Abstract
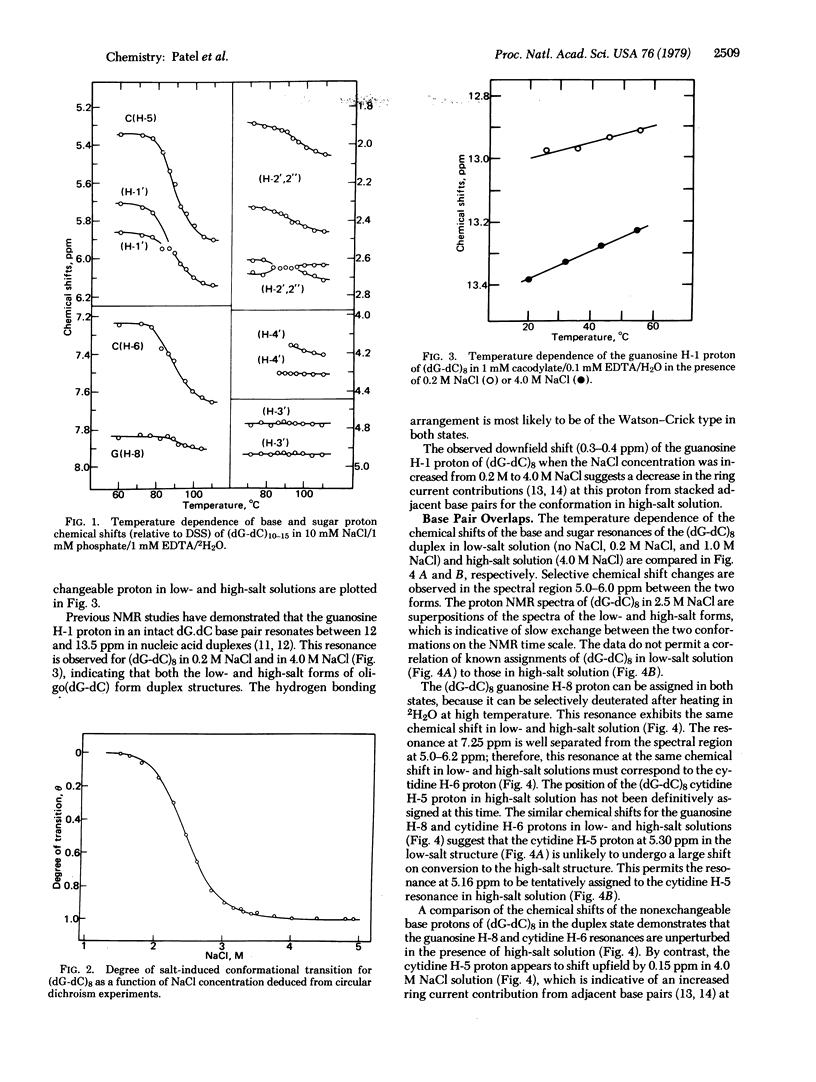
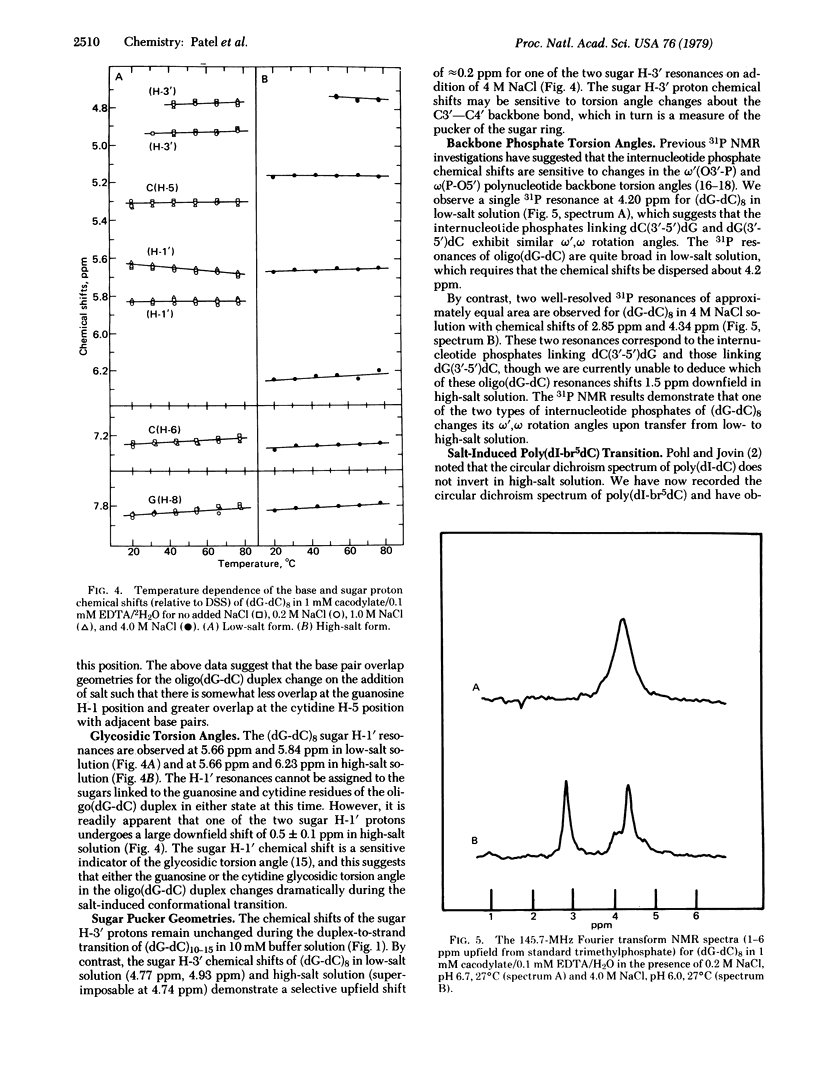
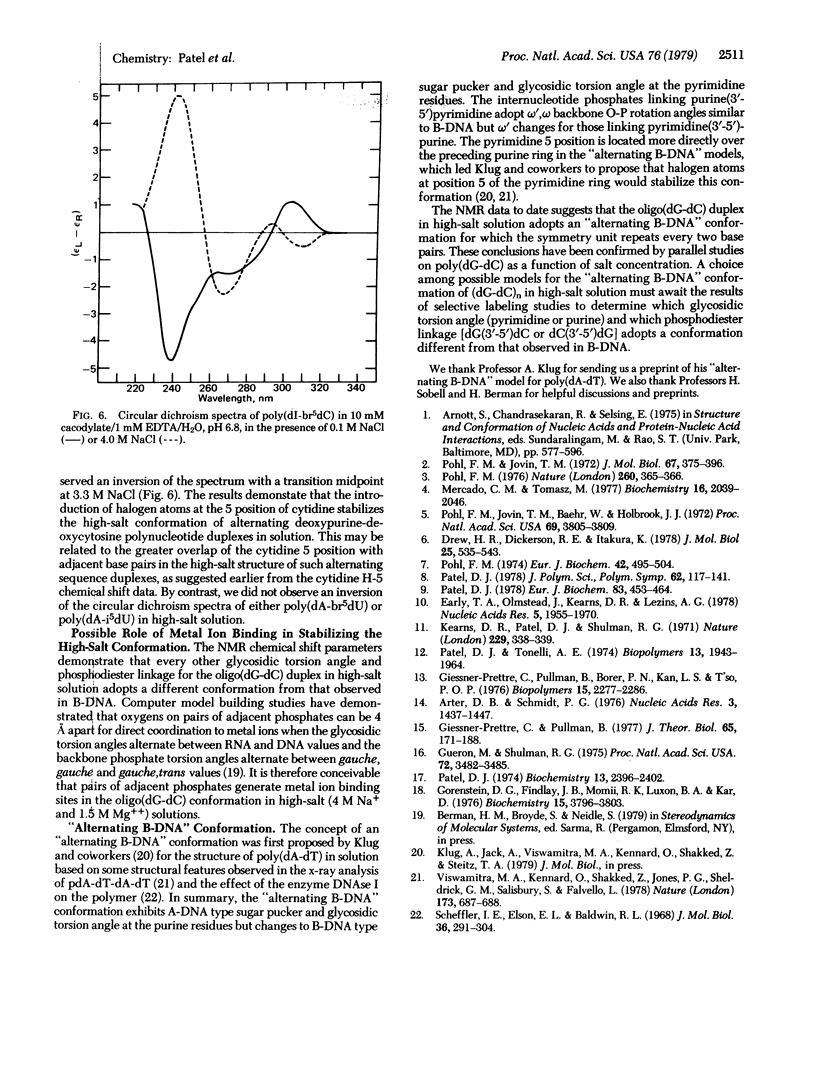
The high resolution 1H and 31P NMR spectra of the (dG-dC)8 duplex have been recorded in low- and high-salt solutions in order to evaluate the structural aspects of the salt-induced transition of oligo(dG-dC) in solution [Pohl, F. M. & Jovin, T. M. (1972) J. Mol. Biol. 67, 375-396]. The NMR data require that the (dG-dC)8 duplex in 4 M NaCl adopt an "alternating B-DNA" conformation for which the symmetry unit repeats every two base pairs. By contrast, the oligomer duplex in low-salt solution is of the regular B-DNA type in solution. The chemical shift parameters for oligo(dG-dC) in high-salt solution demonstrate that every other glycosidic torsion angle and phosphodiester linkage adopts a different conformation from that observed in regular B-DNA. We demonstrate further that the generation of the "alternating B-DNA" structure is facilitated by introduction of halogen atoms at the 5 position of pyrimidine and that this probably reflects the greater overlap of this position with adjacent base pairs in high salt solution. An "alternating B-DNA" model has recently been proposed for alternating deoxy purine-deoxy pyrimidine polynucleotides based on the x-ray structure of pdA-dT-dA-dT [Klug, A., Jack, A., Viswamitra, M.A., Kennard, O., Shakked, Z. & Steitz, T.A. (1979) J. Mol. Biol., in press].
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arter D. B., Schmidt P. G. Ring current shielding effects in nucleic acid double helices. Nucleic Acids Res. 1976 Jun;3(6):1437–1447. doi: 10.1093/nar/3.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E., Itakura K. A salt-induced conformational change in crystals of the synthetic DNA tetramer d(CpGpCpG). J Mol Biol. 1978 Nov 15;125(4):535–543. doi: 10.1016/0022-2836(78)90315-7. [DOI] [PubMed] [Google Scholar]
- Early T. A., Olmsted J., 3rd, Kearns D. R., Lezius A. G. Base pairing structure in the poly d(G-T) double helix: wobble base pairs. Nucleic Acids Res. 1978 Jun;5(6):1955–1970. doi: 10.1093/nar/5.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B., Borer P. N., Kan L. S., Ts'o P. O. Ring-current effects in the Nmr of nucleic acids: a graphical approach. Biopolymers. 1976 Nov;15(11):2277–2286. doi: 10.1002/bip.1976.360151114. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. On the conformational dependence of the proton chemical shifts in nucleosides and nucleotides. I. Proton shifts in the ribose ring of pyrimidine nucleosides as a function of the torsion angle about the glycosyl bond. J Theor Biol. 1977 Mar 7;65(1):171–188. doi: 10.1016/0022-5193(77)90082-0. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Findlay J. B., Momii R. K., Luxon B. A., Kar D. Temperature dependence of the 31P chemical shifts of nucleic acids. A prode of phosphate ester torsional conformations. Biochemistry. 1976 Aug 24;15(17):3796–3803. doi: 10.1021/bi00662a023. [DOI] [PubMed] [Google Scholar]
- Guéron M., Shulman R. G. 31P magnetic resonance of tRNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3482–3485. doi: 10.1073/pnas.72.9.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. R., Patel D. J., Shulman R. G. High resolution nuclear magnetic resonance studies of hydrogen bonded protons of tRNA in water. Nature. 1971 Jan 29;229(5283):338–339. doi: 10.1038/229338a0. [DOI] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Peptide antibiotic-oligonucleotide interactions. Nuclear magnetic resonance investigations of complex formation between actinomycin D and d-ApTpGpCpApT in aqueous solution. Biochemistry. 1974 May 21;13(11):2396–2402. doi: 10.1021/bi00708a025. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Synthetic RNA and DNA duplexes. Premelting, melting and postmelting transitions of alternating inosine-cytosine polynucleotides in solution. Eur J Biochem. 1978 Feb;83(2):453–464. doi: 10.1111/j.1432-1033.1978.tb12111.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Tonelli A. E. Assignment of the proton Nmr chemical shifts of the T-N3H and G-N1H proton resonances in isolated AT and GC Watson-Crick base pairs in double-stranded deoxy oligonucleotides in aqueous solution. Biopolymers. 1974;13(10):1943–1964. doi: 10.1002/bip.1974.360131003. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Thermodynamics of the helix-coil transition of (dG-dC) oligomers. Eur J Biochem. 1974 Mar 1;42(2):495–504. doi: 10.1111/j.1432-1033.1974.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by dAT oligomers. I. Hairpin and straight-chain helices. J Mol Biol. 1968 Sep 28;36(3):291–304. doi: 10.1016/0022-2836(68)90156-3. [DOI] [PubMed] [Google Scholar]
- Viswamitra M. A., Kennard O., Jones P. G., Sheldrick G. M., Salisbury S., Favello L., Shakked Z. DNA double helical fragment at atomic resolution. Nature. 1978 Jun 22;273(5664):687–688. doi: 10.1038/273687a0. [DOI] [PubMed] [Google Scholar]



