Abstract
The glutamate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) are critically involved in the excitatory synaptic transmission, and blocking AMPARs at the spinal level reverses neuropathic pain. However, little is known about changes in the composition of synaptic AMPARs in the spinal dorsal horn after peripheral nerve injury. AMPARs lacking the GluA2 subunit are permeable to Ca2+, and their currents show unique inward rectification. We found that AMPAR-mediated excitatory postsynaptic currents (AMPAR-EPSCs) of spinal dorsal horn neurons exhibited a linear current-voltage relationship in control rats, whereas AMPAR-EPSCs of dorsal horn neurons displayed inward rectification in rats with spinal nerve injury. In nerve-injured rats, compared with control rats, the GluA2 protein level was significantly less in the plasma membrane but was greater in the cytosolic vesicle fraction in the dorsal spinal cord. However, the GluA1 protein levels in these fractions did not differ significantly between nerve-injured and control rats. Blocking N-methyl-d-aspartate receptors (NMDARs) abolished inward rectification of AMPAR-EPSCs of dorsal horn neurons in nerve-injured rats. Furthermore, inhibition of calpain or calcineurin, but not protein kinase C, completely blocked nerve injury–induced inward rectification of AMPAR-EPSCs of dorsal horn neurons. In addition, blocking GluA2-lacking AMPARs at the spinal cord level reduced nerve injury–induced pain hypersensitivity. Our study suggests that nerve injury increases GluA2 internalization and the prevalence of GluA2-lacking AMPARs in the spinal dorsal horn to maintain chronic neuropathic pain. Increased prevalence of spinal GluA2-lacking AMPARs in neuropathic pain is mediated by NMDARs and subsequent stimulation of calpain and calcineurin signaling.
Introduction
Chronic neuropathic pain, caused by a lesion to or dysfunction of the peripheral or central nervous system, is a significant and unmet clinical problem. Conventional analgesics are less effective in alleviating symptoms and are often associated with various adverse effects. The cellular and molecular mechanisms underlying various neuropathic pain conditions are complex and remain to be fully defined. Several changes in the peripheral and central nervous system are closely associated with neuropathic pain, which include augmented primary afferent excitability (Campbell et al., 1988; Matzner and Devor, 1994), increased glutamatergic input to spinal dorsal horn neurons (Wang et al., 2007; Zhang et al., 2009), and diminished γ-aminobutyric acid– and glycine-mediated synaptic inhibition of spinal dorsal horn neurons (Coull et al., 2003; Zhou et al., 2012).
Glutamate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) are predominantly involved in the fast excitatory synaptic transmission and plasticity in the mammalian central nervous system. AMPARs are heterotetrameric cation channels composed of a combinational assembly of four subunits, GluA1 through GluA4. The subunit composition determines the electrophysiological properties of AMPARs and their permeability to Ca2+ (Burnashev et al., 1992; Mayer and Armstrong, 2004). The GluA2 subunit is particularly important for the biophysical properties of AMPARs because an arginine residue (Arg607) in the pore-lining region makes GluA2-containing AMPARs impermeable to Ca2+ (Hollmann et al., 1991; Isaac et al., 2007). In contrast, GluA2-lacking AMPARs have a high Ca2+ permeability and show inward-rectifying currents (Burnashev et al., 1992). We have shown that blocking of spinal AMPARs reverses pain hypersensitivity induced by nerve injury (Chen et al., 2000). However, little is known about changes in the synaptic AMPAR composition in the spinal dorsal horn in neuropathic pain.
Altered AMPAR compositions can significantly change neuronal and synaptic functions in the brain (Plant et al., 2006; Li et al., 2012). Acute inflammatory pain is associated with increased insertion of GluA1-containing AMPARs in the spinal dorsal horn (Galan et al., 2004; Larsson and Broman, 2008), and GluA2 internalization mediated by protein kinase C (PKC) activation is associated with persistent inflammatory pain (Park et al., 2009). In addition, stimulation of N-methyl-d-aspartate receptors (NMDARs) can induce GluA2 internalization in the hippocampus and hypothalamus (Tigaret et al., 2006; Li et al., 2012). Although peripheral nerve injury increases NMDAR activity in spinal dorsal horn neurons (Isaev et al., 2000), the AMPAR plasticity in the spinal cord and its associated functional significance in neuropathic pain have not yet been investigated.
In the present study, we determined whether peripheral nerve injury alters synaptic AMPAR composition in the spinal dorsal horn. We showed that nerve injury causes internalization of the GluA2 subunit and increases the prevalence of synaptic GluA2-lacking AMPARs in spinal dorsal horn neurons. Blocking GluA2-lacking AMPARs at the spinal level reverses nerve injury–induced pain hypersensitivity. We also provide new evidence that increased prevalence of spinal GluA2-lacking AMPARs induced by nerve injury results from activation of NMDARs and subsequent stimulation of calpain and calcineurin activity. These new findings should greatly improve our understanding of signaling mechanisms involved in spinal synaptic plasticity associated with neuropathic pain.
Materials and Methods
Animal Model of Neuropathic Pain.
Male Sprague-Dawley rats (8–10 weeks old; Harlan, Indianapolis, IN) were used in this study. A total of 65 rats were used throughout this study. All the experimental protocols were approved by the Animal Care and Use Committee of The University of Texas M.D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines for the ethical use of animals. We used left L5 and L6 spinal nerve ligation (SNL) in rats as the neuropathic pain model (Kim and Chung, 1992; Chen et al., 2000). In brief, we induced anesthesia with 2–3% isoflurane and then isolated the left L5 and L6 spinal nerves and ligated them tightly with 5-0 silk suture. Sham animals were used as controls, and they underwent similar surgical procedures except nerve ligation. We confirmed tactile allodynia in all SNL rats before they were used for the final electrophysiological and biochemical experiments, which were done 3–4 weeks after surgery.
Intrathecal catheters were implanted in some SNL rats during isoflurane-induced anesthesia 1 week after surgery. In brief, we made a small opening in the atlanto-occipital membrane of the cisterna magna and inserted a PE-10 catheter such that the caudal tip reached the lumbar spinal cord (Chen et al., 2009). The animals were allowed to recover for 5 days after the catheter cannulation.
Assessment of Tactile Allodynia.
To assess pain hypersensitivity in response to an innocuous stimulus (allodynia), rats were individually placed in suspended chambers on a mesh floor. The tactile stimulus producing a 50% likelihood of withdrawal response was calculated by using the modified “up-down” method without using 15.1 g as the cutoff (Chaplan et al., 1994). A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) were applied perpendicularly to the plantar surface of the left hindpaw with sufficient force to bend the filament for 6 seconds. Brisk withdrawal or paw flinching was considered a positive response.
Spinal Cord Slice Preparation and Electrophysiological Recordings.
Lumbar spinal cord slices at the L5–L6 levels were prepared from sham control and nerve-injured rats as we described previously (Li et al., 2002; Zhou et al., 2008, 2011). We removed the lumbar spinal cord through laminectomy during isoflurane-induced anesthesia. We sliced the spinal cord (400 μm) using a vibratome slicer and continuously superfused the slices with artificial cerebrospinal fluid containing (in mM) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3 (bubbled with 95% O2/5% CO2; pH 7.3).
Neurons in the lamina II outer zone of the spinal cord were identified with use of differential interference contrast/infrared illumination on a fixed-stage microscope (BX50WI; Olympus, Tokyo, Japan). We selected these neurons for recording, because they can be visualized in adult spinal cord slices and receive predominantly nociceptive input from unmyelinated primary afferents (Woolf et al., 1992; Pan et al., 2003). All whole-cell recordings were obtained at 34°C by using borosilicate pipettes that were filled with a solution containing (in mM) 110 Cs2SO4, 5 TEA, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, 0.1 spermine, and 10 lidocaine N-ethyl bromide (adjusted to pH 7.2–7.4 with 1 M CsOH, 290–300 mOsm). We included 0.1 mM spermine in the intracellular solution to compensate for a possible loss of endogenous polyamines from intracellular dialysis during whole-cell recordings. Neurons were voltage-clamped at −60 mV, and AMPAR-mediated excitatory postsynaptic currents (AMPAR-EPSCs) were recorded in the presence of 2 μM strychnine, 10 μM bicuculline, and 50 μM dl-2-amino-5-phosphonovaleric acid (AP5). We used electrical stimulation (0.3–0.6 mA, 0.2 millisecond) of the dorsal root to evoke EPSCs. Monosynaptic EPSCs were identified on the basis of the constant latency of evoked EPSCs, and the absence of conduction failure of evoked EPSCs in response to a 20-Hz electrical stimulation as we described previously (Li et al., 2002; Zhou et al., 2008). The input resistance was monitored, and the recording was abandoned if it changed more than 15%. To determine the current-voltage relationship, AMPAR-EPSCs were recorded at various membrane potentials ranging from −70 to +70 mV in 20-mV steps. The rectification index was calculated by dividing the amplitude of AMPAR-EPSCs recorded at +50 mV by that at −50 mV (Li et al., 2012).
AP5 and bicuculline were obtained from Ascent Scientific (Princeton, NJ). IEM-1460 and calpeptin were purchased from Tocris Bioscience (Ellisville, MO). Chelerythrine and FK-506 were obtained from Sigma-Aldrich (St. Louis, MO). All of the drugs were freshly prepared in artificial cerebrospinal fluid before the experiments and delivered by using syringe pumps at the final concentrations indicated.
Western Blot Analysis.
To quantify subcellular protein levels in the dorsal spinal cord tissues, rats were killed with sodium pentobarbital (80 mg/kg i.p.). The rats were then decapitated, and the L5–L6 spinal dorsal horn quadrants were collected. Subcellular fractionation was carried out according to the procedures described previously (Park et al., 2009). The tissue was homogenized in ice-cold buffer containing 10 mM Tris (pH 7.4), 1 mM EDTA, 1 mM Na3VO4, 0.25 M sucrose, and the phosphatase and protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged at 1000g for 10 minutes at 4°C to remove nuclei and large debris. The supernatant was centrifuged at 20,000g for 30 minutes at 4°C to separate the crude plasma membrane and cytosolic fractions. The cytosolic fraction sample was subsequently centrifuged at 150,000g for 1 hour at 4°C, and the pellet was used as the cytosolic vesicle fraction (CVF) samples. The plasma membrane fraction and CVF pellets were prepared by using the lysis buffer containing 20 mM Tris (pH 7.6), 0.5% Nonidet P-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, and the protease inhibitor cocktail (Sigma-Aldrich). The samples were incubated for 30 minutes at 4°C and then centrifuged at 12,000g for 15 minutes at 4°C. The supernatant was collected, and the protein concentration was determined by using the Lowry protein assay. For Western blotting, 50 μg of proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a membrane (Millipore, Billerica, MA). The immunoblots were probed with a rabbit anti-GluA1 antibody (1:1000 dilution) (ab1504; Millipore), a rabbit anti-GluA2 antibody (1:1000 dilution) (ab10529; Millipore), and anti-β-actin antibody (1:5000 dilution) (4967; Cell Signaling Technology, Danvers, MA). The specificity of anti-GluA1 and anti-GluA2 antibodies has been shown previously (Park et al., 2009). An ECL kit (GE Healthcare, Pittsburgh, PA) was used to detect the protein bands, and the band density was then quantified and normalized to that of β-actin (as a loading control).
Data Analysis.
Data are presented as mean ± S.E.M. added. For spinal cord slice recordings, —four to six rats were used in each protocol. The amplitudes of AMPAR-EPSCs were analyzed offline with Clampfit 9.2 (Molecular Devices, Sunnyvale, CA). Data were tested for normal distributions using the D’Agostino-Pearson test. The Student’s t test was used to compare the nerve injury effects on AMPAR-EPSCs and GluA1 and GluA2 protein levels in the spinal cord. One-way analysis of variance (with Tukey’s or Dunnett’s post hoc test) was performed to compare the drug treatment effects on the rectification index of AMPAR-EPSCs and paw withdrawal thresholds. P < 0.05 was considered statistically significant.
Results
Nerve Injury Increases GluA2-Lacking AMPAR Prevalence of Dorsal Horn Neurons.
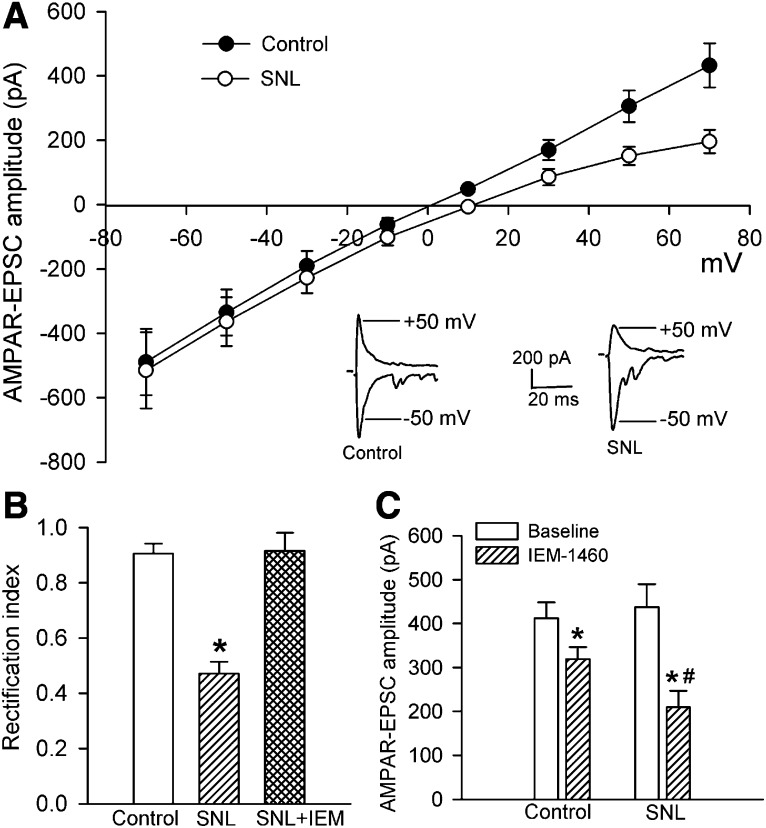
To determine whether nerve injury alters GluA2-lacking and GluA2-containing AMPAR prevalence in the spinal dorsal horn, we examined the current-voltage relationship of AMPAR-EPSCs of lamina II neurons. GluR2-lacking AMPARs are highly permeable to Ca2+ and, because of a voltage-dependent block by intracellular polyamines, exhibit inward rectification at positive holding potentials (Bowie and Mayer, 1995; Isaac et al., 2007). On the basis of this unique biophysical feature, we assessed the I-V relationship of AMPAR-EPSCs in lamina II neurons evoked by electrical stimulation of the dorsal root at various holding potentials. In lamina II neurons of control rats, the AMPAR-EPSCs recorded showed a near-linear I-V relationship (Fig. 1A), indicating that synaptic AMPAR-EPSCs are mediated predominantly by GluA2-containing AMPARs. In contrast, the amplitude of AMPAR-EPSCs of lamina II neurons in SNL rats was reduced at positive membrane potentials (Fig. 1A). The rectification index (I+50 mV/I−50 mV) of AMPAR-EPSCs of lamina II neurons was significantly reduced in SNL rats (0.47 ± 0.04; n = 10 neurons) compared with that in sham control rats (0.90 ± 0.04; n = 13 neurons; P < 0.05; Fig. 1B).
Fig. 1.
Nerve injury induces a switch from predominantly GluA2-containing to GluA2-lacking AMPARs in spinal dorsal horn neurons. (A) Original AMPAR-EPSCs traces (recorded at −50 and +50 mV) and I-V curves of AMPAR-EPSCs of lamina II neurons recorded at holding potentials ranging from −70 to +70 mV in sham control (n = 13 neurons) and SNL rats (n = 10 neurons). (B) Comparison of the rectification index of AMPAR-EPSCs of lamina II neurons in control rats, SNL rats, and SNL rats treated with 100 μM IEM-1460 (n = 8 neurons). (C) Summary data show the effect of 100 μM IEM-1460 on the amplitude of AMPAR-EPSCs of lamina II neurons recorded at a holding potential of −60 mV in control (n = 7 neurons) and SNL rats (n = 8 neurons). *P < 0.05 vs. sham or baseline control group; #P < 0.05 vs. corresponding value in control group.
To determine the contribution of GluA2-lacking AMPARs to synaptic AMPAR activity of dorsal horn neurons, we used IEM-1460, a selective blocker of GluA2-lacking AMPARs (Samoilova et al., 1999; Rossi et al., 2008). Bath application of IEM-1460 (100 μM) for 6–8 minutes normalized the rectification index of AMPAR-EPSCs of lamina II neurons in SNL rats (0.89 ± 0.06; Fig. 1B). We also compared the effect of IEM-1460 on AMPAR-EPSCs recorded with a holding potential at −60 mV in control and SNL rats. Treatment with IEM-1460 (100 μM) decreased the amplitude of AMPAR-EPSCs in lamina II neurons by 21.0 ± 5.8% in sham control rats. The reduction in the amplitude of AMPAR-EPSCs by IEM-1460 (51.4 ± 6.1%) was significantly greater in SNL rats than in control rats (Fig. 1C). Together, these results suggest that nerve injury increases the prevalence of GluA2-lacking synaptic AMPARs in the spinal dorsal horn.
Nerve Injury Increases GluA2 Internalization in the Spinal Cord.
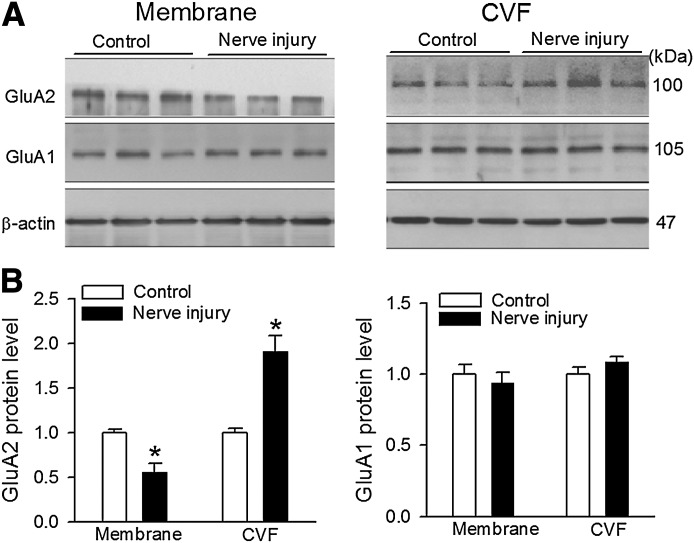
GluA1 and GluA2 are the most abundant AMPAR subunits and are highly concentrated on the postsynaptic membranes of the superficial dorsal horn, although all four AMPAR subunits are present in the spinal cord (Polgar et al., 2008). We thus determined whether nerve injury affects membrane protein levels of GluA1 and GluA2 subunits in the dorsal spinal cord. To this end, we examined the protein amount of GluA1 and GluA2 subunits in the plasma membrane and CVF fractions in the dorsal spinal cords obtained from sham control and SNL rats 3 weeks after surgery. The CVF fraction was used to estimate the GluA1 and GluA2 protein levels present in the cytoplasm (Park et al., 2009; Li et al., 2012). The protein level of GluA2 in the plasma membrane fraction was significantly lower in the SNL group than in the control group (n = 6; Fig. 2). Furthermore, the protein level of GluA2 in the CVF fraction was much higher in the SNL rats than in the sham control rats. In contrast, the protein amount of GluA1 in the plasma membrane and CVF fractions in the spinal cord did not differ significantly between control and SNL groups (n = 6; Fig. 2). These results indicate that nerve injury promotes GluA2 internalization in the spinal dorsal horn.
Fig. 2.
Nerve injury promotes GluA2 internalization in the dorsal spinal cord. Representative gel images (A) and group data (B) show the GluA1 and GluA2 protein band density in the plasma membrane and CVF in the dorsal spinal cords obtained from control and SNL rats (n = 6 samples in each group, 1 sample/rat). *P < 0.05 vs. value in contralateral side.
Nerve Injury Increases the Prevalence of GluA2-Lacking AMPARs of Dorsal Horn Neurons through Calcineurin but Not PKC.
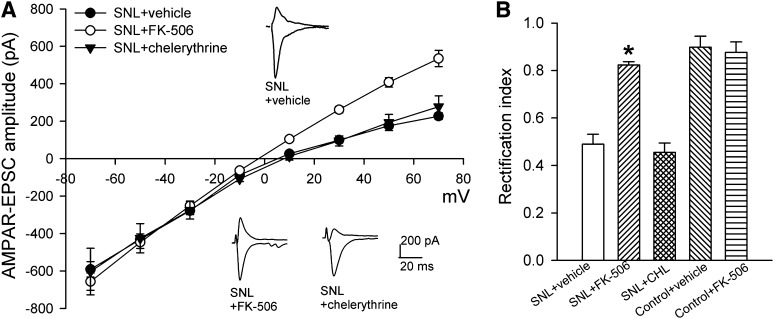
It has been reported that GluA2 internalization in the spinal cord during tissue inflammation requires PKC after NMDAR activation (Park et al., 2009). We thus determined whether PKC mediates nerve injury–induced increases in the prevalence of GluA2-lacking AMPARs of dorsal horn neurons. Chelerythrine is a highly specific PKC inhibitor (Herbert et al., 1990), and we have shown that treatment of spinal cord slices with 10 μM chelerythrine blocks PKC-dependent NMDAR activation (Zhao et al., 2012). In spinal cord slices taken from SNL rats, treatment with the specific PKC inhibitor chelerythrine (10 μM, 2–3 hours) had no significant effect on the inwardly rectifying I-V relationship of AMPAR-EPSCs and the reduced rectification index of AMPAR-EPSCs of lamina II neurons caused by SNL (Fig. 3, A and B).
Fig. 3.
Calcineurin, but not PKC, contributes to the nerve injury–induced switch to GluA2-lacking AMPARs of spinal dorsal horn neurons. (A) Original AMPAR-EPSC traces (recorded at −50 and +50 mV) and summary data show the effects of incubation of spinal cord slices from SNL rats with FK-506 (1 μM; n = 11 neurons), chelerythrine (10 μM; n = 9 neurons), or vehicle (n = 8 neurons) on the I-V relationships of AMPAR-EPSCs of lamina II neurons. (B) Group data show the rectification index of AMPAR-EPSCs of lamina II neurons in SNL rats treated with vehicle, FK-506, or chelerythrine (CHL) and in control rats treated with vehicle (n = 10 neurons) or FK-506 (n = 9 neurons). *P < 0.05 vs. SNL vehicle group.
Calcineurin, a Ca2+-dependent protein phosphatase, plays a role in GluA2 internalization in the brain (Beattie et al., 2000; Lin et al., 2000; Li et al., 2012). Nerve injury increases the activity of calcineurin in the spinal cord through NMDAR activation and Ca2+ influx (Zhou et al., 2012). We next determined the role of calcineurin in nerve injury–induced increases in the prevalence of GluA2-lacking AMPARs of dorsal horn neurons. Incubation of the spinal cord slices from SNL rats with the selective calcineurin inhibitor FK-506 (1 μM, 2–3 hours) (Liu et al., 1991; Li et al., 2012) abolished the inward rectification of AMPAR-EPSCs of lamina II neurons at the positive holding potentials (Fig. 3A). FK-506 also significantly increased the rectification index of AMPAR-EPSCs of these neurons in SNL rats (Fig. 3B). In spinal cord slices from control rats, treatment with FK-506 had no significant effect on the rectification index of AMPAR-EPSCs of lamina II neurons (Fig. 3B). These results suggest that calcineurin, but not PKC, is involved in the increased prevalence of GluA2-lacking AMPARs of dorsal horn neurons caused by nerve injury.
NMDAR Activation and Calpain Signaling Contribute to Increased Prevalence of GluA2-Lacking AMPARs of Dorsal Horn Neurons by Nerve Injury.
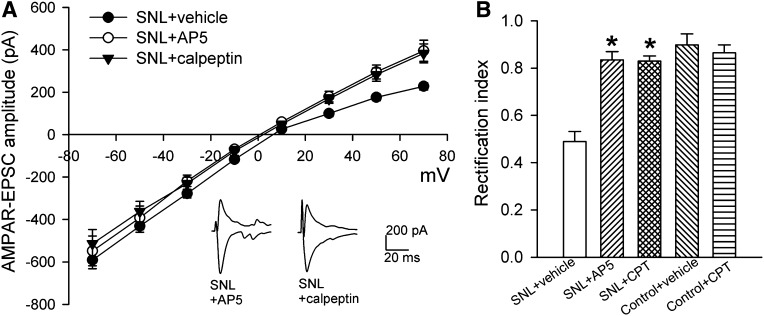
Activation of NMDARs induces internalization of GluA2 in the hippocampus and hypothalamus (Beattie et al., 2000; Li et al., 2012). Because nerve injury potentiates NMDAR activity in spinal lamina II neurons (Isaev et al., 2000), we first determined whether NMDARs contribute to increased prevalence of GluA2-lacking AMPARs of dorsal horn neurons after SNL. Incubation of the spinal cord slices from SNL rats with the specific NMDAR antagonist AP5 (50 μM, 2–3 hours) abolished the inward rectification of AMPAR-EPSCs of lamina II neurons (Fig. 4A). AP5 treatment also normalized the rectification index of AMPAR-EPSCs of lamina II neurons in SNL rats to the value seen in sham control rats (Fig. 4B).
Fig. 4.
NMDARs and calpain are involved in nerve injury–induced switch to GluA2-lacking AMPARs of spinal dorsal horn neurons. (A) Representative AMPAR-EPSC traces (recorded at −50 and +50 mV) and summary data show the effects of incubation of spinal cord slices from SNL rats with AP5 (50 μM; n = 9 neurons), calpeptin (30 μM; n = 8 neurons), or vehicle (n = 8 neurons) on the I-V relationships of AMPAR-EPSCs of lamina II neurons. Note that SNL + vehicle group data were reproduced from Fig. 3A for comparison. (B) Group data show the rectification index of AMPAR-EPSCs of lamina II neurons in SNL rats treated with AP5 or calpeptin (CPT) and in control rats treated with vehicle (n = 10 neurons) or CPT (n = 10 neurons). *P < 0.05 vs. SNL vehicle group.
We recently found that nerve injury increases the activity of calpain, a Ca2+-activated cysteine protease (Molinari and Carafoli, 1997), in the spinal cord through increased NMDAR activity (Zhou et al., 2012). It is not clear how increased NMDAR activity by nerve injury leads to a persistent increase in calcineurin activity in the spinal cord. Calpain can cause sustained increases in calcineurin activity through proteolytic cleavage by removing the autoinhibitory domain of calcineurin A (Wu et al., 2004; Shioda et al., 2006). Thus, we reasoned that nerve injury–induced calpain activation may contribute to increased prevalence of GluA2-lacking AMPARs of dorsal horn neurons in neuropathic pain. To test this hypothesis, we used a specific membrane-permeable inhibitor of calpain, calpeptin (Tsujinaka et al., 1988; Zhou et al., 2012). Incubation of the spinal cord slices from SNL rats with calpeptin (30 μM, 2–3 hours) switched the I-V relationship of AMPAR-EPSCs from inward rectifying to near linear (Fig. 4A). In addition, calpeptin treatment completely normalized the rectification index of AMPAR-EPSCs of lamina II neurons in SNL rats (Fig. 4B). In spinal cord slices from sham control rats, treatment with calpeptin had no significant effect on the rectification index of AMPAR-EPSCs of lamina II neurons (Fig. 4B). Collectively, these data suggest that nerve injury increases the GluA2-lacking AMPAR prevalence of spinal dorsal horn neurons through the NMDAR-mediated calpain and calcineurin signaling.
Increased GluA2-Lacking AMPAR Prevalence at the Spinal Cord Level Contributes to Nerve Injury–Induced Pain Hypersensitivity.
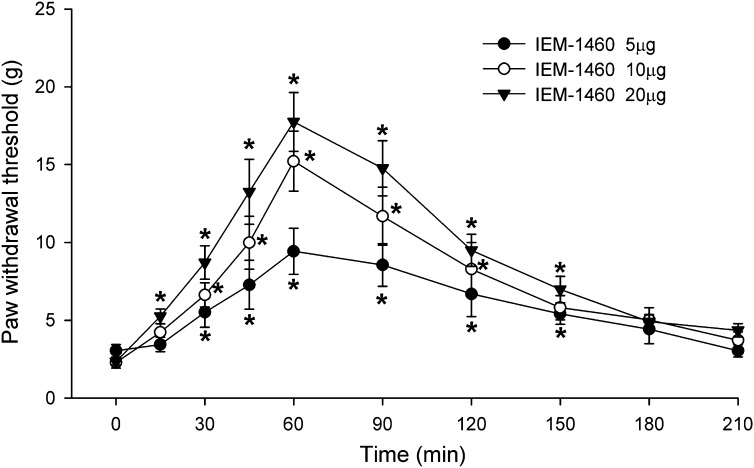
The functional significance of increased GluA2-lacking AMPAR prevalence in the spinal dorsal horn has not been defined previously. In additional SNL rats, we determined whether blocking GluA2-lacking AMPARs at the spinal level attenuates nerve injury–induced pain hypersensitivity. IEM-1460 was dissolved in normal saline and administered in a volume of 5 μl, followed by a 10-μl flush with normal saline. Intrathecal injection of normal saline has no effect on the tactile withdrawal threshold in SNL rats (Chen and Pan, 2005; Cai et al., 2013). In SNL rats, intrathecal injection of 5–20 μg of IEM-1460 dose-dependently increased the withdrawal threshold in response to von Frey filaments applied to the left hindpaw (n = 8 rats in each group; Fig. 5). Animals receiving intrathecal injection of IEM-1460 did not exhibit any motor dysfunction, as judged by the placing-stepping reflex and ambulation behavior. These results suggest that increased GluA2-lacking AMPAR prevalence at the spinal level contributes to maintaining the pain hypersensitivity caused by nerve injury.
Fig. 5.
GluA2-lacking AMPARs at the spinal level contribute to pain hypersensitivity induced by nerve injury. Group data show the time course of the effect of injection of 5, 10, and 20 μg i.t. IEM-1460 on tactile allodynia measured with von Frey filaments applied to the left hindpaw (n = 8 rats in each group). *P < 0.05 vs. baseline control value (time 0).
Discussion
The major objective of our study was to determine possible nerve injury–induced changes in the synaptic AMPAR composition in spinal dorsal horn neurons and the underlying signaling mechanisms. The unique biophysical property of GluA2-lacking AMPAR currents is the inward rectification because of the voltage-dependent block by intracellular polyamines (Bowie and Mayer, 1995; Koh et al., 1995). Tissue inflammation can increase the GluA2-lacking AMPAR prevalence and GluA2 internalization in the spinal dorsal horn (Galan et al., 2004; Vikman et al., 2008; Park et al., 2009). In the present study, we found that AMPAR-mediated EPSCs displayed inward rectification in spinal dorsal horn neurons after SNL. In addition, the selective GluA2-lacking AMPAR blocker IEM-1460 (Rossi et al., 2008; Fortin et al., 2010) produced a significantly greater reduction in the amplitude of AMPAR-EPSCs in dorsal horn neurons in SNL than in sham control rats. IEM-1460 blocked the inward rectification of AMPAR-EPSCs of dorsal horn neurons of SNL rats. These results suggest that peripheral nerve injury increases the GluA2-lacking AMPAR prevalence in spinal dorsal horn neurons.
We also investigated whether the increased GluA2-lacking AMPAR prevalence of dorsal horn neurons after nerve injury might be associated with increased GluA2 internalization. We found that the GluA2 protein level in the plasma membrane of the dorsal spinal cord was much lower in SNL rats than in sham control rats. Notably, the GluA2 protein level in the CVF of the dorsal spinal cord was significantly higher in SNL rats than in control rats. However, the GluA1 protein amounts in the plasma membrane and CVF were similar in SNL and control rats. Our findings suggest that nerve injury potentiates GluA2-lacking synaptic AMPAR activity by promoting GluA2 internalization in the spinal dorsal horn.
GluA2-lacking AMPARs are Ca2+-permeable (Hollmann et al., 1991; Keller et al., 1992) and associated with larger channel conductance than are AMPARs assembled by a combination of GluA1, GluA3, and GluA4 subunits (Oh and Derkach, 2005; Thiagarajan et al., 2005). Because of these functional properties, increased prevalence of GluA2-lacking AMPARs can increase excitatory synaptic strength (Terashima et al., 2004) and is involved in long-term potentiation consolidation in the hippocampus (Plant et al., 2006) and neuronal hyperactivity in the hypothalamus (Li et al., 2012). We showed in the present study that intrathecal injection of IEM-1460 readily reversed tactile allodynia induced by SNL. Our results suggest that the increased GluA2-lacking AMPAR prevalence at the spinal cord level contributes to the maintenance of pain hypersensitivity induced by nerve injury.
We provide new information about the signaling mechanisms involved in increased GluA2-lacking AMPAR prevalence in the spinal cord after nerve injury. It has been reported that increased PKC activation after tissue inflammation can result in GluA2 internalization in the spinal cord (Park et al., 2009). However, we found that inhibition of PKC with chelerythrine failed to change the inward rectification of AMPAR-EPSCs of dorsal horn neurons in SNL rats. It has been shown that PKC mediates primarily GluA2-lacking AMPAR translocation from perisynaptic to synaptic sites in the hippocampus (Yang et al., 2010). The spinal cord NMDAR activity is increased in the spinal cord of SNL rats (Isaev et al., 2000), and NMDAR-triggered Ca2+ influx plays a critical role in GluA2 internalization in the brain (Tigaret et al., 2006; Li et al., 2012). In the present study, we found that blocking NMDARs abolished the inward rectification of AMPAR-EPSCs of dorsal horn neurons caused by nerve injury. Thus, activation of NMDARs, but not PKC, plays a prominent role in the increased prevalence of GluA2-lacking AMPARs of spinal dorsal horn neurons in neuropathic pain.
The Ca2+/calmodulin-dependent phosphatase calcineurin can dephosphorylate proteins involved in the endocytosis process, such as dynamin, synaptojanin, and the adaptor protein AP180 (Clayton et al., 2007), and is involved in GluA2 internalization caused by insulin and NMDAR stimulation (Beattie et al., 2000; Lin et al., 2000; Li et al., 2012). We found in this study that inhibition of calcineurin with FK-506 normalized the I-V relationship of AMPAR-EPSCs of dorsal horn neurons from inwardly rectifying to linear in SNL rats, suggesting that calcineurin plays an important role in increased GluA2-lacking AMPAR prevalence in the spinal cord after nerve injury. In addition, calpain is a family of cysteine proteases that are activated by Ca2+ at neutral pH (Croall and Ersfeld, 2007). Activation of NMDARs induces Ca2+ influx to activate calpain (Adamec et al., 1998; Hewitt et al., 1998) and cleaves the autoinhibitory domain in calcineurin A, which converts calcineurin to a constitutively active form (i.e., no longer requiring Ca2+ and calmodulin for activation) (Wu et al., 2004; Shioda et al., 2006). We have shown that nerve injury increases calpain activity in the spinal dorsal horn through NMDAR activation (Zhou et al., 2012). In the present study, we found that inhibition of calpain completely blocked nerve injury–induced inward rectification of AMPAR-EPSCs of dorsal horn neurons. Thus, our data suggest that calpain may be the missing link between increased NMDARs and persistent calcineurin activation in the spinal dorsal horn after nerve injury, which together could constitute the signaling cascade responsible for nerve injury–induced increases in GluA2-lacking AMPAR prevalence of dorsal horn neurons. Nevertheless, calpain may not be an essential upstream signaling of calcineurin in the regulation of GluA2-lacking AMPAR prevalence after nerve injury, because enhanced NMDAR activity may activate calcineurin via Ca2+/calmodulin-dependent activation without the involvement of calpain.
One of the limitations of this study is that we did not examine whether increases in the prevalence of GluA2-lacking synaptic AMPARs in the spinal dorsal horn are associated with other neuropathic pain conditions such as painful diabetic neuropathy and postherpetic neuralgia. Also, it is not clear what other AMPAR subunits can replace the internalized GluA2 subunits in GluA2-lacking AMPARs in the spinal cord after nerve injury. It is possible that some extrasynaptic Ca2+-permeable AMPARs are present in the membrane (Kopach et al., 2011), which could replace the GluA2-containing AMPARs at postsynaptic sites once they are internalized after nerve injury. Nerve injury may increase the postsynaptic insertion of GluA3 and/or GluA4 subunits in spinal dorsal horn neurons. In addition, the glutamate receptor–interacting protein (GRIP) functions as an AMPAR-targeting and synaptic-stabilizing protein (Dong et al., 1997). Increased calpain activation caused by nerve injury may also result in GRIP degradation (Lu et al., 2001) and disruption of GRIP binding to GluA2 (Lu et al., 2001) to cause GluA2 internalization in the spinal dorsal horn. These unanswered questions should be addressed in future studies.
In summary, our study demonstrates that nerve injury increases the prevalence of GluA2-lacking synaptic AMPARs and GluA2 internalization in the spinal dorsal horn. Furthermore, the NMDAR-calpain-calcineurin signaling pathway is critically involved in the phenotype switch from predominantly GluA2-containing to GluA2-lacking AMPARs in the spinal dorsal horn in neuropathic pain. Thus, the NMDAR activation–mediated calpain and calcineurin signaling plays a critical role in glutamatergic synaptic plasticity in the spinal cord after nerve injury. This new information is important to our understanding of the mechanisms underlying synaptic plasticity associated with neuropathic pain. Reducing GluA2-lacking Ca2+-permeable AMPAR activity in the spinal dorsal horn may represent a potentially important strategy for treating neuropathic pain.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor
- AP5
dl-2-amino-5-phosphonovaleric acid
- CVF
cytosolic vesicle fraction
- EPSC
excitatory postsynaptic current
- FK-506
C44H69NO12
- GRIP
glutamate receptor–interacting protein
- IEM-1460
C19H37N2Br.HBr
- NMDAR
N-methyl-d-aspartate receptor
- PKC
protein kinase C
- SNL
spinal nerve ligation
Authorship Contributions
Participated in research design: Chen, Zhou, Pan.
Conducted experiments: Chen, Zhou, Byun.
Performed data analysis: Chen, Zhou, Byun, Pan.
Wrote or contributed to the writing of the manuscript: Chen, Zhou, Pan.
Footnotes
This work was supported the National Institutes of Health (Grants DE022015 and NS073935) and by the N.G. and Helen T. Hawkins endowment (to H.-L.P.).
References
- Adamec E, Beermann ML, Nixon RA. (1998) Calpain I activation in rat hippocampal neurons in culture is NMDA receptor selective and not essential for excitotoxic cell death. Brain Res Mol Brain Res 54:35–48 [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. (2000) Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3:1291–1300 [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15:453–462 [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. (1992) Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8:189–198 [DOI] [PubMed] [Google Scholar]
- Cai YQ, Chen SR, Pan HL. (2013) Upregulation of nuclear factor of activated T-cells by nerve injury contributes to development of neuropathic pain. J Pharmacol Exp Ther 345:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. (1988) Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32:89–94 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Chen SR, Cai YQ, Pan HL. (2009) Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J Neurochem 110:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Eisenach JC, McCaslin PP, Pan HL. (2000) Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology 92:500–506 [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. (2005) Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured Rats. J Pharmacol Exp Ther 312:120–126 [DOI] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. (2007) Activity-dependent control of bulk endocytosis by protein dephosphorylation in central nerve terminals. J Physiol 585:687–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–942 [DOI] [PubMed] [Google Scholar]
- Croall DE, Ersfeld K. (2007) The calpains: modular designs and functional diversity. Genome Biol 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. (1997) GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386:279–284 [DOI] [PubMed] [Google Scholar]
- Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR. (2010) Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci 30:11565–11575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan A, Laird JM, Cervero F. (2004) In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain 112:315–323 [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. (1990) Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 172:993–999 [DOI] [PubMed] [Google Scholar]
- Hewitt KE, Lesiuk HJ, Tauskela JS, Morley P, Durkin JP. (1998) Selective coupling of mu-calpain activation with the NMDA receptor is independent of translocation and autolysis in primary cortical neurons. J Neurosci Res 54:223–232 [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. (1991) Ca2+ permeability of KA-AMPA—gated glutamate receptor channels depends on subunit composition. Science 252:851–853 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871 [DOI] [PubMed] [Google Scholar]
- Isaev D, Gerber G, Park SK, Chung JM, Randik M. (2000) Facilitation of NMDA-induced currents and Ca2+ transients in the rat substantia gelatinosa neurons after ligation of L5–L6 spinal nerves. Neuroreport 11:4055–4061 [DOI] [PubMed] [Google Scholar]
- Keller BU, Hollmann M, Heinemann S, Konnerth A. (1992) Calcium influx through subunits GluR1/GluR3 of kainate/AMPA receptor channels is regulated by cAMP dependent protein kinase. EMBO J 11:891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363 [DOI] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. (1995) Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol 486:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopach O, Kao SC, Petralia RS, Belan P, Tao YX, Voitenko N. (2011) Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn. Pain 152:912–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Broman J. (2008) Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci 28:7084–7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Byan HS, Pan HL. (2012) Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci 32:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. (2002) Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol 543:807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. (2000) Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci 3:1282–1290 [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. (1991) Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807–815 [DOI] [PubMed] [Google Scholar]
- Lu X, Wyszynski M, Sheng M, Baudry M. (2001) Proteolysis of glutamate receptor-interacting protein by calpain in rat brain: implications for synaptic plasticity. J Neurochem 77:1553–1560 [DOI] [PubMed] [Google Scholar]
- Matzner O, Devor M. (1994) Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol 72:349–359 [DOI] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. (2004) Structure and function of glutamate receptor ion channels. Annu Rev Physiol 66:161–181 [DOI] [PubMed] [Google Scholar]
- Molinari M, Carafoli E. (1997) Calpain: a cytosolic proteinase active at the membranes. J Membr Biol 156:1–8 [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. (2005) Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci 8:853–854 [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. (2003) Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci 23:2911–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, et al. (2009) Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci 29:3206–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9:602–604 [DOI] [PubMed] [Google Scholar]
- Polgár E, Watanabe M, Hartmann B, Grant SG, Todd AJ. (2008) Expression of AMPA receptor subunits at synapses in laminae I-III of the rodent spinal dorsal horn. Mol Pain 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B, Maton G, Collin T. (2008) Calcium-permeable presynaptic AMPA receptors in cerebellar molecular layer interneurones. J Physiol 586:5129–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoilova MV, Buldakova SL, Vorobjev VS, Sharonova IN, Magazanik LG. (1999) The open channel blocking drug, IEM-1460, reveals functionally distinct alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors in rat brain neurons. Neuroscience 94:261–268 [DOI] [PubMed] [Google Scholar]
- Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K. (2006) Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 98:310–320 [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. (2004) Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci 24:5381–5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. (2005) Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47:725–737 [DOI] [PubMed] [Google Scholar]
- Tigaret CM, Thalhammer A, Rast GF, Specht CG, Auberson YP, Stewart MG, Schoepfer R. (2006) Subunit dependencies of N-methyl-D-aspartate (NMDA) receptor-induced alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization. Mol Pharmacol 69:1251–1259 [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T, Mori T. (1988) Synthesis of a new cell penetrating calpain inhibitor (calpeptin). Biochem Biophys Res Commun 153:1201–1208 [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. (2008) Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol 586:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. (2007) Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol 579:849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. (1992) Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 355:75–78 [DOI] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. (2004) Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem 279:4929–4940 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Zhou Q. (2010) Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc Natl Acad Sci USA 107:11999–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. (2009) Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience 158:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YL, Chen SR, Chen H, Pan HL. (2012) Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-D-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem 287:25073–25085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK, Pan HL. (2012) N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl- cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem 287:33853–33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. (2008) Sustained inhibition of neurotransmitter release from non-TRPV1-expressing primary afferents by mu-opioid receptor activation-enkephalin in the spinal cord. J Pharmacol Exp Ther 327:375–382 [DOI] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. (2011) Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J Pharmacol Exp Ther 336:254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]