Abstract
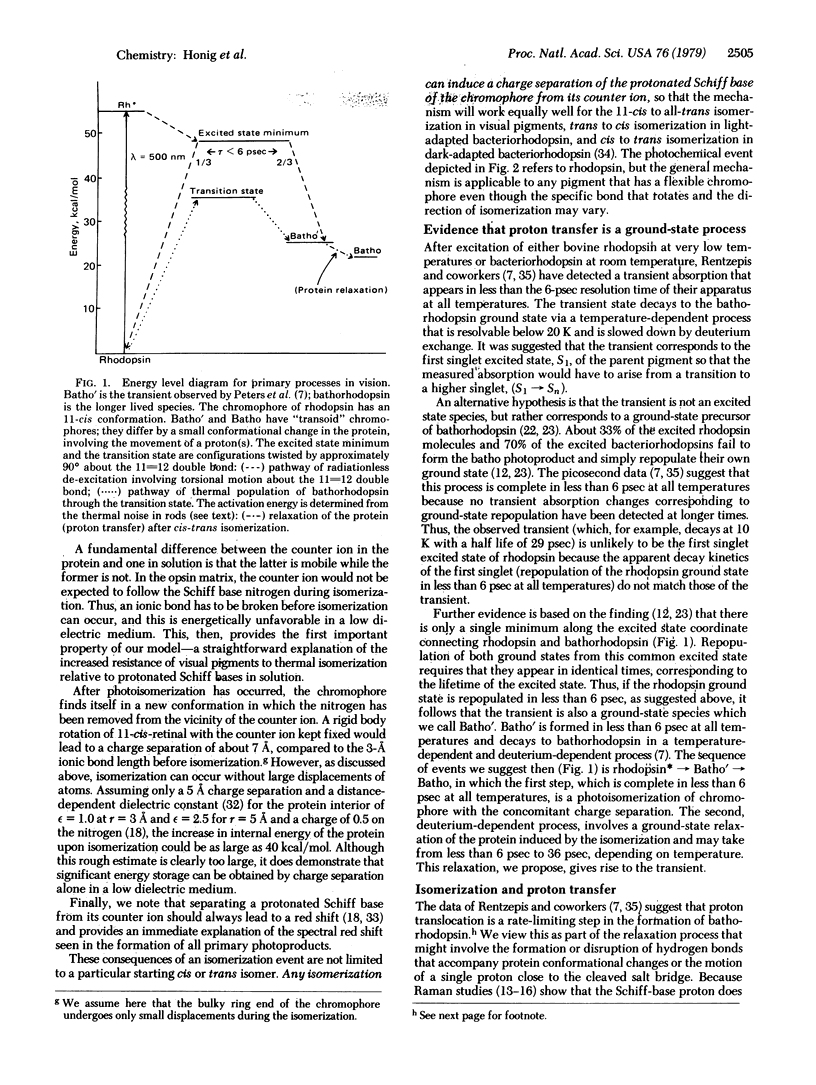
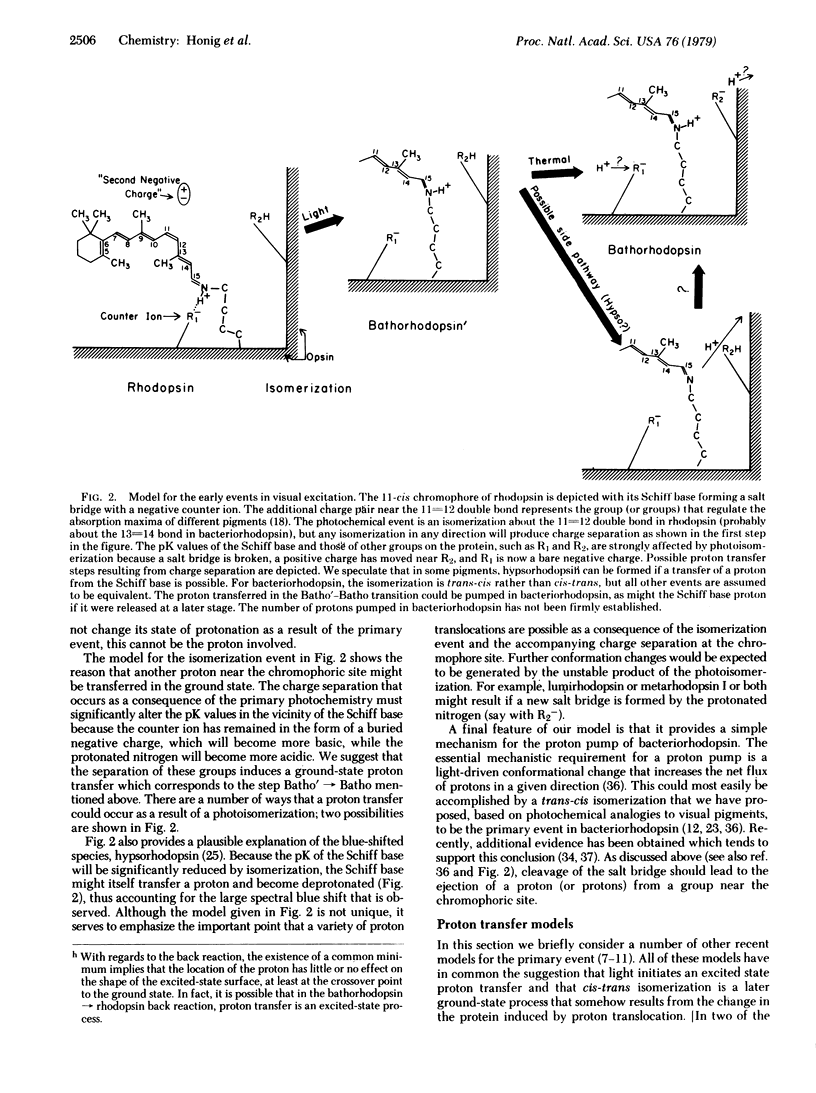
A simple model for the early events in visual pigments and bacteriorhodopsin is proposed. The model makes use of the likelihood that a negatively charged amino acid forms a salt bridge with the positively charged nitrogen of the retinylic chromophore. The photochemical event is a cis-trans isomerization in visual pigments and a trans-cis isomerization in bacteriorhodopsin, which in each case cleaves the salt bridge and thus separates charge in the interior of the protein. We propose that this is how the energy of a photon is transduced into chemical free energy of the primary photoproduct. The use of photoisomerization of a flexible chromophore to achieve charge separation provides a general mechanism which may be applicable to other systems. Our model explains many of the fundamental properties of visual pigments and their photoproducts. First, the extraordinarily low rate of thermally populating the ground state of the primary photoproduct, as determined from psychophysical and electrophysiological measurements, is seen as resulting from the large barrier to thermal isomerization about a double bond, perhaps enhanced by electrostatic attraction in the salt bridge. Second, the increase in energy and the spectral red shift that characterize the primary photochemical events are natural consequences of the separation of charge. Proton-dependent processes detected with picosecond techniques are proposed to be ground-state relaxation processes following the primary photochemical event. Finally, the charged groups of the salt bridge, repositioned by photoisomerization, provide a simple mechanism for vectorial proton translocation in bacteriorhodopsin.
Keywords: charge separation in proteins, rhodopsin photochemistry, purple membrane, proton pumping
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Peters K. S., Rentzepis P. M. Primary intermediates in the photochemical cycle of bacteriorhodopsin. Biophys J. 1978 Sep;23(3):375–382. doi: 10.1016/S0006-3495(78)85456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Dark noise in retinal bipolar cells and stability of rhodopsin in rods. Nature. 1977 Nov 3;270(5632):69–71. doi: 10.1038/270069a0. [DOI] [PubMed] [Google Scholar]
- Aton B., Callender R. H., Honig B. Photochemical cis-trans isomerisation of bovine rhodopsin at liquid helium temperatures. Nature. 1978 Jun 29;273(5665):784–786. doi: 10.1038/273784a0. [DOI] [PubMed] [Google Scholar]
- Blatz P. E., Mohler J. H. Effect of selected anions and solvents on the electron absorption, nuclear magnetic resonance, and infrared spectra of the N-retinylidene-n-butylammonium cation. Biochemistry. 1975 Jun 3;14(11):2304–2309. doi: 10.1021/bi00682a005. [DOI] [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. Molecular aspects of photoreceptor function. Q Rev Biophys. 1975 May;8(2):129–184. doi: 10.1017/s0033583500001785. [DOI] [PubMed] [Google Scholar]
- Eyring G., Mathies R. Resonance Raman studies of bathorhodopsin: evidence for a protonated Schiff base linkage. Proc Natl Acad Sci U S A. 1979 Jan;76(1):33–37. doi: 10.1073/pnas.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. H., Monger T. G., Alfano R. R., Aton B., Callender R. H. Cis-trans isomerisation in rhodopsin occurs in picoseconds. Nature. 1977 Sep 8;269(5624):179–180. doi: 10.1038/269179a0. [DOI] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Bownds D., Yoshizawa T. The chemistry of visual photoreception. Cold Spring Harb Symp Quant Biol. 1965;30:301–315. doi: 10.1101/sqb.1965.030.01.032. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. The stereoisomerization of 11-cis-retinal. J Biol Chem. 1966 Apr 25;241(8):1814–1818. [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Goldschmidt C. R., Ottolenghi M. On the photocycle and light adaptation of dark-adapted bacteriorhodopsin. Biophys J. 1977 Aug;19(2):185–189. doi: 10.1016/S0006-3495(77)85579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf A. Is proton transfer the initial photochemical process in vision? Nature. 1976 Nov 4;264(5581):92–94. doi: 10.1038/264092a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Sulkes M., Lewis A., Marcus M. A. Resonance Raman spectroscopy of squid and bovine visual pigments: the primary photochemistry in visual transduction. Biochemistry. 1978 Oct 31;17(22):4712–4722. doi: 10.1021/bi00615a018. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Iwasa T., Yoshizawa T. Photochemical reaction of bacteriorhodopsin. FEBS Lett. 1976 Dec 15;72(1):33–38. doi: 10.1016/0014-5793(76)80807-1. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Charge stabilization mechanism in the visual and purple membrane pigments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2558–2562. doi: 10.1073/pnas.75.6.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- van der Meer K., Mulder J. J., Lugtenburg J. A new facet in rhodopsin photochemistry. Photochem Photobiol. 1976 Oct;24(4):363–367. doi: 10.1111/j.1751-1097.1976.tb06837.x. [DOI] [PubMed] [Google Scholar]


