Abstract
An Ames test and a 28-day sub-chronic toxicity study in male and female Sprague–Dawley rats were conducted to evaluate the safety of a chicory root extract being investigated as a therapeutic for inflammation. Chicory extract had no mutagenic activity in the Ames test although it was cytotoxic to certain strains of Salmonella at higher doses with and without metabolic activation. For the 28-day rat study, measurements included clinical observations, body weights, food consumption, clinical pathology, gross necropsy and histology. There were no treatment-related toxic effects from chicory extract administered orally at 70, 350, or 1000 mg/kg/day. Since there were no observed adverse effects of chicory extract in these studies, the NOAEL for the extract is 1000 mg/kg/g administered orally for 28 days.
Keywords: Chicorium intybus, Sesquiterpene lactone, Ames, Dietary supplement, Inflammation, Toxicity
1. Introduction
Chicory (Cichorium intybus L.) is a perennial herb in the Asteraceae family with many commercial uses. Historically, chicory was grown by the ancient Egyptians as a medicinal plant, vegetable crop, and for animal forage (Grieve, 1971). Greeks and Romans also grew chicory as a vegetable crop; its use was mentioned by several ancient writers including Horace, Virgil, Ovid, and Pliny the Elder (Grieve, 1971). Today, the roots are dried and roasted and used as a coffee substitute (Bais and Ravishankar, 2001). The chicons (shoots and leaves) are grown for consumption in salads and vegetable dishes. Young and tender roots can also be boiled and eaten. Chicory extracts are added to alcoholic and non-alcoholic beverages (Bais and Ravishankar, 2001). The US imports over 2.3 million kilograms of chicons and 1.9 million kilograms roasted chicory roots for coffee according to 2002 US Department of Commerce tariff and trade data. Chicory extract is Generally Regarded as Safe (GRAS) by the FDA and appears on the Everything Added to Food in the United States (EAFUS) list.
Besides its alimentary use, chicory also has a history of medicinal use. Chicory roots have been used as a digestive aid, diuretic, laxative, and mild sedative (Bais and Ravishankar, 2001). Additionally, hepatoprotective agents have been described in the seeds (Gadgoli and Mishra, 1997). Chicory is recognized as a source of dietary fibers such as inulin and fructo-oligosaccharides, which have health-promoting properties (Bais and Ravishankar, 2001). Its aqueous, ethanolic, and methanolic extracts have been shown to affect cholesterol uptake (Kim, 2000) and tumor development in mice (Hazra et al., 2002), prevent immunotoxicity induced by ethanol (Kim et al., 2002), and have anti-inflammatory properties in vitro and in vivo (Jindal et al., 1975; Cavin et al., 2005).
Studies have linked sesquiterpene lactones, the bitter agents in chicory, to some of the anti-inflammatory health benefits (Hall et al., 1979; Cavin et al., 2005). Sesquiterpene lactones are C15 terpenoid compounds that have a range of biological and pharmaceutical activities (Picman, 1986; Hehner et al., 1998). They have been reported as the active compounds of some well-known medicinal plants, such as Arnica montana (leopard’s bane) (Lyss et al., 1997) and Tanacetum parthenium (feverfew) (Kwok et al., 2001) and have been used clinically for inflammatory conditions including migraines (Palevitch et al., 1997) and arthritis (Knuesel et al., 2002). Studies have shown that sesquiterpene lactones inhibit pro-inflammatory gene expression through inactivation of the transcription factor nuclear factor-κB (NF-κB) (Lyss et al., 1997; Kwok et al., 2001).
In general, sesquiterpene lactones have a reputation for their unpalatable effects partially due to their bitter flavor and implication of some specific sesquiterpene lactones in livestock poisoning. Western bitterweed (Hymenoxys odo-rata), Colorado rubberweed (Hymenoxys richardsonii) and other Hymenoxys species contain the sesquiterpene lactone hymenovin (Ivie et al., 1975a). Hymenovin contains an alpha-methylene-gamma-lactone moiety that disrupts cellular signaling (Cheeke and Shull, 1985). Bitter sneezeweed (Helenium amarum) and other related Helenium species contain sesquiterpene lactones with toxicity at high concentrations (Ivie et al., 1975b). Repin is a sesquiterpene lactone with well-documented neurotoxic effects (Robles et al., 1995, 1998). Repin is found in Russian knapweed (Centaurea repens) and yellow star-thistle (Centaurea solstitialis), which are both naturalized in western North America cattle grazing areas. Horses are most often affected, causing equine nigropallidal encephalomalacia (ENE) which resembles Parkinson’s Disease (Robles et al., 1998).
Although chicory has a long history of human use without reported toxicity, high levels of concentrated chicory sesquiterpene lactones have the potential to produce toxic effects. Since chicory sesquiterpene lactones may be useful as anti-inflammatory agents, we investigated the safety of a chicory root sesquiterpene lactone extract prepared by sequential ethanolic extraction, defatting with n-heptane and extraction with ethyl acetate using the Ames test for mutagenicity and a 28-day sub-chronic toxicity study in rats.
2. Materials and methods
2.1. Extraction procedure
Chicory cv. Sacson roots were provided by Leroux (Lille-Valenciennes, France). A sesquiterpene enriched extract was prepared by the following procedure. Dry chicory roots (1 kg) were extracted in 10 L of 95% ethanol for 24 h at room temperature (24 °C). The resulting ethanolic extract was defatted with n-heptane. A secondary extraction of the defatted ethanolic extract with ethyl acetate created an extract rich in sesquiterpene lactones. Solvents were removed under vacuum, creating a brown syrup that was stored at 4 °C.
2.2. LC–MS analysis
To determine the sesquiterpene content and provide a fingerprint for evaluating stability, chicory extract was separated and analyzed with the Waters (Milford, MA) LC–MS Integrity™ system consisting of a solvent delivery system including a W616 pump and W600S controller, W717 plus auto-sampler, W996 PDA detector and Waters TMD Thermabeam™ electron impact (EI) single quadrupole mass detector. Data were collected and analyzed with the Waters Millennium® v. 3.2 software, linked with the 6th edition of the Wiley Registry of Mass Spectral Data, containing 229,119 EI spectra of 200,500 compounds. Substances were separated on a Phenomenex® Luna C-8 reverse phase column, size 150 × 2 mm, particle size 3 µm, pore size 100 Å, equipped with a Phenomenex® Security-Guard™ pre-column. The mobile phase consisted of two components: Solvent A (0.5% ACS grade acetic acid in double distilled de-ionized water, pH 3–3.5), and Solvent B (100% acetonitrile). The mobile phase flow was adjusted at 0.25 ml/min, and generally a gradient mode was used for all analyses. The gradient points were for time 0.0 min −95% A and 5% B; for time 25.0 min −5% A and 95% B; held isocratic for 2 min and from 27.0 min to 30.0 min. – back to initial conditions of 95% A and 5% B. A column equilibration time of 15 min was set between subsequent injections.
2.3. Bacterial reverse mutation assay
Evaluation of chicory extract for potential mutagenic properties was performed using Salmonella typhimurium strains TA97a, TA98, TA100, TA1535, and Escherichia coli strain WP2 uvrA (328). The extract was dissolved in DMSO and tested at 0, 5, 10, 50, 100, 500, 1000, 2500, and 5000 µg/plate using the standard Ames plate incorporation method (Ames et al., 1975) with and without exogenous metabolic activation using Aroclor 1254-induced S9 as described elsewhere (Maron and Ames, 1983). Positive controls without metabolic activation included for S. typhimurium strain TA97a, 1 µg ICR 191 Acridine mutagen (ICR 191); strain TA98, 25 µg 2-nitrofluorene (2 NF); strains TA100 and TA1525, 2 µg sodium azide (NAAZ); and for E. coli strain WP2 uvrA (328), 2 µg N-ethyl-N-nitro-nitrosoguanidine (ENNG). With metabolic activation, the positive controls were for S. typhimurium strain TA97a, 20 µg 10-dimethyl-1,2-benzanthracene (DMBA); strains TA98, TA100, and TA 1535, 2.5 µg 2-aminoanthracene (2AA); and for E. coli strain WP2 uvrA (328), 12.5–25 µg 2AA. DMSO alone was used as a negative control. All concentrations were tested in triplicate and the assay was repeated once. This was a GLP study conducted by Next Century Inc. (project number 05-04-001) in accordance with FDA, ICH, EPA, and OECD guidelines.
2.4. 28-Day rat toxicity study
A 28-day oral toxicity study was conducted in CRL:CD® (SD) IGS BR rats to determine the potential of chicory extract to produce toxic effects. Forty healthy rats (20 males and 20 females) were selected for the test and equally distributed into eight groups (five males and five females per group). Oral gavage dose levels of 70, 350, and 1000 mg/kg/day of the test article, as well as a vehicle control, were selected for the test.
An appropriate amount of the test or control article was administered daily by oral gavage to each rat for 30 consecutive days. The test article was administered as a 1.4%, 7.0% or 20.0% w/v solution in corn oil. Corn oil was administered at a similar dose volume to the control animals. The test article mixtures were prepared daily. Samples were collected from each dose level and analyzed for changes in stability. The animals were observed daily for viability, signs of gross toxicity, behavioral changes, and weekly for a battery of detailed observations. Body weights were recorded at test initiation (day 1), weekly thereafter, and at terminal sacrifice. Individual food consumption was recorded weekly to coincide with body weight measurements. Blood was sampled from all animals on day 29 of the study for hematology, clinical biochemistry and serology assessments and at terminal sacrifice for determination of prothrombin time and partial thromboplastin time. Gross necropsies were performed on all study rats, and selected organs and tissues were evaluated histologically in the control and high dose level groups. This was a GLP study conducted by Product Safety Labs (study number 17678) in accordance with FDA, ICH, EPA, and OECD guidelines.
2.4.1. Animals
The Sprague–Dawley derived, CD® rat was the system of choice because, historically, it has been a preferred and commonly used species for oral toxicity tests. Fifty CRL:CD® (SD) IGS BR rats (25 males and 25 females) were individually housed in suspended stainless steel caging with mesh floors which conform to the size recommendations in the most recent NIH Guide for the Care and Use of Laboratory Animals. Animal room temperature and relative humidity were 16–23 °C and 38–65%, respectively, and there was a 12 h light/dark cycle. During acclimation, the animals were fed Purina Certified Rodent Meal #5002 ad libitum. During the study, food consumption was measured weekly. All animals were fasted overnight prior to blood collection. Filtered tap water was supplied ad libitum. Pooled serum samples from animals on test were evaluated for the absence of viruses near the end of the in-life portion of the study.
2.4.2. Dose preparation and administration
Individual doses were calculated based on the most recent weekly body weights and were adjusted each week to maintain the targeted dose level for all rats (i.e., mg/kg/day). All doses were administered volumetrically, at a constant volume of 5 ml/kg, after correcting for concentration of the test solution. The control animals received the vehicle only at the same volume as the test groups. The test article was administered as a 1.4%, 7.0%, or 20% w/v solution in corn oil. On each day of dosing, for each concentration, an appropriate amount of the test article was accurately weighed into a 4 oz container. To facilitate the mixing process, approximately 1.5 g of acetone was added to the container, mixed, and then a portion of the total corn oil required was added. This preliminary dilution was maintained on a magnetic stir plate for approximately 30 min to evaporate the acetone and mix the test article with the corn oil. This preparation was then brought to a volume of 50 ml with corn oil. Dose preparations were used within approximately 2 h, and maintained on a magnetic stir plate during administration. Each animal was dosed by oral gavage using a stainless steel ball-tipped gavage needle attached to an appropriate syringe. Dose administration was daily via oral gavage to each rat for 30 consecutive days. The first day of administration was considered day 1 of the study. Dosing was at approximately the same time each day ± 2 h with an exception on the day the hematology and clinical chemistry samples were collected (study day 29). On the day of blood collection, food was returned to the fasted animals for a minimum of 2 h prior to test article administration.
2.4.3. Clinical observations
All animals were observed twice daily for mortality. Cage-side observations were made daily during the study and any abnormal findings recorded. Detailed observations were recorded on day 1 (prior to administration of test article) and weekly thereafter on all animals. These observations were conducted both while handling the animal and with the animal placed in an open field. Observations included, but were not limited to: changes in skin, fur, eyes, and mucous membranes, occurrence of secretions and excretions and autonomic activity (e.g., lacrimation, piloerection, pupil size, unusual respiratory pattern). Changes in gait, posture and response to handling as well as the presence of clonic or tonic movements, sterotypies (e.g., excessive grooming, repetitive circling), or aberrant behavior (e.g., self-mutilation, walking backwards) were also recorded.
2.4.4. Body weight and body weight gain
Individual body weights were recorded twice during the acclimation period, at study initiation (day 1), and weekly thereafter. Mean body weight gains were calculated for each group at each interval and for the overall (days 1–28) testing interval. Animals were also weighed immediately prior to sacrifice (fasted body weight) for calculation of organ to body weight and organ to brain weight ratios.
2.4.5. Food consumption and food efficiency
Individual food consumption was measured, adjusting for spillage, and recorded weekly to coincide with body weight measurements. Mean food consumption was calculated for each sex/dose level during each weekly interval and overall (days 1–28) testing interval. Mean food efficiency was also calculated for each sex/dose level based on body weight gain and food consumption data. Animals were allowed ad libitum access to food throughout the study. Animals were fasted overnight prior to blood collection on day 29 and prior to terminal sacrifice on day 31.
2.4.6. Clinical pathology examinations
All animals were fasted overnight prior to each blood collection. Blood samples for hematology (except prothrombin time and partial thromboplastin time) and clinical chemistry from the test and control groups were collected via orbital sinus bleeding under isoflurane anesthesia on study day 29. Blood samples used to determine the prothrombin time and partial thromboplastin time were collected via the inferior vena cava under isofurane anesthesia for all groups at terminal sacrifice. All blood samples were evaluated for quality by visual examination prior to analysis. Upon completion of clinical chemistry, remaining serum samples were pooled for Serology. Examinations were made by E.I. Dupont de Nemours and Company, and Haskell Laboratory for Health and Environmental Sciences (Table 1).
Table 1.
Summary of hematology and clinical chemistry parameters measured
| Hematology | |
| Erythrocyte count (RBC) | Hemoglobin concentration (HGB) |
| Hematocrit (HCT) | Mean corpuscular volume (MCV) |
| Mean corpuscular hemoglobin (MCH) | Red cell distribution width (RDW) |
| Absolute reticulocyte count (ARET) | Platelet count (PLT) |
| Total white blood cell (WBC) and differential leukocyte count | |
| Clinical Chemistry | |
| Serum aspartate aminotransferase (AST) | Serum alanine aminotransferase (ALT) |
| Sorbital dehydrogenase (SDH) | Alkaline phosphatase (ALKP) |
| Total bilirubin (BILI) | Urea nitrogen (BUN) |
| Blood creatinine (CREA) | Total cholesterol(CHOL) |
| Triglycerides (TRIG) | Fasting glucose (GLUC) |
| Total serum protein (TP) | Albumin (ALB) |
| Globulin (GLOB) | Calcium (CALC) |
| Inorganic phosphorous (IPHS) | Sodium (NA) |
| Potassium (K) | Chloride (CL) |
2.4.7. Necropsy and histopathology
At scheduled sacrifice, all rats were euthanized by exsanguination from the abdominal aorta under isoflurane anesthesia. All male and female rats from each group were sacrificed on day 31. Gross necropsy of all males and females included an initial examination of external surfaces and orifices, as well as the cranial, thoracic and abdominal cavities and their contents. Rats were examined for gross lesions. Tissues of interest and any gross lesions were retained in neutral buffered 10% formalin (NBF).
The liver, kidneys, adrenals, brain, heart, thymus, spleen, uterus, ovaries, testes, and epididymides (of all animals sacrificed by design) were weighed wet as soon as possible after dissection to avoid drying. The following organs and tissues from all animals were preserved in NBF for possible future histopathological examination: all gross lesions, lungs, trachea, brain- including sections of the medulla/pons, cerebellar cortex and cerebral cortex, spinal cord (three levels: cervical, mid-thoracic, and lumbar), thymus, heart, sternum with bone marrow, adrenals, liver, spleen, kidneys, thyroid/parathyroid, ovaries, testes, uterus (with attached urinary bladder, cervix and vagina), esophagus, ileum, cecum, accessory genital organs (epididymides, prostate, and seminal vesicles), peripheral nerve (sciatic), stomach, duodenum, jejunum, colon, rectum, representative lymph node (mesenteric and mandibular), and pancreas and salivary glands. Histological examination was performed on the preserved organs and tissues of the animals from the control groups and test high dose groups. The fixed tissues were trimmed, processed, embedded in paraffin, sectioned, placed on glass microscope slides, and stained with hematoxylin and eosin. Slide preparation and histopathological assessments were performed by Experimental Pathology Laboratories, Inc. (EPL®).
2.4.8. Statistics
Mean and standard deviations were calculated for all quantitative data. Treated and control groups were compared using a one-way analysis of variance (ANOVA), followed by comparison of the treated groups to control by Dunnett’s t-test for multiple comparisons. A 95% confidence level was used to determine statistically significant differences between treated and control groups.
3. Results
3.1. Sesquiterpene lactone content
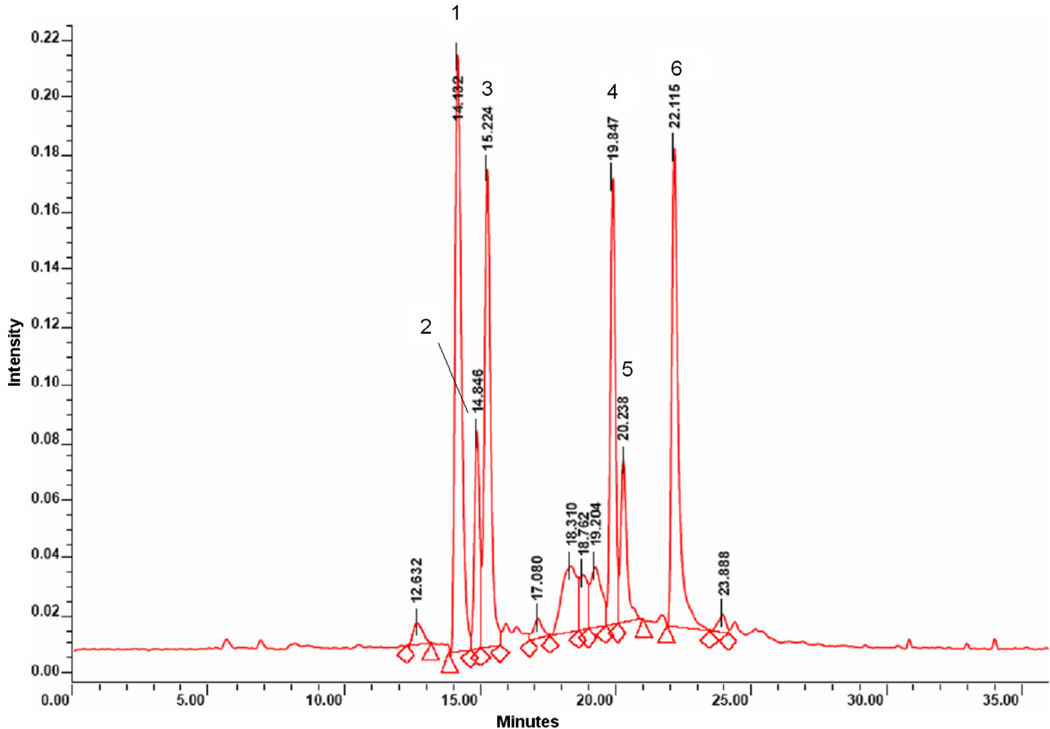
The major peaks shown in the LC–MS chromatogram (Fig. 1) matched the UV spectra and fragmentation patterns of several known sesquiterpene lactones (van Beek et al., 1990). Putative compounds included dihydrolactucin, 14.1 min; lactucin, 14.8 min; a (4-OH-phenyl) acetate ethyl-ester compound, 15.2 min; 8-deoxylactucin,19.8 min; (epi-) jacquinelin, 20.2 min; and dihydrolactucopicrin at 22.1 min. Using peak area as a guide, it was determined the chicory root extract contains approximately 60% sesquiterpene lactones. Doses for the Ames test and 28-day oral toxicity test were analyzed by LC–MS for stability. The peak area and retention times for each of the major sesquiterpene lactones in the prepared doses were identical to the parent test article, confirming the stability of the test article in solution and throughout the testing period.
Fig 1.
HPLC chromatogram of chicory extract showing putative compounds: (1) dihydrolactucin; (2) lactucin; (3) (4-OH-phenyl) acetate ethyl-ester; (4) 8-deoxylactucin; (5) (epi) jacquinelin; (6) dihydrolactucopicrin.
3.2. Bacterial reverse mutation assay
Chicory root extract was evaluated in the bacterial reverse mutation assay (Ames test) using S. typhimurium strains TA97a, TA98, TA100, TA1535, and E. coli strain WP2 uvrA (328) with and without a metabolic activation system. All bacterial strains tested showed appropriate phenotypical characteristics. Extract-related cytotoxicity (reduction in colony numbers) was noted at a concentration of 5000 µ/plate in S. typhimurium strains TA97a and TA100 with metabolic activation (Table 2). In TA97a without metabolic activation, cytotoxicity was noted above 1000 µ/plate and in TA100 without metabolic activation cytotoxicity was noted above 2500 µ/plate. Despite the occurrence of cytotoxicity at higher concentrations, there was no evidence of mutagenicity in any bacterial strain with or without metabolic activation at any concentration.
Table 2.
Ames test observations in Salmonella typhimurium strains TA97a and TA100
| Concentration µg/plate | TA97a |
TA100 |
||
|---|---|---|---|---|
| Colony (#) | SD | Colony (#) | SD | |
| Without metabolic activation | ||||
| 0 | 67.0 | 5.2 | 125.0 | 6.0 |
| 5 | 51.7 | 9.9 | 131.0 | 10.2 |
| 10 | 50.5 | 5.0 | 123.0 | 20.0 |
| 50 | 69.0 | 9.9 | 130.5 | 7.8 |
| 100 | 73.3 | 5.5 | 121.3 | 8.1 |
| 500 | 56.7 | 14.0 | 123.0 | 23.8 |
| 1000 | 36.0 | 15.7 | 123.3 | 12.6 |
| 2500 | 29.0 | 3.0 | 111.7 | 9.5 |
| 5000 | 9.3 | 5.5 | 74.3 | 7.2 |
| With metabolic activation | ||||
| 0 | 82.0 | 20.4 | 136.7 | 9.2 |
| 5 | 68.3 | 30.6 | 131.7 | 2.1 |
| 10 | 56.0 | 28.2 | 131.0 | 2.7 |
| 50 | 87.3 | 15.1 | 133.3 | 10.2 |
| 100 | 83.3 | 15.0 | 124.3 | 10.2 |
| 500 | 56.7 | 14.6 | 114.0 | 10.2 |
| 1000 | 47.7 | 4.5 | 124.3 | 15.8 |
| 2500 | 53.7 | 4.0 | 122.7 | 17.6 |
| 2000 | 44.3 | 2.3 | 116.0 | 10.5 |
3.3. 28-Day rat toxicity study
Rats received 0, 70, 350, or 1000 mg/kg/day of the chicory root extract by oral gavage. There were no extract-related mortalities, and there were no clinical signs in any test or recovery group that were considered to be of toxico-logical significance.
3.3.1. Body weights
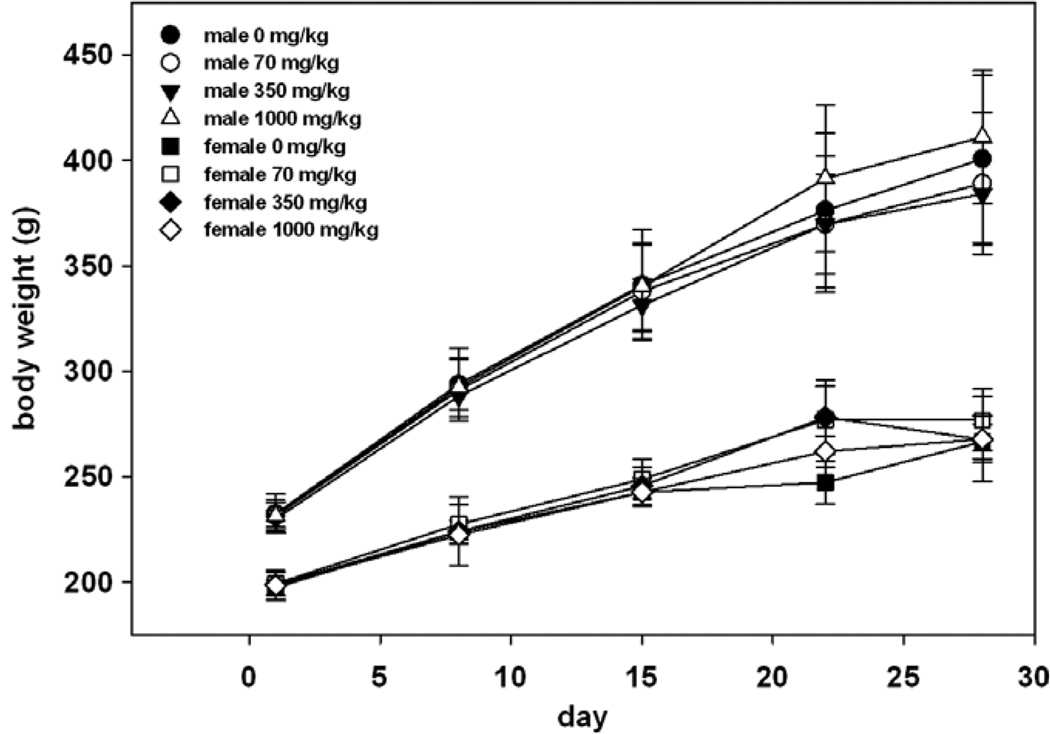
The mean body weight and mean body weight gain for male and female rats at 70, 350 and 1000 mg/kg/day were comparable with control values. A statistically significant increase compared to the control was observed in the body weights of females in the low and mid-doses on day 22 (Fig. 2). This finding was not observed in the high dose group and therefore is considered not to be related to treatment.
Fig 2.
Mean male and female body weights (± standard deviation).
3.3.2. Food consumption
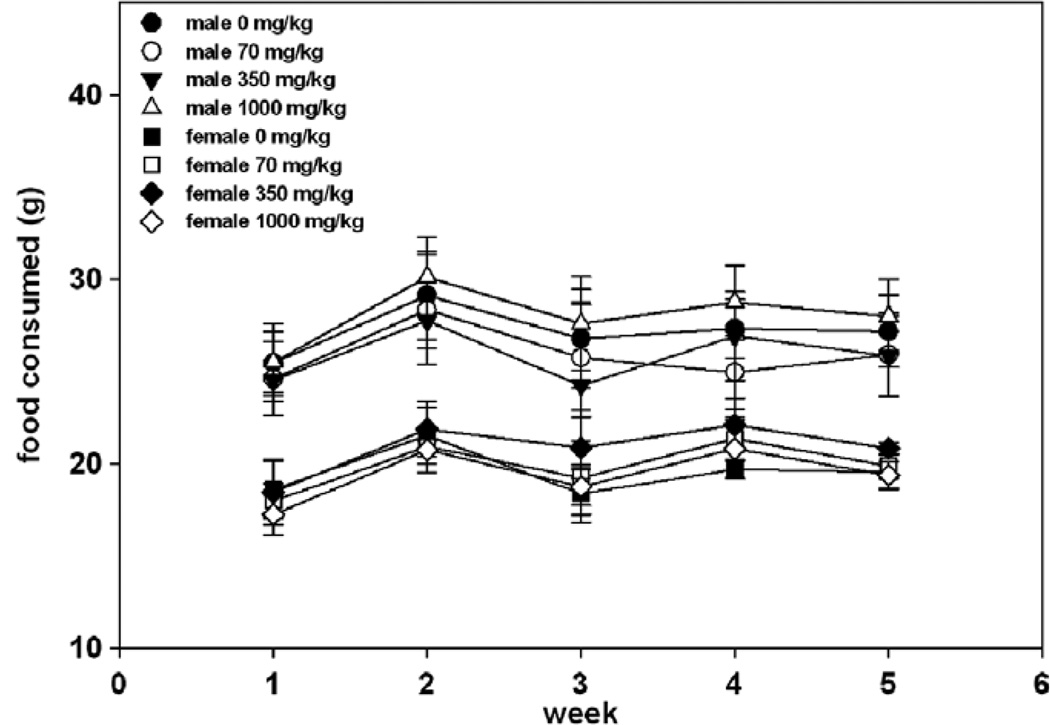
Overall (days 1–28) average daily food consumption and food efficiency for male and female rats at 70, 350 and 1000 mg/kg/day were comparable with control values. A statistically significant increase compared to the control was observed in the food consumption on days 22–28 and overall (0–28) for the females in the mid-dose (Fig. 3). A statistically significant increase compared to the control was observed in the food efficiency on days 15–22 for all groups of treated female rats. On days 22–28, a statistically significant decrease compared to the control was observed in the food efficiency for mid-dose females and a decrease without statistical significance was noted for the high dose females. These findings were of similar magnitude between groups and do not appear to be dose related. Therefore, these results are considered not to be of toxicological significance.
Fig 3.
Mean food consumption (± standard deviation).
3.3.3. Clinical pathology
There were no treatment-related effects in hematology parameters (Table 3). Mean cell volume was increased (105% of the control group, p = 0.03). The MCV of females dosed with 1000 mg/kg/day was 105% of the control group mean. This magnitude of change relative to control was influenced by one control female with a low mean cell volume. Eosinophils were increased (331% of the control group, p = 0.02) in males dosed with 350 mg/kg/day. This change was considered to be unrelated to treatment because it did not occur in a dose-related pattern.
Table 3.
Mean hematology and coagulation values
| Male 0 |
Female 0 |
Male 70 |
Female 70 |
Male 350 |
Female 350 |
Male 1000 |
Female 1000 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| RBC (×106/µL) | 8.57 | 0.31 | 8.54 | 0.33 | 8.68 | 0.33 | 83.43 | 0.31 | 8.37 | 0.19 | 8.36 | 0.10 | 8.35 | 0.22 | 8.27 | 0.37 |
| HGB (g/dL) | 16.4 | 0.4 | 16.0 | 0.3 | 16.5 | 0.5 | 16.0 | 0.6 | 16.4 | 0.1 | 16.2 | 0.4 | 16.2 | 0.4 | 16.1 | 0.4 |
| HCT (%) | 47.8 | 1.2 | 44.8 | 1.1 | 48.1 | 1.8 | 45.4 | 1.9 | 47.1 | 0.5 | 45.5 | 1.2 | 46.7 | 1.5 | 45.5 | 1.5 |
| MCV (fL) | 55.7 | 1.0 | 52.5 | 1.9 | 55.4 | 1.2 | 53.8 | 1.3 | 56.2 | 1.2 | 54.5 | 1.3 | 56.0 | 2.0 | 55.1 | 0.8 |
| MCH (pg) | 19.1 | 0.4 | 18.7 | 0.6 | 19.0 | 0.4 | 19.0 | 0.6 | 19.6 | 0.4 | 19.4 | 0.5 | 19.4 | 0.4 | 19.4 | 0.4 |
| MCHC (g/dL) | 34.3 | 0.4 | 35.7 | 0.5 | 34.3 | 0.4 | 35.3 | 0.5 | 34.9 | 0.4 | 35.6 | 0.7 | 34.6 | 0.7 | 35.2 | 0.4 |
| RDW (%) | 11.1 | 0.3 | 10.5 | 0.3 | 11.3 | 0.6 | 10.7 | 0.4 | 11.0 | 0.2 | 10.4 | 0.2 | 11.3 | 0.4 | 10.4 | 0.3 |
| ARET (×103/µL) | 158.7 | 25.3 | 150.9 | 13.4 | 159.4 | 36.7 | 151.2 | 16.5 | 162.4 | 28.8 | 120.4 | 42.1 | 200.7 | 33.2 | 149.0 | 30.1 |
| PLT (×103/µL) | 539 | 182 | 573 | 211 | 464 | 143 | 544 | 157 | 531 | 64 | 551 | 289 | 641 | 177 | 642 | 363 |
| WBC (×103/µL) | 13.11 | 2.53 | 11.31 | 3.28 | 12.33 | 1.56 | 12.58 | 3.67 | 12.75 | 1.65 | 10.41 | 3.72 | 13.64 | 2.64 | 9.53 | 3.35 |
| ANEU (×103/µL) | 1.52 | 1.05 | 1.66 | 1.14 | 1.61 | 0.35 | 1.74 | 0.71 | 1.91 | 0.58 | 1.11 | 0.43 | 1.55 | 0.43 | 1.94 | 0.84 |
| ALYM (×103/µL) | 11.07 | 1.77 | 9.03 | 2.15 | 10.13 | 1.50 | 10.38 | 2.84 | 10.10 | 1.19 | 8.89 | 3.11 | 11.50 | 2.84 | 7.22 | 3.33 |
| AMON (×103/µL) | 0.35 | 0.06 | 0.32 | 0.17 | 0.30 | 0.09 | 0.24 | 0.10 | 0.29 | 0.03 | 0.20 | 0.11 | 0.26 | 0.06 | 0.29 | 0.16 |
| AEOS (×103/µL) | 0.10 | 0.13 | 0.26 | 0.16 | 0.22 | 0.14 | 0.13 | 0.14 | 0.34 | 0.13 | 0.15 | 0.21 | 0.21 | 0.12 | 0.07 | 0.09 |
| ABAS (×103/µL) | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.04 | 0.01 | 0.01 | 0.02 |
| ALUC (×103/µL) | 0.04 | 0.03 | 0.03 | 0.03 | 0.05 | 0.04 | 0.06 | 0.06 | 0.07 | 0.02 | 0.05 | 0.07 | 0.07 | 0.02 | 0.02 | 0.02 |
| PT (s) | 14.5 | 0.3 | 14.8 | 0.6 | 14.8 | 0.4 | 14.8 | 0.3 | 14.3 | 0.4 | 14.7 | 0.3 | 14.6 | 0.6 | 14.9 | 0.3 |
| APTT (s) | 21.1 | 2.0 | 15.9 | 2.0 | 21.8 | 2.1 | 16.6 | 1.1 | 20.1 | 1.5 | 16.0 | 2.1 | 22.1 | 3.8 | 17.9 | 1.3 |
Dose level (mg/kg): 0, 70, 350, 1000.
There were no treatment-related or statistically significant effects in coagulation parameters. Activated partial thromboplastin time was minimally prolonged in females dosed with 1000 mg/kg/day (not statistically significant). This increase relative to controls was due primarily to one control female with an unusually low clotting time. Therefore, this change in 1000 mg/kg/day females was considered to be unrelated to treatment.
There were no adverse effects on clinical chemistry parameters (Table 4). Cholesterol was significantly (p < 0.05) decreased in females dosed with 350 or 1000 mg/kg/ day. Group means were 76% and 81% of the control group mean, respectively, and thus had a flat dose response. In addition, cholesterol concentrations of individual females were within or above the in-house reference interval for similarly aged rats (95% reference interval for mid-study time point of 90 day studies is 35–69 mg/dL; Haskell Notebook No. E-98560-AN). Therefore, although these changes appear treatment related, they were considered not to be toxicologically significant. Increased sorbitol dehydrogenase in females dosed with 70 mg/kg/day and decreased bilirubin in males dosed with 70 or 350 mg/kg/day were observed. The statistically significant changes in mean clinical chemistry results did not occur in a dose-related pattern were considered to be unrelated to treatment, and thus non-adverse.
Table 4.
Mean clinical chemistry values
| Male 0 |
Female 0 |
Male 70 |
Female 70 |
Male 350 |
Female 350 |
Male 1000 |
Female 1000 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| AST (U/L) | 83 | 9 | 57 | 6 | 72 | 3 | 65 | 5 | 75 | 10 | 70 | 7 | 73 | 7 | 71 | 13 |
| ALT (U/L) | 41 | 5 | 28 | 5 | 35 | 4 | 30 | 4 | 37 | 6 | 33 | 4 | 36 | 5 | 30 | 4 |
| SDH (U/L) | 10.2 | 3.4 | 7.6 | 2.2 | 8.5 | 2.6 | 12.0 | 3.8 | 9.6 | 2.3 | 10.9 | 2.6 | 10.4 | 3.5 | 9.0 | 1.2 |
| ALKP (U/L) | 243 | 54 | 95 | 6 | 238 | 27 | 108 | 27 | 218 | 57 | 113 | 26 | 205 | 22 | 102 | 29 |
| BILI (mg/dL) | 0.14 | 0.01 | 0.14 | 0.03 | 0.11 | 0.02 | 0.13 | 0.02 | 0.11 | 0.02 | 0.13 | 0.03 | 0.12 | 0.01 | 0.14 | 0.03 |
| BUN (mg/dL) | 10 | 1 | 11 | 1 | 10 | 1 | 11 | 2 | 10 | 1 | 12 | 2 | 9 | 2 | 10 | 1 |
| CREA (mg/dL) | 0.25 | 0.04 | 0.27 | 0.02 | 0.25 | 0.02 | 0.30 | 0.03 | 0.24 | 0.03 | 0.30 | 0.03 | 0.25 | 0.02 | 0.27 | 0.02 |
| CHOL (mg/dL) | 51 | 14 | 80 | 6 | 51 | 7 | 68 | 7 | 44 | 10 | 61 | 7 | 51 | 8 | 65 | 12 |
| TRIG (mg/dL) | 48 | 18 | 46 | 12 | 64 | 25 | 41 | 18 | 49 | 12 | 39 | 18 | 47 | 5 | 33 | 13 |
| GLUC (mg/dL) | 108 | 13 | 110 | 18 | 112 | 13 | 115 | 8 | 106 | 7 | 105 | 6 | 105 | 8 | 106 | 10 |
| TP (g/dL) | 5.9 | 0.1 | 6.6 | 0.3 | 6.2 | 0.4 | 6.5 | 0.2 | 6 | 0.1 | 6.3 | 0.2 | 6.0 | 0.2 | 6.5 | 0.4 |
| ALB (g/dL) | 3.3 | 0.1 | 3.7 | 0.2 | 3.4 | 0.1 | 3.7 | 0.1 | 3.4 | 0.1 | 3.5 | 0.1 | 3.3 | 0.1 | 3.7 | 0.3 |
| GLOB (g/dL) | 2.6 | 0.1 | 2.9 | 0.2 | 2.8 | 0.3 | 2.8 | 0.1 | 2.6 | 0.1 | 2.8 | 0.1 | 2.7 | 0.1 | 2.8 | 0.2 |
| CALC (mg/dL) | 10.4 | 0.1 | 10.8 | 0.3 | 10.6 | 0.1 | 10.6 | 0.2 | 10.5 | 0.3 | 10.6 | 0.3 | 0.2 | 0.2 | 10.7 | 0.2 |
| IPHS (mg/dL) | 8.7 | 1.8 | 6.6 | 0.6 | 7.9 | 0.2 | 6.8 | 0.3 | 7.8 | 0.2 | 6.8 | 0.5 | 0.3 | 0.3 | 6.5 | 0.4 |
| NA (mmol/L) | 142.5 | 1.0 | 141.5 | 1.1 | 142.4 | 1.9 | 141.0 | 1.4 | 142.2 | 1.0 | 142.6 | 1.5 | 1.3 | 1.3 | 142.0 | 0.9 |
| K (mmol/L) | 5.70 | 0.95 | 4.67 | 0.30 | 4.90 | 0.18 | 4.58 | 0.42 | 4.80 | 0.59 | 4.73 | 0.35 | 0.26 | 0.26 | 4.54 | 0.36 |
| CL (mmol/L) | 102.5 | 0.9 | 102.4 | 0.9 | 101.9 | 2.4 | 101.2 | 1.0 | 102.7 | 1.1 | 103.2 | 1.3 | 1.7 | 1.7 | 101.9 | 0.9 |
Dose level (mg/kg): 0, 70, 350, 1000.
3.3.4. Necropsy and histopathology
Mean absolute and relative organ weights (organ-to-body and organ-to-brain weight) for all organs evaluated were comparable to respective control values (Table 5) with two exceptions. Organ weight, organ-to-brain weight and organ-to-body weight ratios were comparable to control. A statistically significant increase compared to the control was observed in the organ weight for the adrenals in the low-dose females and in the organ-to-body weight ratio for the liver in the mid-dose males. There were no gross lesions observed in any of the rats from any of the groups. Potential treatment-related histomorphological findings were limited to the testis and epididymis of one male from the 1000 mg/kg dose group. Definitive association between treatment and these lesions in a single high dose animal remains uncertain because these are occasionally similar findings in untreated rats and because of the small group sizes in this study.
Table 5.
Mean organ weights (g)
| Male 0 |
Female 0 |
Male 70 |
Female 70 |
Male 350 |
Female 350 |
Male 1000 |
Female 1000 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body weight | 362.80 | 38.09 | 237.60 | 7.23 | 356.60 | 35.35 | 250.80 | 15.96 | 352.00 | 24.60 | 239.60 | 17.43 | 374.80 | 31.72 | 238.80 | 7.40 |
| Brain | 2.22 | 0.14 | 2.12 | 0.30 | 2.18 | 0.05 | 2.26 | 0.26 | 2.06 | 0.22 | 2.15 | 0.21 | 2.24 | 0.05 | 2.14 | 0.17 |
| Liver | 11.59 | 1.54 | 8.22 | 0.55 | 11.33 | 1.46 | 8.60 | 0.61 | 11.88 | 0.41 | 8.02 | 0.66 | 12.79 | 1.54 | 8.85 | 0.44 |
| Kidneys | 3.49 | 0.55 | 2.37 | 0.53 | 3.08 | 0.39 | 2.59 | 0.60 | 2.95 | 0.19 | 2.40 | 0.79 | 3.32 | 0.21 | 2.48 | 0.21 |
| Thymus | 0.535 | 0.097 | 0.592 | 0.168 | 0.506 | 0.159 | 0.684 | 0.226 | 0.436 | 0.110 | 0.559 | 0.202 | 0.578 | 0.100 | 0.572 | 0.188 |
| Heart | 1.43 | 0.17 | 0.93 | 0.05 | 1.26 | 0.11 | 0.99 | 0.10 | 1.27 | 0.07 | 1.05 | 0.11 | 1.33 | 0.09 | 0.98 | 0.08 |
| Spleen | 0.73 | 0.13 | 0.63 | 0.08 | 0.64 | 0.13 | 0.61 | 0.07 | 0.65 | 0.04 | 0.59 | 0.11 | 0.77 | 0.19 | 0.61 | 0.04 |
| Epididymides/ovaries | 1.259 | 0.279 | 0.173 | 0.045 | 1.104 | 0.113 | 0.171 | 0.066 | 1.277 | 0.192 | 0.174 | 0.054 | 1.262 | 0.187 | 0.138 | 0.042 |
| Testes/utems | 3.34 | 0.21 | 0.58 | 0.13 | 3.21 | 0.33 | 0.57 | 0.09 | 3.32 | 0.13 | 0.58 | 0.18 | 3.39 | 0.24 | 0.50 | 0.08 |
| Adrenals | 0.086 | 0.011 | 0.090 | 0.008 | 0.085 | 0.007 | 0.116 | 0.022 | 0.082 | 0.013 | 0.106 | 0.011 | 0.086 | 0.006 | 0.104 | 0.012 |
Dose level (mg/kg): 0, 70, 350, 1000.
4. Discussion
The current study provides evidence that chicory root extracts rich in sesquiterpene lactones prepared by sequential ethanolic extraction, defatting with n-heptane and extraction with ethyl acetate have no short-term toxicity up to 1000 mg/kg/day. There have been no previous studies formally addressing the toxicity of chicory root sesquiterpene lactone extracts, however several animal studies have been performed using aqueous and ethanolic extracts without reports of toxicity (Gadgoli and Mishra, 1997; Kim, 2000; Roberfroid, 2000; Kim et al., 2002). At the cellular level, an Ames test showed no evidence of mutagenic activity in various strains of E. coli and S. typhimurium exposed to chicory extract. In Salmonella typhimurium, there was some cytotoxicity observed with and without metabolic activation in strains TA97a and TA100 at high concentrations (Table 2). This is not surprising, since it has been previously reported that some sesquiterpene lactones have antimicrobial properties (Ramos et al., 2002).
Administration of chicory extract to rats by oral gavage at 70, 350, or 1000 mg/kg/day for 28 days caused no significant adverse toxicological effects attributable to treatment. Body weights were all comparable to control with the exception on day 22 of females receiving the low and inter-mediate doses (Fig. 2). Since this response was not seen in the high dose group and was not seen on any other day, we conclude it was not an effect of treatment. There were some fluctuations in food consumption and food efficiency, however these differences were uniform between groups and did not show a clear trend in any direction.
There were some slight changes noted in hematology and clinical chemistry parameters. The MCV of females in the high dose group was 105% of the control group; however, this value was influenced by one control female with a low mean cell volume. Other red cell mass parameters were unaffected and therefore this event was determined not to be toxicologically significant. Cholesterol was mildly decreased in females in the intermediate and high dose groups; however, there was a flat dose response and the values were within the in-house reference interval for similarly aged rats. None of the results were considered to be related to treatment.
Organ weight, organ-to-brain weight and organ-to-body weight ratios in treatment groups were comparable to control animals with a few minor exceptions (adrenal gland weights in intermediate dose females; organ-to-body weight ratio in high dose males). One male in the high dose group had a lesion of the testis and epididymis. It is difficult to say whether these findings are of toxicological importance since the same lesions are occasionally seen in control animals. It has been established that sesquiterpene lactones can inactivate sperm by reacting with thiol groups on the outer membrane (Huacuja et al., 1993), however this effect was only studied in vitro and does not necessarily suggest a direct effect on male fertility or male reproductive organs. To our knowledge, there have been no reports of adverse effects of sesquiterpene lactones on male reproductive organs.
In summary, some evidence suggests that chicory root extract containing sesquiterpene lactones is non-toxic. Chicory has been grown and consumed by humans since ancient Egyptian times. The aqueous extract which contains a large amount of carbohydrates is generally regarded as safe (GRAS) and has a history of human use as a coffee additive. The current study showed chicory extract prepared by ethanol extraction, defatting with n-heptane and further extraction with ethyl acetate is non-mutagenic in the performed Ames test. The 28-day rat study showed no treatment-related adverse events, demonstrating that the tested chicory extract is non-toxic to rats, with a NOAEL of 1000 mg/kg/day when administered orally for 28 consecutive days. The combined history of human use of the root and aqueous extract and data from the current study support the use of chicory sesquiterpene lactone root extract in a longer-term test of toxicity in experimental animal models and also support further testing of chicory root extract as a therapeutic agent for inflammatory diseases.
Acknowledgements
Partially supported by Phytomedics Inc., Fogarty International Center of the NIH under U01 TW006674 for the International Cooperative Biodiversity Groups, and Rutgers University and NJ Agricultural Experiment Station.
Abbreviations
- ANOVA
analysis of variance
- EAFUS
everything added to food in the United States
- EPA
Environmental Protection Agency
- FDA
Food and Drug Administration
- GLP
good laboratory practices
- GRAS
generally regarded as safe
- ICH
International Conference on Harmonization
- NBF
neutral buffered formalin
- NIH
National Institutes of Health
- NOAEL
no observed adverse effect level
- OECD
Organization for Economic Co-operation and Development
References
- Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-micro-some mutagenicity test. Mutat. Res. 1975;31:347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Bais HP, Ravishankar GA. Cichorium intybus L.—cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food. Agric. 2001;81:467–484. [Google Scholar]
- Cavin C, Delannoy M, Malnoe A, Debefve E, Touche A, Courtois D, Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005;327:742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Cheeke PR, Shull LR. Natural Toxicants in Feeds and Poisonous Plants. Westport, CT, USA: AVI Publishing Company Inc; 1985. [Google Scholar]
- Gadgoli C, Mishra SH. Antihepatotoxic activity of Cichorium intybus. J. Ethnopharmacol. 1997;58:131–134. doi: 10.1016/s0378-8741(97)00090-1. [DOI] [PubMed] [Google Scholar]
- Grieve M. A Modern Herbal. Mineola, NY: Dover Publications; 1971. [Google Scholar]
- Hall IH, Lee KH, Starnes COJ, Sumida Y, Wu RY, Waddell TG, Cochran JW, Gerhart KG. Anti-inflammatory activity of sesquiterpene lactones and related compounds. J. Pharm. Sci. 1979;68:537–542. doi: 10.1002/jps.2600680505. [DOI] [PubMed] [Google Scholar]
- Hazra B, Sarkar R, Bhattacharyya S, Roy P. Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia. 2002;73:730–733. doi: 10.1016/s0367-326x(02)00232-0. [DOI] [PubMed] [Google Scholar]
- Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J. Biol. Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- Huacuja RL, Carranco A, Guzman SA, Guerrero C. Inactivation of SH groups with sesquiterpene lactones: effects on nuclear decondensation pattern/motility induced by heparin in human spermatozoa. Adv. Contracept. Deliv. Syst. 1993;9:97–106. [PubMed] [Google Scholar]
- Ivie GW, Witzel DA, Herz W, Kannan R, Norman JO, Rushing DD, Johnson JH, Rowe LD, Veech JA. Hymenovin, major toxic constituent of Western bitterweed (Hymenoxys odorata DC.) J. Agric. Food Chem. 1975a;23:841–845. doi: 10.1021/jf60201a051. [DOI] [PubMed] [Google Scholar]
- Ivie GW, Witzel DA, Rushing DD. Toxicity and milk bittering properties of tenulin, the major sesquiterpene lactone constituent of Helenium amarum (bitter sneezeweed) J. Agric. Food Chem. 1975b;23:845–849. doi: 10.1021/jf60201a012. [DOI] [PubMed] [Google Scholar]
- Jindal MN, Patel VR, Patel NB. Pharmacological actions of aqueous and alcoholic extracts of roots of Cichorium intybus Linn. Ind. J. Pharmacol. 1975;7:24–33. [Google Scholar]
- Kim JH, Mun YJ, Woo WH, Jeon KS, An NH, Park JS. Effects of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int. J. Immunopharmacol. 2002;2:733–744. doi: 10.1016/s1567-5769(02)00008-5. [DOI] [PubMed] [Google Scholar]
- Kim M. The water-soluble extract of chicory reduces cholesterol uptake in gutperfused rats. Nutr. Res. 2000;20:1017–1026. [Google Scholar]
- Knuesel O, Weber M, Suter A. Arnica montana gel in osteoarthritis of the knee: an open, multicenter clinical trial. Adv. Ther. 2002;19:209–218. doi: 10.1007/BF02850361. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- Lyss G, Schmidt TJ, Merfort I, Pahl HL. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappaB. Biol. Chem. 1997;378:951–961. doi: 10.1515/bchm.1997.378.9.951. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Palevitch D, Earon G, Carasso R. Feverfew (Tanacetum parthenium) as a prophylactic treatment for migraine: a double-blind placebo-controlled study. Phytother. Res. 1997;11:508–511. [Google Scholar]
- Picman AK. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 1986;14:255–281. [Google Scholar]
- Ramos A, Rivero R, Visozo A, Piloto J, Garcia A. Parthenin, a sesquiterpene lactone of Parthenium hysterophorus L. is a high toxicity clastogen. Mutat. Res. 2002;514:19–27. doi: 10.1016/s1383-5718(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. Chicory fructooligosaccharides and the gastrointestinal tract. Nutrition. 2000;16:677–679. doi: 10.1016/s0899-9007(00)00244-6. [DOI] [PubMed] [Google Scholar]
- Robles M, Aregullin M, West J, Rodriguez E. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta Med. 1995;61:199–203. doi: 10.1055/s-2006-958055. [DOI] [PubMed] [Google Scholar]
- Robles M, Choi BH, Han B, Santa Cruz K, Kim RC. Repin-induced neurotoxicity in rodents. Exp. Neurol. 1998;152:129–136. doi: 10.1006/exnr.1998.6826. [DOI] [PubMed] [Google Scholar]
- van Beek TA, Maas P, King BM, Leclercq E, Voragen AGJ, De Groot A. Bitter sesquiterpene lactones from chicory roots. J. Agric. Food Chem. 1990;38:1035–1038. [Google Scholar]