Abstract
Traumatic injuries, both in the central nervous system (CNS) and peripheral nervous system (PNS), can potentially lead to irreversible damage resulting in permanent loss of function. Investigating the complex dynamics involved in these processes may elucidate the biological mechanisms of both nerve degeneration and regeneration, and may potentially lead to the development of new therapies for recovery. A scientific overview on the biological foundations of nerve injury is presented. Differences between nerve regeneration in the central and PNS are discussed. Advances in microtechnology over the past several years have led to the development of invaluable tools that now facilitate investigation of neurobiology at the cellular scale. Microfluidic devices are explored as a means to study nerve injury at the necessary simplification of the cellular level, including those devices aimed at both chemical and physical injury, as well as those that recreate the post-injury environment.
Keywords: peripheral nerve injury, nerve regeneration, microfluidic chamber
1. Introduction
Nerve injuries can often cause devastating functional disabilities. Fortunately, peripheral nerves hold the potential to regenerate after injury; however, complete repair and exact functional restorations are not possible. Current state-of-the-art treatment for peripheral nervous system (PNS) injuries involves end-to-end suturing of uninjured nerve ends when the injury is small, and the use of autologous nerve grafts when the injury is large. The use of autologous nerve grafts in clinical peripheral nerve repair is associated with donor site morbidity, the need for multiple surgeries, limited tissue availability and inadequate functional reinnervation [1–3]. Regeneration is not inherently possible in the central nervous system (CNS) environment, and hence no pharmacological or technological solutions to the CNS repair and regeneration are available [4,5]. Accordingly, there is a considerable research interest in studying both nerve injury and regeneration in order to elucidate how to promote successful nerve repair. Investigating nerve injury on a cellular scale offers a unique potential for probing the pathophysiology of injury at the single neural cell level and investigating neural responses to the immediate environment. Traditional in vitro cell culture techniques have contributed significantly to our understanding of healthy and diseased neurons [6,7]. However, these techniques do not provide a controlled environment to grow or guide neurons, or enable precise probing of the cells and evaluation of extracellular or environmental interactions. Modern microfluidic technology offers the potential to accurately model or control the changing neuronal microenvironments. Thus, the precision and control supplied by microfluidic technology may be particularly relevant for the study of nerve degeneration and regeneration.
Neurons naturally operate in the microscale as multi-state mechanical, chemical and electrical sensors and actuators. Their operations occur on a level fundamentally familiar to engineers, and in a way that makes interfacing of neuronal cells with microdevices intuitive [8]. Application of microtechnology or microelectromechanical systems (MEMS) has contributed greatly in the past several years, and offers invaluable tools to facilitate neuroscience studies at the cellular scale. This technology has led to the development of laboratory-on-a-chip (LOC) devices, built using microtechnology, incorporating elements such as microscale channels, pumps and valves, and offering precise control or manipulation of the neuronal microenvironment in ways previously unachievable with macro-scale methods [9–12]. In view of their potential, innovative LOC microdevices have been developed to offer a highly structured environment to experiment with neuronal cells. These LOC devices offer spatial control at the level of cell deposition within designed structures, separation of subcellular components through the use of microchannels, and influence over interactions with other cell types [8,13–19]. There is also the benefit of precise control over the amount of reagents or factors added to the cells and low cost associated with the devices owing to the small volume of expensive reagents required for the devices. There has been some application of microtechnology to in vivo implantable devices for nerve regeneration, such as those that incorporate microfluidic chips into nerve implants in order to provide precise delivery of a target drug or enable monitoring of regeneration [20–24]. However, the majority of neuronal LOC devices are in vitro devices, in contrast to in vivo cell or whole-organism devices, and will be the primary focus of this review.
While studying neurons in their healthy state is undoubtedly valuable, experimentation into the mechanistic understanding of the underlying pathways governing axonal injury and regeneration, a research area of great importance, is less developed and would benefit from advances in the LOC technology [25]. Our intent is to give an overview of the biology of nerve injury and explore microfluidic LOC devices that accurately and selectively injure axons or model the post-injury environment and examine their potential in the field of regenerative neuroscience. Utilization of these types of LOC devices will enable a deeper understanding of axonal injury and regeneration mechanisms, and eventually lead to the development of clinically relevant therapies.
2. Biology of nerve injury and regeneration
The physiology of the nervous system presents distinctive challenges to nerve regeneration. An understanding of the general organization and components of the nervous system is necessary for insights into difficulties that may arise during nerve injury and regeneration. The nervous system is categorized into the CNS and the PNS. The CNS consists of the brain and spinal cord, and serves as the control centre, conducting and interpreting signals, whereas the PNS consists of motor and sensory nerves that transmit signals between the CNS and the rest of the body. The nervous system consists mainly of neurons and glial cells. Neurons, the basic functional units, are made up of a soma (cell body), axons that conduct signals away from the soma, and dendrites that relay signals to the soma. Axons contain the majority of the cell's cytoplasm [26]. Glial cells are the support cells of the nervous system, and are much more plentiful than neurons. These cells have some capacity for cell division, unlike neurons which cannot undergo mitosis and proliferation, although they can regenerate or sprout processes under the right conditions [1]. Schwann cells are the glial cells of the PNS, whereas oligodendrocytes, astrocytes and microglia are the glial cells of the CNS. Schwann cells form the myelin sheath that insulates peripheral axons. Schwann cells ensheath axons, and the myelin sheath forms concentric layers around the axon, which become tightly apposed. Oligodendrocytes myelinate axons of the CNS, and in contrast to Schwann cells, can myelinate several axons each. Another important distinction is that in the PNS, Schwann cells are surrounded by a neurilemma, which is a basement membrane (basal lamina) similar to the type found in epithelial layers. These characteristic differences can be found summarized in figure 1. The presence of a basal lamina is one of the distinguishing features of the PNS, as CNS axons do not have this continuous basal lamina surrounding their axons [1,27]. The absence of a basal lamina may contribute to regenerative failure in the CNS, as the basal lamina not only provides access to growth promoting extracellular matrix (ECM) molecules, but may also shield the axons from inhibitory molecules [28].
Figure 1.
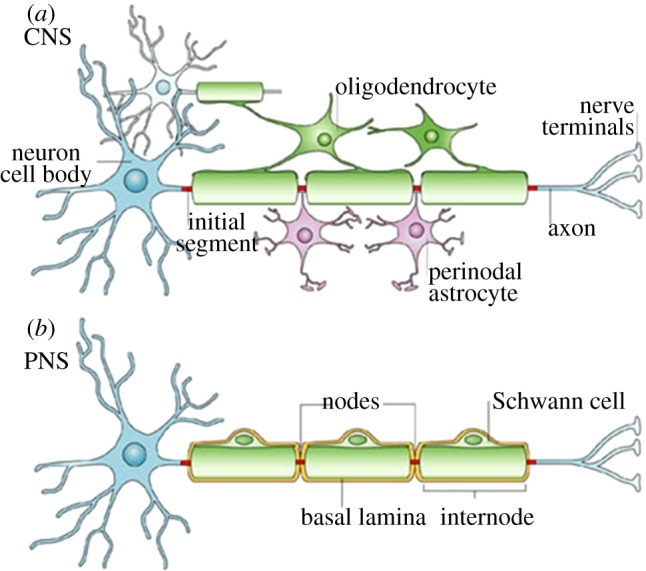
(a,b) A typical CNS neuron is myelinated by oligodendrocytes, which have the capacity to myelinate several cells at once, while a PNS neuron is myelinated by Schwann cells, which myelinate in a one-to-one ratio and are surrounded by basal lamina (adapted with permission from Poliak & Peles [27]).
Upon axonal injury, either through transection or severe compression, axon pathophysiology may proceed through different paths. The axon can undergo a degenerative retraction from the site of injury for a relatively short distance unless the injury is close to the cell body, in which case it proceeds to the cell body where retrograde neuronal degeneration can occur. Otherwise, Wallerian degeneration (WD) of the distal segment occurs, characterized by axonal swelling followed by accumulations of spheroids, and disruption of the cytoskeleton [25]. WD can be described as a series of cellular events that lead to anterograde degeneration of axons and myelin sheaths from the site of the lesion to the nerve endings, whereas the proximal stump undergoes regeneration. A major difference between PNS and CNS neurons is the response to axotomy and injury [1,25,29–32]. In the CNS, regeneration does not occur in the native environment, whereas in the PNS regeneration does occur, although complete functional recovery may not. The inherent ability of PNS neurons to regenerate contrasting with the inability of CNS neurons to regenerate has been a long-standing area of research interest. Injury to the axon reactivates an intrinsic growth capacity in the cell body [33]. In the PNS, in contrast to the CNS, this intrinsic growth capacity is coupled with a locally permissive environment owing to a more successful clearance of axonal and myelin debris from the degeneration process by Schwann cells and macrophages, as well as support from axon guidance cues, such as ECM components, cytokines and growth factors [34]. Figure 2 gives a high-level summary of the differences in CNS and PNS injury and regeneration, demonstrating successful regeneration following injury in the PNS with the aid of Schwann cells, circulating monocytes and macrophages, contrasted with scar formation in the CNS. The following is meant to be an overview of injury and regeneration in order to provide the rationale and context for the development of neuronal LOC devices; further sources can be consulted for an in-depth biological review [1,3,25,28–37].
Figure 2.
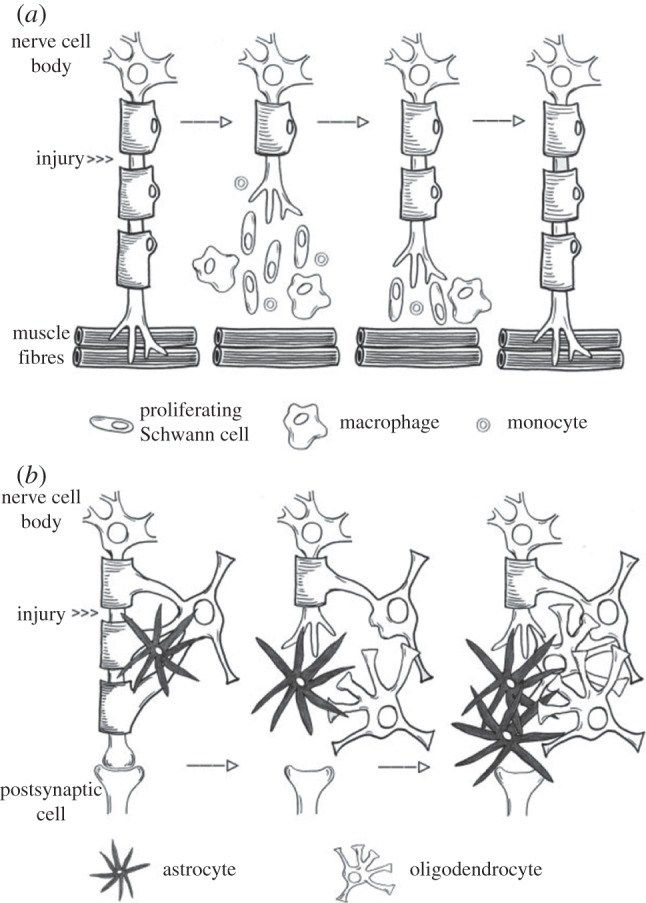
(a) A comparison of PNS regeneration demonstrating a favourable regeneration environment cleared of debris by Schwann cells and macrophages, with (b) the CNS post-injury environment, which includes unsuccessful debris clearance and scar formation (adapted with permission from Burnett & Zager [35] by Schmidt & Leach [1]).
2.1. Peripheral nervous system injury and regeneration
Peripheral nerve injuries (PNIs) can result from stretch-related injuries, lacerations or compressive trauma [35]. While these types of injuries can all lead to WD, there is typically more preservation of continuity of the neuron with stretch-related and compressive injuries, particularly with regard to the basal lamina, although disruption of this continuity can also be seen in extreme cases [29]. This preservation of continuity of the basal lamina can increase the probability of successful regeneration. Laceration models are seen proportionately more often in the literature owing to their ease of reproducibility.
Depending on the extent and site of injury, changes occur at the soma, site of injury and axon segments proximal and distal to the site of injury. Within hours of injury, axons and myelin start to physically fragment and swell, inhibiting signal conduction. In the soma, the nucleus moves towards the periphery of the cell body, and there is an increase in cell volume as production of RNA and regenerative proteins increases [36]. Somal proximity to the site of injury and age of the subject determines neuronal survival; the closer the injury is to the cell body and the older the subject, the more susceptible the neurons will be to apoptosis, with the exception of enhanced sensitivity of neurons to apoptosis in neonates [35,36]. Similar to WD, the proximal segment undergoes minimal degradation, called traumatic degeneration, depending on the extent of injury. If the injury is sufficiently severe, or proximal to the cell body, then this degradation can extend back to the cell body leading to cell death [36]. Schwann cells become active and proliferate, forming dedifferentiated daughter cells that release molecules to help in the degeneration and regeneration process and remove axonal and myelin debris from the site of injury together with macrophages. Distal to the site of injury, focal lesions can trigger WD. These focal lesions need not be transections, but do need to cause a focal block of anterograde axonal transport, such as with a severe compressive injury or transection [31]. Late in the WD process, Schwann cells align themselves in columns along the intact basement membrane, known as the bands of Bunger, serving to guide sprouting axons during regeneration.
The WD process needs to complete before nerve regeneration can occur in severe injuries; however, in mild injuries, depending on proximity to the cell body, regeneration can begin nearly immediately. Regeneration has been found to be dependent on responses from the cell body, depending on a variety of factors including the age of the subject, severity of lesion, distance from the cell body, location of the injury and availability of pathway-derived growth factors [38]. The first signs of regrowth may be visible several weeks post-injury for more severe injuries, or as early as 24 h post-injury for milder injuries [35]. The proximal axon produces multiple sprouts containing growth cones, normally present during development, that initiate regeneration [25,35]. The reformed growth cones can encounter negative cues such as physical barriers, molecular barriers and other inhibitory factors. Physical barriers can include glial cells within the site of injury, whereas molecular barriers include chondroitin sulfate proteoglycans (CSPGs) [39]. The regenerating growth cones become misdirected as they attempt to avoid these barriers, and thus become unsuccessful in making connections to target tissues. The growth cone, similar to that in the developmental state, can also be positively guided towards targets through both soluble and bound tropic cues, including growth factors.
The growth cones have an affinity for laminin and fibronectin, ECM components of the basal lamina of the Schwann cell tubes, and use these for guidance. Once contacted, the regenerating axons preferentially grow within these tubes towards the end organ. Schwann cells along the bands of Bunger also increase production and release of factors such as nerve growth factor that act as stimuli for continued axon regrowth and additional guidance cues [35]. Growth factor signalling has traditionally been known to play roles in development, but the role of such signalling has recently been also extended to regeneration [40]. Far less is known about the role of pathway-derived growth factor signalling in the response of regenerating adult neurons, and the signalling pathways involved in each case may be different [41].
The nature of the site of damage can influence the success of regeneration by affecting growth cones, and can vary based on extent of injury. In a transection, there may be a gap and an extended lesion site, and thus more chances for scarring and space for wandering axons [25]. Outlook for functional recovery after transections that cause a large gap between proximal and distal ends can be negligible. When a nerve repair is performed, there can be scarring or poor matching post-suturing. By contrast, a nerve crush may yield a more favourable setting for regeneration and reinnervation, because the internal structure and surroundings are preserved [25]. Human peripheral axon regeneration rates have been reported to range around 1 mm per day in clinical situations, with further diminishing rates over time [1,25,35]. These rates may vary depending on the extent of injury, as well as the type, with higher rates of regeneration in crush injuries and lower rates in transection injuries; regeneration rates expectedly also vary across species. Axonal regeneration does not always mean functional recovery, as misdirection of regenerating axons is a common cause of poor functional recovery. In vitro studies of PNI and regeneration are valuable in order to elucidate the roles of growth factors in regeneration, as well as to observe how to enhance regeneration rates and functional reinnervation.
2.2. Central nervous system injury and regeneration
CNS axon injury can result from the mechanical forces associated with the rapid deformation of the brain or spinal cord during trauma. Blunt trauma may cause vascular rupture, decrease the integrity of the blood–brain barrier and directly crush nerves or, in the case of severe force, cause complete axotomy [42]. Oedema can also result from trauma, with cytotoxic swelling of neurons, which can, in turn, cause compression of further tissue. Despite the availability of in vivo models, mechanistic understanding of the underlying pathways governing CNS axonal injury remains only partially understood. As regeneration does not occur in the native environment of the CNS, performing injury studies within an in vitro platform may help elucidate the mechanisms underlying both axonal injury and regeneration. These types of injuries also share common features and potentially convergent pathways with other conditions that may result in CNS axonal degeneration, including Alzheimer's, HIV-related dementia and multiple sclerosis [31]. There is a need for complete understanding of the degeneration process, as severity of axon damage plays a large role in eventual outcomes following degeneration [43,44].
Focal injuries result from high impact, rapid events such as blows to the head, or projectile and penetrating blast injury. Focal injuries can cause haematomas or haemorrhaging that, in turn, cause further compression [31]. When an object impacts the head, there is an initial focal contact force, and this force may, in turn, accelerate or decelerate the brain, causing further inertial forces. Focal lesions can trigger WD of distal axons. While dynamic deformation rarely leads to primary axotomy, there does not need to be a complete transection of the axon, as a focal block of axonal transport may be enough to trigger degeneration [31,45]. Diffuse injuries result from inertial forces and rapid head rotations, as would occur during car accidents and falls [46,47]. Diffuse axonal injury (DAI) is multi-focal, with multiple spheroids appearing on individual axons, and is characterized by swollen and disconnected axons. The extent of injury is dependent on the mode, severity, duration and rate of the strain, as well as the distance of the injury from the cell body, anatomical location of the injury and the mechanical properties of the axon [31,48]. Axons are viscoelastic and can withstand varying degrees of stretch deformation under normal activities, but are thought to become brittle under dynamic loading conditions, making them vulnerable to damage [45]. The mechanical properties of the axon can also vary with age, previous injuries and disease. Mechanically stretching cultured axons has been demonstrated to replicate the morphological and structural changes associated with DAI [49].
Damage to the axonal cytoskeleton or a primary axotomy can result from a rapid unidirectional stretch. While it is clear that primary injury results from the direct mechanical strain experienced by cells from injury, the mechanism of the downstream cellular events is not well understood. Changes in molecular gradients occur, as there is sodium influx following injury, which increases swelling and also increases intracellular calcium levels, which may activate proteases for breaking down the cytoskeleton [31,50]. Axonal transport proteins may accumulate in the areas of swelling owing to disruptions in transport, including amyloid precursor protein. Cytoskeletal changes are more apparent upon direct damage to the axolemma, which would occur in severe injury, and it would be beneficial to study these in vitro. Recent evidence has also indicated axon-specific degeneration pathways separate from those related to the cell body, making it beneficial to study axon injury while being able to manipulate the cell body and axon independently [30,31,51,52]. The determination of axon-specific pathways may be important for understanding the cellular and molecular mechanisms for degeneration.
Immediately after injury, CNS axons can display abortive regeneration with an initial outgrowth for up to 0.5 mm, but then come to a stop and die-back and retract or become arrested and form retraction bulbs [25]. It has been demonstrated that CNS neurons can express their intrinsic growth capacity on permissive substrates within the right biological environment, but the growth appears to be too slow for full functional reinnervation [53]. David & Aguayo [54] initially demonstrated that peripheral nerve grafts can be used to promote CNS axon regeneration. It has also been demonstrated that peripheral nerve grafts are the most promising grafts for CNS nerve repair, promoting regeneration from non-permissive white matter to permissive grey matter in spinal cord repair [55]. The natural CNS environment is regarded as unfavourable with inhibitory effects from glial scars, CSPGs and myelin-associated proteins, inadequate inflammatory responses and hindered debris clearance owing to the presence of the blood–brain barrier. Glial scars formed at the site of an injury act as both mechanical and biochemical barriers for regrowing axons. As the name suggests, the scar is often comprised of glial cell types, including reactive astrocytes, microglia, oligodendrocyte precursors and fibroblasts. It also contains growth inhibitory factors such as semaphorins, nephrins, tenascin and CSPGs [48,56,57]. Myelin-associated inhibitors found in the glial scar, such as Nogo-66, myelin-associated glycoprotein and oligodendroctye-associated glycoprotein, have been shown to interact with the Nogo receptor (Ngr) to inhibit neurite outgrowth [58]. By contrast, ECM component molecules such as laminin have been found to promote regeneration [38]. Although the CNS contains microglia, they do not aid in debris clearance to the extent of Schwann cells, which attract macrophages to enhance clearance, as well as produce neurotrophic and neurotropic factors to aid in regeneration [42]. The availability of a model that allows for the study of CNS regeneration and determines the effects of these factors within an injury platform would be valuable.
3. Microfluidic devices for studying nerve injury and regeneration
Traumatic injuries, both in the CNS and PNS, can lead to irreversible damage, resulting in permanent loss of function. Studying the complex dynamics involved in these processes may elucidate the biological mechanisms of nerve regeneration and degeneration, potentially leading to the development of new strategies and therapies for nerve regeneration and recovery. In vivo animal models of trauma have permitted the study of a multitude of complex variables and permit the analysis of behavioural outcome, enabling monitoring of prognosis and determination of functional outcome to various treatment strategies. These in vivo animal experiments include models of instant rotational injury, impact acceleration injury, lateral fluid percussion injury, controlled cortical impact, spinal cord compression and contusion, nerve stretch and complete nerve transection, as previously reviewed [59,60]. These models have provided much insights, but they are highly complex, containing multiple parameters, have potentially low reproducibility, and are time consuming and labour-intensive. More importantly, they do not allow for monitoring axonal regeneration in real-time, or enable study at the reductionist single-cell level necessary for determining precise mechanisms. By contrast, as an alternative parallel experimental system, in vitro models allow the study of biochemical pathways, gene expression levels and phenotypic changes at the level of a single axon, which are extremely relevant in the study of traumatic axonal injuries.
Microfabrication technologies enable the development of powerful platforms to grow and manipulate neurons and in order to model and study axon injuries. These LOC devices can be made using modified semiconductor fabrication technologies, including photolithography, etching and deposition methods, in order to construct microscopic structures in glass, silicon or polymeric materials such as poly(dimethylsiloxane) (PDMS). These platforms can play a role in the development of novel and innovative surgical and repair strategies for damaged PNS axons. Conventional mass-produced metal surgical instruments are affordable, but are not durable and degrade quickly, whereas instruments made of diamond and ceramics are durable but are expensive. MEMS-based technology can enable development of unprecedented Si probes and blades with enhanced performance characteristics and cost of production. For cell culture, LOC devices provide perfusion to cells or enable probing of cells and cell compartments with chemical reagents. Microfluidic platforms further facilitate study at the single-cell resolution of axons isolated from soma through compartmentalization. Composite microfluidic platforms, with compartments for neurons and means to carry out highly parallel experiments constitute high-throughput LOC devices. Such devices enable precise control over cellular microenvironments, require small volumes of reagents, and have potential for automation and multiplexing [15,61]. Microfluidic systems have been broadly used for neuron cell culture, neuron manipulation, neural stem cell differentiation, neuropharmacology, neuroelectrophysiology and neuron biosensors [13,62].
The most well-known microfluidic devices for neuronal study are compartmentalized LOC systems fabricated through PDMS soft lithography. There are several properties that make PDMS an excellent choice for biological studies. As a material, PDMS is inexpensive, flexible and easily fabricated and bonded to other materials. It is also biologically inert, non-toxic to cells, impermeable to water, permeable to gases and optically transparent down to 230 nm, facilitating microscopy [63]. Typically, a master wafer is created using standard photolithography techniques and replicas created from the mould using PDMS soft lithography [12]. Fabrication of these types of devices has been covered elsewhere, and will not be the focus of this review [10,12,64]. LOC platforms facilitating compartmentalization and thus separation of axons from cell body allows precise tailoring and manipulation of the microenvironment of axons, distinct from the cell body, as is the case in many in vivo situations. Compartmentalization enables more in-depth studies of myelination, neurite outgrowth, drug screening and protection, signalling, as well as the study of networks ranging from cellular to organ levels in organotypic cultures [11,12,65].
Microfluidic platforms are highly compatible with incorporation of different injury modalities. Incorporation of an injury platform within a microfluidic culture system would allow for better determination of axon-specific mechanisms in degeneration and regeneration by allowing for independent manipulation of axon and cell body. In addition, locations of injuries respective to cell bodies can be generalized within a range for arrays of axons. Microfluidic platforms can be broadly classified as devices that model chemical and physical injury, and devices that model the regeneration environment. Here, we provide an overview of exemplary injury devices.
3.1. Microfluidic chemical injury devices
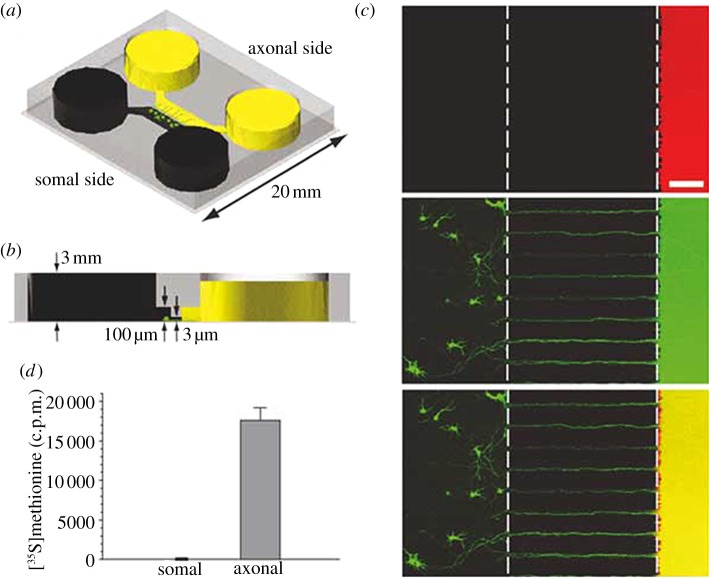
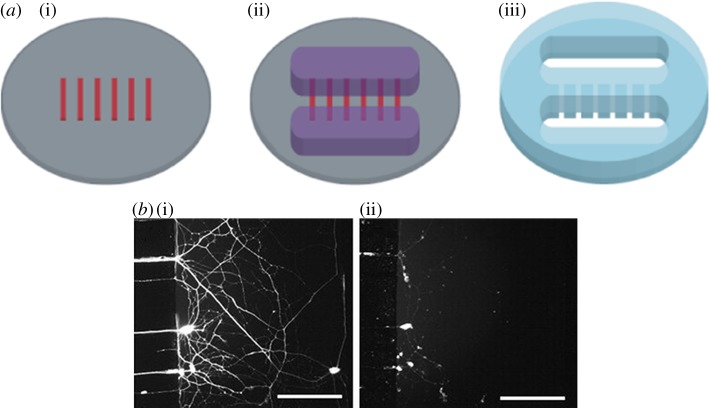
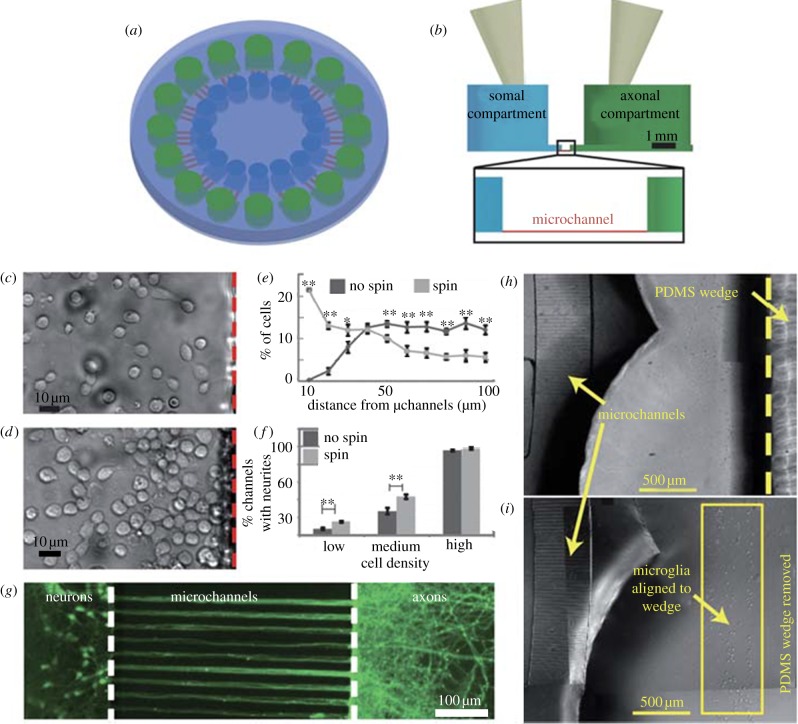
The simplest microfluidic injury devices provide chemical injury. Traumatic injuries to axons of the CNS and PNS can be induced by chemicals such as chemotherapeutics, neurotransmitters in excess (excitotoxicity) and detergents [66–68]. One of the first and most cited microfluidic LOC device designed to study neurons in their various compartment structures (soma, axon, dendrites) was the device created by Jeon's group [12]. Fluidic isolation of the axonal chamber from the somal chamber is achieved through microlitre-level volume differences between the two chambers. Microchannels between the chambers provide high fluidic resistance that leads to a small but sustained flow that counteracts diffusion. This device can be seen in figure 3. Potential neurotoxins can be localized precisely in the axonal compartment. Several studies have used this type of device and its derivatives for examining axon injury and regeneration [9,15,19,69]. These types of devices also include the ability to direct sites of neuronal attachment and neurite outgrowth through micropatterning techniques. Yang et al. [66] used a similar device for examining the localized degenerative effects of paclitaxel on peripheral axons, as can be seen in figure 4. Through utilization of the compartmentalized device, it was determined that paclitaxel caused significant axonal degeneration when applied directly to axons, whereas erythropoietin was successful in protecting axons from this type of chemotherapy-induced distal axon degeneration whether applied to the soma or the axon. Hosmane et al. [70] created a multiplexed circular version of the platform which used centrifugation to enhance axonal throughput through microchannels, and demonstrated increased microglial accumulation to aid in debris clearance near the site of injured CNS axons seen in figure 5. Peyrin et al. [67] developed a three-compartment microfluidic device to study simultaneous axonal degeneration and death mechanisms of CNS axons subjected to axotomy with precise spatio-temporal control. The injury was induced by a brief and isolated flux of detergent in the central compartment. In their proof of concept for the device, they observed rapid Wallerian-like degeneration in the distal axons subjected to axotomy, consistent with in vivo axotomy. Li et al. [68] developed an integrated microfulidic platform to chemically induce axonal injury and study the recovery and regeneration of axon either in co-culture with glial cells in a controllable chamber using valves or treatment with monosialoganglioside, a drug aiding neuronal regeneration. Their results indicated that axons were more resistant to injury upon localized application of acrylamide compared with the soma, and that axons had self-destruct programmes different from the soma, where injury to the soma caused secondary axon collapse.
Figure 3.
A microfluidic compartment device (a) with volume differences between the two chambers (b) to maintain isolation across microchannels. Isolation was demonstrated (c) by localization of Texas red and (d) radioactive S35 methionine (adapted with permission from Park et al. [9]).
Figure 4.
(a) In the schematic, from left to right, we see a representation of the first and second layer of the master wafer and resulting PDMS device. (b) In the images, we see representative axons (i) prior to treatment compared with (ii) after a 24 h treatment with taxol, with axonal degeneration apparent (adapted with permission from Yang et al. [66]).
Figure 5.
A circular microfluidic platform (a) top-down schematic (not to scale) and a (b) side view (to scale) denotes somal (green) and axonal (blue) compartments and microchannels (red). Comparing cell placement in (c) no spun and (d) spun devices shows spinning (e) enhanced cell proximity to microchannels. Spinning also (f) enhanced axonal throughput, with no observable enhancement in (g) high-density cultures with all channels occupied by neurites. (h) A PDMS wedge can be used in the axonal compartment to (i) localize microglia (adapted from Hosmane et al. [70] with permission from the Royal Society of Chemistry).
3.2. Microfluidic physical injury devices
Physical modes include using aspiration, physical cutting, laser ablation techniques, valve-based compression of axons [71–74]. Microfluidic platforms have been used as valuable tools to study axon regeneration in vivo. Many model organisms such as Aplysia californica, Caenorhabditis elegans, Drosophila and zebrafish have been used for in vivo neuron injury and regeneration studies [68,71–73,75–82]. Caenorhabditis elegans in particular provides an interesting paradigm for studying nerve injury and regeneration as its genome has been completely sequenced, and in vivo axotomy for the organism is feasible. The critical step of immobilizing the worm and subjecting it to axotomy has conventionally been done through the use of glue and anaesthetics. These methods can either have unknown toxic effects that are difficult to evaluate or are labour-intensive and of low-throughput. Microfluidic platforms can provide a clever alternative to these techniques. The immobilization can be achieved in several ways such as anaesthetizing, cooling or trapping the worm using deflectable valves [11,83]. Chokshi et al. [72] have developed microfluidic platforms to immobilize single worms on either a short-term or long-term basis to characterize their on-chip behaviour. The immobilization is achieved in one of the two approaches; either CO2 is used to change the microenvironment and cease the worm's movement in a long-term manner, or a deformable membrane is used to mechanically restrict the worm. A behaviour module revitalizes the worm after immobilization through mechanical stimulation, consisting of a saw-shaped microchannel that forces the worm to move in a sinusoidal pattern so that its locomotion can be analysed.
While studying nervous system injury in model organisms such as C. elegans, an enormous volume of screening studies often needs to be done involving massive image acquisition and processing, data acquisition and interpretation. MEMS platforms can be integrated with imaging to increase the performance and throughput of these studies. The potential for automation is high on these platforms because of their small size and scale. A robust and high-throughput performance can be achieved on these platforms [84]. Chung & Lu [71] designed and developed an automated, integrated microfluidic system to perform high-throughput microsurgery. This device is capable of processing multiple worms in parallel without increase in control complexity. The device can be used to simultaneously load worms in one set of channels and perform imaging and laser ablation in the other set. Guo et al. [73] developed a high-throughput microfluidic platform for in vivo nerve regeneration studies that enables precise focusing and nanosurgery of trapped worms and feeding for recovery of the operated worms in two different modules. The device also incorporates an adjustable trap for immobilization of worms at various developmental stages. Highly specific laser ablation techniques can be used to injure the worms once they are steadily immobilized. Using this chip, they observed faster than expected rates of axonal regeneration, and that distal fragments of the severed C. elegans axon regrow in the absence of anaesthetics. Based on the frequency or the repetition rate of laser, the gaps created in the axotomy vary. With high-frequency lasers, 2–5 µm gaps are created, whereas low-frequency lasers result in precise 1–2 µm gaps [75,85]. Other physical modes of injury to axons involve needles to transect individual axons, fluid percussion and microelectrodes [67].
The mechanical and cellular response to injury can be quite complex, but study of such stimuli can be simplified by using controlled cellular injury in vitro models. These models include several advantages over in vivo animal models, including the ability to monitor real-time acute injury responses [48]. Existing in vitro injury models have subjected neural cultures or explants to the forces experienced during traumatic CNS injury, and include stretchable deformable membranes, two-photon laser ablation and hydrodynamic shear-based axotomy through microfluidic channels [67,86–88]. Recent advances in culturing neurons within hydrogels have allowed for the development of three-dimensional cultures that allow for bulk deformation [89].
Mechanotransduction is the study of cellular adaptation to internal and external mechanical stress. Cells elicit a downstream biochemical signal in response to variations in forces acting on a cell. Several tools have been developed to study the combinatorial interplay between mechanical properties and force-induced biochemical changes in cells [90]. The mechanotransduction of CNS and PNS injuries provide powerful insights for development of treatment strategies, and accordingly, several in vitro platforms have been developed to study mechanotransduction in injury. Stretch-induced injury is one of the many modes of mechanotransduction and physical injury observed in vivo during traumatic insult to axons of the CNS and PNS. Several groups have modelled and studied these injuries in vitro. A stretch-induced injury model of rat cortical astrocytes was developed by culturing the cells on a deformable membrane that was subjected to deformation by a positive rapid pressure [91]. The astrocytes were grown in tissue culture wells on flexible silastic bottoms to which a pressure was applied that stretched the membrane, and in turn, the astrocytes in order to study the morphological, physiological and biochemical consequences of stretch-induced injury. The system enabled the study of the extent and degree of injury with precise control over membrane deformation by varying the amplitude and duration of pressure. Cell injury was demonstrated to be proportional to the degree of silastic membrane deformation, with increasing stretch causing mitochondrial swelling, disruption of glial filaments and vacuolization. This is one of the earliest in vitro attempts to study injuries in cells derived from the brain. With the advancement in MEMS, novel platforms could be developed to study the role of mechanotransduction in traumatic axonal injuries. The forced being applied on an axon determines its fate of degeneration, regeneration or stalling in place.
In a more recent study, Hosmane et al. [74] developed a valve-based axon injury platform that enabled compression of CNS axons at the micrometre-scale, seen in figure 6. The valves controlled push-down pads that descended with application of compressed gas. The pressure of the gas was modulated in order to create different levels of injury. Increased pressures, more likely to cause axotomy, were found to promote subsequent axonal regrowth. In another example, Smith et al. [92] applied continuous mechanical tension to axons, and achieved a sustained, rapid growth. The device physically splits integrated neuronal cultures into two halves and separated the halves progressively further apart using a microstepper motor system. In doing so, they achieved a growth rate of 1 mm per day. Transecting axons to induce axonal injury by laser ablation, as discussed previously in whole organisms, is another physical injury technique [69,85,88,93]. Kim et al. [88] developed a neuro-optial microfluidic platform that integrates a microfluidic chip, femtosecond laser for axotomy and mini-incubator to maintain a sterile and appropriate microenvironment for long-term monitoring of events post-injury. An example of the laser ablation achieved within these devices can be seen in figure 7. These injury devices also contained soluble- and surface-bound inhibitors within the injury compartment in order to better mimic the regeneration environment in vivo. Sretavan et al. developed a microdevice to assist the axon regeneration after injury. This device included the development of a silicon nitride knife with ultra-sharp knife edge with a 20 nm radius of curvature produced using MEMS technology. This knife was used for cutting regions of damaged axons individually. These damaged segments were replaced with healthy donor axon segments through dielectrophoresis, which was used to manipulate and line up the donor axon segments in the region of interest. The axon membranes of the segments were successfully electrofused.
Figure 6.
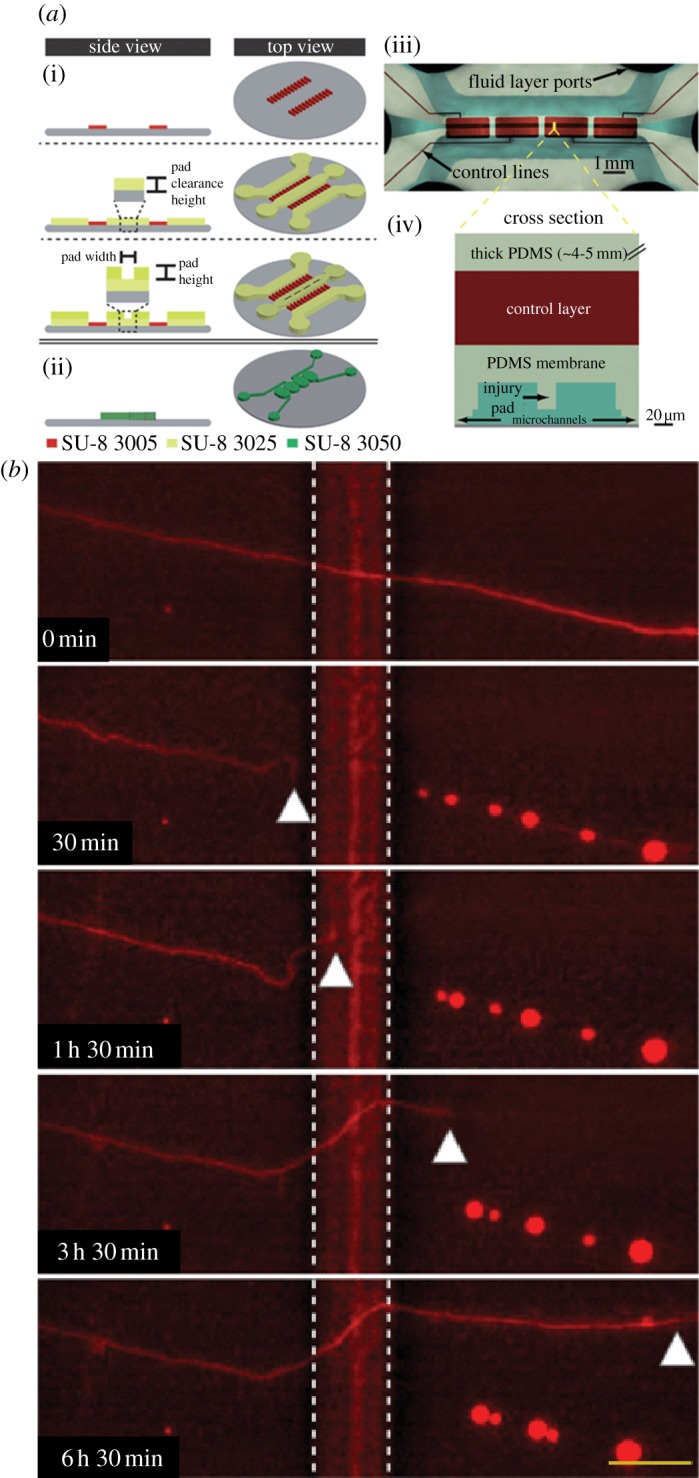
(a) Details the valve-based injury platform with the (i) cell layer process flow and (ii) control layer process flow, followed by (iii) top down and (iv) cross-section images to visualize control layer (red) versus cell layer (blue). (b) Below, axon compression, degeneration, and regrowth are monitored real-time within a representative device, with degeneration apparent at an image from 30 min, and the start of regrowth apparent in the 1 h and 30 min image (adapted from Hosmane et al. [74] with permission from the Royal Society of Chemistry).
Figure 7.
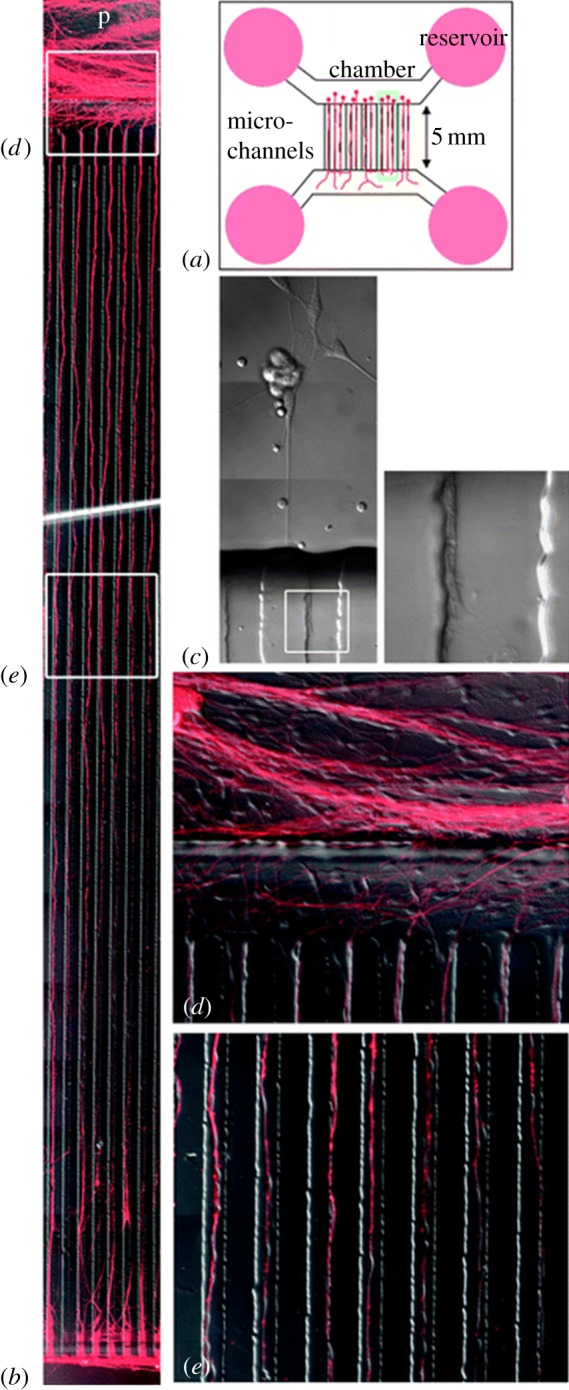
(a) Schematic of the device can be seen, along with (b) representative images of DRG growth through microchannels, and a higher magnification of the same. (c) A representative axon bundle cut with a fem- tosecond laser demonstrates high thermal confinement of the site of injury. Axons can be seen (d) entering and (e) traveling through microchannels in higher resolution images (adapted from Kim et al. [88] with permission from the Royal Society of Chemistry).
3.3. Microfluidic devices that recreate the regeneration environment
One of the major obstacles for regeneration is the distance or gap between the proximal extending axon and the distal stump. In bridging this gap, axons have to circumvent the non-permissive substrates for neurite growth, whereas certain growth factors and other transient molecules may aid positive guidance. Recent studies have shown that targeting a specific group of extracellular inhibitory factors in itself was insufficient to promote long-distance regeneration of CNS axons. Hur et al. [65] aimed to promote regeneration by directly targeting the growth cone through pharmacological inhibition or genetic silencing of non-muscle myosin II (NMII). As part of this study, the axonal compartment was coated with inhibitory chondroitin sulfate proteoglycans, and the effect of applying blebbistatin, a specific inhibitor of NMII ATPase activity, was examined. As can be seen in figure 8, application of blebbistatin allows axons to overcome inhibitory cues. The inhibition of NMII causes reorganization of microtubules and actin in the growth cone in a way that allows for rapid axon extension, both over permissive and inhibitory substrates.
Figure 8.
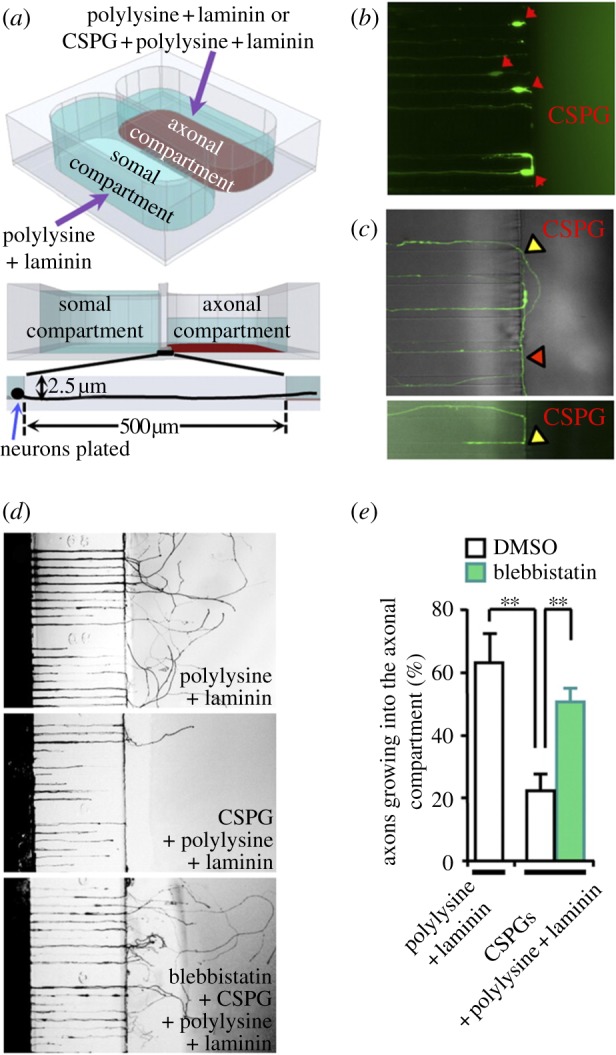
Inhibition of NM II allows axons to overcome inhibitory substrates in the axonal compartment of a microfluidic device. (a) Schematic of the two-compartment chamber is given. (b,c) Representative images of DRG neurons facing the inhibitory chamber demonstrate inability to breach the border. (d,e) Blebbistatin was locally applied to the axonal side, demonstrating an ability to overcome inhibitory cues (adapted with permission from Hur et al. [65]).
Over the time course post-injury, chemical gradients of several neurotrophic factors are established; these gradients may significantly influence the growth dynamics of axons post-injury and may even play a role in determining the fate of axons towards regeneration or degeneration. Establishing gradients in neuronal culture may deliver powerful insights about regrowth dynamics. MEMS platforms, owing to their operating dimensions and small scale, make it feasible to establish stable gradients over time. For example, gradients can be established on these platforms by exploiting the surface tension differences between two ports connected by a microchannel. This same principle is applied to develop and demonstrate a passive pump in microfluidic devices [94].
LOC devices that mimic the repulsive or attractive chemical cues present in the regeneration environment provide insights into how to best guide regrowing axons towards appropriate targets. In the event of injury, neurites need to perform the non-trivial tasks of reorganizing and re-establishing existing connections. Kothapalli et al. [95] developed a novel microfluidic device to study neurite guidance under the influence of chemogradients. The neurons were cultured in microchannels on a physiological three-dimensional environment of collagen type I, and gradients were established of chemoattractants such as netrin 1 and chemorepellents such as slit 2. The gradients developed were stable up to 48 h. This time frame allowed for the qualitative and quantitative study of neurite turning, providing valuable insights into the development, maintenance and reorganization of complex neural networks. The ability to monitor and guide regrowing axons can enhance our efforts in promoting functional recovery.
4. Conclusion
Nerve injury is a widely observed, but difficult to study phenomenon, particularly in vivo. The current standard treatment for PNI, such as end-to-end surgical reconnection, or reconnection with an autologous nerve graft, are also highly limited. While in the periphery nerve regeneration occurs, functional recovery may not. In the CNS, the outlook is starker, as no treatments are currently available. A more complete understanding of the neurobiology of nerve injury and regeneration in both of these systems may improve surgical- or biomaterial- or scaffold-based repair outcomes and functional recovery. Therefore, in vitro methods are of interest so as to very precisely and microscopically observe nerve injury and develop and test different repair strategies. Continuing advances in the field of microtechnology enable the creation of devices capable of studying regeneration at the reductionist cellular scale, allowing for the ability to tease out mechanisms that may be lost at the complex in vivo setting. Chemical injury can be easily achieved within a microfluidic platform, whereas physical injury is accomplished through the incorporation of other technologies such as lasers, nanoscale ultra-sharp knives and valve-based compression. The post-regeneration environment can also be modelled, through chemical modifications of the device surface or alteration of fluid flows. While the strength of microfluidic devices are in their reductionist, highly controllable environments, currently available microfluidic devices do not perfectly mimic the in vivo environment, particularly as most operate at the two-dimension level, and many effects may not translate from the cell level to in vivo. Large surface-to-volume ratios and particularly miniscule amounts of media may lead to cell viability issues owing to evaporation and potential difficulty in maintaining cell culture conditions, if not tightly managed. The devices have promoted the formation of interdisciplinary and collaborative research teams, but wide-scale adoption by neuroscientists, although expected, has not yet occurred. Despite these concerns, microfluidic devices continue to look promising for investigating axonal regeneration. A variety of LOC devices discussed in this review point to a vibrant field where novel platform technologies are facilitating cellular discoveries, and basic research is promoting the development of novel platform technologies. Next-generation devices are expected to better mimic the three-dimensional in vivo regeneration environment, as well as incorporate other advances in other fields such as optogenetics and biosensors, in order to extend fundamental findings from cellular studies and take a step closer to realizing clinical therapies for enhancing nerve regeneration in both the PNS and CNS.
Acknowledgements
The authors acknowledge Bhaskar Chennuri and David Wu for their help in preparation of this manuscript.
Funding statement
The authors acknowledge the Neuroengineering Training Initiative (NETI), NIH grant no. 5T32EB003383-09, for supporting R.S.
References
- 1.Schmidt CE, Leach JB. 2003. Neural tissue engineering: strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 5, 293–347. ( 10.1146/annurev.bioeng.5.011303.120731) [DOI] [PubMed] [Google Scholar]
- 2.Hadlock T, Elisseeff J, Langer R, Vacanti J, Cheney M. 1998. A tissue-engineered conduit for peripheral nerve repair. Arch. Otolaryngol. Head Neck Surg. 124, 1081–1086. ( 10.1001/archotol.124.10.1081) [DOI] [PubMed] [Google Scholar]
- 3.Thomas M, Brushart M. 2011. Nerve repair. New York, NY: Oxford University Press. [Google Scholar]
- 4.Bellamkonda RV. 2006. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials 27, 3515–3518. ( 10.1016/j.biomaterials.2006.02.030) [DOI] [PubMed] [Google Scholar]
- 5.Sretavan DW, Chang W, Hawkes E, Keller C, Kliot M. 2005. Microscale surgery on single axons. Neurosurgery 57, 635 ( 10.1227/01.NEU.0000175545.57795.ac) [DOI] [PubMed] [Google Scholar]
- 6.Abu-Rub M, McMahon S, Zeugolis DI, Windebank A, Pandit A. 2010. Spinal cord injury in vitro: modelling axon growth inhibition. Drug Disc. Today 15, 436–443. ( 10.1016/j.drudis.2010.03.008) [DOI] [PubMed] [Google Scholar]
- 7.Song H, Ming G, Poo M. 1997. cAMP-induced switching in turning direction of nerve growth cones. Nature 388, 275–279. ( 10.1038/40864) [DOI] [PubMed] [Google Scholar]
- 8.Pearce TM, Williams JC. 2006. Microtechnology: meet neurobiology. Lab Chip 7, 30–40. ( 10.1039/b612856b) [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. 2006. Microfluidic culture platform for neuroscience research. Nat. Protoc. 1, 2128–2136. ( 10.1038/nprot.2006.316) [DOI] [PubMed] [Google Scholar]
- 10.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. 1998. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 70, 4974–4984. ( 10.1021/ac980656z) [DOI] [PubMed] [Google Scholar]
- 11.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. 2000. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116. ( 10.1126/science.288.5463.113) [DOI] [PubMed] [Google Scholar]
- 12.Taylor A, Blurton-Jones M, Rhee S, Cribbs D, Cotman C, Jeon N. 2005. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605. ( 10.1038/nmeth777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Ren L, Li L, Liu W, Zhou J, Yu W, Tong D, Chen S. 2009. Microfluidics: a new cosset for neurobiology. Lab Chip 9, 644–652. ( 10.1039/b813495b) [DOI] [PubMed] [Google Scholar]
- 14.Gross PG, Kartalov EP, Scherer A, Weiner LP. 2007. Applications of microfluidics for neuronal studies. J. Neurol. Sci. 252, 135–143. ( 10.1016/j.jns.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 15.Gross PG, Kartalov EP. 2010. Microfluidics for nanoneuroscience. Microfluidic devices in nanotechnology, pp. 1–46. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 16.Taylor AM, Jeon NL. 2010. Micro-scale and microfluidic devices for neurobiology. Curr. Opin. Neurobiol. 20, 640–647. ( 10.1016/j.conb.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 17.Brunello C, et al. 2013. Microtechnologies to fuel neurobiological research with nanometer precision. J. Nanobiotechnol. 11, 11 ( 10.1186/1477-3155-11-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soe AK, Nahavandi S, Khoshmanesh K. 2012. Neuroscience goes on a chip. Biosens. Bioelectron. 35, 1–13. ( 10.1016/j.bios.2012.02.012) [DOI] [PubMed] [Google Scholar]
- 19.Park JW, Kim HJ, Kang MW, Jeon NL. 2013. Advances in microfluidics-based experimental methods for neuroscience research. Lab Chip 13, 509–521. ( 10.1039/c2lc41081h) [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Kotake N, Mabuchi K, Takeuchi S. 2009. Regeneration-type nerve electrode using bundled microfluidic channels. Electron. Commun. Jpn. 92, 29–34. ( 10.1002/ecj.10059)21572940 [DOI] [Google Scholar]
- 21.Bennet D, Kim S. 2011. Implantable microdevice for peripheral nerve regeneration: materials and fabrications. J. Mater. Sci. 46, 4723–4740. ( 10.1007/s10853-011-5510-z) [DOI] [Google Scholar]
- 22.Stieglitz T, et al. 2012. Miniaturized neural interfaces and implants. In Proc. SPIE 8251, Microfluidics, BioMEMS, and Medical Microsystems X 82510A-A ( 10.1117/12.912526) [DOI] [Google Scholar]
- 23.Yue Z, Moulton SE, Cook M, O'Leary S, Wallace GG. 2013. Controlled delivery for neuro-bionic devices. Adv. Drug Deliv. Rev. 65, 559–569. ( 10.1016/j.addr.2012.06.002) [DOI] [PubMed] [Google Scholar]
- 24.Zhong Y, Bellamkonda RV. 2008. Biomaterials for the central nervous system. J. R. Soc. Interface 5, 957–975. ( 10.1098/rsif.2008.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reier P, Lane M. 2008. Degeneration, regeneration, and plasticity in the nervous system. Neurosci. Med. 2008, 691–727. ( 10.1007/978-1-60327-455-5_46) [DOI] [Google Scholar]
- 26.Ducker TB, Kempe LG, Hayes GJ. 1969. The metabolic background for peripheral nerve surgery. J. Neurosurg. 30, 270–280. ( 10.3171/jns.1969.30.3part1.0270) [DOI] [PubMed] [Google Scholar]
- 27.Poliak S, Peles E. 2003. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4, 968–980. ( 10.1038/nrn1253) [DOI] [PubMed] [Google Scholar]
- 28.Höke A. 2006. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2, 448–454. ( 10.1038/ncpneuro0262) [DOI] [PubMed] [Google Scholar]
- 29.Müller HW, Stoll G. 1998. Nerve injury and regeneration: basic insights and therapeutic interventions. Curr. Opin. Neurobiol. 11, 557–562. ( 10.1097/00019052-199810000-00019) [DOI] [PubMed] [Google Scholar]
- 30.Hilliard MA. 2009. Axonal degeneration and regeneration: a mechanistic tug-of-war. J. Neurochem. 108, 23–32. ( 10.1111/j.1471-4159.2008.05754.x) [DOI] [PubMed] [Google Scholar]
- 31.Coleman M. 2005. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898. ( 10.1038/nrn1788) [DOI] [PubMed] [Google Scholar]
- 32.Stoll G, Müller HW. 1999. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 9, 313–325. ( 10.1111/j.1750-3639.1999.tb00229.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe N, Cavalli V. 2008. Nerve injury signaling. Curr. Opin. Neurobiol. 18, 276–283. ( 10.1016/j.conb.2008.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Yu W, Strickland S. 2007. Peripheral regeneration. Annu. Rev. Neurosci. 30, 209–233. ( 10.1146/annurev.neuro.30.051606.094337) [DOI] [PubMed] [Google Scholar]
- 35.Burnett MG, Zager EL. 2004. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg. Focus 16, 1–7. ( 10.3171/foc.2004.16.5.2) [DOI] [PubMed] [Google Scholar]
- 36.Maggi SP, Lowe J, 3rd, Mackinnon SE. 2003. Pathophysiology of nerve injury. Clin. Plast. Surg. 30, 109–126. ( 10.1016/S0094-1298(02)00101-3) [DOI] [PubMed] [Google Scholar]
- 37.Bähr M, Bonhoeffer F. 1994. Perspectives on axonal regeneration in the mammalian CNS. Trends Neurosci. 17, 473–479. ( 10.1016/0166-2236(94)90136-8) [DOI] [PubMed] [Google Scholar]
- 38.Tucker BA, Rahimtula M, Mearow KM. 2006. Laminin and growth factor receptor activation stimulates differential growth responses in subpopulations of adult DRG neurons. Eur. J. Neurosci. 24, 676–690. ( 10.1111/j.1460-9568.2006.04963.x) [DOI] [PubMed] [Google Scholar]
- 39.Webber CA, Xu Y, Vanneste KJ, Martinez JA, Verge VM, Zochodne DW. 2008. Guiding adult mammalian sensory axons during regeneration. J. Neuropathol. Exp. Neurol. 67, 212–222. ( 10.1097/NEN.0b013e3181654972) [DOI] [PubMed] [Google Scholar]
- 40.Cui Q. 2006. Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol. Neurobiol. 33, 155–179. ( 10.1385/MN:33:2:155) [DOI] [PubMed] [Google Scholar]
- 41.Liu RY, Snider WD. 2001. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J. Neurosci. 21, RC164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N, Wen X. 2009. Neural tissue engineering and regenerative medicine. Fundam. Tissue Eng. Regen. Med. 2009, 271–288. ( 10.1007/978-3-540-77755-7_21) [DOI] [Google Scholar]
- 43.De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL. 1998. Axonal damage correlates with disability in patients with relapsing–remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121, 1469–1477. ( 10.1093/brain/121.8.1469) [DOI] [PubMed] [Google Scholar]
- 44.Mattson MP. 2004. Pathways towards and away from Alzheimer's disease. Nature 430, 631–639. ( 10.1038/nature02621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang-Schomer MD, Patel AR, Baas PW, Smith DH. 2010. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 24, 1401–1410. ( 10.1096/fj.09-142844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gennarelli TA. 1993. Mechanisms of brain injury. J. Emergency Med. 11, 5. [PubMed] [Google Scholar]
- 47.Smith DH, Meaney DF. 2000. Axonal damage in traumatic brain injury. Neuroscientist 6, 483–495. ( 10.1177/107385840000600611) [DOI] [Google Scholar]
- 48.LaPlaca M, Simon C, Prado G, Cullen D. 2007. CNS injury biomechanics and experimental models. Prog. Brain Res. 161, 13–26. ( 10.1016/S0079-6123(06)61002-9) [DOI] [PubMed] [Google Scholar]
- 49.Smith DH, Wolf JA, Lusardi TA, Lee VMY, Meaney DF. 1999. High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J. Neurosci. 19, 4263–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pike BR, Zhao X, Newcomb JK, Posmantur RM, Wang KKW, Hayes RL. 1998. Regional calpain and caspase-3 proteolysis of spectrin after traumatic brain injury. Neuroreport 9, 2437–2442. ( 10.1097/00001756-199808030-00002) [DOI] [PubMed] [Google Scholar]
- 51.Coleman MP, Perry VH. 2002. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 25, 532–537. ( 10.1016/S0166-2236(02)02255-5) [DOI] [PubMed] [Google Scholar]
- 52.Fischer LR, Glass JD. 2007. Axonal degeneration in motor neuron disease. Neurodegen. Dis. 4, 431–442. ( 10.1159/000107704) [DOI] [PubMed] [Google Scholar]
- 53.Bray GM, Villegas-Pérez MP, Vidal-Sanz M, Carter DA, Aguayo AJ. 1991. Neuronal and nonneuronal influences on retinal ganglion cell survival, axonal regrowth, and connectivity after axotomy. Ann. NY Acad. Sci. 633, 214–228. ( 10.1111/j.1749-6632.1991.tb15613.x) [DOI] [PubMed] [Google Scholar]
- 54.David S, Aguayo AJ. 1981. Axonal elongation into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science 214, 931–933. ( 10.1126/science.6171034) [DOI] [PubMed] [Google Scholar]
- 55.Cheng H, Cao Y, Olson L. 1996. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273, 510–513. ( 10.1126/science.273.5274.510) [DOI] [PubMed] [Google Scholar]
- 56.Fitch MT, Silver J. 2008. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301. ( 10.1016/j.expneurol.2007.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fawcett JW, Asher RA. 1999. The glial scar and central nervous system repair. Brain Res. Bull. 49, 377–391. ( 10.1016/S0361-9230(99)00072-6) [DOI] [PubMed] [Google Scholar]
- 58.Filbin MT. 2003. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713. ( 10.1038/nrn1195) [DOI] [PubMed] [Google Scholar]
- 59.Wang HC, Ma YB. 2010. Experimental models of traumatic axonal injury. J. Clin. Neurosci. 17, 157–162. ( 10.1016/j.jocn.2009.07.099) [DOI] [PubMed] [Google Scholar]
- 60.Fouad K, Dietz V, Schwab ME. 2001. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res. Rev. 36, 204–212. ( 10.1016/S0165-0173(01)00096-0) [DOI] [PubMed] [Google Scholar]
- 61.Chao T-C, Ros A. 2008. Microfluidic single-cell analysis of intracellular compounds. J. R. Soc. Interface 5(Suppl. 2), S139–SS50. ( 10.1098/rsif.2008.0233.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majumdar D, Gao Y, Li D, Webb DJ. 2011. Co-culture of neurons and glia in a novel microfluidic platform. J. Neurosci. Methods 196, 38–44. ( 10.1016/j.jneumeth.2010.12.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sia SK, Whitesides GM. 2003. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 24, 3563–3576. ( 10.1002/elps.200305584) [DOI] [PubMed] [Google Scholar]
- 64.Xia Y, Whitesides GM. 1998. Soft lithography. Annu. Rev. Mater. Sci. 28, 153–184. ( 10.1146/annurev.matsci.28.1.153) [DOI] [Google Scholar]
- 65.Hur E-M, et al. 2011. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc. Natl Acad. Sci. USA 108, 5057–5062. ( 10.1073/pnas.1011258108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang IH, Siddique R, Hosmane S, Thakor N, Höke A. 2009. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Exp. Neurol. 218, 124–128. ( 10.1016/j.expneurol.2009.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kilinc D, Peyrin JM, Soubeyre V, Magnifico S, Saias L, Viovy JL, Brugg B. 2011. Wallerian-like degeneration of central neurons after synchronized and geometrically registered mass axotomy in a three-compartmental microfluidic chip. Neurotox. Res. 19, 149–161. ( 10.1007/s12640-010-9152-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L, et al. 2012. Spatiotemporally controlled and multi-factor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal. Chem. 84, 6444–6553. ( 10.1021/ac3013708) [DOI] [PubMed] [Google Scholar]
- 69.Kim HJ, Park JW, Park JW, Byun JH, Vahidi B, Rhee SW, Jeon NL. 2012. Integrated microfluidics platforms for investigating injury and regeneration of CNS axons. Ann. Biomed. Eng. 40, 1268–1276. ( 10.1007/s10439-012-0515-6) [DOI] [PubMed] [Google Scholar]
- 70.Hosmane S, Yang IH, Ruffin A, Thakor N, Venkatesan A. 2010. Circular compartmentalized microfluidic platform: study of axon–glia interactions. Lab Chip 10, 741–747. ( 10.1039/b918640a) [DOI] [PubMed] [Google Scholar]
- 71.Chung K, Lu H. 2009. Automated high-throughput cell microsurgery on-chip. Lab Chip 9, 2764–2766. ( 10.1039/b910703g) [DOI] [PubMed] [Google Scholar]
- 72.Chokshi TV, Ben-Yakar A, Chronis N. 2009. CO2 and compressive immobilization of C. elegans on-chip. Lab Chip 9, 151–157. ( 10.1039/b807345g) [DOI] [PubMed] [Google Scholar]
- 73.Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, Chronis N, Ben-Yakar A. 2008. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat. Methods 5, 531–533. ( 10.1038/nmeth.1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosmane S, Fournier A, Wright R, Rajbhandari L, Siddique R, Yang IH, Ramesh KT, Venkatesan A, Thakor N. 2011. Valve-based microfluidic compression platform: single axon injury and regrowth. Lab Chip 11, 3888–3895. ( 10.1039/c1lc20549h) [DOI] [PubMed] [Google Scholar]
- 75.Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. 2007. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc. Natl Acad. Sci. USA 104, 15 132–15 137. ( 10.1073/pnas.0707001104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chokshi TV, Bazopoulou D, Chronis N. 2010. An automated microfluidic platform for calcium imaging of chemosensory neurons in Caenorhabditis elegans. Lab Chip 10, 2758–2763. ( 10.1039/c004658b) [DOI] [PubMed] [Google Scholar]
- 77.Gabel CV, Antoine F, Chuang C-F, Samuel AD, Chang C. 2008. Distinct cellular and molecular mechanisms mediate initial axon development and adult-stage axon regeneration in C. elegans . Development 135, 1129–1136. ( 10.1242/dev.013995) [DOI] [PubMed] [Google Scholar]
- 78.Zou Y, Chiu H, Zinovyeva A, Ambros V, Chuang C-F, Chang C. 2013. Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science 340, 372–376. ( 10.1126/science.1231321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiu H, Alqadah A, Chuang C-F, Chang C. 2011. C. elegans as a genetic model to identify novel cellular and molecular mechanisms underlying nervous system regeneration. Cell Adhes. Migration 5, 387–394. ( 10.4161/cam.5.5.17985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN. 2012. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 26, 1612–1625. ( 10.1101/gad.193243.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin SM, O'Brien GS, Portera-Cailliau C, Sagasti A. 2010. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development 137, 3985–3994. ( 10.1242/dev.053611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. 2009. Axon regeneration requires a conserved MAP kinase pathway. Science 323, 802–806. ( 10.1126/science.1165527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeng F, Rohde CB, Yanik MF. 2008. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab Chip 8, 653–656. ( 10.1039/B804808H) [DOI] [PubMed] [Google Scholar]
- 84.Chung K, Crane MM, Lu H. 2008. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637–643. ( 10.1038/nmeth.1227) [DOI] [PubMed] [Google Scholar]
- 85.Vogel A, Venugopalan V. 2003. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 103, 577–644. ( 10.1021/cr010379n) [DOI] [PubMed] [Google Scholar]
- 86.Morrison B, III, Saatman KE, Meaney DF, McIntosh TK. 1998. In vitro central nervous system models of mechanically induced trauma: a review. J. Neurotraum. 15, 911–928. ( 10.1089/neu.1998.15.911) [DOI] [PubMed] [Google Scholar]
- 87.Chung R, Staal J, McCormack G, Dickson T, Cozens M, Chuckowree J, Quilty MC, Vickers JC. 2005. Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy. J. Neurotraum. 22, 1081–1091. ( 10.1089/neu.2005.22.1081) [DOI] [PubMed] [Google Scholar]
- 88.Kim Y, Karthikeyan K, Chirvi S, Davé DP. 2009. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab Chip 9, 2576–2581. ( 10.1039/b903720a) [DOI] [PubMed] [Google Scholar]
- 89.Cullen DK, LaPlaca MC. 2006. Neuronal response to high rate shear deformation depends on heterogeneity of the local strain field. J. Neurotraum. 23, 1304–1319. ( 10.1089/neu.2006.23.1304) [DOI] [PubMed] [Google Scholar]
- 90.Kurth F, Eyer K, Franco-Obregón A, Dittrich PS. 2012. A new mechanobiological era: microfluidic pathways to apply and sense forces at the cellular level. Curr. Opin. Chem. Biol. 16, 400–408. ( 10.1016/j.cbpa.2012.03.014) [DOI] [PubMed] [Google Scholar]
- 91.Ellis E, McKinney J, Willoughby K, Liang S, Povlishock J. 1995. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J. Neurotraum. 12, 325–339. ( 10.1089/neu.1995.12.325) [DOI] [PubMed] [Google Scholar]
- 92.Smith DH, Wolf JA, Meaney DF. 2001. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 7, 131–139. ( 10.1089/107632701300062714) [DOI] [PubMed] [Google Scholar]
- 93.Wu T, Mohanty S, Gomez-Godinez V, Shi LZ, Liaw L-H, Miotke J, Meyer RL, Berns MW. 2011. Neuronal growth cones respond to laser-induced axonal damage. J. R. Soc. Interface 9, 535–547. ( 10.1098/rsif.2011.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker GM, Beebe DJ. 2002. A passive pumping method for microfluidic devices. Lab Chip 2, 131–134. ( 10.1039/B204381E) [DOI] [PubMed] [Google Scholar]
- 95.Kothapalli CR, van Veen E, de Valence S, Chung S, Zervantonakis IK, Gertler FB, Kamm RD. 2011. A high-throughput microfluidic assay to study neurite response to growth factor gradients. Lab Chip 11, 497–507. ( 10.1039/c0lc00240b) [DOI] [PubMed] [Google Scholar]