Abstract
Study Objectives:
Sleep and sleep disordered breathing (obstructive sleep apnea [OSA]) are known to affect the growth hormone/insulin-like growth factor (GH/IGF) axis. There are few relevant population studies in this area, particularly in the elderly. We conducted this study to investigate the relationship between sleep (architecture and OSA) and circulating IGF-I (insulin-like growth factor-1), IGFBP-1 (insulin-like growth factor binding protein-1), and IGFBP-3 (insulin-like growth factor binding protein-3) levels in an elderly population.
Design Setting:
Cross-sectional analysis of participants from the year 9 visit of the Cardiovascular Health Study (CHS) who were enrolled in the Sleep Heart Health Study (SHHS).
Patients or Participants:
1,233 elderly participants from the CHS and SHHS.
Measurements and Results:
The mean age of males (n = 526) and females (n = 697) was 77 years. The mean value of IGF-I (ng/mL) in males was 112.4 vs. 97.1 in females (p < 0.01). Mean IGFBP-1 and IGFBP-3 levels were higher in females than males (p < 0.01). As expected, slow wave sleep was better preserved in females compared to males (22% total sleep time vs. 9% total sleep time, p < 0.01). Furthermore, as expected, OSA (apneahypopnea index [AHI] ≥ 5/h) was more prevalent in males compared to females (60% vs. 46%, p < 0.01). Multivariable linear regression was used to determine the relationship between objective sleep parameters and circulating IGF-I, IGFBP-1, and IGFBP-3 levels, with adjustment for age, sex, race, BMI, diabetes, estrogen use, progestin use, and physical activity. We did not detect a significant association between slow wave sleep (SWS) (per 5 min) and IGF-I, IGFBP-1, and IGFBP-3 levels (ng/mL). We found no significant linear association between OSA (AHI ≥ 5/h) and IGF-I, IGFBP-1, and IGFBP-3 levels. Gender-stratification of the entire cohort did not alter these findings. Sensitivity analyses excluding diabetics revealed that moderate OSA (AHI ≥ 5 and < 15) is inversely associated with IGFBP-3 levels in women.
Conclusions The relationship between SWS and GH/IGF system is not significant in the elderly. Furthermore, OSA does not appear to adversely influence the GH/IGF axis, as reported in younger individuals. Whether our study findings are due to diminished GH/IGF-I axis activity in elderly needs further investigation by replication in other large population based elderly cohorts.
Citation:
Shah N; Rice T; Tracy D; Rohan T; Bůžková P; Newman A; Kaplan RC. Sleep and insulin-like growth factors in the cardiovascular health study. J Clin Sleep Med 2013;9(12):1245-1251.
Keywords: Insulin-like growth factors, IGF, IGFBP-3, slow wave sleep, sleep apnea, sleep, elderly, GH/IGF axis
The growth hormone/insulin-like growth factor (GH/ IGF) system comprises a complex endocrine system that is involved in embryonic development, growth, and normal adult physiology. A key mediator of this system, insulin-like growth factor-1 (IGF-I), is important in modulating cell proliferation, differentiation, and survival/apoptosis, and also has insulin-like metabolic effects. GH is the main signal controlling liver production of IGF-I, with reciprocal inhibitory effects of IGF-I on GH secretion. Physiologic regulation of circulating IGF-I is controlled mainly by growth hormone (GH). GH has a pulsatile secretion that varies markedly in a diurnal fashion, with most GH produced during slow wave sleep (SWS) or stage N3 sleep (formerly stages 3 and 4). While GH is known to be pulsatile and the pulsatile secretion seems to be important in some GH effects, it appears that IGF-I levels are more strongly associated with basal GH levels than with pulsatile GH.1
BRIEF SUMMARY
Current Knowledge/Study Rationale: While it is known that sleep and sleep disordered breathing may affect the growth hormone/insulin-like growth factor axis, there are few relevant population studies in this area specifically involving elderly individuals. We conducted this study to investigate the relationship between sleep (architecture and obstructive sleep apnea) and circulating insulin-like growth factor-1, insulin-like growth factor binding protein-1, and insulin-like growth factor binding protein-3 levels in an elderly population.
Study Impact: Due to the substantial dependence of the growth hormone/ insulin-like growth factor axis on age, it is important to understand the impact of sleep architecture and sleep disordered breathing on circulating insulin-like growth factor-1 and insulin-like growth factor-binding protein levels among elderly patients. This study helps understand the independent impact of sleep architecture and sleep disordered breathing on the growth hormone/insulin-like growth factor axis in an elderly population.
Prior studies in the Cardiovascular Health Study (CHS) and other cohorts have implicated circulating levels of IGF-I and insulin-like growth factor binding proteins (e.g., insulin-like growth factor binding protein-1 [IGFBP-1], insulin-like growth factor binding protein-3 [IGFBP-3], which affect bioactivity of IGF-I), in risk of cancer, cardiovascular diseases, functional status, and mortality.2–4
While it is known that sleep and sleep disordered breathing may affect the GH/IGF factor axis,5–7 there are few relevant population studies in this area. Prior studies have shown there to be an inverse relationship between the severity of sleep disordered breathing (as measured by the apnea-hypopnea index [AHI]) and IGF-I, and that this relationship appears to be independent of BMI and age.5–7 Due to the substantial dependence of the GH/ IGF axis on age, it is important to understand the impact of sleep architecture and sleep disordered breathing on circulating IGF-I and IGFBP levels among elderly patients for several reasons. First, understanding this relationship may help in determining the cause of altered levels of IGF-I in some older adults.8 Second, IGF binding proteins have also been linked with circadian regulatory mechanisms9,10 and these IGF-BPs are of proven relevance to risk of diabetes and death in older adults.11–13,14 Third, sleep quality, duration, and sleep disordered breathing have been implicated as a risk factor for diabetes and metabolic disorder, and effects of sleep on IGF-I and related mediators may possibly explain this association.15–21 Fourth, emerging evidence suggests a positive influence of diminished GH/IGF-I axis on longevity (via improved cancer and diabetes related outcomes).22–24 Whether this is partially mediated by the influence of sleep architecture and sleep disordered breathing on the GH/IGF-I axis is unknown.
We therefore investigated the relationship between sleep architecture and IGF-I, IGFBP-1, and IGFBP-3 levels in an elderly population of CHS participants who were also involved in the Sleep Heart Health Study (SHHS). We examined the relationship between obstructive sleep apnea (OSA) and its associated characteristics, namely hypoxemia and arousal, and circulating IGF-I, IGFBP-1, and IGFBP-3 levels among elderly participants. Due to the major impact of gender on SWS,25 sleep disordered breathing prevalence,26 and on the GH/IGF axis,27–29 we present our primary analyses in a gender stratified manner.
METHODS
Study Population
The CHS is a longitudinal cohort study that enrolled 5,888 adults, 65 years and older, from 4 US communities. It consists of an original cohort of 5,201 individuals recruited during 1989-1990 and an additional cohort of African American individuals recruited during 1992-1993. Participants were invited to repeated examinations for collection of data and blood specimens. The examinations were conducted annually through 1999, and again in 2005-2006 on surviving participants. As part of the year 9 CHS visit cycle (1998), a total of 1,350 CHS participants were enrolled into the SHHS. Briefly, the SHHS is a multicenter prospective cohort study evaluating the natural cardiovascular consequences of sleep disordered breathing.30,31 The study includes 6,441 participants ≥ 40 years of age from multiple population-based cohorts. All SHHS participants underwent a baseline examination that included portable sleep monitoring. Our study sample for the present analyses consisted of 1,233 older adults who had objective sleep measurement as part of the SHHS evaluation, as well as circulating IGF-I and IGFBP-3 levels measured as part of an ancillary study to CHS.
All participants provided informed consent, and institutional review board approvals were obtained at all participating institutions.
Sleep Measurements
The SHHS as described above collected objective sleep data on 6,441 participants (1995-1998) who underwent a full-montage unattended polysomnogram, which provided measurements pertaining to sleep architecture and sleep disordered breathing. Additionally, they completed questionnaires about sleep habits. Apnea was defined as an absence or near absence of airflow ≥ 10 seconds. Hypopnea was defined as 70% decrease in airflow plus ≥ 4% desaturation lasting > 10 seconds. The AHI was calculated as the number of apnea and hypopnea events divided by total sleep time in hours. Hypoxemia variables included average oxygen saturation, lowest oxygen saturation, and T90 (time spent with oxygen saturation less than 90%). Sleep fragmentation was assessed using the arousal index variable. In the SHHS, the arousal index was defined as the total number of arousals per hour of sleep. We excluded arousal index data from sleep studies coded as “sleep wake only” in order to account for technical issues related to EEG scoring of arousals. All sleep studies were scored by the SHHS reading center according to Rechtschaffen and Kales criteria.32
IGF and Other Measurements
Physical and cognitive function tests, questionnaires, laboratory panels, and other key covariates were collected during the CHS study examinations. Laboratory measurements were performed using blood collected using standard procedures after an overnight fast and stored at -70°C. Measurements of IGFBP-1, IGFBP-3, and IGF-I were performed at the Jewish General Hospital (Montreal, Canada) after an extraction step using ELISA methods (Diagnostics Systems Laboratory, Webster, TX). Assay coefficient of variation was 4% to 6% for IGF-I concentration, and 3% to 5% for IGFBP-3 concentration.33 Other laboratory measurements such as fasting glucose were measured at the CHS Central Laboratory using standard methods.
Clinical Variables
Participants were considered to have diabetes at baseline if they reported use of insulin or an oral hypoglycemic agent or if they had fasting serum glucose ≥ 7.0 mmol/L (126 mg/dL). Participants were considered to have impaired fasting glucose if their fasting serum glucose level was 110 to 125 mg/dL and they were not on insulin or oral hypoglycemic agents. BMI was calculated as the measured weight in kilograms divided by the square of measured height in meters. Physical activity was measured in the CHS using a validated questionnaire-the Modified Minnesota Leisure-Time Activities Questionnaire.34,35 The questionnaire was administered by trained interviewers, and inquired about the frequency and duration of participation in 15 leisure-time activities in the 2 weeks preceding a physical examination.
Analysis
Descriptive statistics including demographic, clinical, and sleep characteristics are reported as mean or median (± standard deviation or range, as appropriate) and are stratified by gender. Skewed variables were log transformed. IGF concentrations (IGF-I, IGFBP-1, and IGFBP-3) are similarly described. Gender-based comparisons of the above noted variables were made using χ2, t-test, or Wilcoxon rank sum analyses, as appropriate. We used linear regression to model sleep variables as predictive of IGF-I, IGFBP-1, and IGFBP-3 levels. Models were adjusted for known determinants of circulating IGF-I, IGFBP-1, and IGFBP-3 concentrations, including sex, race, BMI, diabetes mellitus, physical activity, and use of sex hormones. Because of known gender dimorphism in regulation of the IGF system, we also stratified these analyses by sex. To better assess the relationship between OSA and IGF-I, IGFBP-1, and IGFBP-3, the regression analysis was examined in a restricted sample of non-diabetics and obese participants (obesity defined as a BMI ≥ 30 kg/m2).
RESULTS
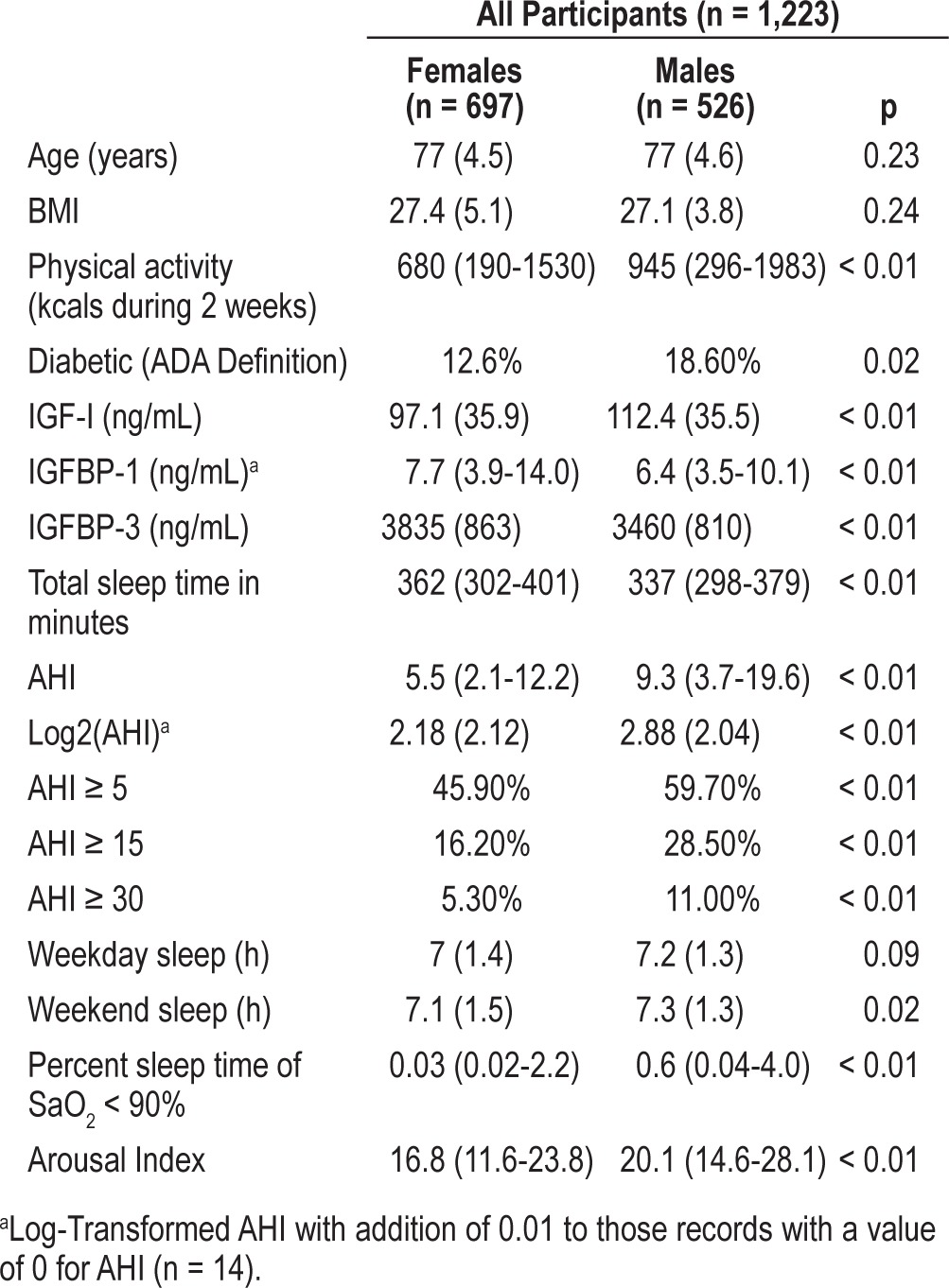
The study population (n = 1,223) had a mean age of 77.5 years and included 697 women and 526 men. Mean IGF-I levels were higher among men than women (112.4 ng/mL vs. 97.1 ng/mL, p < 0.01); however, mean IGFBP-3 levels were higher among women (3835 ng/mL vs. 3460 ng/mL, p < 0.05). Overall, women in our cohort had better sleep quality than men. SWS was better preserved in females than males, with females spending 76 min on average in SWS compared to males who spent 29 min (p < 0.01). The mean AHI in females was 5.5/h vs. 9.3/h in males (p < 0.01). Since the distribution of the AHI variable in our study sample is not normal, we report the log-transformed AHI with addition of 0.01 to those records with a value of 0 for AHI (n = 14). The prevalence of OSA (AHI ≥ 5) in males was 60% and in females was 46% (p < 0.01). The prevalence of moderate OSA (AHI ≥ 15) was 29% in males, and16% in females (p < 0.01). Women also had a lower arousal index than men (16.8 vs. 20.1, p < 0.01), suggesting less sleep fragmentation among women (Table 1).
Table 1.
Baseline characteristics of the study sample
Tables 2, 3, and 4 show the association between various sleep parameters and IGF-I, IGFBP-1, and IGFBP-3 levels. Table 2 shows the entire cohort, and Tables 3 and 4 show gender-stratified results. We quantified associations as Beta (B), reflecting change in IGF-I, IGFBP-1, or IGFBP-3 for each “unit” change (5 min) in stage 2, slow wave, or REM sleep. After adjustment for age, gender, race, BMI, diabetes, estrogen use, progestin use, and physical activity, there were no significant associations between measures of sleep disordered breathing and IGF-I, IGFBP-1, and IGFBP-3 levels. Sleep architecture was also not significantly associated with IGF-I and IGFBP levels. Time spent in stage 1 sleep (5-min increments) was not significantly associated with IGF-I, IGFBP-1, or IGFBP-3 levels. Time spent in stage 2 sleep (5-min increments) was inversely associated with IGF-I levels (B = -0.2, CI = -0.4, 0, p = 0.03) but not with IGFBP-1 or IGFBP-3 levels. Time spent in SWS was also not significantly associated with IGF-I, IGFBP-1, or IGFBP-3 levels in the multivariate model. Time spent in REM sleep was significantly associated with IGFBP-3 levels (B = 10.5, CI 1.2, 19.9, p = 0.03) but not with IGF-I or IGFBP-1 levels. Beta (B) in the above mentioned analyses reflects change in IGF-I, IGFBP-1, or IGFBP-3 for each “unit” change (5 min) in stage 2, slow wave, or REM sleep.
Table 2.
Multivariable associations of sleep measures with IGF-I, IGFBP-1 and IGFBP-3 for all participants
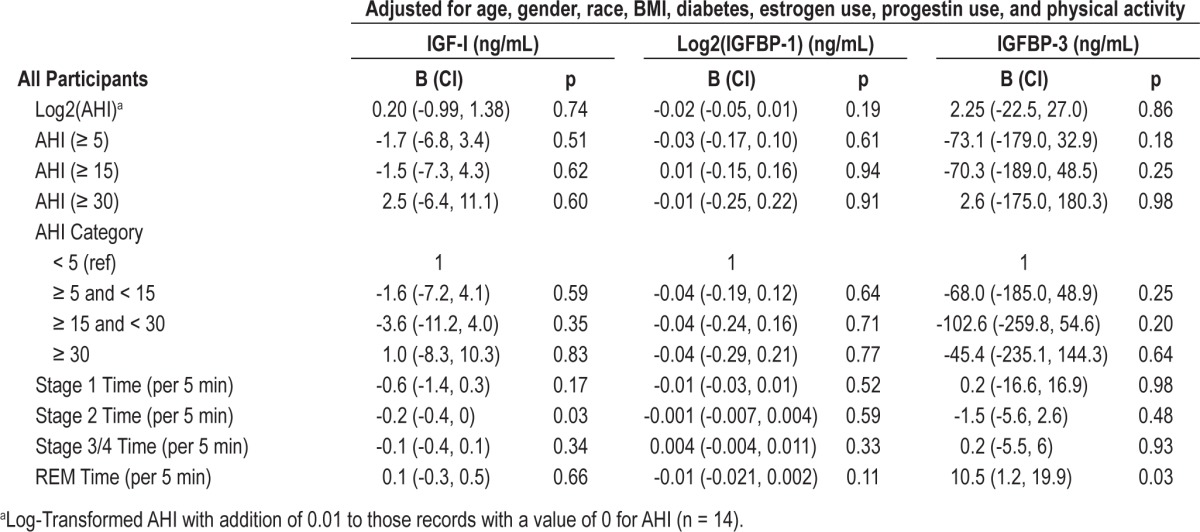
Table 3.
Multivariable associations of sleep measures with IGF-I, IGFBP-1 and IGFBP-3 for females
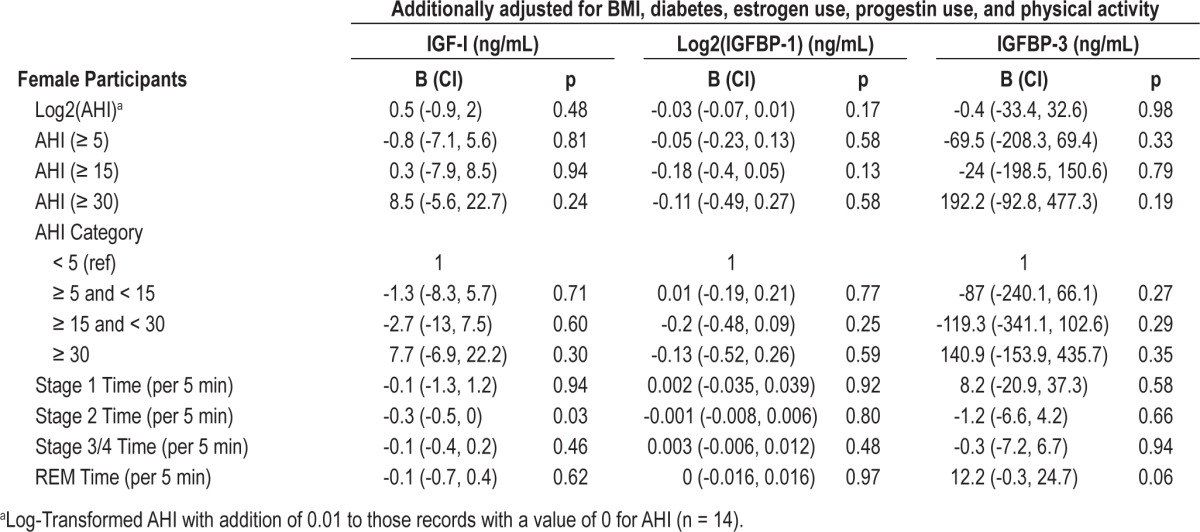
Table 4.
Multivariable associations of sleep measures with IGF-I, IGFBP-1 and IGFBP-3 for males
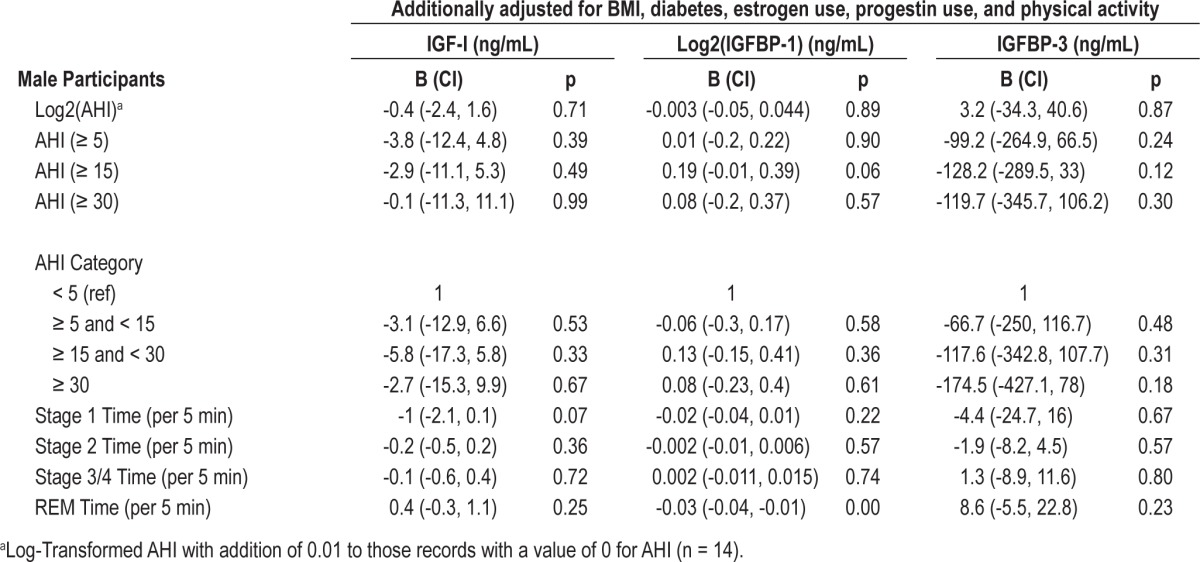
Table 3 shows the association between sleep measures and IGF-I, IGFBP-1, and IGFBP-3 levels among female participants. After adjustment for age, race, BMI, diabetes, estrogen use, progestin use, and physical activity, there were no associations between measures of sleep disordered breathing and IGF-I, IGFBP-1, or IGFBP-3 levels. The association between time spent in REM sleep and IGFBP-3 levels was similar among females compared to the entire cohort, albeit with attenuated level of statistical significance (B = 12.2, CI -0.3, 24.7, p = 0.06).
Table 4 shows the association between sleep measures and IGF-I, IGFBP-1, and IGFBP-3 levels among male participants. After adjustment for age, race, BMI, diabetes, estrogen use, progestin use, and physical activity, there were no associations between measures of sleep disordered breathing and IGF-I, IGFBP-1, or IGFBP-3 levels. The association between stage 2 sleep and IGF-I levels was not significant in males (B = -0.2, CI -0.5, 0.2, p = 0.36). Similarly, the association between REM sleep and IGFBP-3 was not significant in males (B = 8.6, CI = -5.5, 22.8, p = 0.23). However, unlike females, there is a significant inverse association between REM sleep (5-min increments) and IGFBP-1 (B = -0.03, CI = -0.04, -0.01, p < 0.01).
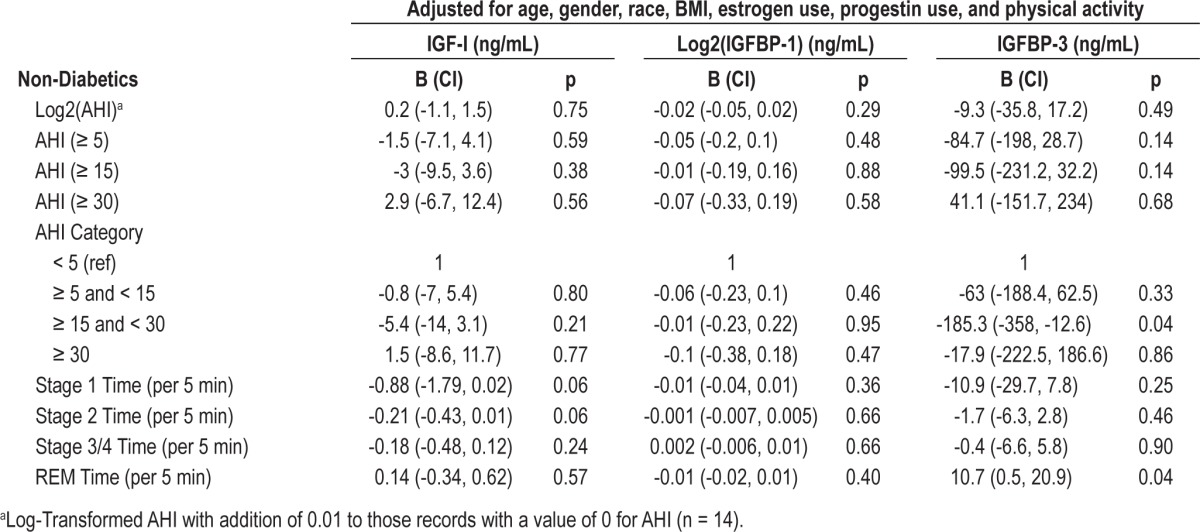
Table 5 shows the association between sleep measures and IGF-I, IGFBP-1, and IGFBP-3 levels in non-diabetic individuals. Beta (B) in Table 5 is changed in IGF-I, IGFBP-1, and IGFBP-3 levels (ng/mL) for each unit of the predictor variable. After adjustment for age, gender, race, BMI, diabetes, estrogen use, progestin use, and physical activity, there were no significant associations between SWS or sleep disordered breathing and IGF-I, IGFBP-1, or IGFBP-3 levels. However, moderate OSA was inversely associated with IGFBP-3 levels (B = -185, CI = -358, -13, p = 0.04). When we stratified this non-diabetic cohort by gender, this association persisted only in females (B = -256, CI = -504, -8, p = 0.04) and not in males (B = -136, CI = -375, 104, p = 0.8) (Data not shown). None of the other sleep disordered breathing variables were associated with IGF-I, IGFBP-1, or IGFBP-3 levels in this gender stratified analysis of non-diabetic individuals. Finally, we investigated the relationship between OSA and IGF-I and IGFBP-3 levels in a restricted sample of obese patients (n = 270 with BMI ≥ 30 kg/m2). In this sample of obese patients, we found no significant association between OSA and IGF-I levels (data not shown).
Table 5.
Multivariable associations of sleep measures with IGF-I, IGFBP-1 and IGFBP-3 for non-diabetic participants
DISCUSSION
This study evaluated the relationship between sleep (architecture and disordered breathing) and the GH/IGF-I system in an elderly population of patients who were enrolled in the CHS and who also participated in the SHHS. The major findings of our study are: (a) No significant association was found between SWS and circulating IGF-I, IGFBP-1, or IGFBP-3 levels after adjusting for confounding variables (b) No significant association was detected between measures of sleep disordered breathing and circulating IGF-I, IGFBP-3 or IGFBP-3 levels. (c) Among non-diabetic females, an inverse association was detected between moderate sleep apnea (AHI of 15-30/h) and IGFBP-3 after adjustment for confounding variables.
Numerous studies27,36,37 support a strong relationship between SWS and increased GH secretion. Increased SWS has also been associated with increased IGF-I levels.38 In our study of elderly individuals, to the contrary, we found no significant correlation between SWS and IGF-I, IGFBP-1, or IGFBP-3 levels. Based on our study findings, we conclude that the relationship between SWS and the GH/IGF-I axis is not as robust in the elderly as among younger individuals. We found weak yet significant associations between non-SWS and circulating IGF-I and IGFBP-3 levels (Tables 2–5). For instance, we found that increased time in stage 2 sleep is associated with reduced IGF-I levels, suggesting an adverse influence of increased stage 2 sleep on IGF-I levels. There was evidence of gender differences, with significance only among females and not males. This suggests that in females, increased stage 2 sleep is associated with reduced IGF-I levels. Similarly, we saw a significant association between time in REM sleep and IGFBP-3 levels, suggesting a positive influence of REM sleep (surrogate for consolidated sleep) on the GH/IGF axis. Gender did not appear to modify this relationship.
We also did not detect a significant association between sleep disordered breathing and IGF-I, IGFBP-1, or IGFBP-3 levels; an association that has been described in prior studies.5–7 Ursavas et al. has reported a negative correlation between measures of OSA (arousal index, AHI, average desaturation) and IGF-I levels.7 In that study, the mean age of the study sample was 48.8 years among the control participants and 52 years among those with sleep apnea. In comparison, the mean age of our study sample is 77.5 years. Therefore, our study is better designed to assess the relationship between sleep and GH/IGF axis in the elderly. Our study is further strengthened by adjusting our multivariable models for important confounding variables including age, BMI, physical activity, and use of sex hormones. We also conducted gender-stratified analyses in addition to sensitivity analyses, excluding obese and diabetic individuals in order to better assess the relationship between sleep and GH/IGF axis. In doing so, we found that although the association between sleep disordered breathing and IGFBP-3 was not significant in the entire cohort or the gender-stratified cohort; there was a significant association in female non-diabetics, where moderate sleep disordered breathing was inversely associated with IGFBP-3 levels. This was not seen in male non-diabetic participants. Therefore, our study demonstrates that the associations between sleep disordered breathing and IGF-I, IGFBP-1, and IGFBP-3 are not significant in an elderly cohort and that sleep disordered breathing likely does not adversely affect the GH/ IGF axis (as seen in younger individuals). Our study suggests an inverse association between moderate sleep apnea and IGFBP-3 among female non-diabetics. However, this finding should be interpreted with caution, as a similar association was not seen with mild or severe sleep apnea.
A limitation of our study is that sleep and IGF measures were not collected on the same day but rather within one year of each other. However, in additional analyses we found strong correlation among IGF-1 levels within individuals between two study visits conducted 12 months apart (r = 0.86). Thus, because IGF-I levels are relatively stable over the short term in the elderly, the measured IGF-I levels used in this study are likely strongly correlated with the measurements that would have been obtained on the same day as the sleep examination. Although we adjusted for potential confounding variables in our regression models, residual confounding by unmeasured variables is possible. In addition, given multiple influences in the elderly that result in relatively low levels of IGFs, it is possible that we may not have had adequate statistical power to detect relatively subtle effects of sleep variables on IGF levels. On the other hand, our study has numerous strengths. It is the first study of its kind to assess an association between sleep architecture and OSA and circulating IGF levels in a population-based sample of elderly participants. The methods of measurement for sleep and IGFs were rigorous as part of two large epidemiological studies namely, the CHS and the SHHS that mutually shared roughly a third of their sample. We were able to adjust for important confounding variables including physical activity which further strengthens our study findings. Finally, our robust sample size with adequate numbers of males and females allowed us to conduct several important secondary data analyses among gender-stratified diabetes and obesity-restricted samples.
In summary, our study did not detect an expected (as seen in younger individuals) significant positive association between SWS and circulating IGF-I, IGFBP-1, or IGFBP-3 levels in our elderly cohort of participants. We also did not observe a significant relationship between various indices of OSA and circulating IGF-I, IGFBP-1, and IGFBP-3 levels among the elderly (as expected among younger individuals). Collectively, our study findings suggest that the relationship between SWS and the GH/IGF system is weaker in the elderly, and aging appears to dilute the adverse influence of sleep disordered breathing on the GH/IGF system. Future studies are needed to clarify the role of aging on the relationship between sleep and GH/IGF system in order to better understand the implications of circulating levels of IGF-I and IGFBPs in risk of cancer, cardiovascular diseases, functional status, and mortality.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, 01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm. In addition, this work was supported by SHHS grant HL53934 (for CHS sites of SHHS, i.e. Pittsburgh and Sacramento). Finally, Dr. Shah has received research grant funding from the American Sleep Medicine Foundation (2011-current). Work was performed at the Albert Einstein College of Medicine, Bronx, NY.
REFERENCES
- 1.Faje AT, Barkan AL. Basal, but not pulsatile, growth hormone secretion determines the ambient circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab. 2010;95:2486–91. doi: 10.1210/jc.2009-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biernacka KM, Perks CM, Holly JM. Role of the IGF axis in prostate cancer. Minerva Endocrinol. 2012;37:173–85. [PubMed] [Google Scholar]
- 3.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–44. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 4.Empen K, Lorbeer R, Volzke H, et al. Association of serum insulin-like growth factor I with endothelial function: Results from the population-based Study of Health in Pomerania (SHIP) Eur J Endocrinol. 2010;163:617–23. doi: 10.1530/EJE-10-0563. [DOI] [PubMed] [Google Scholar]
- 5.Gianotti L, Pivetti S, Lanfranco F, et al. Concomitant impairment of growth hormone secretion and peripheral sensitivity in obese patients with obstructive sleep apnea syndrome. J Clin Endocrinol Metab. 2002;87:5052–7. doi: 10.1210/jc.2001-011441. [DOI] [PubMed] [Google Scholar]
- 6.Johnsen SP, Hundborg HH, Sorensen HT, et al. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90:5937–41. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- 7.Ursavas A, Karadag M, Ilcol YO, et al. Low level of IGF-1 in obesity may be related to obstructive sleep apnea syndrome. Lung. 2007;185:309–14. doi: 10.1007/s00408-007-9026-x. [DOI] [PubMed] [Google Scholar]
- 8.Sherlock M, Toogood AA. Aging and the growth hormone/insulin like growth factor-I axis. Pituitary. 2007;10:189–203. doi: 10.1007/s11102-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 9.Chu LW, Zhu Y, Yu K, et al. Correlation between circadian gene variants and serum levels of sex steroids and insulin-like growth factor-I. Cancer Epidemiol Biomarkers Prev. 2008;17:3268–73. doi: 10.1158/1055-9965.EPI-08-0073. [DOI] [PubMed] [Google Scholar]
- 10.Amaral IP, Johnston IA. Circadian expression of clock and putative clock-controlled genes in skeletal muscle of the zebrafish. Am J Physiol Regul Integr Comp Physiol. 2012;302:R193–206. doi: 10.1152/ajpregu.00367.2011. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Salojin KV, Mi QS, et al. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology. 2004;145:627–38. doi: 10.1210/en.2003-1274. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich N, Schneider H, Dorr M, et al. All-cause mortality and serum insulin-like growth factor I in primary care patients. Growth Horm IGF Res. 2011;21:102–6. doi: 10.1016/j.ghir.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Rajpathak SN, He M, Sun Q, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61:2248–54. doi: 10.2337/db11-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgers AM, Biermasz NR, Schoones JW, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–20. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- 15.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 16.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw JE, Punjabi NM, Wilding JP, Alberti KG, Zimmet PZ. International Diabetes Federation Taskforce on Epidemiology and Prevention. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 19.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 20.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87:201–23. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9:366–76. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitale G, Brugts MP, Ogliari G, et al. Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians' offspring. Aging (Albany NY) 2012;4:580–9. doi: 10.18632/aging.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 27.Van Cauter E. Slow wave sleep and release of growth hormone. JAMA. 2000;284:2717–8. [PubMed] [Google Scholar]
- 28.Veldhuis JD, Iranmanesh A. Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: predominant impact of age, obesity, gonadal function, and sleep. Sleep. 1996;19(10 Suppl):S221–4. doi: 10.1093/sleep/19.suppl_10.s221. [DOI] [PubMed] [Google Scholar]
- 29.Veldhuis JD. Gender differences in secretory activity of the human somatotropic (growth hormone) axis. Eur J Endocrinol. 1996;134:287–95. doi: 10.1530/eje.0.1340287. [DOI] [PubMed] [Google Scholar]
- 30.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 31.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 33.Sanders JL, Ding V, Arnold AM, et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol A Biol Sci Med Sci. 2013 Jun 28; doi: 10.1093/gerona/glt088. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 35.Folsom AR, Jacobs DR, Jr, Caspersen CJ, Gomez-Marin O, Knudsen J. Test-retest reliability of the Minnesota Leisure Time Physical Activity Questionnaire. J Chronic Dis. 1986;39:505–11. doi: 10.1016/0021-9681(86)90195-5. [DOI] [PubMed] [Google Scholar]
- 36.Van Cauter E, Copinschi G. Interrelationships between growth hormone and sleep. Growth Horm IGF Res. 2000;10(Suppl B):S57–62. doi: 10.1016/s1096-6374(00)80011-8. [DOI] [PubMed] [Google Scholar]
- 37.Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl A):S10–7. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Prinz PN, Moe KE, Dulberg EM, et al. Higher plasma IGF-1 levels are associated with increased delta sleep in healthy older men. J Gerontol A Biol Sci Med Sci. 1995;50:M222–6. doi: 10.1093/gerona/50a.4.m222. [DOI] [PubMed] [Google Scholar]


