significant oxidative chemistry of ⋅NO with VP-16.
In our previous study we showed that the reaction of ⋅NO/NO species with VP-16 initiates through the obligate intermediacy of the 4′-phenoxyl radical, with subsequent formation of o-quinone and short-lived VP-16 nitrogen oxide species (Sinha et al., 2013). ⋅NO reacts with phenolic compounds (Janzen et al., 1993; Yenes and Messeguer, 1999; Cudic and Ducrocq, 2000), and the initiator in these reactions is believed to be the autoxidation of ⋅NO (Liu et al., 1998; Espey et al., 2001) with the formation of the ⋅NO2 radical, which can attack the electron-rich phenolic OH group to generate the phenoxyl radical (Hogg et al., 1996; Goss et al., 1999).
Interaction of VP-16 with Topo II has been shown to cause both single- and double-strand DNA breaks (Long et al., 1984; Glisson et al., 1986; Sinha et al., 1988), which in the absence of DNA repair results in cell death. Indeed, a significant decrease in VP-16–dependent DNA damage and cell death has been observed in cell lines where the activity of Topo II had been compromised (Glisson et al., 1986; Sinha et al., 1988). Our previous studies clearly showed that products of VP-16 exposed to ⋅NO (in the presence of O2) were no longer cytotoxic to HL60 cells and lacked sufficient ability to induce Topo II–dependent DNA damage (Sinha et al., 2013).
To further address the relevance of this ⋅NO chemistry observed in vitro, iNOS-expressing human melanoma A375 cells were used to directly elucidate the role of ⋅NO in the cytotoxicity of VP-16. Our present studies clearly show that inhibition of formation of ⋅NO (and ⋅NO-related species) by L-NIL increased caspase-3–dependent apoptosis by VP-16 (2-fold), with a concomitant increase (2.5- to 3.5-fold) in VP-16 cytotoxicity to A375 tumor cells (Fig. 2, A and B; Table 1). Induction of iNOS in A375 cells with LPS further decreased VP-16 cytotoxicity in A375 cells. Interestingly, inhibition of ⋅NO/⋅NO-related species by L-NIL resulted in sensitization of cells to VP-16. These studies taken together strongly suggest that ⋅NO/⋅NO-related species react with VP-16 and generate products that are significantly less cytotoxic to A375 cells.
Coculture studies performed with LPS-activated macrophage RAW cells (which generate large quantities of ⋅NO/⋅NO-related species) show a further (>15-fold) decrease in VP-16 cytotoxicity to A375 cells. This is a very significant finding, as this decrease in VP-16 cytotoxicity in the presence of exogenously generated ⋅NO from macrophage cells is quite large compared with that found with endogenously generated ⋅NO from iNOS catalysis in A375 cells (2.5- to 3.5-fold). Our findings would suggest that even in those cancer cells expressing low levels of iNOS, infiltrating macrophages would further increase VP-16 resistance by increasing detoxification of VP-16 via ⋅NO-dependent chemistry. In tumors, the presence of macrophages is well established, and they play significant roles in tumor progression, neovascularization, and poor survival (Jenkins et al., 1995; Lewis and Pollard, 2006; Pollard, 2008; Ambs and Glynn, 2011).
Nitric oxide has been reported to stabilize Bcl2, resulting in decreased cytotoxicity of cis-platinum in A375 cells (Tang and Grimm, 2004; Chanvorachote et al., 2006), thus raising the possibility of a general apoptotic resistance mechanism for ⋅NO independent of direct VP-16 reactions. However, our current study shows that endogenously/exogenously formed ⋅NO (or ⋅NO-derived species) reacts with VP-16 to generate less active metabolites of VP-16, resulting in a decrease in both Topo II–mediated DNA damage and cytotoxicity. In contrast, L-NIL had no significant effects on Adr-induced apoptosis or cytotoxicity in A375 cells, which would indicate that Bcl2 did not play a role in VP-16 activity.
Inhibition of cellular ⋅NO formation by L-NIL caused an increase in intracellular unmodified VP-16, resulting in increased DNA damage and cytotoxicity. Our observations with Adr further confirm that L-NIL did not affect apoptosis nor did it significantly affect its cytotoxicity. Similar observations were also noted with Camptothecin, a Topo I–active drug, where the presence of L-NIL had no significant effect on caspase-3 activity or its cytotoxicity in A375 cells (unpublished observations), lending support to the conclusion that L-NIL did not modulate VP-16 pharmacology by nonspecific effects on caspase-3 or Topo II activities. We conclude, therefore, that iNOS catalysis within A375 tumor cells was sufficient to directly detoxify active VP-16 via ⋅NO/⋅NO2-dependent oxidative reactions.
⋅NO readily partitions into a hydrophobic medium, such as the interior of membranes (Liu et al., 1998). This, in combination with a relatively high rate of ⋅NO generation from iNOS catalysis, facilitates ⋅NO autoxidation and formation of ⋅NO2 (Hogg et al., 1996; Goss et al., 1999; Moller et al., 2007). The organic solubility of VP-16 may likewise localize and enhance interactions with reactive nitrogen oxides. The occurrence of both augmented iNOS catalysis (Ambs and Glynn, 2011) and alterations in cancer cell plasma membrane architecture (Zhuang et al., 2002; Moller et al., 2007; Miersch et al., 2008) will significantly influence iNOS-dependent detoxification of VP-16 and resistance toward cancer cells.
While in human patients VP-16 is exclusively metabolized by Cyp3A4 in the liver to its dihydroxy derivative, it is possible that VP-16 could also be metabolized by Cyp3A4 in A375 melanoma cells to the catechol derivative and o-quinone. The roles of Cyp3A4 in A375 cells were not investigated in our present study, but inhibition of o-quinone formation by L-NIL would suggest that ⋅NO was involved in its formation in A375 cells. The formation of the o-quinone was rapid and decreased significantly with time, suggesting binding to proteins and DNA. The formation of VP-16-o-quinone by ⋅NO-dependent pathways is very significant in light of the role of the quinone in the induction of acute myeloid leukemia in patients treated with VP-16. It has been reported that VP-16 metabolites increase Topo II–dependent cleavage near leukemia-associated MLL translocation breakpoints (Lovett et al., 2001). Furthermore, Vlasova et al. (2011) have shown that the VP-16 radical formed from 1-electron oxidation can redox cycle, leading to enhanced Topo II–mediated strand breaks and MLL gene translocation. Our previous studies have shown that VP-16 is readily oxidized by ⋅NO chemistry to its phenoxyl radical, an obligatory intermediate in the formation of the VP-16-o-quinone (Sinha et al., 2013).
We conclude from our findings that ⋅NO oxidative chemistry occurs in A375 cancer cells and can detoxify VP-16. Our results provide insight into the pharmacology and anticancer activities of VP-16 in tumors that may ultimately contribute to increased resistance and treatment failure. VP-16 is currently used for the treatment of a variety of cancers (e.g., lung, melanoma, and breast) that are known to express iNOS (Chen et al., 2008; Grimm et al., 2008). It is tempting to suggest that the use of VP-16 and related anticancer drugs capable of reacting with ⋅NO/⋅NO2 may be ill-advised for patients harboring cancers with intensive iNOS activity. Furthermore, in tumors that do not express significant amounts of iNOS, infiltrating macrophages may further detoxify VP-16 and render tumor cells resistant to VP-16, as shown in our studies with melanoma cells.
Acknowledgments
The authors thank Mary J. Mason and Dr. Ann G. Motten for their careful review of the manuscript, and Drs. Michael Waalkes and Fiona Summers for their critical review of the manuscript.
Abbreviations
- Adr
adriamycin
- P450
cytochrome P450
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high-performance liquid chromatography
- iNOS
inducible nitric-oxide synthase
- L-NIL
N6-(1-iminoethyl)-l-lysine hydrochloride
- LPS
lipopolysaccharide
- MTT
microculture tetrazolium toxicity
- ⋅NO
nitric oxide
- ⋅NO2
nitrogen dioxide
- PBS
phosphate-buffered saline
- VP-16
etoposide
- VP-16⋅
VP-16-phenoxyl radical
- Topo II
topoisomerase II
Authorship Contributions
Participated in research design: Sinha, Kumar, Bhattacharjee, Mason.
Conducted experiments: Sinha, Kumar, Bhattacharjee
Contributed to new reagents or analytic tools: Kumar, Bhattacharjee.
Performed data analysis: Sinha, Kumar, Bhattacharjee, Espey.
Wrote or contributed to writing of the manuscript: Sinha, Mason.
Footnotes
This research was supported in part by the Intramural Research Program of the National Institutes of Health [National Institute of Environmental Health Sciences].
References
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48:589–601 [PubMed] [Google Scholar]
- Ambs S, Glynn SA. (2011) Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle 10:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Lardinois O, Bonini MG, Bhattacharjee S, Stadler K, Corbett J, Deterding LJ, Tomer KB, Kadiiska M, Mason RP. (2009) Site-specific carboxypeptidase B1 tyrosine nitration and pathophysiological implications following its physical association with nitric oxide synthase-3 in experimental sepsis. J Immunol 183:4055–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanvorachote P, Nimmannit U, Stehlik C, Wang L, Jiang BH, Ongpipatanakul B, Rojanasakul Y. (2006) Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res 66:6353–6360 [DOI] [PubMed] [Google Scholar]
- Chen GG, Lee TW, Xu H, Yip JHY, Li M, Mok TSK, Yim AP. (2008) Increased inducible nitric oxide synthase in lung carcinoma of smokers. Cancer 112:372–381 [DOI] [PubMed] [Google Scholar]
- Chin MP, Deen WM. (2010) Prediction of nitric oxide concentrations in melanoma. Nitric Oxide 23:319–326 [DOI] [PubMed] [Google Scholar]
- Cook JA, Krishna MC, Pacelli R, DeGraff W, Liebmann J, Mitchell JB, Russo A, Wink DA. (1997) Nitric oxide enhancement of melphalan-induced cytotoxicity. Br J Cancer 76:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudic M, Ducrocq C. (2000) Transformations of 2,6-diisopropylphenol by NO-derived nitrogen oxides, particularly peroxynitrite. Nitric Oxide 4:147–156 [DOI] [PubMed] [Google Scholar]
- Curtis JF, Reddy NG, Mason RP, Kalyanaraman B, Eling TE. (1996) Nitric oxide: a prostaglandin H synthase 1 and 2 reducing cosubstrate that does not stimulate cyclooxygenase activity or prostaglandin H synthase expression in murine macrophages. Arch Biochem Biophys 335:369–376 [DOI] [PubMed] [Google Scholar]
- Doroshow JH. (1983) Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res 43:4543–4551 [PubMed] [Google Scholar]
- Espey MG, Miranda KM, Thomas DD, Wink DA. (2001) Distinction between nitrosating mechanisms within human cells and aqueous solution. J Biol Chem 276:30085–30091 [DOI] [PubMed] [Google Scholar]
- Evig CB, Kelley EE, Weydert CJ, Chu Y, Buettner GR, Burns CP. (2004) Endogenous production and exogenous exposure to nitric oxide augment doxorubicin cytotoxicity for breast cancer cells but not cardiac myoblasts. Nitric Oxide 10:119–129 [DOI] [PubMed] [Google Scholar]
- Fan Y, Schreiber EM, Giorgianni A, Yalowich JC, Day BW. (2006) Myeloperoxidase-catalyzed metabolism of etoposide to its quinone and glutathione adduct forms in HL60 cells. Chem Res Toxicol 19:937–943 [DOI] [PubMed] [Google Scholar]
- Gantchev TG, Hunting DJ. (1998) The ortho-quinone metabolite of the anticancer drug etoposide (VP-16) is a potent inhibitor of the topoisomerase II/DNA cleavable complex. Mol Pharmacol 53:422–428 [DOI] [PubMed] [Google Scholar]
- Glisson B, Gupta R, Smallwood-Kentro S, Ross WE. (1986) Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res 46:1934–1938 [PubMed] [Google Scholar]
- Goss SPA, Singh RJ, Hogg N, Kalyanaraman B. (1999) Reactions of *NO, *NO2 and peroxynitrite in membranes: physiological implications. Free Radic Res 31:597–606 [DOI] [PubMed] [Google Scholar]
- Grimm EA, Ellerhorst J, Tang CH, Ekmekcioglu S. (2008) Constitutive intracellular production of iNOS and NO in human melanoma: possible role in regulation of growth and resistance to apoptosis. Nitric Oxide 19:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim N, Roman J, Nemec J, Sinha BK. (1986) Peroxidative free radical formation and O-demethylation of etoposide (VP-16) and teniposide (VM-26). Biochem Biophys Res Commun 135:215–220 [DOI] [PubMed] [Google Scholar]
- Haim N, Nemec J, Roman J, Sinha BK. (1987a) In vitro metabolism of etoposide (VP-16-213) by liver microsomes and irreversible binding of reactive intermediates to microsomal proteins. Biochem Pharmacol 36:527–536 [DOI] [PubMed] [Google Scholar]
- Haim N, Nemec J, Roman J, Sinha BK. (1987b) Peroxidase-catalyzed metabolism of etoposide (VP-16-213) and covalent binding of reactive intermediates to cellular macromolecules. Cancer Res 47:5835–5840 [PubMed] [Google Scholar]
- Henwood JM, Brogden RN. (1990) Etoposide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in combination chemotherapy of cancer. Drugs 39:438–490 [DOI] [PubMed] [Google Scholar]
- Hickok JR, Thomas DD. (2010) Nitric oxide and cancer therapy: the emperor has NO clothes. Curr Pharm Des 16:381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N, Singh RJ, Goss SPA, Kalyanaraman B. (1996) The reaction between nitric oxide and alpha-tocopherol: a reappraisal. Biochem Biophys Res Commun 224:696–702 [DOI] [PubMed] [Google Scholar]
- Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. (1995) Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 92:4392–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen EG, Wilcox AL, Manoharan V. (1993) Reactions of nitric oxide with phenolic antioxidants and phenoxyl radicals. J Org Chem 58:3597–3599 DOI: 10.1021/jo00066a001. [Google Scholar]
- Kagan VE, Kuzmenko AI, Tyurina YY, Shvedova AA, Matsura T, Yalowich JC. (2001) Pro-oxidant and antioxidant mechanisms of etoposide in HL-60 cells: role of myeloperoxidase. Cancer Res 61:7777–7784 [PubMed] [Google Scholar]
- Kalyanaraman B, Perez-Reyes E, Mason RP. (1980) Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs. Biochim Biophys Acta 630:119–130 [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Nemec J, Sinha BK. (1989) Characterization of free radicals produced during oxidation of etoposide (VP-16) and its catechol and quinone derivatives. An ESR Study. Biochemistry 28:4839–4846 [DOI] [PubMed] [Google Scholar]
- Katki AG, Kalyanaraman B, Sinha BK. (1987) Interactions of the antitumor drug, etoposide, with reduced thiols in vitro and in vivo. Chem Biol Interact 62:237–247 [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–612 [DOI] [PubMed] [Google Scholar]
- Lepoivre M, Flaman JM, Bobé P, Lemaire G, Henry Y. (1994) Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. Relationship to cytostasis induced in tumor cells by cytotoxic macrophages. J Biol Chem 269:21891–21897 [PubMed] [Google Scholar]
- Liu LF. (1989) DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem 58:351–375 [DOI] [PubMed] [Google Scholar]
- Liu LF, Rowe TC, Yang L, Tewey KM, Chen GL. (1983) Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem 258:15365–15370 [PubMed] [Google Scholar]
- Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr (1998) Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci USA 95:2175–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike JD, Horwitz SB. (1976) Effect of VP-16-213 on the intracellular degradation of DNA in HeLa cells. Biochemistry 15:5443–5448 [DOI] [PubMed] [Google Scholar]
- Long BH, Musial ST, Brattain MG. (1984) Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16-213 and VM26: a quantitative structure-activity relationship. Biochemistry 23:1183–1188 [DOI] [PubMed] [Google Scholar]
- Lovett BD, Strumberg D, Blair IA, Pang SK, Burden DA, Megonigal MD, Rappaport EF, Rebbeck TR, Osheroff N, Pommier YG, et al. (2001) Etoposide metabolites enhance DNA topoisomerase II cleavage near leukemia-associated MLL translocation breakpoints. Biochemistry 40:1159–1170 [DOI] [PubMed] [Google Scholar]
- Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, Mutus B. (2008) Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem 283:18513–18521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR, Jr, Denicola A. (2007) Membrane “lens” effect: focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol 20:709–714 [DOI] [PubMed] [Google Scholar]
- Myers CE, Mimnuagh E, Yeh G, Sinha BK. (1987) Biochemical Mechanism of Tumor Cell Kill by the Anthracyclines in Anthracyclines and Anthracenedione-Based Anticancer Agents (Lown W, ed) pp 527–569, Elsevier, NY [Google Scholar]
- Pollard JW. (2008) Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 84:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Politi PM, Sinha BK, Myers CE. (1988) Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res 48:4766–4769 [PubMed] [Google Scholar]
- Ranguelova K, Suarez J, Magliozzo RS, Mason RP. (2008) Spin trapping investigation of peroxide- and isoniazid-induced radicals in Mycobacterium tuberculosis catalase-peroxidase. Biochemistry 47:11377–11385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, Hailemichael Y, Jayaraman P, Myers JN, Grimm EA, et al. (2010) Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res 16:1834–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha BK. (1995) Topoisomerase inhibitors: therapeutic potentials. Drugs 49:11–19 [DOI] [PubMed] [Google Scholar]
- Sinha BK, Politi PM, Eliot HM, Kerrigan D, Pommier Y. (1990) Structure-activity relations, cytotoxicity and topoisomerase II dependent cleavage induced by pendulum ring analogues of etoposide. Eur J Cancer 26:590–593 [DOI] [PubMed] [Google Scholar]
- Sinha BK. (1980) Binding specificity of chemically and enzymatically activated anthracycline anticancer agents to nucleic acids. Chem Biol Interact 30:67–77 [DOI] [PubMed] [Google Scholar]
- Sinha BK, Trush MA, Kennedy KA, Mimnaugh EG. (1984) Enzymatic activation and binding of adriamycin to nuclear DNA. Cancer Res 44:2892–2896 [PubMed] [Google Scholar]
- Sinha BK, Katki AG, Batist G, Cowan KH, Myers CE. (1987) Differential formation of hydroxyl radicals by Adriamycin in sensitive and resistant MCF-7 human breast tumor cells: implications for the mechanism of action. Biochemistry 26:3776–3781 [DOI] [PubMed] [Google Scholar]
- Sinha BK, Haim N, Dusre L, Kerrigan D, Pommier Y. (1988) DNA strand breaks produced by etoposide (VP-16,213) in sensitive and resistant human breast tumor cells: implications for the mechanism of action. Cancer Res 48:5096–5100 [PubMed] [Google Scholar]
- Sinha BK, Bhattacharjee S, Chatterjee S, Jiang JJ, Motten AG, Kumar A, Espey MG, Mason RP. (2013) Role of nitric oxide in the chemistry and anticancer activity of etoposide (VP-16,213). Chem Res Toxicol 26:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CH, Grimm EA. (2004) Depletion of endogenous nitric oxide enhances cisplatin-induced apoptosis in a p53-dependent manner in melanoma cell lines. J Biol Chem 279:288–298 [DOI] [PubMed] [Google Scholar]
- Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, Degraff W, Gamsom J, Vodovotz Y, Russo A, et al. (1997) Nitric oxide and some NO donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide 1:88–94 [DOI] [PubMed] [Google Scholar]
- Wink DA, Vodovotz Y, Cook JA, Krishna MC, Kim S, Coffin D, DeGraff W, Deluca AM, Liebmann J, Mitchell JB. (1998) The role of nitric oxide chemistry in cancer treatment. Biochemistry (Mosc) 63:802–809 [PubMed] [Google Scholar]
- van Maanen JMS, de Vries J, Pappie D, van den Akker E, Lafleur VM, Retèl J, van der Greef J, Pinedo HM. (1987) Cytochrome P-450-mediated O-demethylation: a route in the metabolic activation of etoposide (VP-16-213). Cancer Res 47:4658–4662 [PubMed] [Google Scholar]
- Vlasova II, Feng W-H, Goff JP, Giorgianni A, Do D, Gollin SM, Lewis DW, Kagan VE, Yalowich JC. (2011) Myeloperoxidase-dependent oxidation of etoposide in human myeloid progenitor CD34+ cells. Mol Pharmacol 79:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WM, Liu LZ, Loizidou M, Ahmed M, Charles IG. (2002) The role of nitric oxide in cancer. Cell Res 12:311–320 [DOI] [PubMed] [Google Scholar]
- Yenes S, Messeguer A. (1999) A study of the reaction of different phenol substrates with nitric oxide and peroxynitrite. Tetrahedron 55:14111–14122 DOI: 10.1016/S0040-4020(99)00877-7. [Google Scholar]
- Zhuang L, Lin JQ, Lu ML, Solomon KR, Freeman MR. (2002) Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res 62:2227–2231 [PubMed] [Google Scholar]