Abstract
The risk of fragility fractures exponentially increases with aging. Reduced mass and strength of both bone in osteoporosis and skeletal muscle in sarcopenia play a key role in the age-related incidence of fragility fractures. Undernutrition is often observed in the elderly, particularly in those subjects experiencing osteoporotic fractures, more likely as a cause than a consequence. Calcium (Ca), inorganic phosphate (Pi), vitamin D, and protein are nutrients that impact bone and skeletal muscle integrity. Deficiency in the supply of these nutrients increases with aging. Dairy foods are rich in Ca, Pi, and proteins and in many countries are fortified with vitamin D. Dairy foods are important souces of these nutrients and go a long way to meeting the recommendations, which increase with aging. This review emphaszes the interactions between these 4 nutrients, which, along with physical activity, act through cellular and physiological pathways favoring the maintenance of both bone and skeletal muscle structure and function.
Keywords: dairy, Ca, inorganic phosphate, vitamin D, protein, bone and skeletal muscle health, elderly
Key teaching points:
Preventing bone loss and risk of falling are key to reducing age-related increases in fragility fracture.
Ca and vitamin D are needed to reduce the risk of hip fracture.
Increasing Pi intake stimulates the renal reabsorption and the overall retention of Ca.
Protein intake through the production of insulin-like growth factor-I (IGF-I) positively interacts with vitamin D metabolism and the Ca-Pi economy.
Interactions of Ca, Pi, protein, and vitamin D reduce bone resorption and increase bone formation, attenuating age-related bone loss.
Mechanical loading of skeletal muscle acts in concert with amino acids and IGF-I on skeletal mass and strength.
INTRODUCTION
The delimitation of the specific roles of nutrients in foods influencing the structure and function of human body organs or systems in health and diseases is a challenge, in part due to the rapidity of new information to shape our understanding. The musculoskeletal system faces a similar challenge. Bone and skeletal muscle acquisition during development and maintenance, in terms of mass and strength, during adulthood are under the influence of environmental factors, among which mechanical and nutritional factors play pivotal roles. The nutrients that have the greatest physiological impact, via well-defined specific mechanisms on bone and skeletal muscle throughout life, are calcium (Ca), vitamin D, inorganic phosphate (Pi), and protein.
This article focuses on how the interactions among Ca, Pi, vitamin D, and protein can positively or even synergistically impact on mechanical loading to favor skeletal health during adulthood. We also report the dramatic consequences of their insufficient supply on bone and skeletal muscle integrity with the associated risk of fragility fracture that markedly increases with aging. Then, based on both physiological and clinical information, practical recommendations for securing adequate intake are presented.
Dietary Patterns
Foods rather than nutrients are chosen and consumed. Hence, the appropriate and logical approach to examining the relationship between diet and diseases such as osteoporosis is through food surveys that can provide dietary patterns related to bone mineral density and fragility fracture risk [1,2]. From dietary patterns emerge the concept of food groups that usually include bread, other cereals, and potatoes; fruit and vegetables; meat, fish, and alternatives; milk and dairy products; and fatty and sugary foods [2]. Furthermore, the dietary pattern concept has provided the base for setting recommendations regarding the proportion of each food group that should be eaten to optimize the general health of a population [1,2].
Food consumption is affected by a whole range of factors or drivers, including the availability, accessibility, and choice of foods [3]. These factors may in turn be influenced by geography, demography, disposable income, socioeconomic status, urbanization, marketing, religion, culture, and consumer attitudes [3,4].
Nutrient Profiling of Foods: The Contribution of Dairy Products
A diet can be energy rich but nutrient poor [5], and this type of consumption can result in nutrient deficits, as observed even in developed countries such as the United States, among children, the elderly, and low-income populations [6]. Dietary guidance needs to identify foods that are nutrient rich, affordable, and appealing [7]. In this respect, the contribution to energy and nutrient intakes of major food groups can be established. This assessment allows for identifying the relative nutrient-percalorie cost so that foods that are affordable, appealing, as well as nutrient rich are in dietary guidelines [8]. A nutrient profiling analysis of the U.S. diet shows that milk and milk products are foods of low energy with substantial micronutrient density and appreciable affordability [7].
The food group approach provides very useful information for setting up overall dietary recommendations with the aim of improving various health components. However, there is the necessity to first identify which nutrients are essential for musculoskeletal health—that is, Ca, phosphorus in the form of inorganic phosphate (Pi), vitamin D, and protein—and then determine how these essential nutrients interact in the development and maintenance of bone and muscle.
BONE AND SKELETAL MUSCLE HEALTH
Bone
Bone Composition and Cellular Remodeling Activity
Bone is a composite material, which is essentially made of mineral (60%), an organic matrix (30%), and water (10%). The mineral phase of bone is an analog of the naturally occurring hydroxyapatite {Ca10(PO4)6(OH)2} crystal [9]. The basic building block of the bone matrix fiber network is type I collagen, a triple-helical molecule, whereas noncollagenous proteins comprise 10%–15% of the total bone protein content [9]. The function of the mineral phase is to strengthen the collagen fiber network, thus providing adequate resistance to mechanical loading. Taking into account these chemical and mechanical characteristics, the structural integrity/composition of the bone tissue is clearly dependent upon the dietary supply of Ca, Pi, and protein. The essentiality of these nutrients for bone integrity can be fully appreciated by the severe consequences observed following their selective/individual dietary deficiency. Thus, a limitation in Ca, Pi, or protein while the intakes of the other two nutrients are maintained at normal levels leads to severe bone structural deficiency associated with loss of bone strength, as observed in diseases such as osteoporosis or osteomalacia [10,11]. In order to play their structural role in bone, these three nutrients require a normal vitamin D status (see Interactions section for the interplay between Ca, Pi, protein, and vitamin D in bone metabolism). Besides being integrated as part of bone material, through the mediating activity of certain amino acids, dietary Ca, Pi, and protein exert an important impact on bone forming and resorbing cells (for review, see Bonjour [12,13]). Overall, their combination tends to reduce bone resorption and stimulate bone formation.
Resorption and formation of bone occur through the process of remodeling during adulthood, under control of three types of bone cells. Osteoblasts are responsible for the formation of the organic matrix, essentially made of collagen proteins, and the deposition onto the collagen fibers of Ca and Pi, the two main bone mineral crystal components. Osteoclasts resorb both the mineral and matrix of the bone tissue, and this must occur prior to formation. Osteocytes, which are derived from the mature osteoblasts, are the most abundant cells in bone. They influence both the osteoblast and osteoclast functions by forming an interconnected network in bone [14].
Undernutrition in Elderly and Osteoporosis
Undernutrition is often observed in the elderly population [15–17]. They have insufficient vitamin D supply from both skin and dietary sources, as well as low Ca and protein intakes; these are associated with reduced bone mineral mass and increased risk of fragility fracture [16,18–22] (Fig. 1). Inadequately low vitamin D and Ca supply leads to a decrease in the intestinal Ca and Pi absorption. A major effect of vitamin D through its active metabolite 1,25-dihydroxycholecalciferol (1,25[OH]D) is to stimulate the active components involved in the translocation of Ca and Pi across the intestinal epithelial cells [23]. Insufficient vitamin D supply resulting in inadequately low intestinal Ca absorption leads to the overproduction of parathyroid hormone (PTH), which, in turn, increases bone resorption [24].
Fig. 1.
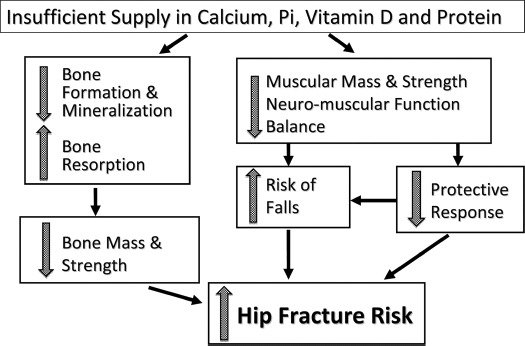
Undernutrition and pathogenesis of hip fracture risk. Insufficiency in the 4 nutrients—Ca, Pi, vitamin D, and protein—can contribute to a reduction in both mass and strength of bone and skeletal muscle. The risk of falling is worsened by impairment in neuromuscular function and abnormalities in gait and balance. This dysfunction reduced the protective response that may prevent falling when stumbling. Undernutrition also decreases the soft tissue pad around the hip, thus increasing the impact of a fall on the proximal femur.
The decrease in protein intake leads to a decline in the circulating level of the bone anabolic factor insulin-like growth factor-I (IGF-I) [25]. In protein malnutrition not only is the production of IGF-I reduced but so is its bone anabolic action [26], explaining, at least in part, the decline in bone formation [13].
Deficiencies in the supply of vitamin D, Ca, and Pi are associated with defective bone structural integrity, as expressed by either a prominent defect in mineral deposition that characterizes the pathological condition of osteomalacia or by a loss of the entire mineralized organic matrix, an important feature of osteoporosis. Many observational and interventional studies have reported on the beneficial effects of at least one of these nutrients on bone mineral density with evidence, in some reports, of a reduction in osteoporotic fracture risk [22, 27–30]. The reduction in fragility fractures observed with vitamin D supplementation in the elderly can be ascribed not only to its preventive effect on bone loss [28,31] but also to a reduction in the risk of falling [32]. Likewise, with increased protein intake, the reduction in osteoporotic fracture, as observed in a large prospective study carried out in a population at risk of experiencing hip fracture [33], may also be ascribed, at least in part, to a beneficial effect on neuromuscular functions reducing the risk and consequence of falling [13,25,34].
Preventing Bone Loss and Risk of Falling Are Key to Reducing Fracture Incidence with Aging
With aging, there is an exponential increase in the prevalence and incidence of fractures due to two determinants. First there is an intrinsic weakness of the bones to mechanical loading. This is due both to a loss in the mass of the material contained within the periosteal envelop and to a deterioration of the cortical and trabecular microstructure, as specified in the conceptual definition of osteoporosis [35]. The second determinant is the increased risk and consequence of falling. In the elderly, postural instability, reduced muscle mass and strength (i.e., sarcopenia), and hip subcutaneous fat pad thinness increase both the propensity to fall and the subsequent risk of fracture. This is because of the greater mechanical force impacting an already fragile bony structure. These skeletal and extraskeletal factors can be positively influenced by adequate measures improving both nutritional status and physical mobility.
Influence of Dairy Products on Bone Health
With respect to potential impact of foods on bone metabolism, dairy products are of particular interest. Dairy foods contain nutrients including Ca, Pi, and protein in appreciable amounts compared to their recommended allowances. The beneficial effect of these 3 nutrients on bone organic matrix formation and mineralization while exerting an inhibitory effect on bone resorption has been well documented (for recent reviews, see Bonjour [12,13]).
Vitamin D levels in dairy foods are variable and insufficient to meet body needs, recently estimated at least at 20 μg (800 IU) per day in healthy adults [36–38]. The 2011 report from the Institute of Medicine recommended 15 μg (600 IU) and 20 μg (800 IU) per day in subjects aged 51–70 years and ≥71 years, respectively [39]. The widespread strategy in countries such as Canada and the United States has been to require vitamin D fortification of dairy products such as fluid milk as well as allow fortification of dairy products and other foods (e.g., cereal flours, fruit juice) [40,41]. A systematic review and meta-analysis indicated that vitamin D–fortified foods improved circulating 25-hydroxyvitamin D (25OHD) concentrations in community-dwelling adults [42].
It has been the practice to ascribe the positive effect of dairy consumption on bone health to Ca alone (e.g., Matkovic et al. [43]). Indeed, early observations of protein and Pi suggested either negative or neutral effects of these nutrients on Ca balance or bone mass and strength [44]. However, there is now robust evidence that dietary protein and Pi can enhance Ca balance by stimulating its intestinal absorption and renal tubular reabsorption, respectively, and thereby can substantially contribute to the positive effect of dairy foods on bone health (see Interactions section).
Numerous clinical studies have reported on the relation between dairy product consumption and bone variables including bone turnover markers, bone mineral density, measured as areal or volumetric, or fracture in adult subjects (for review, see Heaney [45]). Several recent interventional studies have been published on the beneficial effects of vitamin D fortified dairy products on bone remodeling and/or bone mineral density in postmenopausal women [46–50] and in the elderly [51,52].
Both pathophysiological and clinical data explain how, when taken separately, Ca, Pi, vitamin D, and protein improve bone maintenance in adults. An inadequate dietary intake of Ca or an inadequate supply of vitamin D influences the secretion of PTH, which, when constantly stimulated, leads to increased bone remodeling with greater resorption than formation. (The prevailing increased bone resorption resulting from constant overproduction or infusion of PTH contrasts with its anabolic action when the hormone is intermittently administered. Hence, the use of daily injections of active PTH sequences such as its amino-terminal 1–34 [Teriparatide], in the treatment of osteoporosis.) Deficiency of vitamin D reduces the capacity of the intestinal mucosa to adequately absorb Ca and Pi. This impairment in the intestinal absorption of the two main bone crystal components can best be explained by a decline in the renal production of the active form of vitamin D, namely, 1,25(OH)D. The resulting reduced circulating Ca × Pi product impairs bone mineralization, and inadequate levels of Ca and Pi can also impair the activity of bone-forming cells [12].
Selective deficiency in protein intake has severe consequences on bone integrity, with reduced bone formation and increased bone resorption leading to increased skeletal fragility. Furthermore, inadequate protein intake affects the neuromuscular system, impairing coordination movement and reducing muscle mass and strength. Besides being an essential component of bone matrix and muscle fibers, in bone cell and in skeletal myocytes, protein-derived essential amino acids stimulate the production of the growth factor IGF-I. Low protein intake is associated with low IGF-I, osteoporosis, and sarcopenia. The serum level of IGF-I decreases with aging [22]. Prospective studies have documented that a low circulating IGF-I level is associated with an increased risk of osteoporotic fractures [53,54].
INTERACTIONS
Interactions between Ca and Vitamin D
Combined daily vitamin D (800 IU or 20 μg) and Ca supplementation (1200 mg of Ca as triphosphate) has been shown to reduce the incidence of hip and other nonvertebral fractures in elderly French nursing home residents who were at high risk for fracture (mean age 84 years, Ca intake ≤ 600 mg/d, and initial mean serum level of 25OHD < 10 ng/ml [<25 nmol/L]) within 18–36 months [55]. Another study has replicated these results in the United States [56]. Though neither study measured the rate of falling, the reduction in nonvertebral fracture rate observed in the French study [55] as early as 6 months following the onset of the intervention suggests that the beneficial effect of the combined treatment was not merely due to the prevention of bone loss but rather to an early effect of the vitamin D supplementation on the propensity to falling [57]. In fact, vitamin D was shown to reduce the risk of falling as early as 3 months after the onset of its administration in the elderly [58,59]. Evidenced from a comparative meta-analysis of randomized controlled trials [28], oral vitamin D appears to reduce the risk of hip fracture only when Ca supplementation is added (Fig. 2).
Fig. 2.
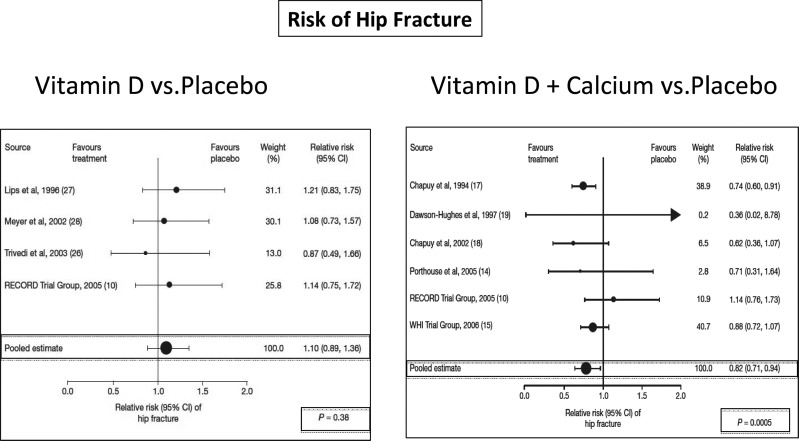
Need for additional Ca to reduce the risk of hip fracture with vitamin D supplementation. Evidence from a comparative meta-analysis of randomized controlled trials that oral vitamin D appears to reduce the risk of hip fracture only when Ca supplementation is added. The figure shows two forest plots of the risk of hip fracture between vitamin D and either placebo/no-treatment groups (left panel) or between vitamin D and Ca and placebo/no-treatment groups (right panel). The pooled estimate of the relative risk was statistically significant only with the combination of vitamin D and Ca. Adapted from Boonen et al. [28].
Interactions between Protein Intake, Ca-Phosphate Economy, and Vitamin D Metabolism
Dietary protein is required to promote bone formation. As for any other organ, amino acids are required for the synthesis of intracellular and extracellular proteins and other nitrogen-containing compounds. Through their amino acid content, proteins can also influence Ca-Pi economy and bone metabolism [13]. Dietary protein stimulates the formation of IGF-I (Fig. 3); this effect can be observed independent of dietary energy supply [13].
Fig. 3.
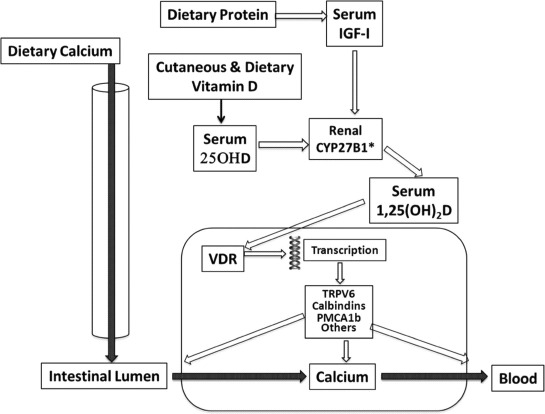
Schematic interaction of vitamin D and dietary protein on renal 1,25(OH)D production and thereby on intestinal Ca absorption. The transfer of dietary Ca from the intestinal lumen to the blood is stimulated by 1,25(OH)D, the renal production of which depends on its substrate, 25OHD, and IGF-I. The mechanism of intestinal Ca translocation depends of the presence of the vitamin D receptor (VDR), which in turn influences the transcription of several factors that are implicated in the transport of Ca across either the luminal or basolateral membranes or through the intracellular compartment of the enterocyte. CYP27B1: 25 hydroxyvitamin D-1α hydroxylase, TRPV6 = transient receptor potential cation channel, subfamily V, member 6, Calbindins = Ca binding proteins, PMCA1b = plasma membrane Ca ATPase 1b (Color figure available online.)
At the bone level, some amino acids such as arginine are capable of stimulating the local production of IGF-I by osteoblastic cells [60]. This effect was associated with an increase in osteoblastic cell proliferation and collagen synthesis. Note that IGF-I is probably the main mediator in the anabolic effect of PTH [61], as documented in a randomized controlled trial carried out in women with osteoporosis [62].
A dietary protein-mediated increase in the circulating level of IGF-I enhances the renal production of 1,25(OH)D (Fig. 3), which in turn stimulates the intestinal absorption of both Ca and Pi. IGF-I also increases the tubular reabsorption of Pi. Through this dual renal activity of IGF-I, the concentration of Ca and Pi in the systemic extracellular compartment increases and thereby positively influences the process of bone mineralization. This indirect positive effect of protein, via the IGF-I–1,25(OH)D connected endocrine system on intestinal Ca absorption, is associated with a direct stimulatory effect of amino acids such as arginine and lysine on Ca translocation from the luminal to the contraluminal side of the intestinal mucosa. The overall effect of protein intake on enhancing intestinal absorption accounts for the associated increased calciuria. Recent studies showed that the increased urinary Ca excretion associated with a high-protein diet does not result in negative skeletal Ca balance reflecting bone loss [34]. Despite experimental and clinical evidence to the contrary [63], it has been alleged that dietary protein is deleterious for bone health by inducing chronic metabolic acidosis and osteoporosis. Reviews and meta-analyses based on scientific arguments demonstrated that nutrition-induced acid–base changes do not influence Ca balance and thereby are not implicated in osteoporosis development or progression [22,34,64–68]. Further, there is no consistent evidence for superiority of plant-based protein over animal protein on Ca metabolism, bone loss prevention, and risk reduction of fragility fracture [22].
The favorable effect of increasing dietary protein on bone mineral density or content is better sustained when the supply of both Ca and vitamin D is adequate [19,69,70]. However, in post-menopausal women with low Ca intake (600 vs 1500 mg/day), a relatively high protein consumption (20% vs 10% of energy intake) enhanced Ca retention [71]. Similarly, in healthy older women and men, a protein supplement increasing the intake from ∼0.8 to 1.6 g/kg, when exchanged isocalorically for carbohydrates, was associated with higher circulating levels of IGF-I and lowered levels of urinary N-telopeptide, a marker of bone resorption [69]. This result is compatible with high dietary protein preventing bone loss in elderly.
Interactions between Ca and Inorganic Phosphate
Bone contains about 99% and 80% of the whole-body Ca and P (phosphorus), respectively [72]. The Ca/P mass ratio in bone is 2.2, close to that measured in human milk.
The initial step of Ca-Pi crystal nucleation takes place within matrix vesicles that bud from the plasma membrane of osteogenic cells and migrate into the extracellular skeletal compartment [73]. They are endowed with a transport system that accumulates Pi inside matrix vesicles, followed by the influx of Ca ions [73]. This process leads to the formation of an impure form of hydroxyapatite and its subsequent association with the organic matrix collagen fibrils (Fig. 4). In addition to this structural role, both Ca and Pi positively influence the activity of bone forming and resorbing cells [12,74]. Pi plays a role in the maturation of osteocytes, which are implicated in bone mineralization and systemic Pi homeostasis because they produce fibroblast growth factor 23, a hormonal regulator of renal Pi reabsorption and 1,25(OH)D production [75–77]. In contrast to their tight association in bone formation and resorption, renal Ca and Pi reabsorptions are independent, driven by distinct molecular mechanisms. Each has different extraskeletal functions in cellular metabolism [12]. At both the renal and intestinal levels, interactions of Ca and Pi are implicated in the acquisition and maintenance of bone, as well as in osteoporosis management. In the kidney, increased Pi intake reduces urinary Ca and increase Ca balance (Fig. 5). During growth and adulthood, administration of Ca-Pi in a ratio close to that of dairy products leads to positive effects on bone health. In contrast, when pharmaceutical mineral supplements are used to induce large differences between luminal Ca and Pi concentrations, this may have adverse effects on bone health. In patients with osteoporosis treated with anabolic agents, a Ca-Pi supplement appears to be preferable to carbonate or citrate Ca salt [12].
Fig. 4.
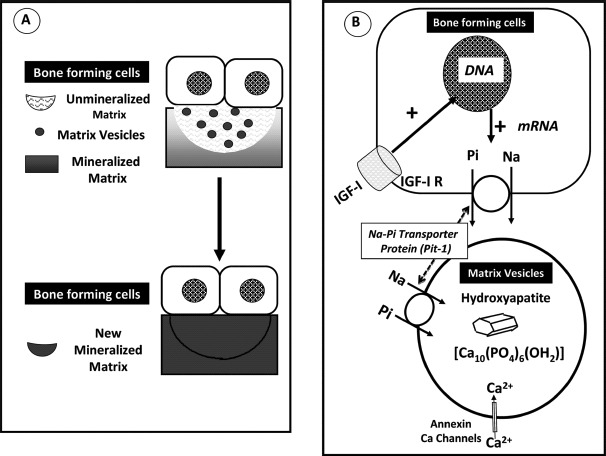
Bone mineralization process involving the interaction of Pi and Ca. (A) Bone forming cells, either osteoblasts or epiphyseal chondrocytes form vesicles, which bud from the plasma membrane and migrate in the nonmineralized organic matrix. (B) Matrix vesicles have the capacity to accumulate Pi through an Na-dependent Pi transporter, the driving force, and Ca through annexin channels. This accumulation leads to the formation of an impure form of hydroxyapatite crystal and its subsequent association with the collagen fibrils of the organic matrix. The bone forming cells are endowed with IGF-I receptor. The binding of IGF-I to this receptor stimulates the protein expression (mRNA) of the Na-dependent Pi transporter, which migrates to the plasma membrane of the bone forming cells. Adapted from Bonjour [12] (Color figure available online.)
Fig. 5.
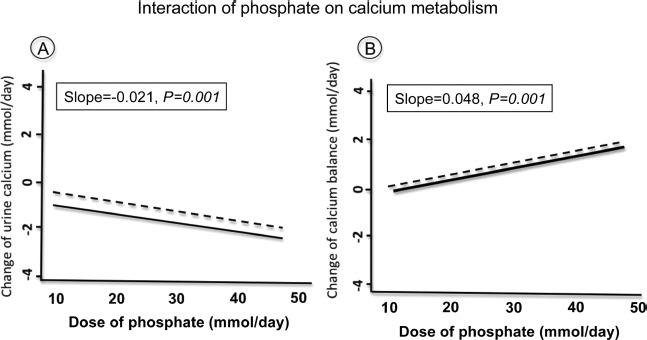
Influence of phosphate on the urinary (A) and balance (B) of Ca in healthy adults. Results from a meta-analysis of intervention studies indicate that higher phosphate intakes were associated with decreased urine Ca (A) and increased Ca retention (B). The change in slope was not different whether the Ca intake was low (dotted regression line) or high (continuous regression line), indicating the absence of interaction in relation with the amount of dietary Ca. Adapted from Fenton et al. [66].
SKELETAL MUSCLE
Muscle strength plays an important role in reducing the risk of falling and thus the risk of fractures. Skeletal muscle mass corresponds to about 40% of body mass. The total mass of protein contained in skeletal muscle for a man with a body weight (b.w.) of 70 kg is about 5.0 kg [72]. Comparatively, in bone the total amount of protein, essentially present as collagen, is about 2.0 kg. In the whole body, about 50% of proteins are present in both skeletal muscle and bone. The whole-body protein pool is constantly renewed at a mean daily rate of about 2% [78–80].
In addition to bone loss, the decline of skeletal muscle mass and strength—that is, sarcopenia, beginning around 45–55 years—is considered as one of the most important factors implicated the progression of disability with aging [81–83]. Sarcopenia is estimated to affect 30% of individual aged 60 years and older and more than 50% of those age 80 years and older [84]; it results from an imbalance between protein synthesis and degradation. Muscle quality refers to strength per cross-sectional area or strength per unit muscle mass and is considered a more meaningful indicator of muscle function than strength alone. Increased fatty infiltration of skeletal muscle (myosteatosis) with age reduces muscle strength, increases the propensity to fall, and was recently reported to predict the occurrence of hip fracture [85]. Reduction in both quality of muscle unit performance and motorneuron number [84] contributes to the significant progressive decline in physical ability with aging [86]. Several mechanisms contribute to the age-related decrease in muscle mass and strength, including altered hormonal status, inflammatory processes, reduced physical activity, and undernutrition, most often resulting from low dietary intake of energy and protein [84].
Beneficial Interactions between Nutrients and Physical Activity on Skeletal Muscle Health
Role of Protein Supply on Muscle Performance
Sarcopenia can be counteracted by adequate interventions, particularly by the combination of adequate nutritional intake and exercise training [87]. Improving intakes of protein can prevent or at least reduce the progression of muscle loss [87].
A prospective observational study over 3 years showed that protein intake was positively associated with preservation of lean mass in women and men aged 70–79 years [88]. Individuals with the highest quintile of daily protein intake (1.1 g/kg b.w.) lost 40% less total body and appendicular lean mass than those in the lowest quintile (0.7 g/kg b.w.) [88]. Thus, a daily protein intake well above the recommended daily allowances currently set at 0.8 g/kg b.w. for adults would reduce the risk of sarcopenia in older adults [88]. It has been proposed that dietary protein requirements should be increased from 0.8 to 1.0–1.2 g/kg b.w. per day for optimal skeletal muscle and bone health in elderly people [34].
Several relatively short-term interventional studies have investigated the skeletal muscle anabolic response to various protein supplementations in the elderly [89]. Compared to the bone response, the impact of protein supplementation and/or increased resistance exercise on skeletal muscle mass and strength can be expected to occur much more rapidly, taking into account the difference in protein metabolism between the two tissues. Fasting muscle protein synthesis rates do not seem to substantially differ between young adults and healthy elderly [90]. When age differences have been reported, they might, at least partly, be ascribed to substantial deterioration in health condition or nutritional status in elderly subjects. In addition, the elderly may have markedly limited physical activity [90]. Most research has been focused on disturbances in protein skeletal muscle synthesis in response to the main anabolic stimuli; that is, food intake and physical activity [90].
The regular performance of resistance exercises and the habitual ingestion of adequate amounts of dietary protein are two important ways for older people to slow down the progression of the age-related loss of skeletal muscle mass and function as well as improve balance and physical functioning capabilities [91–93] (Fig. 6).
Fig. 6.
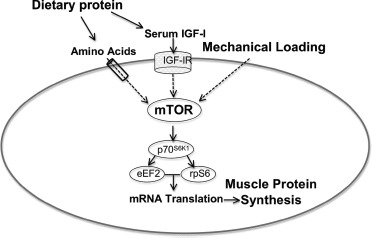
Interaction between dietary protein and mechanical loading on skeletal muscle cell. Dietary protein increases serum amino acids and IGF-I, which, through transporter and receptor localized in the skeletal muscle sarcolemma exert, through a complex intracellular pathway, an anabolic effect. Contractile forces resulting from mechanical loading also stimulate a complex molecular cascade. These pathways impact on the mammalian target of rapamycin complex (mTOR), which enhances several translation factors (70-kDa ribosomal protein S6, eukaryotic elongation factor 2, ribosomal protein S6). This simplified illustration schematizes the combined effects of dietary proteins, through amino acids and IGF-I, and mechanotransduction-mediated anabolic cell signaling on skeletal muscle mass and strength. Adapted from Pasiakos [93] (Color figure available online.)
Over the last decade, studies have reported the effects of protein supplementation on skeletal muscle health in middle-aged and/or elderly subjects [94–102]. Interventions varied from 10 to 72 weeks, in both women and men aged between 48 and 84 years. The supplementation was given as whole proteins such as casein or whey or as a mixture of amino acids, with or without creatine or carbohydrates, and given either before or after a resistance training program. The magnitude of the responses to protein or amino acid cocktail supplementation in terms of increased skeletal muscle mass and strength was variable [94–102]. The baseline level of protein consumption may have contributed to variable responses, because subjects who were already consuming adequate protein demonstrated no further augmentation in skeletal muscle mass and/or strength in response to additional protein [103].
Among other determinants are the initial degree of sarcopenia; the health condition of the study participants, whether bedridden patients, frail, or physically fit elderly were studied; the association or not of regular resistance exercise to the dietary intervention; and when strength training exercise was applied, whether the supplementation was taken before or after the physical activity sessions. The skeletal muscle response to protein and/or resistance training could be influenced by antioxidant intakes as opposed to the level of the oxidative stress [104,105]. The daily timing distribution of protein supplementation can also influence skeletal muscle response [83,96]. Ingestion of ≍25–30 g of high-quality protein (≍10 g of essential or indispensable amino acids) at each meal (breakfast, lunch, and dinner) maximally stimulates skeletal muscle protein synthesis in both young and older individuals [83].
The “quality” of the supplementation is another factor that may influence the magnitude of the skeletal muscle mass and strength response [106]. Ingestion of protein hydrolysate, as opposed to its intact protein, tends to enhance amino acid incorporation into skeletal muscle proteins [90]. Postprandial amino acid availability due to variations in intestinal digestion and absorption may explain difference between these two forms of protein supplementation [90]. Branched-chain amino acids, particularly leucine, increase the rate of protein synthesis through changes in signaling pathways including mammalian target of rapamycin complex and other protein phosphorylation involved in skeletal muscle anabolism [107–110]. In addition, by stimulating insulin secretion, leucine indirectly favors skeletal muscle protein anabolism [111]. Leucine content can considerably differ according to protein sources and is highest in whey isolate, milk, and muscle and lowest in wheat [106]. Dietary supplement of indispensable amino acids is a potent stimulus for muscle protein synthesis and there is a dose–response relationship between circulating indispensable amino acids concentration and skeletal muscle protein synthesis [106].
A short-term study showed that diets enriched with branched-chain amino acids (leucine, isoleucine) compared to aromatic amino acids (phenylalanine, histidine) differently affected Ca metabolism and circulating IGF-I levels [112]. These distinct effects might be related to unequal signaling activity of these two chemically different types of amino acids on the extracellular Casensing receptor (CaR) [113]. To our knowledge, it is not known whether this amino acid–activated Casensing receptor would be involved in skeletal muscle function, like the physiologically well-identified Ca channels or pumps localized in structures such as the sarcolemma or sarcoplasmic reticulum [114]. Finally, in a 6-month randomized double-blind placebo-controlled trial with 6-month follow-up in patients with recent hip fracture, a protein supplement of 20 g of casein per day increased serum IGF-I and isometric muscle strength of the biceps [115]. This response was associated with attenuation of proximal femur bone loss, improved clinical outcome, and shortened stay in rehabilitation hospitals [115].
Interaction of Vitamin D and Protein on Muscle Performance
Muscle weakness is classically a clinical feature of severe vitamin D deficiency. Muscle weakness is preferentially localized to the proximal muscles around shoulder and pelvic girdle. It is manifest with difficulty in walking, standing up from a chair, and/or climbing stairs [11]. Several studies reported an association between low vitamin D status and reduced muscle performance and postural instability [116–122]. Nevertheless, it is still uncertain how vitamin D would mechanistically act at the cellular and molecular levels to improve muscle mass and strength, thus explaining the functional observed gain in postural stability and reduction in falling in response to normalization of the serum level of 25OHD. Identification of how vitamin D and/or its metabolites might directly act on skeletal myocytes at the cellular and molecular levels remains less convincing compared to the well-documented intestinal effects of 1,25(OH)D [23].
Whatever the mechanism of action of vitamin D on muscle mass and performance, it is not conceivable that correction of its deficiency in malnutritioned elderly could be fully beneficial with regard to the risk of falling without an adequate supply of protein. Further clinical investigation is needed to document this positive interaction between the supply of vitamin D and protein on muscle mass and function in frail elderly.
Bone Nutrient Requirements for Adults
The recommended intakes for Ca, phosphorus, vitamin D, and protein are presented in Table 1. They are specified for France [123], the United Kingdom [124,125], the United States, and Canada [39,126,127] because there are some substantial differences from country to country, particularly for Ca and vitamin D.
Table 1.
Bone Nutrient Recommendations for Healthy Adultsa
| Bone Nutrients | Government Recommendations for Healthy Adults over 50 Years |
|---|---|
| Calcium (mg/d) | |
| France ANC | 1200 |
| UK RNI | 700 |
| United States and Canada RDA | 1200 (F; M > 70 years)
1000 (M 50–70 years) |
| Phosphorus (mg/d) | |
| France ANC | 750 |
| UK RNI | 550 |
| United States and Canada RDA | 700 |
| Vitamin D (IU/d) | |
| France ANC | 200 (51–75 years)
400–600 (75 + years) |
| UK DRV | ND (51–65 years)
400 (65 + years) |
| United States and Canada RDA | 600 (51–70 years)
800 (71 + years) |
| Protein | |
| France ANC (g/kg b.w./d) | 1.0 |
| UK RNI (g/d) | 53.3 (M 50 + years)
46.5 (W 50 + years) |
| United States and Canada RDA (g/kg b.w./d) | 0.8 |
ANC = Apports nutritionnels conseillés, RNI = reference nutrient intake, RDA = recommended dietary allowance, DRV = dietary reference value, b.w. = body weight.
See text for references to the listed country-specified nutrient requirements.
CONCLUSIONS
Dairy foods provide nutrients—Ca, Pi, and protein—which, in adequate vitamin supply, positively interact on several physiological mechanisms involved in the maintenance of bone health and the prevention of osteoporosis. There is also an important interaction between dietary protein and mechanical loading on skeletal muscle mass and function, thus contributing to the prevention of sarcopenia. Thus, in the elderly the risk of fragility fracture can be attenuated by appropriate nutritional measures and adapted regular physical activity.
REFERENCES
- 1.Hannan MT, Tucker KL. The influence of food groups upon bone health. In: New SA, Bonjour JP, editors. Nutritional Aspects of Bone Health. Cambridge, UK: The Royal Society of Chemistry; 2003. pp. 403–420. [Google Scholar]
- 2.New SA. Food groups and bone health. In: Holick MF, Dawson-Hughes B, editors. Nutrition and Bone Health. Totowa, NJ: Humana Press Inc.; 2004. pp. 235–248. [Google Scholar]
- 3.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkesworth S, Dangour AD, Johnston D, Lock K, Poole N, Rushton J, Uauy R, Waage J. Feeding the world healthily: the challenge of measuring the effects of agriculture on health. Philos Trans R Soc Lond B Biol Sci. 2010;365:3083–3097. doi: 10.1098/rstb.2010.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92:1181–1188. doi: 10.3945/ajcn.2010.29300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 7.Drewnowski A. The contribution of milk and milk products to micronutrient density and affordability of the U.S. diet. J Am Coll Nutr. 2011;30:422S–428S. doi: 10.1080/07315724.2011.10719986. [DOI] [PubMed] [Google Scholar]
- 8.Drewnowski A, Fulgoni V., III Nutrient profiling of foods: creating a nutrient-rich food index. Nutr Rev. 2008;66:23–39. doi: 10.1111/j.1753-4887.2007.00003.x. [DOI] [PubMed] [Google Scholar]
- 9.Robey PG, Boskey AL. The composition of bone. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, DC: The American Society of Bone and Mineral Research; 2008. pp. 32–38. [Google Scholar]
- 10.Weaver CM, Heaney RP. Nutrition and osteoporosis. In: Rosen CJ, Compston JE, Lian JB, editors. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, DC: The American Society of Bone and Mineral Research; 2009. pp. 206–208. [Google Scholar]
- 11.Lips P, van Shoor NM, Bravenhoer N. Vitamin D-related disorders. In: Rosen CJ, Compston JE, Lian JB, editors. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, DC: The American Society for Bone and Mineral Research; 2009. pp. 329–335. [Google Scholar]
- 12.Bonjour JP. Calcium and phosphate: a duet of ions playing for bone health. J Am Coll Nutr. 2011;30:438S–448S. doi: 10.1080/07315724.2011.10719988. [DOI] [PubMed] [Google Scholar]
- 13.Bonjour JP. Protein intake and bone health. Int J Vitam Nutr Res. 2011;81:134–142. doi: 10.1024/0300-9831/a000063. [DOI] [PubMed] [Google Scholar]
- 14.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82:2–8. doi: 10.1136/pgmj.2005.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abellan van Kan G, Gambassi G, de Groot LC, Andrieu S, Cederholm T, Andre E, Caubere JP, Bonjour JP, Ritz P, Salva A, Sinclair A, Vellas B, Dayde J, Deregnaucourt J, Latge C. Nutrition and aging. The Carla Workshop. J Nutr Health Aging. 2008;12:355–364. doi: 10.1007/BF02982667. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony PS, Charlton KE, Maggio M, Tsai AC, Vellas B, Sieber CC. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734–1738. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 18.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 19.Bell J, Whiting SJ. Elderly women need dietary protein to maintain bone mass. Nutr Rev. 2002;60:337–341. doi: 10.1301/002966402320583406. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75:773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 21.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–644. doi: 10.1093/aje/155.7.636. [DOI] [PubMed] [Google Scholar]
- 22.Bonjour JP. Dietary protein: an essential nutrient for bone health. J Am Coll Nutr. 2005;24:526S–536S. doi: 10.1080/07315724.2005.10719501. [DOI] [PubMed] [Google Scholar]
- 23.Rizzoli R, Bonjour JP. Physiology of calcium and phosphate homeostasis. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism: Principles and Clinical Applications. San Diego, CA: Academic Press; 2006. pp. 345–360. [Google Scholar]
- 24.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 25.Bonjour JP, Schurch MA, Chevalley T, Ammann P, Rizzoli R. Protein intake, IGF-1 and osteoporosis. Osteoporos Int. 1997;7(suppl 3):S36–S42. doi: 10.1007/BF03194340. [DOI] [PubMed] [Google Scholar]
- 26.Bourrin S, Ammann P, Bonjour JP, Rizzoli R. Dietary protein restriction lowers plasma insulin-like growth factor I (IGF-I), impairs cortical bone formation, and induces osteoblastic resistance to IGF-I in adult female rats. Endocrinology. 2000;141:3149–3155. doi: 10.1210/endo.141.9.7633. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D, Specker B, Orav JE, Wong JB, Staehelin HB, O'Reilly E, Kiel DP, Willett WC. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86:1780–1790. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 28.Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–1423. doi: 10.1210/jc.2006-1404. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Staehelin HB. Importance of vitamin d and calcium at older age. Int J Vitam Nutr Res. 2008;78:286–292. doi: 10.1024/0300-9831.78.6.286. [DOI] [PubMed] [Google Scholar]
- 30.Caroli A, Poli A, Ricotta D, Banfi G, Cocchi D. Invited review: dairy intake and bone health: a viewpoint from the state of the art. J Dairy Sci. 2011;94:5249–5262. doi: 10.3168/jds.2011-4578. [DOI] [PubMed] [Google Scholar]
- 31.Heaney RP. Vitamin D and calcium interactions: functional outcomes. Am J Clin Nutr. 2008;88:541S–544S. doi: 10.1093/ajcn/88.2.541S. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 33.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999;69:147–152. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 34.Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J Am Geriatr Soc. 2009;57:1073–1079. doi: 10.1111/j.1532-5415.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group. Geneva, Switzerland: Author; 1994. WHO Technical Report Series No 843. [PubMed] [Google Scholar]
- 36.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 37.Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD, Cole DE, Atkinson SA, Josse RG, Feldman S, Kline GA, Rosen C. Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. CMAJ. 2010;182:E610–E618. doi: 10.1503/cmaj.080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stahelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 39.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80:1710S–1716S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell S, Cranney A, Horsley T, Weiler HA, Atkinson SA, Hanley DA, Ooi DS, Ward L, Barrowman N, Fang M, Sampson M, Tsertsvadze A, Yazdi F. Efficacy of food fortification on serum 25-hydroxyvitamin D concentrations: systematic review. Am J Clin Nutr. 2008;88:1528–1534. doi: 10.3945/ajcn.2008.26415. [DOI] [PubMed] [Google Scholar]
- 42.Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012;142:1102–1108. doi: 10.3945/jn.112.158014. [DOI] [PubMed] [Google Scholar]
- 43.Matkovic V, Kostial K, Simonovic I, Buzina R, Brodarec A, Nordin BE. Bone status and fracture rates in two regions of Yugoslavia. Am J Clin Nutr. 1979;32:540–549. doi: 10.1093/ajcn/32.3.540. [DOI] [PubMed] [Google Scholar]
- 44.Heaney RP, Recker RR. Effects of nitrogen, phosphorus, and caffeine on calcium balance in women. J Lab Clin Med. 1982;99:46–55. [PubMed] [Google Scholar]
- 45.Heaney RP. Dairy and bone health. J Am Coll Nutr. 2009;28(suppl 1):82S–90S. doi: 10.1080/07315724.2009.10719808. [DOI] [PubMed] [Google Scholar]
- 46.Manios Y, Moschonis G, Trovas G, Lyritis GP. Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: the Postmenopausal Health Study. Am J Clin Nutr. 2007;86:781–789. doi: 10.1093/ajcn/86.3.781. [DOI] [PubMed] [Google Scholar]
- 47.Bonjour JP, Brandolini-Bunlon M, Boirie Y, Morel-Laporte F, Braesco V, Bertiere MC, Souberbielle JC. Inhibition of bone turnover by milk intake in postmenopausal women. Br J Nutr. 2008;100:866–874. doi: 10.1017/S0007114508937429. [DOI] [PubMed] [Google Scholar]
- 48.Manios Y, Moschonis G, Panagiotakos DB, Farajian P, Trovas G, Lyritis GP. Changes in biochemical indices of bone metabolism in post-menopausal women following a dietary intervention with fortified dairy products. J Hum Nutr Diet. 2009;22:156–165. doi: 10.1111/j.1365-277X.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- 49.Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Post-menopausal Health Study. Br J Nutr. 2010;104:100–107. doi: 10.1017/S000711451000019X. [DOI] [PubMed] [Google Scholar]
- 50.Bonjour JP, Benoit V, Rousseau B, Souberbielle JC. Consumption of vitamin D- and calcium-fortified soft white cheese lowers the biochemical marker of bone resorption TRAP 5b in post-menopausal women at moderate risk of osteoporosis fracture. J Nutr. 2012;142:698–703. doi: 10.3945/jn.111.153692. [DOI] [PubMed] [Google Scholar]
- 51.Bonjour JP, Benoit V, Pourchaire O, Ferry M, Rousseau B, Souberbielle JC. Inhibition of markers of bone resorption by consumption of vitamin D and calcium-fortified soft plain cheese by institutionalised elderly women. Br J Nutr. 2009;102:962–966. doi: 10.1017/S0007114509371743. [DOI] [PubMed] [Google Scholar]
- 52.Bonjour JP, Benoit V, Pourchaire O, Rousseau B, Souberbielle JC. Nutritional approach for inhibiting bone resorption in institutionalized elderly women with vitamin D insufficiency and high prevalence of fracture. J Nutr Health Aging. 2011;15:404–409. doi: 10.1007/s12603-011-0003-y. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K. Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res. 1997;12:1272–1279. doi: 10.1359/jbmr.1997.12.8.1272. [DOI] [PubMed] [Google Scholar]
- 54.Garnero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet. 2000;355:898–899. doi: 10.1016/s0140-6736(99)05463-x. [DOI] [PubMed] [Google Scholar]
- 55.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 56.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 57.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 59.Bischoff-Ferrari HA, Conzelmann M, Stahelin HB, Dick W, Carpenter MG, Adkin AL, Theiler R, Pfeifer M, Allum JH. Is fall prevention by vitamin D mediated by a change in postural or dynamic balance? Osteoporos Int. 2006;17:656–663. doi: 10.1007/s00198-005-0030-9. [DOI] [PubMed] [Google Scholar]
- 60.Chevalley T, Rizzoli R, Manen D, Caverzasio J, Bonjour JP. Arginine increases insulin-like growth factor-I production and collagen synthesis in osteoblast-like cells. Bone. 1998;23:103–109. doi: 10.1016/s8756-3282(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 61.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 62.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 63.Bonjour JP. Nutritional disturbance in acid-base balance and osteoporosis: an hypothesis that disregards the essential homeostatic role of the kidney. Br J Nutr. 2013. in press. [DOI] [PMC free article] [PubMed]
- 64.Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr. 2008;88:1159–1166. doi: 10.1093/ajcn/88.4.1159. [DOI] [PubMed] [Google Scholar]
- 65.Fenton TR, Eliasziw M, Tough SC, Lyon AW, Brown JP, Hanley DA. Low urine pH and acid excretion do not predict bone fractures or the loss of bone mineral density: a prospective cohort study. BMC Musculoskelet Disord. 2010;11:88. doi: 10.1186/1471-2474-11-88. doi: 10.118b/1471-2471-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. Phosphate decreases urine calcium and increases calcium balance: a meta-analysis of the osteoporosis acid-ash diet hypothesis. Nutr J. 2009;8:41. doi: 10.1186/1475-2891-8-41. doi: 10.118b/1475-2891-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J Bone Miner Res. 2009;24:1835–1840. doi: 10.1359/jbmr.090515. [DOI] [PubMed] [Google Scholar]
- 68.Fenton TR, Tough SC, Lyon AW, Eliasziw M, Hanley DA. Causal assessment of dietary acid load and bone disease: a systematic review & meta-analysis applying Hill's epidemiologic criteria for causality. Nutr J. 2011;10:41. doi: 10.1186/1475-2891-10-41. doi: 10.118b/1475-2891-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawson-Hughes B, Harris SS, Rasmussen H, Song L, Dallal GE. Effect of dietary protein supplements on calcium excretion in healthy older men and women. J Clin Endocrinol Metab. 2004;89:1169–1173. doi: 10.1210/jc.2003-031466. [DOI] [PubMed] [Google Scholar]
- 70.Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr. 2000;19:83S–99S. doi: 10.1080/07315724.2000.10718088. [DOI] [PubMed] [Google Scholar]
- 71.McLean RR, Qiao N, Broe KE, Tucker KL, Casey V, Cupples LA, Kiel DP, Hannan MT. Dietary acid load is not associated with lower bone mineral density except in older men. J Nutr. 2011;141:588–594. doi: 10.3945/jn.110.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diem K, Lentner C. “Tables scientifiques. Basel, Switzerland: Ciba-Geigy SA; 1972. [Google Scholar]
- 73.Caverzasio J, Bonjour JP. Characteristics and regulation of Pi transport in osteogenic cells for bone metabolism. Kidney Int. 1996;49:975–980. doi: 10.1038/ki.1996.138. [DOI] [PubMed] [Google Scholar]
- 74.Bonewald LF. Ostecytes. In: Rosen CJ, editor. Primer on The Bone Metabolic Diseases and Disorders of Mineral Metabolism. Hoboken, NJ, USA: American Society for Bone and Mineral Research; 2008. pp. 22–27. [Google Scholar]
- 75.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 76.Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–443. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, Feng JQ. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2011;26:1047–1056. doi: 10.1002/jbmr.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 79.Garlick PJ, McNurlan MA, Ballmer PE. Influence of dietary protein intake on whole-body protein turnover in humans. Diabetes Care. 1991;14:1189–1198. doi: 10.2337/diacare.14.12.1189. [DOI] [PubMed] [Google Scholar]
- 80.McNurlan MA, Essen P, Heys SD, Buchan V, Garlick PJ, Wernerman J. Measurement of protein synthesis in human skeletal muscle: further investigation of the flooding technique. Clin Sci (Lond) 1991;81:557–564. doi: 10.1042/cs0810557. [DOI] [PubMed] [Google Scholar]
- 81.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 82.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 83.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 85.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berger MJ, Doherty TJ. Sarcopenia: prevalence, mechanisms, and functional consequences. Interdiscip Top Gerontol. 2010;37:94–114. doi: 10.1159/000319997. [DOI] [PubMed] [Google Scholar]
- 87.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 88.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 89.Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, Josse R, Lips P, Morales Torres J, Rizzoli R, Yoshimura N, Wahl DA, Cooper C, Dawson-Hughes B. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. 2013;24:1555–1566. doi: 10.1007/s00198-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 90.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009;106:2040–2048. doi: 10.1152/japplphysiol.91551.2008. [DOI] [PubMed] [Google Scholar]
- 91.Campbell WW. Synergistic use of higher-protein diets or nutritional supplements with resistance training to counter sarcopenia. Nutr Rev. 2007;65:416–422. doi: 10.1111/j.1753-4887.2007.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 92.Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26:696S–703S. doi: 10.1080/07315724.2007.10719650. [DOI] [PubMed] [Google Scholar]
- 93.Pasiakos SM. Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients. 2012;4:740–758. doi: 10.3390/nu4070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrews RD, MacLean DA, Riechman SE. Protein intake for skeletal muscle hypertrophy with resistance training in seniors. Int J Sport Nutr Exerc Metab. 2006;16:362–372. doi: 10.1123/ijsnem.16.4.362. [DOI] [PubMed] [Google Scholar]
- 95.Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. 2010;14:155–159. doi: 10.1007/s12603-009-0124-8. [DOI] [PubMed] [Google Scholar]
- 96.Candow DG, Chilibeck PD, Facci M, Abeysekara S, Zello GA. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol. 2006;97:548–556. doi: 10.1007/s00421-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 97.Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging. 2008;12:208–212. doi: 10.1007/BF02982622. [DOI] [PubMed] [Google Scholar]
- 99.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29:18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 100.Holm L, Olesen JL, Matsumoto K, Doi T, Mizuno M, Alsted TJ, Mackey AL, Schwarz P, Kjaer M. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol. 2008;105:274–281. doi: 10.1152/japplphysiol.00935.2007. [DOI] [PubMed] [Google Scholar]
- 101.Onambele-Pearson GL, Breen L, Stewart CE. Influences of carbohydrate plus amino acid supplementation on differing exercise intensity adaptations in older persons: skeletal muscle and endocrine responses. Age (Dordr) 2010;32:125–138. doi: 10.1007/s11357-009-9129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Solerte SB, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, Locatelli E, Schifino N, Giustina A, Fioravanti M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 104.Chaput JP, Lord C, Cloutier M, Aubertin Leheudre M, Goulet ED, Rousseau S, Khalil A, Dionne IJ. Relationship between antioxidant intakes and class I sarcopenia in elderly men and women. J Nutr Health Aging. 2007;11:363–369. [PubMed] [Google Scholar]
- 105.Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem. 2010;21:1–13. doi: 10.1016/j.jnutbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 106.Millward DJ, Layman DK, Tome D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87:1576S–1581S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 107.Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136:269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- 108.Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr. 2006;136:277S–280S. doi: 10.1093/jn/136.1.277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 110.Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr. 2006;136:264S–268S. doi: 10.1093/jn/136.1.264S. [DOI] [PubMed] [Google Scholar]
- 111.Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–279. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dawson-Hughes B, Harris SS, Rasmussen HM, Dallal GE. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int. 2007;18:955–961. doi: 10.1007/s00198-006-0320-x. [DOI] [PubMed] [Google Scholar]
- 113.Conigrave AD, Brown EM, Rizzoli R. Dietary protein and bone health: roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu Rev Nutr. 2008;28:131–155. doi: 10.1146/annurev.nutr.28.061807.155328. [DOI] [PubMed] [Google Scholar]
- 114.Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008;104:296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]
- 115.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- 116.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 117.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 118.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 119.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–633. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dam TT, von Muhlen D, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20:751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mastaglia SR, Seijo M, Muzio D, Somoza J, Nunez M, Oliveri B. Effect of vitamin D nutritional status on muscle function and strength in healthy women aged over sixty-five years. J Nutr Health Aging. 2011;15:349–354. doi: 10.1007/s12603-010-0287-3. [DOI] [PubMed] [Google Scholar]
- 122.Boersma D, Demontiero O, Mohtasham Amiri Z, Hassan S, Suarez H, Geisinger D, Suriyaarachchi P, Sharma A, Duque G. Vitamin D status in relation to postural stability in the elderly. J Nutr Health Aging. 2012;16:270–275. doi: 10.1007/s12603-011-0345-5. [DOI] [PubMed] [Google Scholar]
- 123.Martin AD. “Apports Nutritionnels Conseillés pour la Population Française [Recommended dietary intakes for the French population] Paris, France: TEC&DOC; 2001. [Google Scholar]
- 124.Department of Health. Dietary reference values (DRV) for food energy and nutrients for the United Kingdom. Reports on Public Health and Medical Subjects. London, UK: H.M. Stationary Office; 1991. [Google Scholar]
- 125.Commitee on Medical Aspects of Food and Nutrition Policy. “Nutrition and Bone Health: with Particular Reference to Calcium and Vitamin D. London, UK: The Stationary Office; 1998. [PubMed] [Google Scholar]
- 126.Institute of Medicine Food and Nutrition Board. “Dietary References Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 127.Food and Nutrition Board, Institut of Medicine of the National Academies. “Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids (Macronutrients) Washington, DC: The National Academic Press; 2005. [Google Scholar]


