Abstract
Study Objectives:
The objective of this study was to identify if hyperarousal is a 24-hour phenomenon in insomnia by comparing sleep during napping between good sleepers (GS) and Insomnia sufferers (INS) (subdivided into paradoxical “PARA-I” and psychophysiological “PSY-I”) following a mentally challenging battery of cognitive tests.
Design:
Cross-sectional comparisons of GS, PSY-I, and PARA-I.
Setting:
Participants slept for 4 consecutive nights in the laboratory where PSG was recorded. Upon awakening on mornings 2 and 3, cognitive testing (lasting 90-120 min) was administered, followed by a 20-minute nap.
Participants:
Fourteen PSY-I, 12 PARA-I, and 23 GS completed the study, comprising home questionnaires, clinical interviews, night PSG recordings, cognitive testing, and nap PSG recordings. All participants were between 25 and 50 years of age and met inclusion criteria for PSY-I, PARA-I, or GS.
Interventions:
N/A.
Measurements and Results:
On objective nap parameters, GS had a longer total sleep time (TST; p = 0.008) and better sleep efficiency (SE; p = 0.009), than PSY-I and PARA-I, and both groups of INS were awake significantly longer than GS (p = 0.003). Also, PARA-I took significantly more time than GS to fall asleep (p = 0.014). Subjectively reported sleepiness was comparable across the three groups. Positive relationships were observed between SE over the night and SE over the nap the following day.
Conclusions:
Results show that GS sleep better than INS during naps following prolonged cognitive testing, suggesting that, in INS, hyperarousal predominates over mental fatigue resulting from these tests. These results may parallel what is observed at night when INS experience increased cognitive load but are unable to fall asleep.
Citation:
Pérusse AD; Turcotte I; St-Jean G; Ellis J; Hudon C; Bastien CH. Types of primary insomnia: is hyperarousal also present during napping? J Clin Sleep Med 2013;9(12):1273-1280.
Keywords: Insomnia, napping, hyperarousal
Primary insomnia is one of the most prevalent sleep disorders.1 In fact, between 30% and 48% of the general population occasionally reports insomnia related symptoms, and more than 13% suffers from chronic primary insomnia.2,3 Important consequences resulting from this sleep disorder include fatigue, daytime sleepiness, confusion, sudden mood changes, and cognitive alterations.2 The International Classification of Sleep Disorders Second Edition (ICSD-2)4 differentiates 11 types of insomnia; paradoxical insomnia (PARA-I) and psychophysiological insomnia (PSY-I) being the most prevalent types. PARA-I is characterized by misperceptions in sleep quality and quantity. Individuals suffering from PARA-I complain about sleep difficulties although objective sleep measures (polysomnography; PSG) appear to be normal.5 On the other hand, PSY-I is characterized by “relatively” good perceptions of sleep duration and quality along with “real” sleep onset and/ or sleep maintenance difficulties and/or early morning awakenings.1 The maintenance of PSY-I results from the conditioning between sleep related stimuli (e.g. bedroom) and anxious thoughts concerning possible sleep disturbances.6,7 This conditioning contributes to the elevated cognitive activation that is typically reported in insomnia sufferers (INS).8 Irrespective of insomnia types, a difficulty in napping is one of the core features of insomnia.9–14 Thus, napping difficulties might be similar to sleep difficulties observed in INS during the night. There is also a possibility that napping reflects the hyperarousal phenomenon that characterizes insomnia. These hypotheses remain to be tested.
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study was done to determine if there are differences in napping characteristics between GS and INS. These measures will allow us to identify if hyperarousal is a 24-hour phenomenon in INS.
Study Impact: In this study, INS were more hyperaroused during their naps than GS, suggesting that the high level of hyperarousal characterizing INS influences their functioning not only during the night, but also during the day. Also, this study contributes to a better understanding of the phenomenon of hyperarousal and results confirm once more that insomnia is a 24-hour problem in the hyperarousal domain.
There are several models which have attempted to explain insomnia. One of the most popular is the neurocognitive model of insomnia.15 In this model, the authors state that in order to diminish sleep difficulties, INS tend to develop maladaptive behaviors, such as increasing the time spent in bed and going to bed earlier.15 These strategies are not efficient since they contribute to the elevation of somatic, cognitive and cortical activations.16 Cognitive activation is characterized by intrusive thoughts before sleep and cortical activation is measured by cortical activity across different frequency bands. The hyper-arousal of somatic, cognitive and cortical functions contributes to alterations in sensorial and information processing and the formation of long-term memories. Although the neurocognitive model of insomnia15 has been supported by numerous studies measuring quantitative EEG during the night,17,18 it has not yet been validated during naps. Therefore, a study on nap characteristics in insomnia would allow us to identify if hyperarousal is a phenomenon that influences not only nocturnal sleep, but also diurnal sleep. Several studies using a multiple sleep latency test (MSLT) protocol reported data on objective sleep during naps in insomnia, but these variables predominantly relate to sleep onset latency. While some studies failed to find significant differences in MSLT sleep-onset latencies between primary INS and good sleepers (GS),19,20 others showed that INS had longer MSLT sleep-onset latencies than GS,13,14 even though INS reported higher levels of sleepiness.12 Other studies found that following sleep deprivation, INS had longer sleep onset latencies during daytime naps compared to GS.9–11 Previous results thus tend to imply that hyperarousal is a 24-hour phenomenon in insomnia.
It is also possible that the degree of hyperarousal during naps in INS is influenced by activities completed before napping. Knowing the impact of cognitive testing on napping could contribute to the development of new strategies to help INS nap more efficiently when managing their sleep disorder and its associated consequences. To date, the link between activities completed before a nap and hyperarousal is unknown. In the present study, a battery of mentally challenging tasks was administered to participants before their naps. These tasks would most likely contribute to mental fatigue prior going to sleep since they lasted for 90 to 120 minutes and required a high and constant level of concentration. As such, we believe that mentally exhausting tasks before napping may serve as an analogy of insomnia in GS and/or increase cognitive load in INS, which should, according to the neurocognitive model,15 contribute to the exacerbation of hyperarousal and delay sleep onset. To our knowledge, prolonged cognitive testing has never been administered to INS, as well as GS, before a nap. However, in some studies, cognitive tasks were completed before bedtime at night.21,22 In general, it was observed that after cognitive testing, GS took significantly longer to fall asleep than those who did not complete them. Nonetheless, these studies failed to observe significant between group differences on other sleep parameters such as total sleep time and sleep stage distribution.
Finally, the relationship existing between sleep parameters during the night and the corresponding nap in insomnia has been seldom studied. In one study, it was reported that the shorter the objective total sleep time was during the night, the longer it took for INS to fall asleep during the day and the greater their daytime alertness was,14 suggesting that hyperarousal predominates over sleepiness in insomnia. Another study failed to find significant positive relationships, in a population of GS, between nocturnal sleep variables and sleep variables over a nap the next day.23 Therefore, it would be interesting to investigate the relationship between subjective sleepiness before a nap and objective sleep variables over a nap protocol in INS since studies on this component are limited.12 This would allow us to determine if subjective sleepiness contributes to the level of hyperarousal typically observed in INS. There is a possibility that the subjective perception of sleepiness is enough to exacerbate the level of hyperarousal, contributing to a diminution in the quality and quantity of sleep.
Even though sleep and nap difficulties have commonly been reported in PARA-I and PSY-I alike, significant differences between these two categories of insomnia still exist in their clinical presentation. To date, naps have rarely been studied in a population of INS and when they have been, types of INS were undifferentiated. Thus, napping difficulties reported in previous studies were generalized to all types of insomnia, independently of the specific classifications. However, it is possible that one of the types of insomnia (PSY-I or PARA-I) do not face napping difficulties, especially when considering that the objective nocturnal sleep of PARA-I often mirrors that of GS.5 Therefore, individuals suffering from PARA-I and PSY-I should be classified and divided into two independent groups. This clustering would provide a more representative understanding of napping in insomnia.
Objectives and Hypotheses
This study aims primarily at determining if there are significant differences in objective sleep parameters (sleep onset latency [SOL], wake after sleep onset [WASO], number of awakenings, total sleep time [TST], total wake time [TWT], and sleep efficiency [SE]) during naps among three groups of sleepers: PSY-I, PARA-I, and GS after completing a cognitively demanding battery of tests. It was assumed that this battery of tests would contribute to a state of mental exhaustion and/or an increase in cognitive load since testing lasted for a long period (90-120 min) and required an elevated and constant level of concentration. Mental exhaustion should facilitate sleep during napping, whereas high cognitive loading should delay sleep onset by exacerbating the hyperarousal level already present in INS. Therefore, since PSY-I and PARA-I should be more cognitively loaded after testing, we hypothesized that they would have poorer sleep during naps compared to GS, suggesting that hyperarousal predominates over mental exhaustion. Since PARA-I and GS usually have similar sleep profiles, objective sleep parameters of naps would be worse for PSY-I than PARA-I. Therefore, this study seeks empirical validation of the neurocognitive model of insomnia during napping.
This study also aimed at determining the influence of nocturnal sleep parameters on the ability to nap the next day. We suggested that a negative relationship would exist between nocturnal SE and SE during a nap for GS. In fact, the better the participant slept during the night, the harder it would be for him/ her to fall asleep during a morning nap, since sleep homeostasis has been reset. Conversely, since PSY-I and PARA-I should be more hyperaroused than GS, we hypothesized that a positive relationship would exist between SE of the nocturnal sleep and the nap, confirming previous findings.14 Consequently, the less they slept, the harder it would be for them to fall asleep during a nap the next day.
Finally, this research will allow us to determine if the three groups differ on subjective sleepiness (The Stanford Sleepiness Scale [SSS])24 following cognitive testing. Since PSY-I should have the poorest objective sleep parameters on nights before cognitive testing and PARA-I should have the feeling of a bad night's sleep, both groups should be more tired, and therefore should report higher levels of sleepiness than GS. Independently of sleepers' group, a positive relationship would also exist between the SE during nap and scores on the SSS.
METHODS
Participants
Participants were divided in 3 groups: 14 PSY-I, 12 PARA-I, and 23 GS. All participants were aged between 25 and 50 years. To be included in the PSY-I group, participants had to meet the following criteria: (a) a subjective complaint of insomnia characterized by difficulties initiating and/or maintaining sleep; (b) the insomnia must have been present ≥ 3 nights a week for > 6 months; (c) a complaint of ≥ 1 daytime consequence attributed to insomnia; (d) distress or significant difficulties in social and/or occupational functioning; and (e) SE ≤ 85%. Participants in the PARA-I group had to meet the same inclusion criteria as those of the PSY-I group, but their objective SE had to be ≥ 85% and their TST had to exceed 390 minutes. An important discrepancy also had to be present between subjective and objective sleep variables: TST (≥ 60 min discrepancy) and SE (≥ 15% discrepancy). For this study, GS had to report sleeping ≥ 7 h per night, satisfaction with their sleep, and no subjective sleep complaints. In addition to not meeting criteria for insomnia, GS had to report not using sleep-promoting agents and having a subjective SE ≥ 85%.
Exclusion criteria for all participants were: (a) a significant medical disorder, (b) major psychopathology, (c) other sleep disorders, (d) strong dependency to tobacco, (e) ongoing psychological treatment, (f) use of a medication known to affect sleep, (g) score ≥ 23 on the Beck Depression Inventory (BDI),25 or (h) a score ≥ 15 on the Beck Anxiety Inventory (BAI).26 These criteria were consistent with those of the ICSD-2 and those of Bastien and colleagues.27
Procedure
All participants were recruited through media advertisements as well as email sent to the Laval's university community. Following a brief telephone screening interview, eligible participants were sent a set of questionnaires to evaluate psychological symptoms (BAI and BDI) and sleep difficulties (Insomnia Severity Index [ISI],16 Dysfunctional Beliefs and Attitudes About Sleep [DBAS-16]28 and 2 weeks of sleep diaries16) that they completed at home. Those who met the inclusion criteria for any of the 3 groups were invited to the sleep laboratory for a clinical interview. Upon arrival to the sleep laboratory, informed consent was obtained. The Structured Clinical Interview for DSM-IV (SCID-IV)29 was administered to rule out major psychopathologies and the Insomnia Diagnosis Interview (IDI)16 to explore the nature of insomnia symptoms. These evaluations were conducted respectively by a graduate student in a clinical psychology program (GSJ) and a sleep specialist (CHB). Participants meeting the study criteria underwent four consecutive nights of PSG recordings in the sleep laboratory.
The mornings following nights 2 and 3, participants completed a battery of cognitive tests lasting between 90 to 120 minutes. The battery was composed of the following event-related potentials paradigms: go/no-go, distraction, and distraction delay. This procedure was approved by the ethics comity of the Centre de Recherche de l'Institut Universitaire en Santé Mentale de Québec (CER; # 183).
Go/NoGo Protocol
During this test, 2 types of auditory stimuli were presented to participants. Stimulus 1 was standard and frequent in occurrence and stimulus 2 was rare and either easy “target 1” or difficult “target 2.” Sounds all had the same duration of 40 ms, a rising time of 2 ms, and an intensity of 70 dB. The inter-stimulus interval varied from 1.3 to 1.7 seconds. Four conditions were presented to participants: (1) Go easy consists of stimulus 1 and target 1; (2) Go difficult consists of stimulus 1 and target 2; (3) NoGo easy (same stimuli as Go easy); and (4) NoGo difficult (same stimuli as Go difficult). Each condition consisted of 200 trials. Instructions differed for each condition: (A) Go conditions: participants have to detect target sounds and ignore standard ones; (B) NoGo conditions: they have to ignore target sounds and detect standard ones.
Distraction
This test consisted of 7 white letters appearing one after the other on a black computer screen. The stimuli were presented for 800 ms each, with an inter-stimulus interval of 200 ms. Participants were instructed to memorize those letters, and after the last one appeared they had to write the letters in the correct order. There were 2 different conditions, totalling 15 trials each, each trial lasting approximately 7 seconds. During the first condition, office-like noises (e.g., telephone, background noises of people chatting) were played while the letters appeared. During the second condition no noises were played.
Distraction Delay
This test is similar to the distraction paradigm, except participants had to memorize numbers instead of letters. There was a 10-sec delay before they were allowed to write down the numbers. There were three conditions consisting of 20 trials each, each trial lasting approximately 17 seconds. In the first condition, while waiting, participants heard a one-syllable non-relevant verbal sound. During the second, the sounds comprised 2 different syllables and there were 5 syllables in the third condition.
After cognitive tests, participants completed the SSS. Altogether, cognitive testing lasted between 90 to 120 min, including a 10-min break halfway through the protocol. Tests were followed by a 20-min nap opportunity during which PSG was recorded. Participants were instructed to try napping and were allowed out of bed if not asleep after 15 min (all participants stayed in bed for 20 min). This procedure was followed on both experimental days.
Measures
To evaluate psychological symptoms, the BAI, BDI, and the SCID-IV were used. To portray sleep difficulties, at-home questionnaires; the ISI and DBAS-16 were completed by participants. Adequate psychometric properties have been reported for both questionnaires in previous studies.28,30,31 Also, the IDI was used to evaluate the presence of insomnia and its contributing factors. The SSS was completed on mornings 2 and 3 after cognitive testing. This scale was used to evaluate the level of sleepiness of participants after a cognitive demand and just before napping opportunity.
Prior to the nights in the laboratory, participants completed a 2-week sleep diary.16 The sleep diary assesses subjective sleep quality, so participants had to report their sleep habits, such as the number of awakenings, the length of each awakening, the time spent in bed. From these raw data, the following subjective variables were derived: SOL, the amount of time it took to fall asleep; WASO, the amount of time spent awake after sleep onset; frequency of awakenings (FNA), the number of awakenings during the night; TWT, obtained by the sum of SOL and WASO; TST, the subtraction between the time in bed (TIB) and TWT; and SE, the ratio of TST over TIB.
PSG Recordings
PSG was recorded during 4 nights and 2 naps. The same montage was used for every recording. A standard PSG montage was used including electroencephalography (EEG; F3, F4, Fz, C3, C4, Cz, P3, P4, Pz, O1, and O2), electromyography (EMG; electrodes on chin), electrocardiography (ECG; electrode on heart) and electro-oculography (EOG; one electrode on the supraorbital ridge of the right eye and another on the infraorbital ridge of the left eye) recordings. Reference electrodes were fixed on the mastoids and the ground was on the forehead. On the first night, leg EMG (electrodes on tibialis) and breathing devices were used to detect breathing disorders and limb movements. The inter-electrode impedance was maintained < 5 kΩ. To amplify the signal from the electrodes, a Grass Model 15A54 amplifier system (Astro-Med Inc., West Warwick, USA; gain 10000; bandpass 0.3-100 Hz) was used, and PSG signals were digitized at a sampling rate of 512 Hz with the commercial product Harmonie (Stellate system, Montreal, Canada). PSG recordings during sleep and nap were visually scored (Luna, Stellate system, Montreal, Canada) by experienced sleep technicians using Rechtschaffen and Kales' criteria32 at 20-sec epochs.
In the present study, the objective sleep variables of interest were: SOL, defined as time from lights out to the first epoch of stage 1 sleep; WASO, the time spent awake after sleep onset; TWT, total time spent awake during the nap; TST, the time spent sleeping from lights out to lights on; number of awakenings after sleep onset and; and SE, the ratio of TST over TIB.
Statistical Analyses
One-way ANOVAs were used to compare groups on socio-demographic variables, psychological characteristics, and subjective sleep variables from the sleep diary. Independent samples t-tests were then performed on significant main effects. Repeated measures ANOVAs were used to compare groups on objective sleep parameters of nights (duration of each sleep stage and SOL) and on objective sleep parameters of naps (SOL, WASO, number of awakenings, and TWT). Bonferroni post hoc analyses were then performed on significant main effects. Repeated measures ANCOVAs were computed to compare groups on the other sleep parameters of nights (WASO, TST, TWT, and SE) and naps (SE and TST). Age was used as a covariate since it was significantly different between groups, and it was correlated with WASO (night 2: R = 0.39, p = 0.006; night 3: R = 0.29, p = 0.045), TWT (night 2: R = 0.37, p = 0.009; night 3: R = 0.27, p = 0.059), SE (nap 1: R = -0.30, p = 0.032; nap 2: R = -0.28, p = 0.065; night 2: R = -0.40, p = 0.005; night 3: R = -0.28, p = 0.051), and TST (nap1: R = -0.30, p = 0.041; nap 2: R = -0.26, p = 0.080; night 2: R = -0.42, p = 0.003; night 3: R = -0.35, p = 0.014). A Sidak correction was then performed on significant main effects of groups. Bilateral Pearson correlations were computed between SE of night and SE of its corresponding nap and between the SSS score and SE during the nap. Significance levels were set at 0.05.
Variables of participants who did not fall asleep during naps were included in the above statistical analyses; since all participants stayed in bed for the full 20 min, a value of 20 was attributed for SOL for those who did not sleep.
RESULTS
Socio-demographic, Psychological Measures, and Subjective Sleep Variables
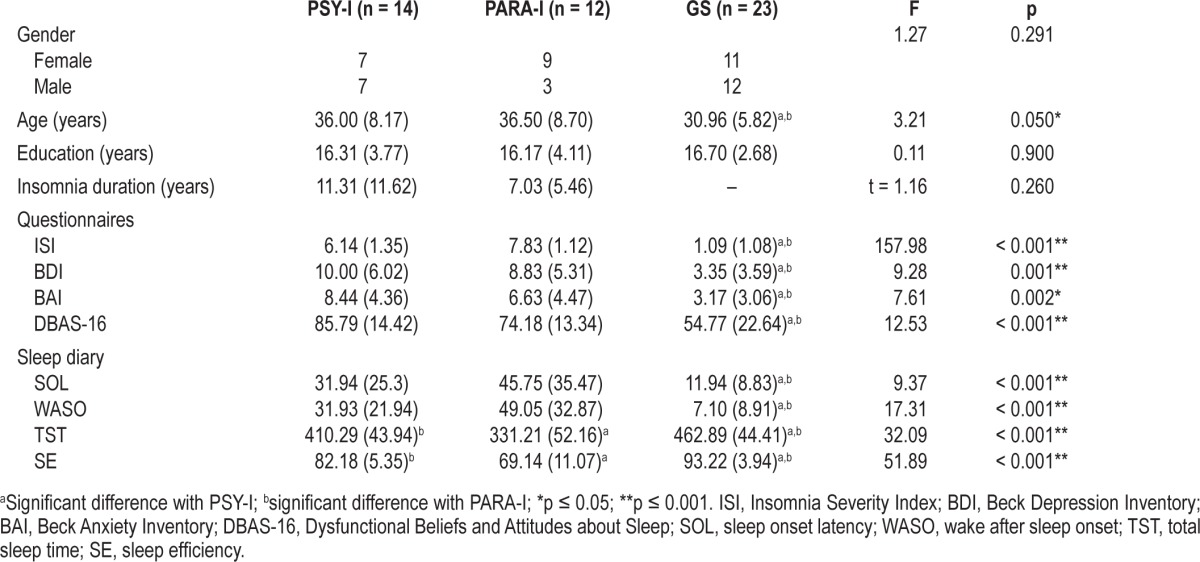
Statistical analyses showed that PSY-I, PARA-I and GS were similar in gender (p = 0.291), and education (p = 0.900). GS were significantly younger than PSY-I and PARA-I (p = 0.050), age varying between 25 and 49. There was no significant difference between INS groups concerning the duration of insomnia (p = 0.260), ranging from 0.25 to 30 years. Analyses also revealed that the severity of insomnia symptoms measured by the ISI varied between 0 and 9 and was significantly greater in PSY-I and PARA-I than GS (p < 0.001). Both groups of INS reported more depressive symptoms (BDI scores ranging from 0 to 20 [p = 0.001]), and anxiety symptoms (BAI scores ranging from 0 to 15 [p = 0.002]), than GS; and scores on the DBAS-16 were significantly higher for PSY-I and PARA-I than GS (p < 0.001), with scores ranging from 17 to 108. Finally, analyses revealed significant differences among groups for all variables on the sleep diary (p < 0.001), values for SOL varying from 1.72 to 116.79 min, from 0 to 105.36 min for WASO, from 223 to 552 min for TST, and from 51.90% to 99.90% for SE. Again, INS reported longer SOL and WASO while reporting shorter TST and lower SE than GS. Therefore, subjectively, INS had poorer sleep quality and quantity than GS. Table 1 illustrates means and SDs for each of the above variables.
Table 1.
Means (SD) of sociodemographic, psychological data and subjective sleep variables of psychophysiological INS (PSY-I), paradoxical INS (PARA-I), and good sleepers (GS)
Objective Sleep Parameters and Subjective Sleepiness Measures
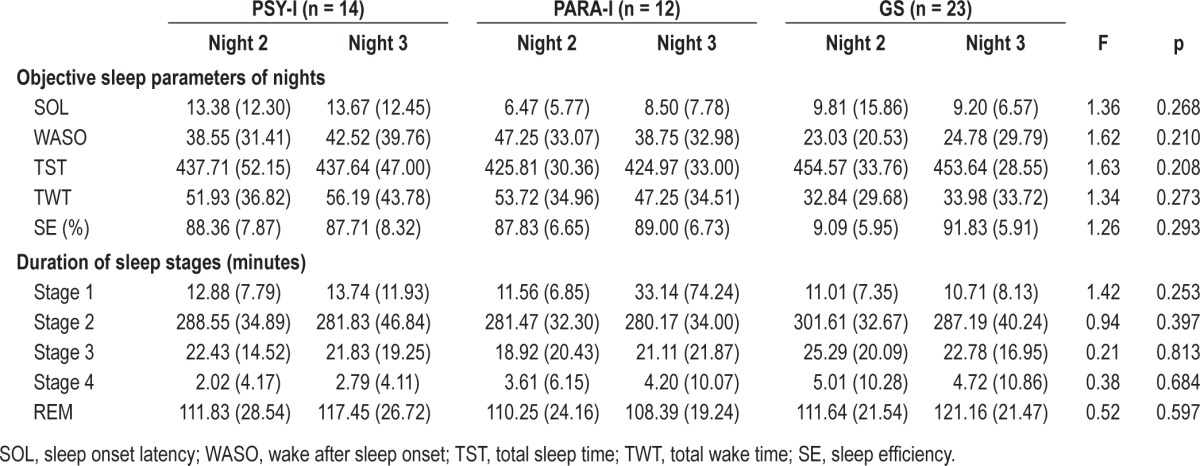
No significant differences between groups were found for all objective sleep parameters of nights (0.208 ≥ p ≥ 0.293); SOL ranging from 0.67 to 75.33 min, SE from 68% to 97%, WASO from 3.33 to 151.33 min, TST from 349 to 519.33 min, and TWT from 6.33 to 131 min. For the duration of sleep stages, no effect of groups was found for any stages (0.253 ≥ p ≥ 0.813). The duration of stage 1 varied from 0 to 267 min, from 147.33 to 360 min for stage 2, from 0 to 68.67 min for stage 3, from 0 to 49.67 for stage 4, and from 65.67 to 169.33 for REM sleep. Since no significant differences were found between groups for objective sleep parameters on either night, no sleep patterns between groups could be identified. See Table 2 for more details on objective sleep parameters of both nights.
Table 2.
Means (SD) of polysomnographic objective sleep parameters of nights of psychophysiological INS (PSY-I), paradoxical INS (PARA-I), and good sleepers (GS)
On objective sleep parameters of naps (naps were treated separately), analyses revealed main effect of groups for SOL (p = 0.008), values ranging from 0 to 20 minutes. Post hoc analyses indicated that PARA-I had a significantly longer SOL than GS (p = 0.014), and the difference between PSY-I and GS was marginally significant (p = 0.078), PSY-I having a longer SOL than GS. A significant difference was also found for TWT (p = 0.003), with PSY-I (p = 0.019), and PARA-I (p = 0.010) spending significantly more time awake during their naps than GS. Values of TWT varied from 0.33 to 20 minutes. No main effects of groups were observed for WASO (p = 0.110) and number of awakenings (p = 0.427), with WASO varying from 0 to 19.67 min and number of awakenings from 0 to 3.
When controlling for age, analyses showed a significant group effect for TST (p = 0.008), where TST was significantly shorter for PSY-I (p = 0.021) and PARA-I (p = 0.034) than GS. Values of TST ranged between 0 to 20 min. Finally, values of SE varied from 0 to 98% and was significantly different between groups (p = 0.009), SE being significantly higher for GS than PSY-I (p = 0.025) and PARA-I (p = 0.036). Conversely, when analyses compared objective measures from the first nap with those of the second one, no main effect of naps was found. Therefore, objective nap measures were similar on both napping opportunities. In sum, analyses on objective sleep parameters of naps showed that GS had a better capacity to nap than PARA-I and PSY-I. See Table 3 for more comprehensive details on objective parameters of naps.
Table 3.
Means (SD) of polysomnographic objective sleep parameters of naps and subjective sleepiness of psychophysiological INS (PSY-I), paradoxical INS (PARA-I), and good sleepers (GS)
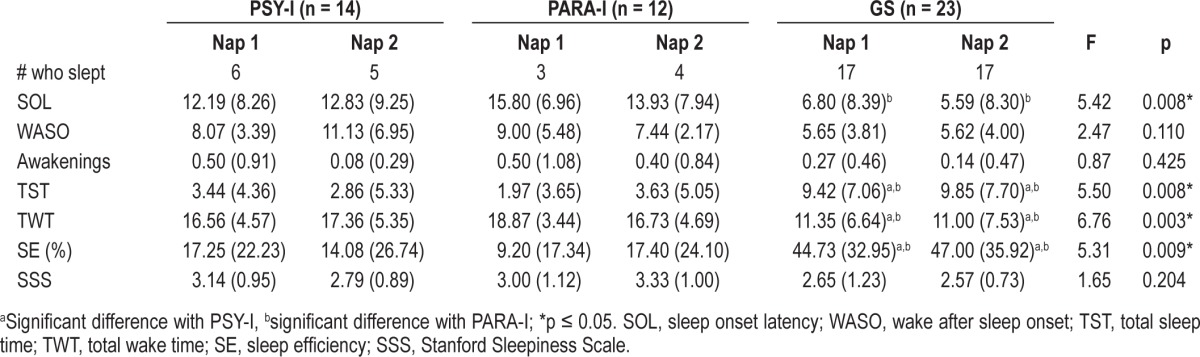
For the subjective sleepiness measure, analyses revealed no effect of groups for the SSS (p = 0.204), and scores varied from 1 to 5. Table 3 illustrates means and SDs for subjective sleepiness before naps from the SSS.
Correlations between Objective and Subjective Measures
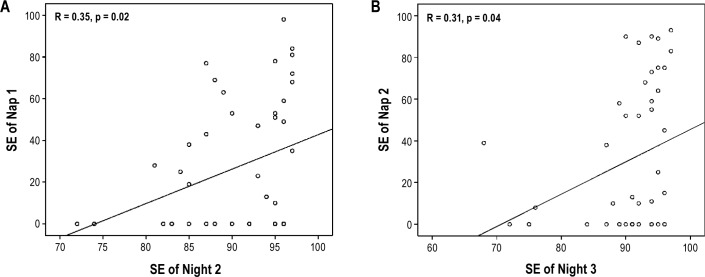
Bilateral Pearson correlation between SE of night 2 and SE of nap 1 was significant (R = 0.35, p = 0.015). Also, SE of night 3 and SE of nap 2 were significantly positively correlated (R = 0.31, p = 0.038). For both bilateral Pearson correlations, Mahalanobis distances confirmed the absence of bivariate outliers at a critical value of p ≤ 0.001. Figure 1 illustrate these relationships on scatterplots. When analyses were computed on each group separately, no significant correlations were found between night 2 and nap 1 (PSY-I: R = 0.38, p = 0.201; PARA-I: R = 0.22, p = 0.500; GS: R = 0.24, p = 0.276) as well as between night 3 and nap 2 (PSY-I: R = 0.40, p = 0.179; PARA-I: R = 0.26, p = 0.467; GS: R = 0.15, p = 0.509).
Figure 1.
(A) Correlation between sleep efficiency (SE) of night 2 and SE of nap 1. (B) Correlation between SE of night 3 and SE of nap 2.
No significant correlation was found between the SSS and SE of nap 1 (R = 0.22, p = 0.313) as well as with SE of nap 2 (R = 0.33, p = 0.129). Mahalanobis distances revealed no bivariate outliers for both correlations at the critical value of p ≤ 0.001. When bilateral Pearson correlations were performed on each group independently, no significant correlations were found between SSS and SE of nap 1 (PSY-I: R = 0.18, p = 0.568; PARA-I: R = -0.22, p = 0.517; GS: R = 0.22, p = 0.313) or between SSS and SE of nap 2 (PSY-I: R = 0.19, p = 0.543; PARA-I: R = 0.25, p = 0.483; GS: R = 0.33, p = 0.129).
DISCUSSION
In the present study, GS and INS, classified in psychophysiological and paradoxical types, were compared on sleep parameters and characteristics during naps following a mentally challenging battery of cognitive tests. Socio-demographic data revealed that both groups of INS were significantly older than GS. Until now, there has been no data available to our knowledge to illustrate the impact of age on PSG recordings variables during a single nap. However, a review paper on MSLT revealed that age contributed to a significant increase in MSLT latency.33 Since groups were significantly different in age, this was factored in our statistical design. Therefore, we could ensure the significant difference of age between groups did not contribute to the significant differences between groups found for some nap parameters.
Results failed to show significant differences between GS, PSY-I, and PARA-I on objective sleep parameters during both nights of PSG recording. These results might be explained by the fact that INS usually sleep better in the laboratory than at home and that GS have a poorer sleep quality in the laboratory. Therefore, sleep patterns of these two populations during laboratory PSG recordings tend to be similar, and the differences that actually exist between them are attenuated. The poorer quality of sleep obtained by GS during laboratory nights might result in some kind of partial sleep deprivation, which could explained why GS slept better during their naps following prolonged cognitive testing compared to both groups of INS. In fact, GS fell asleep significantly faster, their TST was longer, their TWT was shorter, and their SE was greater than PSY-I and PARA-I. Conversely, our results also suggest that for INS, hyperarousal appears to predominate over mental exhaustion following cognitive testing. One explanation might be that completing the battery of cognitive tests increased the cognitive load, which in turn contributed to hyperactivation of cognitive functions in INS, and prevented them from falling asleep. As for GS, prolonged cognitive testing most likely did not contribute to cognitive arousal but more to mental fatigue, as they slept relatively well during naps. Results obtained for objective sleep parameters during naps support the neurocognitive model of insomnia stating that cognitive arousal contributes to poor sleep in insomnia.15 These results may parallel what is observed at night when INS experience cognitive loading and are unable to fall asleep. This finding suggests that the neurocognitive model is not only applicable to nighttime sleep but also to napping, and it could be an explanation for the inability to nap characterizing INS.15,34
Data from the SSS completed at the end of cognitive testing and before napping support previous observations and confirm the present hypothesis, that hyperarousal contributes to the inability to nap. In fact, even though the analyses did not reach significance for the SSS, PSY-I had higher scores than PARA-I and GS, and the scores of PARA-I on the SSS were greater than GS. These results suggest that after prolonged cognitive testing PSY-I reported being the sleepiest, followed by PARA-I and then GS. So, both groups of INS subjectively reported being sleepy, but they were, in general, unable to nap, which suggests again that hyperarousal predominates over sleepiness in insomnia. That said, it could be suggested that GS were as mentally exhausted as PSY-I and PARA-I after testing, but since they were not as cognitively aroused, they slept better. There is also the possibility that partial sleep deprivation explains napping abilities in GS.
We found significant positive correlations between SE during the night and SE of the nap on the next day. As such, it appears that the better participants slept during the night, the better was their ability to nap the next day. The opposite is also true; a low SE for the night led to a low SE during nap on the following day. These results are difficult to reconcile with the literature presented earlier and with other results. Still, nightly and daily sleep efficiencies varied together only when the total sample was taken as a whole and not when groups were studied independently. It is possible that the few observations in each group lead to a lack of power, hence a lack of within-group significant relationship between night and day. Nonetheless, it is also possible than instead of varying with the present night of sleep, daily SE might vary with the SE of the subsequent night of sleep. As is often acknowledged in CBT-I (in the sleep restriction module and/or sleep hygiene instructions16), napping during the day may well influence or borrow on the following night of sleep in INS but not on the nocturnal SE of GS. Our sample had more GS than INS as a whole and might just reflect this last statement. Still, this hypothesis remains to be tested in a larger sample and also on subsequent days and nights.
Alternatively, one might argue that for INS, a greater sleep pressure would build up as a result of the quality of sleep during the night, which would equate to a better nap opportunity the next day. However, the fact that INS tend to increase their time spent in bed in order to increase their sleeping time would most likely contribute to an elevation of hyperarousal, which would diminish the nap opportunity. Additionally, a 20-minute window was used for the naps, similar to a MSLT protocol. Maybe it is not long enough to fall asleep when participants are tired; the time required to fall asleep might be higher in this case. There is also the possibility that SE during the nap would have been normal after a bad night sleep if participants were allowed to nap as long as they wish and if the time pressure to fall asleep was removed. However, the observation obtained in the present study confirmed results previously found.14
In general, the results have shown no significant differences between PSY-I and PARA-I for nap parameters. Even though diagnostic criteria for PSY-I and PARA-I are different,4 there is a possibility that the level of hyperarousal during the day is similar for both groups of INS, which would explain why no significant differences were found between these two groups for objective nap parameters. Also, if levels of hyperarousal are independent of the amount of nocturnal sleep obtained objectively, it would explain the subjective reports of poor sleep in PARA-I.5 Nonetheless, the distinction between PSY-I and PARA-I is not as clear when hyperarousal is taken into account. Future studies on objective nap parameters in INS should take this into consideration and combine PSY-I and PARA-I since there is a possibility that hyperarousal influences to a comparable extent the quality of naps in both types of insomnia. However, this hypothesis also remains to be tested.
The small number of participants in each group limits the interpretations of our results. Therefore, we have to be careful when generalizing and a replication with a larger sample is warranted. Also, to ensure participants actually experienced mental fatigue after completing the battery of cognitive tests, a scale of mental exhaustion should have been used. This would have allowed determining as to whether mental exhaustion contributed to the difficulty in napping in INS. Moreover, we also assume that cognitive testing had an impact on sleep characteristics of naps, but it is possible that the same results would have been obtained in the absence of mentally exhausting tests. Therefore, the presence of a nap not preceded by cognitive testing would have been useful to identify if the results obtained were influenced by the tests or if they had no impact on naps. It would have allowed us to determine if diagnosis alone was sufficient to explain between-group differences on sleep parameters during naps or if prolonged cognitive tests contributed to the results.
To conclude, it seems that INS, independent of type, are more hyperaroused than GS during napping. This observation suggests that the high level of hyperarousal characterizing insomnia influences their functioning not only during the night, but also during the day. Finally, this study contributes to a better understanding of the phenomenon of hyperarousal and gives some insights for future research in the field of insomnia. Additionally, these results confirm once more that insomnia is a 24-hour problem, particularly in the hyperarousal domain. Nonetheless, more studies need to explore nap parameters in a population of INS in order to support these results.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the Canadian Institutes of Health Research (CIHR; # 49500, 86571) and les Fonds de Recherche en Santé du Québec (FRSQ; # 23028). The authors have indicated no financial conflicts of interest. The study was conducted at Laboratoire de Neurosciences Comportementales Humaines du Centre de Recherche de l'Institut Universitaire en Santé Mentale de Québec, Québec, Canada.
ACKNOWLEDGMENTS
The authors thank Sonia Petit for analysing PSG recordings and all the research assistants who helped in cognitive testing and data entry.
REFERENCES
- 1.Bastien CH. Insomnia: Neurophysiological and neuropsychological approaches. Neuropsychol Rev. 2011;21:22–40. doi: 10.1007/s11065-011-9160-3. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, LeBlanc M, Bélanger L, Ivers H, Mérette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56:540–8. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed; diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 5.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 6.Espie CA. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorders in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 7.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 8.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: toward a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. The consequences of a week of insomnia II: patients with insomnia. Sleep. 1998;21:359–68. [PubMed] [Google Scholar]
- 11.Bonnet MH, Arand DL. Activity, arousal, and the MSLT in patients with insomnia. Sleep. 2000;23:1–8. [PubMed] [Google Scholar]
- 12.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: Stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepanski E, Zorick F, Roehrs T, Young D, Roth T. Daytime alertness in patients with chronic insomnia compared with asymptomatic control subjects. Sleep. 1988;11:54–60. doi: 10.1093/sleep/11.1.54. [DOI] [PubMed] [Google Scholar]
- 15.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM. Insomnia: psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 17.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 18.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 19.Seidel WF, Ball S, Cohen S, Patterson N, Yost D, Dement WC. Daytime alertness in relation to mood, performance, and nocturnal sleep in chronic insomniacs and noncomplaining sleepers. Sleep. 1984;7:230–8. doi: 10.1093/sleep/7.3.230. [DOI] [PubMed] [Google Scholar]
- 20.Sugerman JL, Stern JA, Walsh JK. Daytime alertness in subjective and objective insomnia: some preliminary findings. Biol Psychiatry. 1985;20:741–50. doi: 10.1016/0006-3223(85)90153-2. [DOI] [PubMed] [Google Scholar]
- 21.Gross RT, Borkovec TD. Effects of a cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behav Ther. 1982;13:112–6. [Google Scholar]
- 22.Wuyts J, De Valck E, Vandekerckhove M, et al. The influence of pre-sleep cognitive arousal on sleep onset processes. Int J Psychophysiol. 2012;83:8–15. doi: 10.1016/j.ijpsycho.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 23.McDevitt EA, Alaynick WA, Mednick SC. The effect of nap frequency on daytime sleep architecture. Physiol Behav. 2012;107:40–4. doi: 10.1016/j.physbeh.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- 26.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 27.Bastien CH, Guimond S, St-Jean G, Lemelin S. Signs of insomnia in borderline personality disorder individuals. J Clin Sleep Med. 2008;4:462–70. [PMC free article] [PubMed] [Google Scholar]
- 28.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): Validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JBW, Gibbon M, First MB, et al. The structured clinical interview for DSM-III (SCID). 2. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 30.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 33.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal B. The clinical use of the MSLT and MWT. Sleep. 2005;28:123–44. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 34.Moul DE, Nofzinger EA, Pilkonis PA, Houck PR, Miewald JM, Buysse DJ. Symptom report in chronic severe insomnia. Sleep. 2002;25:548–58. [PubMed] [Google Scholar]