Abstract
Study Objectives:
We assessed whether excessive daytime sleepiness was associated with coronary plaque phenotype and subsequent adverse cardiovascular events.
Methods:
Prospective cohort study. Intravascular ultrasound (IVUS) examination of the culprit coronary stenosis was performed. The Epworth Sleepiness Scale (ESS) questionnaire was administered, and the patients were divided into 2 groups—(1) sleepier and (2) less sleepy—based on the ESS score. Adverse cardiovascular outcomes were defined as cardiac death, myocardial infarction, stroke, unplanned revascularization, or heart failure admission.
Results:
One hundred seventeen patients undergoing urgent or non-urgent coronary angiography were recruited. Compared with the less sleepy group (ESS ≤ 10, n = 87), the sleepier group (ESS > 10, n = 30) had higher serum levels of total cholesterol and of low-density-lipoprotein cholesterols (p < 0.05 for both). The IVUS examinations indicated coronary stenoses were longer in the sleepier group than in the less sleepy group (p = 0.011). The cumulative incidence of adverse cardiovascular events at 16-month follow-up was higher in the sleepier than the less sleepy group (12.5% versus 6.9%, p = 0.03). Cox regression analysis adjusting for age and smoking showed increased hazard of adverse cardiovascular events in sleepier group as compared to less sleepy group (HR = 3.44, 95% CI 1.01-11.72).
Conclusion:
In patients presenting with coronary artery disease, excessive daytime sleepiness based on ESS > 10 was associated with longer culprit lesions and future adverse cardiovascular events.
Citation:
Lee CH; Ng WY; Hau W; Ho HH; Tai BC; Chan MY; Richards AM; Tan HC. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med 2013;9(12):1267-1272.
Keywords: Sleepiness, coronary artery disease, intravascular ultrasound, outcomes
Excessive daytime sleepiness is a prevalent health problem that affects 18% of the general population in the United States.1 This condition increases the risk of road traffic accidents and causes serious economic consequences due to poor work performance.2,3 In the clinical context, excessive daytime sleepiness is a common but nonspecific symptom that accompanies neurological, psychiatric, kidney, and cardiopulmonary disorders. Excessive sleepiness is also a side effect of certain medications and a consequence of insufficient sleep duration. The pathological consequences of excessive daytime sleepiness are complex and remain incompletely understood. Previous epidemiological studies have found that elderly people who reported having frequent excessive daytime sleepiness had a higher risk of subsequent adverse cardiac and cerebrovascular events than those who reported never having excessive daytime sleepiness. This pattern remained evident even after adjusting for confounding factors.4–7
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are limited data on the prognostic implication of excessive daytime sleepiness in patients with coronary artery disease. In this prospective observational study, we determined the association between excessive daytime sleepiness (ESS > 10), coronary plaque phenotype, and subsequent adverse cardiovascular outcomes.
Study Impact: The patients were classified into sleepier and less sleepy groups according to their baseline ESS scores (> 10 versus ≤ 10), and we found that the sleepier group was associated with longer lesion lengths. After adjusting for potential confounders, the hazard of adverse cardiovascular events was 3-fold in the sleepier as compared to the less sleepy groups.
In most of these previous studies,4–6 excessive daytime sleepiness was self-reported as a qualitative variable, and some degree of misclassification or overlapping with fatigue could not be excluded. The Epworth Sleepiness Scale (ESS) was developed more than 20 years ago to gain a more objective measure of sleepiness, and is now the most widely used measure of excessive daytime sleepiness.8 The ESS is frequently administered to patients to estimate their risk of obstructive sleep apnea and thus guide decisions regarding overnight polysomnography. The questionnaire also enables the evaluation of responses to treatment for patients diagnosed with obstructive sleep apnea. The ESS has been validated against in-laboratory polysomnography on community individuals in general practice settings.9,10
However, it remains unknown whether excessive daytime sleepiness, as measured by high ESS scores, can predict adverse outcomes in patients with cardiovascular conditions. In this prospective observational study, we determined the association between excessive daytime sleepiness (ESS > 10), coronary plaque phenotype, and subsequent adverse cardiovascular outcomes in a cohort of patients presenting with symptomatic coronary artery disease. Specifically, we targeted patients in whom a stenosis was detected by coronary angiography so that an intravascular ultrasound (IVUS) catheter could be used to assess their plaque phenotype.
METHODS
Study Design and Patients
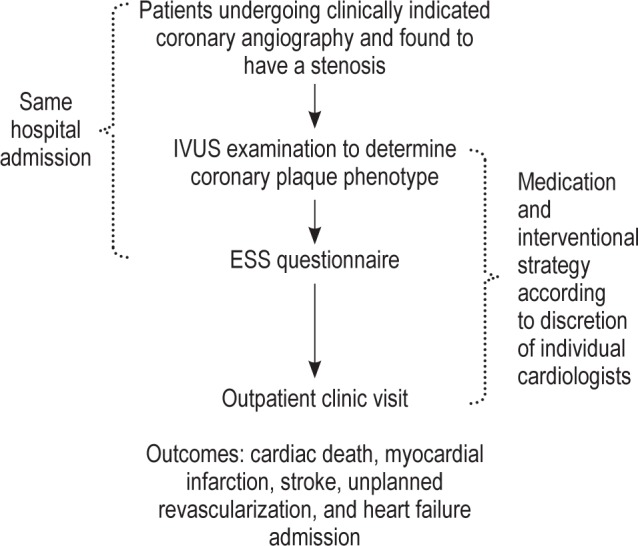
This prospective study was conducted at 2 tertiary centers (the National University Heart Centre and Tan Tock Seng Hospital) in Singapore between February 2011 and March 2013. The participants were selected from among patients aged 21 years and older who were undergoing coronary angiography for stable coronary artery disease or acute coronary syndromes, including myocardial infarction with or without ST-segment elevation, and had been found to have ≥ 1 significant (> 50% visual estimation) de novo stenosis in a native coronary artery. Criteria for exclusion from the study included known obstructive sleep apnea (based on previous polysomnography), cardiogenic shock (systolic blood pressure < 90 mm Hg), chronic renal failure with dialysis treatment, previous treatment of the target vessel, being a foreign patient who could not attend the outpatient clinic for follow-up, or inability to provide informed consent. The angiographic exclusion criteria were angiographically visible residual thrombus despite thrombus aspiration or thrombectomy, a heavily calcified lesion, a tortuous vessel, chronic total occlusion, a significant left main lesion, or a distal lesion that was too small to accomodate an IVUS catheter. The National Healthcare Group Domain Specific Review Board approved the study's research protocol (reference: C/2010/00341), and informed consent was obtained from the subjects. Recruited patients underwent IVUS examination of the culprit lesions. During the same hospital admission, the ESS questionnaire was administered to the patients to determine their degree of excessive daytime sleepiness. A flow chart of this study is shown in Figure 1.
Figure 1. Study flowchart.
Intravascular Ultrasound Examination
Consenting patients were formally enrolled into the study after identification of the culprit lesion by coronary angiography. A guiding catheter was used to selectively cannulate the ostium of the target coronary artery. Immediately after advancing the guidewire, but before balloon predilation, a 20-MHz, 3.5-French phased-array IVUS catheter (Eagle Eye Gold, Volcano Corp., Rancho Cordova, CA, USA) was inserted into the mid- to distal segment of the target coronary artery. The IVUS catheter was automatically pulled back to the ostium of the guiding catheter using a motorized pull-back device at a speed of 0.5 mm/s (R-100 Pullback Device, Volcano Corp.). All images were recorded in digital format on a DVD for subsequent offline quantitative analysis by 2 investigators (CHL, WH), who were blinded to the ESS data. Any discrepancies between the analyses of the 2 investigators were resolved by consensus.
Intravascular Ultrasound Analyses
Conventional gray-scale quantitative IVUS analyses were performed according to the IVUS expert consensus document.11 The following parameters were measured in the culprit lesions in the target coronary artery: (i) external elastic membrane area (mm2), (ii) minimal lumen diameter (mm), (iii) minimal lumen area (mm2), and (iv) plaque burden (mm2).
The virtual-histology-IVUS images were analyzed according to the published guidelines to determine the tissue composition of the lesions.11 The 4 virtual-histology-IVUS plaque components were color-coded and displayed on the virtual-histology-IVUS console, with fibrous tissue shown in green, fibro-fatty tissue in light-green, dense calcium in white, and the necrotic core in red. Volumetric measurements using Simpson's rule were performed over the entire region of interest by tracing the external elastic membrane and the lumen border. The volumetric values for each of the 4 plaque components and the culprit lesion plaque volume were then automatically calculated using VIAS 3.0 offline analysis software (Volcano Corp.).12 Thin-cap fibroatheroma was defined as a plaque with > 10% confluent necrotic core, with > 30 degrees of the necrotic core abutting the lumen in ≥ 3 consecutive frames.13
Epworth Sleepiness Scale and Data Collection
Patients presenting with ST-elevation myocardial infarction were treated with urgent percutaneous coronary intervention. These patients were not only under time pressure, but were in pain and distress, and may have been medicated with opiates that affect decision-making. To standardize the protocol, the ESS questionnaire was administered to all patients after the angiography and intervention procedures. The recruited patients were approached by a dedicated research assistant and asked to complete the ESS questionnaire in the cardiac ward. The ESS is a validated questionnaire that asks subjects to rate their likelihood of falling asleep in several common situations. In this questionnaire, patients rate their perceived likelihood of falling asleep in 8 everyday situations, and their answers give a score of between 0 and 24 points. Based on the ESS score, the recruited patients who completed the ESS were divided into either (1) the sleepier group (ESS > 10) or (2) the less sleepy group (ESS ≤ 10).
The following demographic and clinical information were collected prospectively: ethnicity, gender, age, height, weight, body mass index, clinical presentation (such as ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, unstable angina, or stable angina), cardiovascular risk factors (such as smoking, diabetes mellitus, hypertension, hyperlipidemia, or family history of premature coronary artery disease), previous cardiovascular conditions (such as previous myocardial infarction, percutaneous coronary intervention, or coronary artery bypass surgery), laboratory investigation results, and detailed angiographic findings.
Clinical Follow-Up
After hospital discharge, all of the recruited patients made regular visits to an outpatient clinic. The physicians conducting the outpatient clinic were not part of the study team, and were blinded to the results of the ESS data. The cardiovascular outcome data were collected by a dedicated research assistant via telephone calls or clinic chart reviews, and all of the information was entered prospectively. Adverse cardiovascular events were defined as cardiac death, myocardial infarction, stroke, unplanned revascularization, or heart failure requiring hospitalization. To verify the events, source documents of the patients who had experienced adverse cardiovascular events were reviewed by the investigators.
Statistical Analysis
We summarized the distribution of the continuous normally distributed clinical and laboratory procedural characteristics of the patients using the mean and standard deviation. The differences in the means of the sleepier and less sleepy groups were compared using an independent sample t test. For skewed continuous variables, the median and interquartile ranges were used to summarize the distribution, with comparisons between groups made with the Mann-Whitney test. For categorical variables, the Fisher exact test was used to compare differences in proportions.
For the analysis of time-to-adverse events, each participant's survival time was calculated from the date of index admission to the date when an adverse event first occurred. Patients who did not have an adverse event were censored on February 15, 2013. The Kaplan-Meier cumulative incidence curves were plotted and the difference in cumulative incidence of adverse event rates between the sleepier and less sleepy groups was compared using the log rank test. The Cox proportional hazards regression was then applied to adjust for body mass index and smoking in the time-to-adverse-event analysis. All of the statistical analyses were carried out using STATA version 10.0 (StataCorp LP, College Station, TX, USA), assuming a two-sided test with a 5% level of significance.
RESULTS
Baseline Demographic and Clinical Characteristics
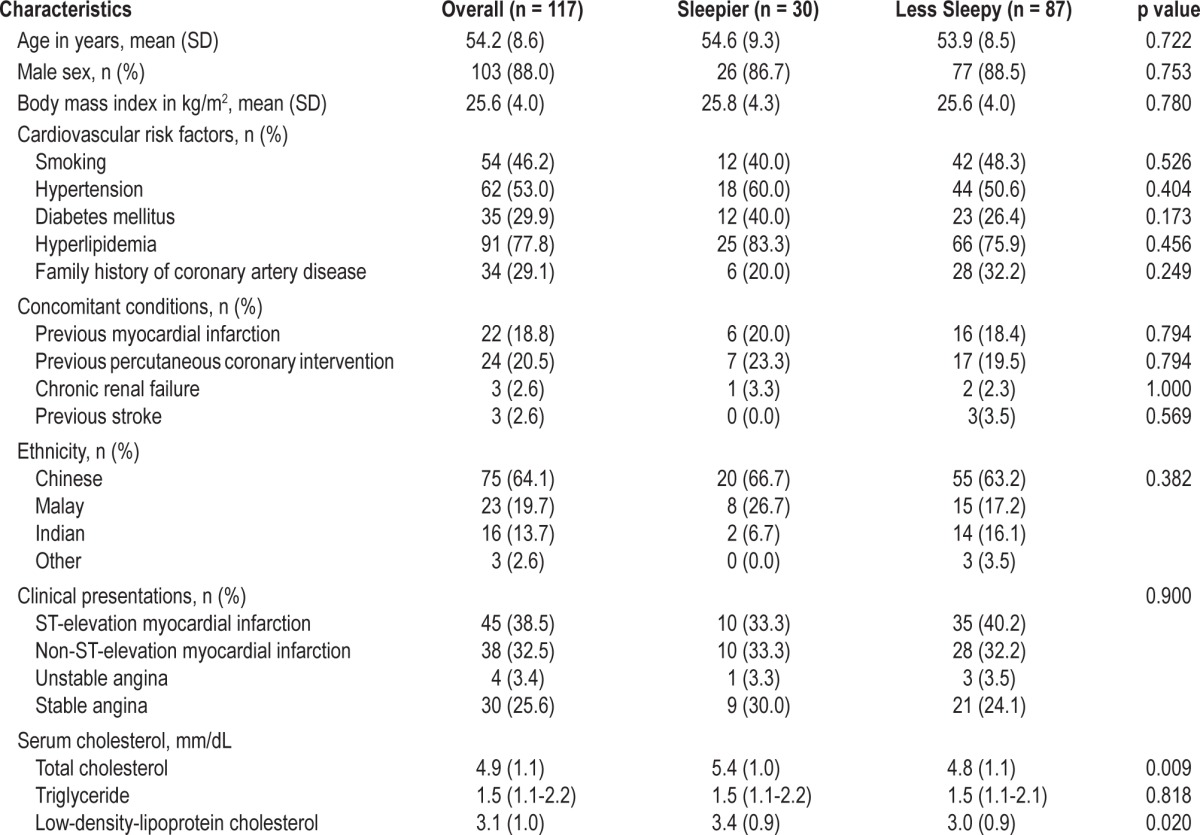
Among the 118 recruited patients, 117 completed the ESS and formed the study population. The distribution of the ESS scores is shown in Figure 2. The median ESS score was 8 (interquartile range 5-11). The baseline demographic and clinical characteristics of the sleepier (ESS > 10, n = 30) and less sleepy (ESS ≤ 10, n = 87) groups are shown in Table 1. The majority of the study patients were male. About 30% of the recruited patients had diabetes mellitus, but none had undergone previous coronary artery bypass grafting. The most common clinical presentations were ST-elevation myocardial infarction (n = 45) and non-ST-elevation myocardial infarction (n = 38). Compared with the less sleepy group, the sleepier group had higher serum levels of total and low-density-lipoprotein cholesterols. There were no significant differences between the sleepier and less sleepy groups with respect to full blood count, renal biochemistry, peak creatine kinase levels (for patients with acute myocardial infarction), or glycosylated hemoglobin levels (for patients with diabetes mellitus) (data not shown).
Figure 2. Distribution of Epworth Sleepiness Scale score.
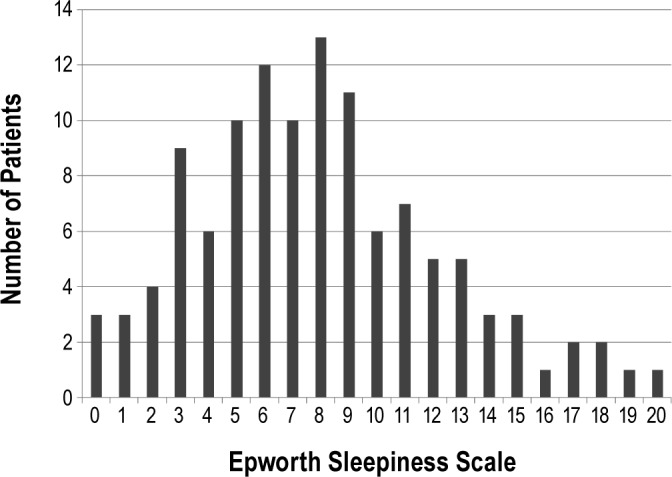
Table 1.
Patient demographics and clinical characteristics
Angiographic and Intravascular Ultrasound Characteristics
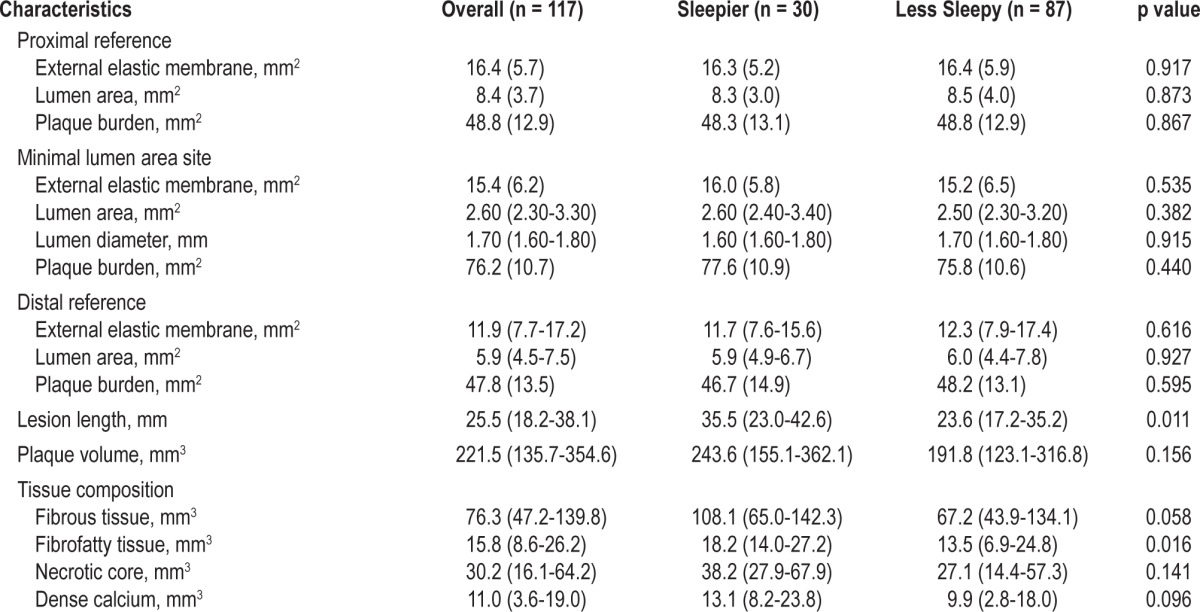
There were no significant differences between the sleepier and less sleepy groups in the angiographic complexity of their coronary artery disease. Likewise, the incidence of multivessel coronary artery disease, location of culprit lesions, and left ventricular ejection fraction were similar between the 2 groups. The incidence of treatment for coronary artery disease using drug-eluting stents was similar between the sleepier and less sleepy groups. The IVUS findings are shown in Table 2. The culprit lesions were longer and had more fibrofatty tissues in the sleepier than in the less sleepy group. Slightly more than half of the culprit lesions were thin-cap fibroatheroma, with no difference in lesion type between the sleepier and less sleepy groups (data not shown).
Table 2.
Intravascular ultrasound findings
Adverse Cardiovascular Events
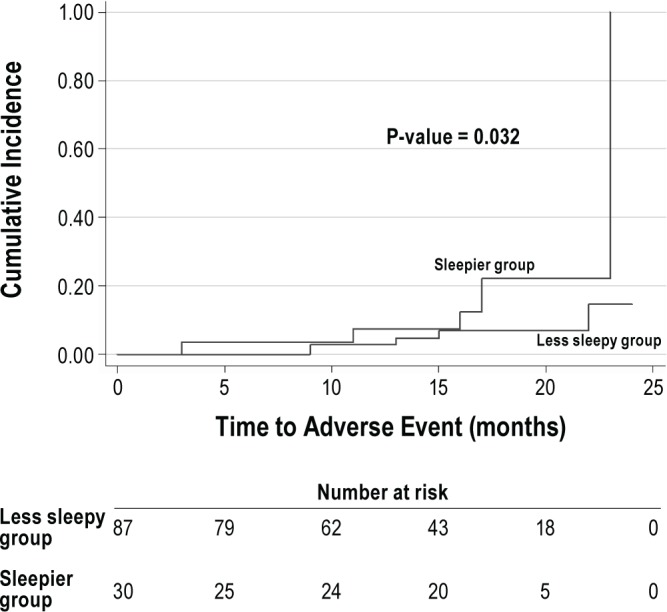
The median follow-up duration was 16 months (95% CI: 14.4-17.6). Complete data on follow-up were available for all 117 patients. Among the 30 patients in the sleepier group, 9 patients developed 10 adverse cardiovascular events. These included 2 cardiac deaths, 2 myocardial infarctions, 4 target vessel revascularizations, 1 stroke, and 1 heart failure admission. Among the 87 patients in the less sleepy group, 2 patients developed 4 adverse cardiovascular events. These included 2 myocardial infarctions and 2 target vessel revascularizations. The Kaplan-Meier cumulative incidence of adverse cardiovascular events is shown in Figure 3. The event rate at 16 months was higher in the sleepier than the less sleepy group (12.5% versus 6.9%, p = 0.03). The Cox regression analysis adjusting for age and smoking showed increased hazard of adverse event in sleepier group as compared to less sleepy group (HR = 3.44, 95% CI 1.01-11.72).
Figure 3. Kaplan-Meier cumulative incidence curves.
DISCUSSION
We report the relationships between excessive daytime sleepiness, coronary plaque phenotype, and incidence of subsequent adverse cardiovascular events in a cohort of patients presenting with symptomatic coronary artery disease. A total of 117 patients underwent an IVUS interrogation of coronary stenosis and answered the ESS questionnaire during the same hospital admission. All of the recruited patients were treated according to standard clinical practice. The patients were classified into sleepier and less sleepy groups according to their baseline ESS scores (> 10 versus ≤ 10), and we found that the sleepier group was associated with longer lesion lengths. After adjusting for potential confounders, the hazard of adverse cardiovascular events was 3-fold in the sleepier as compared to the less sleepy groups.
The evaluation of excessive daytime sleepiness is often performed in the diagnosis of obstructive sleep apnea. In the general population, the association between excessive daytime sleepiness and obstructive sleep apnea has been clearly demonstrated. However, in patients with cardiovascular disorders, this association has proven to be weak.14–16 A possible factor that could explain in this discrepancy is the presence of “transient” obstructive sleep apnea in patients presenting with acute cardiovascular conditions. In addition, myocardial ischemia or heart failure alone can lead to fatigue and excessive daytime sleepiness, even in the absence of obstructive sleep apnea.17,18 Cardiac medications such as β-blockers can also lead to excessive daytime sleepiness. As a result of these entangled relationships, the true prognostic implication of excessive daytime sleepiness in patients presenting with cardiovascular disease has been inadvertently neglected.
Excessive daytime sleepiness is among the most frequent sleep complaints in the general population, yet the data on the effects of excessive daytime sleepiness on subsequent cardiovascular outcomes are limited. In the 1990s, the Cardiovascular Health Study reported that excessive daytime sleep was associated with subsequent cardiovascular mortality in otherwise healthy elderly women.6 A recent French study of over 7,000 healthy community-dwelling elderly subjects found that frequent excessive daytime sleepiness was independently associated with future vascular events, stroke, and cardiovascular mortality.4,5 However, in the aforementioned studies, objective tools such as ESS were not used, and subjects with pre-existing cardiovascular disorders were excluded.4–6 The aim of this study was thus to extend these results by exploring the association between baseline excessive daytime sleepiness and subsequent cardiovascular outcomes in patients presenting with coronary artery disease. We hypothesized that excessive daytime sleepiness is associated with complex coronary plaque phenotype and future adverse cardiovascular events.
Based on a median ESS scores (> 10 versus ≤ 10), we divided the patients into sleepier and less sleepy groups. The incidence of adverse cardiovascular events in the sleepier group was significantly higher than in the less sleepy group in our cohort of middle-aged (mean age 54) and predominantly male patients (88%), even after adjustment for potential confounding factors. The exact mechanisms for this association remain incompletely understood, but heightened sympathetic drive and elevated levels of circulating catecholamines, oxidative stress, endothelial dysfunction, and systemic inflammation have been reported as possible mechanisms.19–22 Our findings corroborate those of previous studies on healthy elderly people. If our observations are verified in larger-scale studies, then conducting the ESS questionnaire could be a simple and inexpensive tool to risk-stratify patients presenting with symptomatic coronary artery disease.
One of the strengths of this study is its use of IVUS rather than coronary angiography to assess the coronary plaque phenotype. As an intrinsic attempt to preserve the lumen and perfusion, the coronary arteries often develop outward remodeling in response to accumulations of atherosclerotic plaques. Thus, the early phase of coronary atherosclerosis is often undetectable by conventional coronary angiography, which depicts the lumen but not the vessel wall. In contrast, IVUS is an intra-coronary imaging technique that provides a three-dimensional tomographic view, and is superior to conventional coronary angiography in the assessment of coronary plaque phenotype.23
This study included patients with relatively severe forms of coronary artery disease, as is evidenced by the high proportion of patients with diabetes mellitus (30%), previous myocardial infarction (19%), and previous percutaneous coronary intervention (21%). In addition, 70% of the patients presented with acute myocardial infarction (ST- or non-ST- elevation myocardial infarction). Rather than resulting from selection bias, this pattern represents the current trend in the management of coronary artery disease. Recent data have suggested that in patients with stable angina, routine invasive management with angiography and percutaneous coronary intervention does not provide any incremental benefit compared with medical therapy alone.24 Thus, in recent years there have been fewer patients with stable angina undergoing coronary angiography, and acute myocardial infarction represents the most common indication of coronary angiography in our institutions.
This study has several limitations. This is a preliminary and hypothesis generating study due to the relatively small study size. The ESS scores were based on the patients' own responses, which were not validated by other family members. However, the number of family members differed among the patients, and the responses given by different family members may vary, which would make such validation unreliable. Also, Singapore is a multiethnic country, and its population includes Chinese, Malays, and Indians. The ESS was administered in English, and although English is the common language among people living in Singapore, it is not the mother tongue for many of them. The levels of language proficiency may have influenced the participants' understanding and interpretation of the ESS questionnaire. Further, the physicians conducting the outpatient clinic visits were blinded to the ESS data, but had access to the IVUS images. Although IVUS is a highly specialized interventional field that most outpatient physicians do not refer to, we could not exclude the possibility that some outpatient physicians may have modified their treatment after reviewing the IVUS images, and that such shifts in treatment may have ultimately changed the natural history of the patients' conditions. Finally, all of the patients in this study underwent clinically indicated coronary angiography, because angiography is a prerequisite for IVUS examinations. Thus, it may not be possible to extrapolate the results of this study to patients who are treated in more conservative ways.
In conclusion, we found that excessive daytime sleepiness (ESS > 10) was associated with longer lesion length in patients presenting symptomatic coronary artery disease. As of the completion of a 16-month follow-up period, the sleepier group was associated with significantly more adverse cardiovascular events than the less sleepy group. In the future, large-scale studies are warrented that target the association between the ESS and a diagnosis of obstuctive sleep apnea, and the effects of coronary artery disease treatment on the degree of excessive daytime sleepiness.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by the Academic Research Fund (grant number R172-000-239-112) of the Ministry of Education, Singapore. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Miss Pei-Ee Lee and Miss Venesa Loh with the recruitment of patients.
REFERENCES
- 1.Swanson LM, Arnedt JT, Rosekind MR, et al. Sleep disorders and work performance: findings from the 2008 National Sleep Foundation Sleep in America poll. J Sleep Res. 2011;20:487–94. doi: 10.1111/j.1365-2869.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 2.Hillman DR, Murphy AS, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 3.Connor J, Norton R, Ameratunga S, et al. Driver sleepiness and risk of serious injury to car occupants: population-based case control study. BMJ. 2002;324:1125. doi: 10.1136/bmj.324.7346.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blachier M, Dauvilliers Y, Jaussent I, et al. Excessive daytime sleepiness and vascular events: the Three City Study. Ann Neurol. 2012;71:661–7. doi: 10.1002/ana.22656. [DOI] [PubMed] [Google Scholar]
- 5.Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the Three City Study. Stroke. 2009;40:1219–24. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- 6.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 7.Boden-Albala B, Roberts ET, Bazil C, et al. Daytime sleepiness and risk of stroke and vascular disease: findings from the Northern Manhattan Study (NOMAS) Circ Cardiovasc Qual Outcomes. 2012;5:500–7. doi: 10.1161/CIRCOUTCOMES.111.963801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 9.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Kang HW, Lee LH. The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. Eur Arch Otorhinolaryngol. 2012;269:1143–7. doi: 10.1007/s00405-011-1808-3. [DOI] [PubMed] [Google Scholar]
- 11.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 12.García-García HM, Mintz GS, Lerman A, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5:177–89. doi: 10.4244/eijv5i2a29. [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 14.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 15.Albuquerque FN, Calvin AD, Sert Kuniyoshi FH, et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–73. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capodanno D, Cumbo M, Marchese A, et al. Daytime sleepiness does not predict sleep apnoea in patients with coronary artery disease. Int J Cardiol. 2011;151:248–50. doi: 10.1016/j.ijcard.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 17.Skinner MA, Choudhury MS, Homan SD, et al. Accuracy of monitoring for sleep-related breathing disorders in the coronary care unit. Chest. 2005;127:66–71. doi: 10.1378/chest.127.1.66. [DOI] [PubMed] [Google Scholar]
- 18.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med. 2012;8:21–6. doi: 10.5664/jcsm.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin M, Thompson J, Miller C, et al. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 20.Mullington JM, Haack M, Toth M, et al. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusardi P, Mugellini A, Preti P, et al. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–5. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee CH. Intravascular ultrasound guided percutaneous coronary intervention: a practical approach. J Interv Cardiol. 2012;25:86–94. doi: 10.1111/j.1540-8183.2011.00651.x. [DOI] [PubMed] [Google Scholar]
- 24.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]


