Abstract
Study Objective:
Determine whether a salivary biomarker of physical fatigue, referred to as the fatigue biomarker index (FBI), can discriminate a control group from a sleep deprived group when saliva is collected under controlled conditions. The study expands on previous work examining changes in the composition of saliva during periods of prolonged exercise.
Methods:
Thirty (30) young adults (14 Control [CON]; 16 Sleep Deprived [SDEP]) were monitored for mood state (Profile of Mood States [POMS]), cognitive performance (Stroop Color-Conflict Tests), and salivary biomarkers of physical fatigue over a 48-h period with sampling at 3-h intervals. Trials lasted from 06:00 on day 1 (time = -3 h) to 09:00 on day 3 (time = 48 h). Levels of salivary biomarkers were calculated from liquid chromatography-mass spectrometry (LC-MS) data. Statistical comparisons were made using Wilcoxon rank sum tests with a Bonferroni correction to limit type 1 error. Receiver-operator characteristic (ROC) analysis was used to evaluate the ability of the various parameters to distinguish the SDEP population from the CON population.
Results:
Longitudinal analysis demonstrated significant between-group differences in all three parameters. ROC analysis demonstrated that cognitive performance tests and salivary biomarkers of physical fatigue distinguish the SDEP population from the CON population.
Conclusions:
A previously identified salivary biomarker of physical fatigue may provide an alternative method for discriminating sleep deprived from rested individuals. The salivary biomarker of physical fatigue holds promise as an objective measure of sleep deprivation, perhaps eventually removing the reliance on self-reported sleep diaries and/or repeated polysomnographs for longitudinal tracking of sleep quality and/or diagnosis of sleep disorders.
Citation:
Michael DJ; Valle B; Cox J; Kalns JE; Fogt DL. Salivary biomarkers of physical fatigue as markers of sleep deprivation. J Clin Sleep Med 2013;9(12):1325-1331.
Keywords: Sleep deprivation, fatigue, biomarkers, mass spectrometry, chromatography, liquid, analytical chemistry methods
There are relatively few objective methods for the diagnosis and longitudinal monitoring of sleep disorders.1,2 At present, clinicians rely primarily on patients' self-reported levels of sleepiness, as well as expensive, time-consuming, and relatively intrusive overnight polysomnographs (PSG).3 Other objective methods proposed for use in sleep medicine include actigraphy, electroencephalography (EEG), multiple sleep latency test (MSLT), reaction times, pupillography, melatonin levels, metabolic rate, body temperature, heart rate, and heart rate variability.2,4–6 While each of these techniques has proven promising, none has yet been adopted for the routine diagnosis and monitoring of all sleep disorders. Given the wide range of sleep disorders and the large number of other diseases which also alter sleep,2,7 it is clear that there is a need for additional objective measures of sleep health.
Insufficient sleep impairs a number of specific functions, as well as an individual's general quality of life. Symptoms arising from sleep loss include increased propensity to fall asleep in inappropriate settings, inability to concentrate, impaired cognitive ability, slowed reaction time, reduced vigilance, headache, mood changes, and fatigue.8,9 Numerous studies have demonstrated that the levels of impairment arising from acute and chronic sleep deprivation are similar to those observed in individuals with elevated blood alcohol content.10–14 The social and economic consequences associated with untreated or improperly treated sleep disorders are significant.15,16
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previously, we described changes in the peptide composition of saliva during periods of prolonged physical exertion. Here, we determined whether similar changes in the peptide composition of saliva are associated with sleep and/or sleep deprivation.
Study Impact: At present, daytime sleepiness is assessed either by self-report, e.g. Epworth Sleepiness Scale, or by expensive and time-consuming clinical tests, e.g., multiple sleep latency test. An inexpensive and objective method to measure sleepiness would significantly improve a clinician's ability to diagnose and/or treat sleep disorders.
The need for objective methods to diagnose and/or track sleep disorders is especially important in circumstances when individuals are motivated not to report truthfully either their amount of sleep or their level of fatigue. For example, financial pressure might motivate an individual to misreport sleep and/or fatigue, such as in a career field where pay is dependent upon hours worked, e.g., an airline pilot or long-haul truck driver. Alternatively, social pressure might motivate an individual to misreport sleep and/or fatigue, such as for an individual who is part of team, e.g., a member of an elite military unit or sports team. In these circumstances, self-reported values of sleep and/or fatigue might differ significantly from true levels, thereby endangering the individual and others around them.
Recently, we have reported that physical fatigue changes the composition of saliva. Specifically, we found that the ratio of two endogenous salivary peptides changed significantly as individuals became more fatigued due to prolonged physical exercise.17 The amino acid sequences of both heptapeptides were determined in our previous work. One peptide had the sequence GGHPPPP (657.7 Da), while the other peptide had the sequence ESPSLIA (715.8 Da). Both of these peptides arise naturally from the family of proteins known as the salivary proline-rich proteins (PRPs).18 The specific ratio of peptides we reported, termed the fatigue biomarker index or FBI, was calculated as the abundance of GGHPPPP divided by the abundance of ESPSLIA. When the ratio is constructed in this way, the value of the FBI decreases as fatigue increases, much like pH levels decrease as the concentration of protons in solution increases. Our primary interest in developing this technology was to provide the US military with an objective measure of fatigue arising from physical activity. The value of this technology in military settings was highlighted by a previous study showing that an individual's FBI value at the start of training is useful as part of a model for predicting the outcome of training for candidates entering Special Forces training within the United States Air Force.19
Here, we report findings from a preliminary investigation of changes in FBI values during periods of sleep deprivation. Previously, we used data from the same study to investigate changes in cognitive performance20,21 and heart rate variability.20 While this study was designed to mimic typical conditions experienced in a military, rather than civilian, setting and included only a relatively small group of young, healthy adults, the results are promising and suggest that the small peptide composition of saliva can be used to monitor an individual's sleep.
METHODS
Selection Criteria
We recruited men between the ages of 18 and 35 years for inclusion in the study. Potential subjects included healthy (asymptomatic) college students, ROTC cadet trainees, and recreational (non-varsity) athletes self-reporting as healthy and fit enough to enter basic military or first responder training, non-smoking, free of disease, and not taking any psycho-tropic medications or dietary supplements that would alter neural or metabolic function. Subjects meeting our eligibility criteria were asked to read and sign an informed consent document approved by the Committee for the Protection of Human Subjects in Research of the University of Texas at San Antonio. This committee approved this study and provided oversight of all human research procedures.
Experimental Design
All protocols were conducted in the Exercise Biochemistry and Metabolism Laboratory of the Department of Health and Kinesiology at The University of Texas at San Antonio. Consented subjects completed a basic medical history screening form, a standard physical examination, skinfold body fat analysis, and a clinical graded treadmill stress test with electrocardiogram to identify preexisting heart conditions that could compromise safe participation and determine aerobic fitness level via indirect calorimetry (TrueMax 2400, ParvoMedics Sandy, UT). Final selection of subjects was dependent upon normal clinical results and history as determined by a participating physician indicating eligibility for safe inclusion in the study. During the health screening session consented subjects were familiarized with all testing planned for the subsequent 48-h protocol.
Eligible subjects (n = 35) were randomly assigned to 1 of 2 experimental groups: (1) control (CON; n = 16) or (2) sleep deprived (SDEP; n = 19). (Note: Analysis of saliva samples was limited to subjects who completed the entire study [CON = 14; SDEP = 16]). The 48-h protocol took place ≥ 1 week following the health screening and familiarization session. The aim of the protocol was to increase fatigue gradually and safely over the course of 48 h. In the present study, we employed a modified version of a 24-h protocol we developed in which participants experienced sleep deprivation and were evaluated for cognitive performance and fatigue level every 3 h.21
All participants reported to the laboratory at 06:00 following an 8-h fast that excluded caffeine or other stimulants. They immediately received a small standardized breakfast (375 kcal) with water. For the purpose of analyzing data in this study, we define 06:00 as Time = -3 h. Data for the 48-h period were collected every 3 h from 09:00 on day one to 09:00 on day 3 (17 data collection points total). Every data collection point was 2 h post-prandial and post-fluid ingestion. Total dietary food and fluid intakes were controlled and provided at levels considered normal for the subject's age, weight, and daily activity level, allowing subjects to remain hydrated (data not shown).
Participants assigned to the CON group were allowed to sleep between the hours of 22:00-09:00, although they were awoken at 00:00, 03:00, and 06:00 and remained awake for approximately 1 h for data collection. Participants assigned to the SDEP group were monitored throughout the 48-h period and were not allowed to sleep. Schedules for data collection, as well as food and water intake, were identical for both groups. During non-sleep hours between data collection periods, all participants maintained a controlled but fixed daily schedule of very light activities (e.g., watching movies, studying, or reading).
Every 3 h during the 48-h period, subjects were weighed (in shorts and shirt, sans shoes). Before the subsequent collection of data, subjects then sat quietly for 20 min.
After the quiet period, subjects completed the Profile of Mood States22 (POMS) survey for assessment of fatigue level and Stroop Color-Conflict Test23 (Stroop tests) for assessment of cognitive performance. While the Stroop tests are known to be influenced by learning effects, they were selected for their ease of administration to groups of the size used in this study. The most relevant POMS factor for our investigation was that of “fatigue.” This factor is determined by the sum of Likert-style scoring of seven subjective feelings (“worn out,” “listless,” “fatigued,” “exhausted,” “sluggish,” “weary,” “bushed”). No time limit was given to complete the POMS survey. No performance feedback was provided to subjects for the survey. For the Stroop tests, subjects were instructed to read aloud as many items as possible in 45 s during each of 3 conditions (word, color, incongruent color-word pairs). Instructions for the Stroop tests were repeated at every data collection point. The number of correct responses for each 45-s test was recorded and no performance feedback was provided to subjects for the tests. The Stroop tests comprise 3 separate tests: color, word, and color-word. We added results from the 3 Stroop tests to calculate a “cumulative cognitive performance” score. We have previously demonstrated that the POMS fatigue factor tracks the decline in Stroop test cognitive performance during a 24-h fatiguing protocol including combinations of sleep deprivation, exercise, caloric restriction, and dehydration.21
Immediately after each data collection period, subjects were fed a small sandwich, raw vegetables, and cookies (300 kcal) with 0.4 L of water. The same meal was provided every 3 h to avoid possible digestion-related fluctuations in vagal tone following a large meal.
Saliva
Saliva samples (∼10 mL) were collected by passive drooling of clear saliva. Samples were placed on ice after collection and transferred to a -80°C freezer for storage until time of analysis. The salivary analyses have been published in detail.17 Briefly, raw saliva was processed through a series of molecular-weight-cutoff filters selected to remove components of saliva greater than ∼10 kDa. The amount of protein in the remaining solution was quantified (bicinchoninic acid [BCA] assay), so that a fixed amount of protein could be injected per sample (4 μg). To target the peptide components, we used a mass-specific tagging approach to label free amines in solution. Specifically, samples were labeled with light and heavy isotopes of acetic anhydride. The difference in mass of the 2 isotopes arises from the presence of protons or deuterons in all positions of both methyl groups in the acetic anhydride. By labeling 2 different aliquots of the same sample separately with the light and heavy variants of acetic anhydride, it was possible to identify those components of saliva with a free amine. These components appeared as pairs of ions separated by predictable masses in a mixture of the differently labeled samples. Once samples were labeled and analyzed by liquid chromatography with mass spectrometric detection, we quantified levels of a previously identified biomarker of physical fatigue,17 referred to as the fatigue biomarker index (FBI). As a ratio of ion intensities for 2 different salivary peptides, the FBI is resistant to trivial sources of change, e.g., the amount of material injected.
Statistical Analysis
All statistical analyses were completed using R (R24; version 2.9.0; cran.r-project.org). Wilcoxon rank sum tests were used for pairwise comparisons between groups at each time point (Figure 1) and across averaged data (Figure 2), with a Bonferroni correction applied to limit the risk of committing type 1 error. Receiver-operator characteristic (ROC) curves were also used to assess cross-sectional data using the pROC package for R.25 Our a priori significance level was p < 0.05.
Figure 1. Sleep deprivation led to significant changes in self-reported fatigue level, cognitive performance, and salivary biomarkers of physical fatigue.
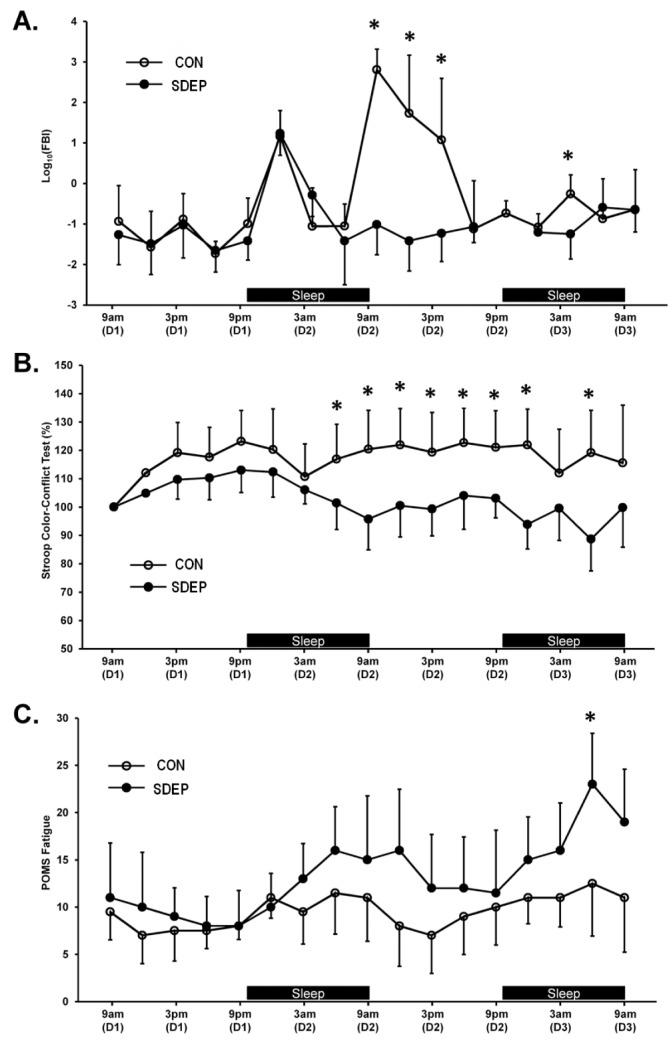
Subjects were evaluated every 3 h during the course of the study. (A) Levels of the salivary biomarker of physical fatigue remained similar across groups through the first hours of the study, and then changed significantly during the hours after the first night of sleep. Both groups showed a significant increase around midnight on the first day of the study, while only the CON group showed a similar, though muted, increase on the second day of the study. (B) Cumulative scores on the Stroop tests, shown as percentages relative to the subject's initial scores, remained similar across groups until the early morning hours of the second day. Scores for individuals in the SDEP group dropped significantly after the first evening, whereas scores for individuals in the CON group remained relatively constant. The initial upward slope seen in both groups is likely to do a learning effect. The absence of significant differences during these early time points suggests that the size of the learning effect was similar for both groups. (C) Self-reported fatigue levels drifted higher with time in both groups. Previous analysis using linear mixed-effects modeling showed a significant positive slope for both groups. With the statistical approach used here, groups did not differ significantly until late on the second day. *indicates significant difference between groups.
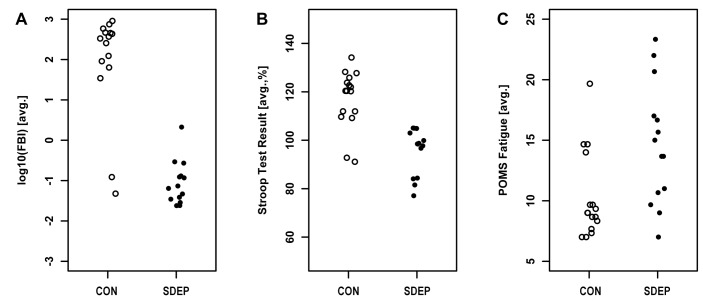
Figure 2. Salivary biomarkers of physical fatigue efficiently detected the effects of sleep deprivation.
Values for self-reported fatigue level, cognitive performance and salivary biomarkers of physical fatigue are shown by group for the data collected between 09:00 and 15:00 on the second day of the study. Each point represents average data for one individual. Receiver-operator characteristic (ROC) analysis suggested that salivary biomarkers of physical fatigue can identify sleep-deprived patients with reasonable specificity and selectivity (area under curve, 92%). Self-reported fatigue levels and cognitive performance tests also performed reasonably (area under curve, 77% and 88%, respectively).
RESULTS
Longitudinal Analysis
By using a 3-h sampling interval, this study provided relatively high resolution temporal data to evaluate changes in parameters of interest, namely self-reported fatigue level, cognitive performance, and salivary biomarkers of physical fatigue. Self-reported fatigue levels were derived from the profile of mood states (POMS) survey; cognitive data came from the Stroop color-conflict tests; and salivary biomarkers of physical fatigue were measured according to the previously described fatigue biomarker index (FBI). Longitudinal data are shown for all 3 parameters and both groups in Figure 1. While significant group differences were observed for all 3 parameters, the patterns of change differed. Significant between-group differences appeared first in the Stroop tests in the early morning hours after the first overnight period. Significant changes in the FBI followed soon after, while significant differences in POMS fatigue level were not observed until the early morning hours of the second overnight period. Whereas the significant difference in cognitive performance remained relatively stable after the first overnight period, differences in salivary biomarkers of physical fatigue were transient, lasting 6 to 9 h after the first overnight period of sleep deprivation. A significant difference in salivary biomarkers of fatigue reoccurred during the second overnight period.
Cross-Sectional Analysis
In addition to analyzing changes in parameters as a function of time, we also evaluated the ability of each of the parameters to discriminate the sleep deprived population from the control population. To make the plots shown in Figure 2, data from the window just after the first overnight period (D2-09:00 through D2-15:00) were averaged for each subject, and then plotted by group. We used this window because it is the most relevant testing window for a future clinical test, assuming testing during traditional U.S. business hours (09:00-17:00). significant between-group differences were observed for 2 (FBI and Stroop) of the 3 parameters. Receiver-operator characteristic (ROC) analysis suggested that cognitive performance tests and salivary biomarkers of physical fatigue performed well when trying to distinguish members of the SDEP and CON groups.
DISCUSSION
Summary
Here, we have compared the impact of sleep deprivation on three different parameters: self-reported fatigue level, cognitive performance, and salivary biomarkers of physical fatigue. The effects of sleep deprivation on the first two parameters have been described extensively elsewhere by us20,21 and by others,9 while salivary biomarkers of physical fatigue have been described only recently,17,19 and it was not yet known how they would be affected by sleep deprivation. We observed significant longitudinal changes in all three parameters, and two of the parameters, cognitive performance tests and salivary biomarkers of physical fatigue, also performed well in cross-sectional tests.
Possible Influence of Circadian Rhythms on the Fatigue Biomarker Index
During the initial part of the trial, the pattern of change for the FBI was similar for both the control and sleep deprived arms of the study. Unexpectedly, both traces included a significant increase in FBI values, suggesting a significant decrease in fatigue level, near midnight during the transition from day 1 to day 2 (Figure 1A). A similar, but blunted, increase is seen in the control arm at close to the same time in the second night (day 2-day 3 transition). These unexpected results suggest that the FBI may be affected by circadian rhythms, the hierarchy of oscillators that regulates a wide range of human behavior and physiology.26 In humans, the circadian system includes both a central pacemaker, the suprachiasmatic nucleus (SCN), and a series of peripheral components.26 The operation of these oscillators has been described at the cellular and molecular level, and a number of studies have demonstrated the tight relationship between the circadian clock, hormone secretion, and metabolism.26–29 In saliva, there is evidence of large-amplitude circadian rhythms for concentrations of several inorganic ions.30 Other components of saliva including cortisol,31 melatonin,32,33 and a variety of metabolites29,34 also follow circadian rhythms. In contrast, the circadian rhythms for salivary proteins in unstimulated saliva appear to be smaller in amplitude with a wide spread in acrophases, thereby removing significant oscillations from population data.30
Of the salivary components examined to date, melatonin has proven most promising as a direct measure of circadian rhythm.33 Specifically, the dim light melatonin onset (DLMO) marker appears to be a robust measure of circadian rhythm even in the presence of confounding factors,32 perhaps leading to its eventual use in clinical settings for the identification of circadian rhythm sleep disorders.35 In contrast, levels of cortisol in serum and saliva are known to be influenced by a number of factors in addition to circadian rhythms, including a variety of acute and chronic stressors such as insomnia, obstructive sleep apnea, depression, and chronic fatigue.36,37 While the exact relationship between measures of salivary cortisol and measures of sleep remains unclear,38 there have been a number of intriguing findings. Of particular note is a previous report describing an association between cortisol and fatigue/physical symptoms.39 Specifically, a population study of older adults identified significant associations of fatigue/physical symptoms with two different measures of cortisol, wakeup cortisol and cortisol awakening response (CAR). The reported association between low wakeup cortisol levels and increased fatigue/physical symptoms39 later the same day agrees with observations of diminished morning cortisol levels in studies of individuals with chronic fatigue syndrome (CFS).40,41 In the case of some sleep disorders, dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis has even been suggested to have a causative role.36 For example, nocturnal salivary cortisol levels are consistently elevated in insomnia,42 providing support for the hypothesis that insomnia arises from hyperarousal of the HPA axis. In other sleep disorders, such as obstructive sleep apnea,36 changes in cortisol are believed to be secondary. However, the wide range of external factors influencing salivary cortisol levels may limit their utility in the diagnosis of sleep disorders.
At present, the mechanism(s) regulating the generation of the peptides of the FBI remains unknown, and it remains unclear whether levels of these peptides might also change in response to other stressors besides sleep deprivation. Given the potential presence of circadian rhythms and the lack of understanding about other potential influences affecting levels of these peptides, FBI measurements should be interpreted carefully.
Other Measures of Sleepiness
Given the increasing and varying needs of researchers, clinicians, patients, regulators, lawmakers, and insurers, several other approaches to quantifying sleepiness are also under development.43–45 Methods to quantify sleepiness have arisen from various behavioral, electrophysiological, genetic, proteomic, and metabolomic studies of sleep and sleep deprivation. Methods of quantifying sleepiness have been proposed based on changes in behavioral factors such as response time6,46 and other measures of attention, as well as various electrophysiological factors such as changes in the power for a particular type of electroencephalographic wave.47 Other methods for measuring sleepiness have focused on changes in the composition of serum,48 saliva,49 cerebrospinal fluid,50,51 exhaled breath, and exhaled breath condensate.52 Inflammatory factors such as IL-6, TNF-α, von Willebrand factor, and C-reactive protein53,54 have drawn considerable attention. A gene expression study identified salivary amylase as a biomarker of sleep drive in both fruit flies (Drosophila melanogaster) and humans.49 Indeed, heterozygote individuals with a single copy of the c.22G > A (rs73598374) polymorphism for adenosine deaminase differ significantly from homozygote individuals with respect to vulnerability to sleep loss, exhibiting both a reduction in sustained attention and an elevation in salivary α-amylase activity during periods of prolonged waking.55 Although many of these measures of sleepiness appear promising, the lack of specificity among many, if not all, of the sleep reporter and regulatory substances identified to date has led some to suggest that proper measurement of sleepiness will ultimately require simultaneous monitoring of numerous analytes.54 While the peptide components of the FBI are more likely to be sleep reporter than sleep regulatory molecules, they might still be well-suited for inclusion in a multi-analyte approach to measuring sleepiness.
Potential Clinical Applications for Salivary Biomarkers of Physical Fatigue
Despite the limitations discussed above, the promising results presented here suggest that salivary biomarkers of physical fatigue might provide useful clinical information with respect to patient sleep health. Potential uses include diagnosing sleep disorders and/or longitudinally monitoring fatigue arising from sleep deprivation. The former would help clinicians make diagnoses based on objective measures, and the latter would allow physicians to select the most effective treatment strategy for various sleep disorders. Salivary biomarkers of physical fatigue might be especially well-suited for diagnosing specific types of sleep disorder. For example, diagnosis of paradoxical insomnia, which is characterized by a significant mismatch between self-reported sleep data and objective measures of sleep obtained from polysomnographs,4 would be much easier if clinicians had a rapid, inexpensive method to evaluate a patient's sleep history. In other sleep disorders, such as periodic limb movement, arousals from sleep are not perceived by patients suggesting that self-reported sleep data will not be accurate,56 highlighting the need for objective measures of sleep. The technology described here might also be well-suited to phenotype patients with various polymorphisms associated with enhanced vulnerability to sleep loss, much like the use of sAA described above.55 Overall, the FBI provides clinicians with an additional objective measure to guide the diagnosis and/or treatment of sleep disorders.
Limitations, Future Studies, and Conclusion
The present study aimed to examine changes in salivary biomarkers of physical fatigue during a period of sleep deprivation. While the data are promising and suggest that salivary biomarkers of physical fatigue may be a useful and objective method to monitor sleep deprivation, the study also has a number of limitations. For example, the sample size is relatively small, and the participants were young and relatively healthy. While subjects were asked about their sleep history, the study did not include a comprehensive evaluation of subjects to exclude all sleep disorders. In addition, our choices of instruments to evaluate cognitive performance and self-reported fatigue level—Stroop tests and POMS survey, respectively— may not represent the most sensitive tools for detecting changes related to sleep loss. Future studies will aim to study salivary changes related to sleep in subjects of much more varied demographic background and health condition.
In conclusion, we report here that the loss of sleep leads to significant changes in levels of a salivary biomarker of physical fatigue. If these preliminary findings are confirmed in future studies, salivary biomarkers of physical fatigue hold promise of providing clinicians, regulators, and patients with a fast, convenient, and relatively inexpensive method to diagnose and/or monitor sleep disorders.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Funding for this study was provided by the U.S. Army through a Phase-II STTR grant to Hyperion Biotechnology and the University of Texas San Antonio (RDE-COM, STTR W911SR-07-C-006).
REFERENCES
- 1.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. The international classification of sleep disorders, revised: diagnostic and coding manual. Chicago, IL: American Academy of Sleep Medicine; 2001. [Google Scholar]
- 3.Cooke JR, Ancoli-Israel S. Normal and abnormal sleep in the elderly. Handb Clin Neurol. 2011;98:653–65. doi: 10.1016/B978-0-444-52006-7.00041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 5.Mitler MM, Miller JC. Methods of testing for sleepiness. Behav Med. 1996;21:171–83. doi: 10.1080/08964289.1996.9933755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinges DF, Powell JW. Microcomputer analysis of performance on a portable, simple visual RT task sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–55. [Google Scholar]
- 7.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–85. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 8.National Highway Traffic Safety Administration. Drowsy driving and automobile crashes; report and recommendations from the national center on sleep disorders research/NHTSA expert panel on driver fatigue and sleepiness. 1998. DOT HS 808 707.
- 9.Orzel-Gryglewska J. Consequences of sleep deprivation. Int J Occup Med Environ Health. 2010;23:95–114. doi: 10.2478/v10001-010-0004-9. [DOI] [PubMed] [Google Scholar]
- 10.Arnedt JT, Wilde GJ, Munt PW, MacLean AW. How do prolonged wakefulness and alcohol compare in the decrements they produce on a simulated driving task? Accid Anal Prev. 2001;33:337–44. doi: 10.1016/s0001-4575(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 11.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 12.Lamond N, Dawson D. Quantifying the performance impairment associated with fatigue. J Sleep Res. 1999;8:255–62. doi: 10.1046/j.1365-2869.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- 13.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med. 2000;57:649–55. doi: 10.1136/oem.57.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falleti MG, Maruff P, Collie A, Darby DG, McStephen M. Qualitative similarities in cognitive impairment associated with 24 h of sustained wakefulness and a blood alcohol concentration of 0.05% J Sleep Res. 2003;12:265–74. doi: 10.1111/j.1365-2869.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 15.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the united states. Sleep. 2007;30:263–73. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 17.Michael DJ, Daugherty S, Santos A, Ruby BC, Kalns JE. Fatigue biomarker index: An objective salivary measure of fatigue level. Accid Anal Prev. 2012;45(Suppl):68–73. doi: 10.1016/j.aap.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Carlson DM. Salivary proline-rich proteins: biochemistry, molecular biology and regulation of expression. Crit Rev Oral Biol Med. 1993;4:495–502. doi: 10.1177/10454411930040033401. [DOI] [PubMed] [Google Scholar]
- 19.Kalns J, Baskin J, Reinert A, et al. Predicting success in the tactical air combat party training pipeline. Mil Med. 2011;176:431–7. doi: 10.7205/milmed-d-10-00110. [DOI] [PubMed] [Google Scholar]
- 20.Fogt DL, Cooke WH, Kalns JE, Michael DJ. Linear mixed-effects modeling of the relationship between heart rate variability and fatigue arising from sleep deprivation. Aviat Space Environ Med. 2011;82:1104–9. doi: 10.3357/asem.3014.2011. [DOI] [PubMed] [Google Scholar]
- 21.Fogt DL, Kalns JE, Michael DJ. A comparison of cognitive performance decreases during acute, progressive fatigue arising from different concurrent stressors. Mil Med. 2010;175:939–44. doi: 10.7205/milmed-d-10-00093. [DOI] [PubMed] [Google Scholar]
- 22.San Diego, CA: Educational and Industrial Testing Services; 1971. Manual for the profile of mood states. [Google Scholar]
- 23.Frankenhaeuser M. Behavior and circulating catecholamines. Brain Res. 1971;31:241–62. doi: 10.1016/0006-8993(71)90180-6. [DOI] [PubMed] [Google Scholar]
- 24.R development core team. R: A language and environment for statistical computing. 2010. http://www.R-project.org R Foundation for Statistical Computing.
- 25.Robin X, Turck N, Hainard A, et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006:R271–7. doi: 10.1093/hmg/ddl207. 15 Spec No 2. [DOI] [PubMed] [Google Scholar]
- 27.Sahar S, Sassone-Corsi P. Regulation of metabolism: The circadian clock dictates the time. Trends Endocrinol Metab. 2012;23:1–8. doi: 10.1016/j.tem.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minami Y, Kasukawa T, Kakazu Y, et al. Measurement of internal body time by blood metabolomics. Proc Natl Acad Sci U S A. 2009;106:9890–5. doi: 10.1073/pnas.0900617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–9. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawes C. Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva. J Physiol. 1975;244:535–48. doi: 10.1113/jphysiol.1975.sp010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 32.Pandi-Perumal SR, Smits M, Spence W, et al. Dim light melatonin onset (DLMO): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Mirick DK, Davis S. Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomarkers Prev. 2008;17:3306–13. doi: 10.1158/1055-9965.EPI-08-0605. [DOI] [PubMed] [Google Scholar]
- 34.Bertram HC, Eggers N, Eller N. Potential of human saliva for nuclear magnetic resonance-based metabolomics and for health-related biomarker identification. Anal Chem. 2009;81:9188–93. doi: 10.1021/ac9020598. [DOI] [PubMed] [Google Scholar]
- 35.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30:1460–83. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–14. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 37.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Garde AH, Karlsson B, Hansen AM, Persson R, Akerstedt T. Sleep and salivary cortisol. In: Kristenson M, Garvin P, Lundberg U, editors. The role of saliva cortisol measurement in health and disease. Oak Park, IL: Bentham Science Publishers; 2012. pp. 116–28. http://dx.doi.org/10.2174/97816080534211120101. [Google Scholar]
- 39.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103:17058–63. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nater UM, Maloney E, Boneva RS, et al. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab. 2008;93:703–9. doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- 41.Roberts AD, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry. 2004;184:136–41. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- 42.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Quan SF. Finding a research path for the identification of biomarkers of sleepiness. J Clin Sleep Med. 2011;7(5 Suppl):S4–5. doi: 10.5664/JCSM.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan SF, Shaw PJ, Naidoo N, Haeggstrom E, Krueger JM, Church GM. Panel discussion: Can there be a biomarker for sleepiness? J Clin Sleep Med. 2011;7(5 Suppl):S45–8. doi: 10.5664/JCSM.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czeisler CA. Impact of sleepiness and sleep deficiency on public health--utility of biomarkers. J Clin Sleep Med. 2011;7(5 Suppl):S6–8. doi: 10.5664/JCSM.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balkin TJ. Behavioral biomarkers of sleepiness. J Clin Sleep Med. 2011;7(5 Suppl):S12–5. doi: 10.5664/JCSM.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: An independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7(5 Suppl):S16–8. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller MA, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 49.Seugnet L, Boero J, Gottschalk L, Duntley SP, Shaw PJ. Identification of a biomarker for sleep drive in flies and humans. Proc Natl Acad Sci U S A. 2006;103:19913–8. doi: 10.1073/pnas.0609463104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Rodriguez JE, Lin L, Iranzo A, et al. Decreased hypocretin-1 (orexin-a) levels in the cerebrospinal fluid of patients with myotonic dystrophy and excessive daytime sleepiness. Sleep. 2003;26:287–90. doi: 10.1093/sleep/26.3.287. [DOI] [PubMed] [Google Scholar]
- 52.Carpagnano GE. Exhaled breath analysis and sleep. J Clin Sleep Med. 2011;7(5 Suppl):S34–7. doi: 10.5664/JCSM.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller MA. Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med. 2011;7(5 Suppl):S31–3. doi: 10.5664/JCSM.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clinton JM, Davis CJ, Zielinski MR, Jewett KA, Krueger JM. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med. 2011;7(5 Suppl):S38–42. doi: 10.5664/JCSM.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachmann V, Klaus F, Bodenmann S, et al. Functional ada polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb Cortex. 2012;22:962–70. doi: 10.1093/cercor/bhr173. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein MZ. Practical geriatrics: Insomnia in late life. Psychiatr Serv. 2001;52:1573–5. doi: 10.1176/appi.ps.52.12.1573. [DOI] [PubMed] [Google Scholar]