Abstract
Narcolepsy with cataplexy is rare in children under 5 years of age. There is limited information on safe and effective treatment of cataplexy in young children. We describe successful treatment of cataplexy in a 3-year-old using venlafaxine and subsequently followed for over 2 years.
Citation:
Ratkiewicz M; Splaingard M. Treatment of cataplexy in a three-year-old using venlafaxine. J Clin Sleep Med 2013;9(12):1341-1342.
Keywords: Cataplexy, toddler, venlafaxine
Narcolepsy with cataplexy is an uncommon disorder in the general population, but is exceedingly rare in children under 5 years of age. In a database review of over 1200 cases, only 2.1% of patients had onset of symptoms of narcolepsy prior to 5 years of age, and even fewer (1.1%) had cataplexy.1 Venlafaxine, a serotonin and noradrenaline reuptake inhibitor, has been used to treat a variety of disorders including cataplexy in older children and adults, ADHD and autism spectrum disorders.2,3 We report the successful use of venlafaxine to treat cataplexy in a 3-year-old boy.
REPORT OF CASE
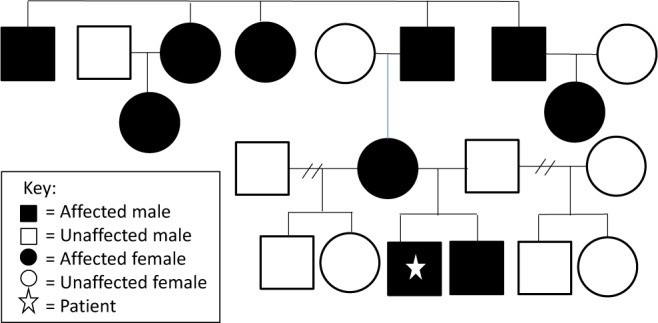
This 6-year-old boy was first evaluated at 7 months of age for suspected cataplexy. His mother, who has narcolepsy with cataplexy diagnosed at 10 years of age, reported that he developed a “head drop” only when he was tickled by his father. Several attempts to elicit a cataplexy event in the clinic were unsuccessful. Observation was recommended due to limited symptoms and age. As a toddler, he slept a minimum of 12 hours each night. Family history (see Figure 1) was remarkable for numerous family members with narcolepsy with cataplexy. His evaluation at 2 years of age included a brain MRI, which showed enlarged symmetric ventricles without evidence of obstruction, and an otherwise structurally normal brain. Polysomnogram and an extended montage video electroencephalogram (EEG) done with tickling was normal. By age 3, daily episodes of “head nodding” occurred when he laughed on the playground at preschool. Subsequently, an episode of cataplexy was precipitated with tickling in the clinic that was videotaped. Initial therapy was venlafaxine 5 mg (0.34 mg/kg) daily in the morning. A compounded liquid suspension was used to provide accurate dosing. One week later symptoms were unchanged, so his dose was increased to 6 mg daily (0.41 mg/kg/day). The frequency of cataplexy was then reduced to only 2 episodes over a 3-week period, and no adverse effects were observed. His dosage was then increased to 7 mg daily (0.47 mg/kg) and at a clinic visit 1 month later, he had not had any episodes of cataplexy. At subsequent clinic visits over the following 2 years, he continued to do well without any episodes of cataplexy and no adverse effects of the medication. His dose was adjusted for weight to maintain a dose of 0.4 mg/kg/ day. His growth remained at the 25th percentile for weight and length with normal developmental milestones, normal blood pressures, and no evidence of precocious puberty. Modified Epworth Sleepiness Scale score remains 11-13 and he continues to nap periodically without excessive daytime sleepiness. He was found to be HLA DQB1*0602 positive. Mother refused lumbar puncture for hypocretin analysis. On his first MSLT, done recently at 6 years of age, while on venlafaxine, he slept during 5 out of 5 naps with a mean sleep onset latency of 8.3 minutes with 1 SOREM.
Figure 1. Family pedigree of narcolepsy with cataplexy.
DISCUSSION
This case demonstrates that venlafaxine is a well-tolerated and effective treatment for cataplexy in a young child. Similar findings in a group of six children between the ages of 7 and 12 have been reported. They all had a good response to therapy without significant adverse effects at doses between 37.5 mg and 150 mg per day, which are considerably higher than the dose required to achieve relief of symptoms in our patient, even when accounting for the difference in weight.5 Aran and colleagues looked retrospectively at a variety of medications, including venlafaxine, to treat narcolepsy. In their study, continuation of venlafaxine was moderate and higher than fluoxetine, tricyclic antidepressants, or other selective serotonin reuptake inhibitors. They also found venlafaxine to be primarily helpful for cataplexy,1 which is consistent with the effects experienced by our patient. Accurate dosing in a small child can be a challenge, as there is not a commercially available liquid suspension and the smallest tablet is 37.5 mg. A compounded liquid formulation is currently being prescribed for our patient, which has previously been shown to chemically stable for 15 days when refrigerated.4 Based on our experience, venlafaxine can be used safely and efficaciously in young children with narcolepsy with cataplexy.
DISCLOSURE STATEMENT
This was not an industry supported study. The child described in the case was evaluated in the Sleep Clinic at Nationwide Children's Hospital. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Aran A, Einen M, Lin L, et al. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollander E, Kaplan A, Cartwright C, Reichman D. Venlafaxine in children, adolescents, and young adults with autism spectrum disorders: an open retrospective clinical report. J Child Neurol. 2000;15:132–5. doi: 10.1177/088307380001500214. [DOI] [PubMed] [Google Scholar]
- 3.Findling R, Greenhill L, McNamara N, et al. Venlafaxine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:433–45. doi: 10.1089/cap.2007.0119. [DOI] [PubMed] [Google Scholar]
- 4.Kervela J, Castagnet S, Chiadmi F, et al. Assessment of stability in extemporaneously prepared venlafaxine solutions. Eur J Hosp Pharm. 2009;15:30–2. [Google Scholar]
- 5.Moller L, Ostergaard J. Treatment with venlafaxine in six cases of children with narcolepsy with cataplexy and hypnagogic hallucinations. J Child Adolesc Psychopharmacol. 2009;19:197–201. doi: 10.1089/cap.2008.036. [DOI] [PubMed] [Google Scholar]


