Abstract
Background
The local-regional failure of advanced oral squamous cell carcinoma (OSCC) after surgery results from the re-growth of residual tumor cells that may be stimulated by epidermal growth factor receptor (EGFR) ligands during the wound-healing process.
Methods
The level of EGFR ligands in human drain fluids from OSCC resection and remote flap donor site were determined. A mouse model of microscopic residual OSCC was established and treated with cetuximab to measure tumor growth, survival, and cervical lymph node metastases. A mouse model of wound-healing was also established to assess the effect of an EGFR antibody on the wound-healing process.
Results
EGFR ligands are found in sites from OSCC resection. EGFR targeted therapy can delay tumor re-growth in a microscopic residual disease model of OSCC without significant effects on local wound-healing.
Conclusion
These results provide a strong rationale for clinical evaluation of this approach to treat patients with local-regionally advanced OSCC.
Keywords: recurrence of oral squamous cell carcinoma, epidermal growth factor receptor (EGFR) ligands, postoperative EGFR inhibition, wound healing, in vivo model of microscopic residual disease
INTRODUCTION
Patients with oral squamous cell carcinomas (OSCC) still have a poor prognosis with a current 5-year survival of only 50% despite advances in surgery and radiation therapy (RT) 1. One of the most critical prognostic parameters is local regional failure, which can occur in up to 50% of patients 2-4. While the current standard of care for patients with local-regionally advanced OSCC is surgery and post-operative RT (PORT), administered along with chemotherapy, the local-regional failure rate for patients with OSCC can exceeds 50% for high-risk patients despite maximally tolerated doses of PORT and chemotherapy 5.
Post-operative local-regional failure results from residual tumor cells that were not expunged by treatment 6. Typically the time interval between surgery and PORT is 4-8 weeks to allow for healing and recovery 6 but during this interval residual tumor cells may repopulate in the growth factor-rich wound 6-11. Thus, surgery itself may induce the expression of growth factors such as EGF and related ligands that can stimulate the growth of residual tumor cells 9. For the treatment of residual disease, the use of PORT has been practiced for several decades, and the incorporation of concurrent chemotherapy to maximally tolerated doses is supported by data from two large randomized trials 12, 13. However, residual tumor cells are thought to have limited response to adjuvant therapy and poorer local-regional control 14, 15. To address this concern, an “early intervention” clinical trial, RTOG-0024, using early postoperative chemotherapy followed by concurrent chemoradiotherapy after surgical resection of high risk head and neck squamous cell carcinoma (HNSCC) was conducted and this strategy was found to be both feasible and tolerable 6.
The EGFR pathway plays an important role in the regulation of cellular proliferation, differentiation and survival 16. This receptor is over-expressed in more than 90% of HNSCC specimens 17, and a higher level of EGFR expression is associated with reduced survival 18-20. Therefore, EGFR targeted treatment strategies have been developed and have shown to be effective in treating patients with HNSCC. The most widely studied EGFR targeting agent is cetuximab (Erbitux, ImClone Systems), an anti-EGFR monoclonal antibody that is approved by the Food and Drug Administration for the treatment of patients with HNSCC 21-23. Cetuximab has also been found in a recent study to inhibit the growth of cultured head and neck cancer cells that are stimulated by the addition of surgical wound catheter drainage fluid from head and neck cancer patients 10. Therefore, we have hypothesized that EGF and transforming growth factor (TGF) -α are present in the wounds of patients who have undergone resection of head and neck cancers and could be stimulating the early repopulation of residual tumor cells and that inhibition of EGFR signaling in this setting could inhibit this tumor re-growth and thereby improve treatment outcomes.
In the present study, we sought to evaluate the levels of the EGF and TGF-α in drain fluids from head and neck surgery patients and determined whether cetuximab (ImClone Systems), an anti-EGFR monoclonal antibody, can inhibit tumor progression and recurrence in an in vivo OSCC model of post-operative microscopic residual disease and how cetuximab affects surgical wound healing.
MATERIALS AND METHODS
Wound drainage fluids from patients with OSCC
Wound drainage fluids were collected according to a protocol approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Informed consent was provided according to the Declaration of Helsinki. We collected paired sample of wound drainage fluids 1-3 days post-operatively from the cancer operative bed and the free flap donor site in 11 patients with SCC of the oral tongue and/or floor of mouth, who underwent cancer resection and free flap reconstruction.
Human cytokine assay
The human cytokine/chemokine Milliplex MAP kit (Millipore, Bedford, MA) was used to test samples for the presence of EGF and TGF-α. Briefly, 25 μL of anti-cytokine antibody-labeled beads (prepared in Millipore assay buffer) were added to each well of a 96-well plate and washed twice with Millipore wash buffer. Samples (25 μL/well) or standards (25 μL/well) were then added to each well, and the plate was sealed before shaking overnight at 4°C. The wells were washed two times in Millipore wash buffer, and as before, 25 μL of detection antibody was added to each well, and the plate was sealed and incubated for 1 hour at room temperature. Following two more washes in Millipore wash buffer, 25 μL of streptavidin-phycoerythrin was added to each well and the plate sealed and incubated for 30 min at room temperature. The plate was then washed two times in Millipore wash buffer and the beads resuspended in 150 μL of sheath fluid (Luminex Corporation, Austin, TX). Samples were loaded into the Bio-Rad Bio Plex system machine (Bio-Rad Laboratories, Hercules, CA) and the cytokine concentration in each sample was calculated automatically from a 5PL standard curve using the Bio-Plex Manager software version 5.0 (Bio-Rad Laboratories).
Animals and maintenance
Eight- to 12-week-old male athymic nude mice and BALB/c male mice were purchased from the National Cancer Institute (Bethesda, MD). Athymic nude mice were used for the microscopic residual disease model, and BALB/c mice were used for the wound-healing model. The mice were kept in a specific pathogen–free facility and were fed irradiated mouse chow and autoclaved reverse osmosis–treated water. The facilities were approved by the American Association for Accreditation of Laboratory Animal Care and met all current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and the National Institutes of Health. Animal procedures were done according to a protocol approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
Cell lines and reagents
The OSC-19 cell line, established in Japan from a cervical lymph node metastatic tumor of a patient with well-differentiated SCC of the tongue 24, was used in the study. OSC-19 cells were retrovirally infected with the green fluorescent protein and the luciferase gene (OSC-19-luc) as described previously 25. The murine SCC7 cell line is a rapidly dividing squamous cell carcinoma that spontaneously arose from the skin of a C3H/HeJ mouse 26. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), L-glutamine, sodium pyruvate, nonessential amino acids, and vitamin solution. Adherent monolayer cultures were maintained on plastic plates and incubated at 37°C in 5% carbon dioxide and 95% oxygen. The cultures were Mycoplasma-free and maintained for no longer than 12 weeks after they were recovered from frozen stocks. Cetuximab (C225/Erbitux) and rat-antimouse EGFR antibody (ME-1) were provided by Imclone (New York, NY).
An in vivo model of microscopic residual disease of OSCC
To establish an in vivo model of microscopic residual disease of OSCC, 5 × 103 of OSC-19-luc cells were suspended in 30 μL of serum-free DMEM and injected into the tongues of athymic nude mice, as described previously 27.
Tumor growth was monitored by bioluminescence imaging every 7-8 days using Living Image software 3.2 (Xenogen, Alameda, CA) as described previously 25. Animals were first imaged 3 days after cell inoculation and then a partial thickness incision of the anterolateral tongue at the site of the previous tumor cell injection was made to simulate a post-operative wound bed harboring microscopic-only residual cancer cells. The mice were then randomized into two groups to receive either phosphate-buffered saline (PBS) as placebo (n=10), or cetuximab (n=15) by intraperitoneal injection twice per week for 3 weeks.
We euthanized mice by CO2 asphyxiation 90 days after the injection of OSC-19-luc cells or sooner if they lost more than 20% of their pre-injection body weight or became moribund. The mouse tumors were resected and fixed in formalin and embedded in paraffin for immunohistochemical and hematoxylin-and-eosin staining. Cervical lymph nodes were also resected, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and evaluated for metastases.
Western blotting
SCC7 cells were plated and incubated in DMEM with 10% FBS overnight. The next day, cells were washed with PBS and incubated in serum-free DMEM for 24 hours. Cells were then treated for 120 minutes with 0-100 nM ME-1. Next, EGF (50 ng/mL) was added for 15 minutes, after which the cells were washed in chilled (4°C) PBS and the plates kept on ice. Total cell lysates were obtained and subjected to Western blot analysis as previously described 28.
The membranes were blocked for 1 hour at room temperature with 5% bovine serum albumin in 0.1% Tween 20 in tris-buffered saline (TBS-T) and incubated overnight at 4°C in anti-EGFR (Upstate Biotechnology, Inc., Lake Placid, NY; 1:500) or anti-phospho-EGFR (Cell Signaling, Beverly, MA; 1:500) in the membrane-blocking solution described above. Next, the membranes were washed with TBS-T and incubated for 1 hour at room temperature in horseradish peroxidase-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) to detect EGFR and phospho-EGFR. Membranes were then analyzed using the SuperSignal West chemiluminescent system (Pierce Biotechnology, Rockford, IL). To verify equal loading of proteins, membranes were stripped and reprobed with anti-β-actin (1:5000).
Wound-healing mouse model
To see the effect of EGFR inhibition on wound-healing, we established a wound-healing mouse model. BALB/C mice were anesthetized by intraperitoneal (i.p.) injection of pentobarbital 50 mg/kg, and the hair on the back was shaved. First, 5 mm full-thickness incisions were made in the skin of the dorsum of the mouse using a sterile biopsy punch. The incision was placed between the shoulder blades of the mouse to prevent the mouse from reaching the wound and then was closely inspected and any bleeding stopped using electrocautery. The animals were randomized to receive either ME-1 (40 mg/kg) or PBS as placebo by intraperitoneal 3 times a week. For histological assessment of the quality of wound healing, the wound edges were closed with 5-0 vicryl surgical suture (Ethicon Inc., Somerville, NJ) and the wounds were excised on 3, 7, 14, and 21 days, and fixed in 10% formalin. Fixed skin samples were embedded in paraffin, and 10 μm sections were prepared, mounted, and stained with hematoxylin and eosin. Histologic sections from each wound were analyzed and graded by who was blinded as to the identity of each sample. Histological assessment was done using a grading scales for the quality of inflammation (Grade 0, no inflammation; Grade 1, mild; Grade 2, mild to moderate; Grade 3, moderate; Grade 4, severe) and fibrosis (Grade 0, no fibrosis; Grade 1, mild; Grade 2, moderate; Grade 3, prominent) as described previously 29. To assess the effect of EGFR inhibition on wound healing, wound size was measured with microcalipers twice a week until complete wound closure.
Statistical analysis
Quantitative data for the presence of cytokines and from histological assessment for inflammation and fibrosis were compared using unpaired Student's t tests. Fisher's exact test was used to analyze associations between treatment groups and tumor formation or cervical lymph node metastases. The time to wound closure in the control group was compared to that in the ME-1 treatment group using the two-tailed t tests. Fisher's exact test was used to analyze associations between treatment groups and incidence of cervical lymph node metastases and tumor formation. Overall survival and disease-free survival were analyzed by the Kaplan-Meier method and compared using log-rank tests. Analysis was performed with Prism 5.01 software (GraphPad Software, San Diego, CA). For all comparisons, P < 0.05 was considered statistically significant.
RESULTS
Epidermal growth factor receptor ligands are increased in drain fluids from head and neck surgical resection beds
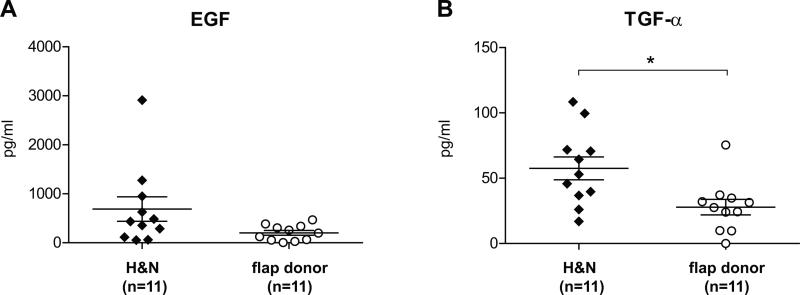
In order to determine whether tissue factors are released in the post-surgical wound that could stimulate residual tumor cells at the resection site, i.e., microscopic residual disease; secreted fluid was harvested post-operatively from wound catheters at the head and neck cancer resection site. The concentrations of EGF and TGF-α in drain fluids (DFs) from the head and neck cancer resection and control flap donor sites were determined using a fluorescence-based multiplex bead immunoabsorption assay (Figure 1). The median concentration of TGF-α (53.0 pg/mL) was significantly higher in DF from the resection sites than in DF from the flap donor sites (27.5 pg/mL, P=0.0106). The concentration of EGF was also higher in DF from the resection sites (median: 434.3 pg/mL) compared with DF from the flap donor site (median: 196.8 pg/mL), but the difference did not reach statistical significance (P=0.0710).
Figure 1.
Cytokine levels of drain fluids (DFs) from head and neck and flap donor site wounds, serum, and saliva. DFs were collected on postsurgical day 1or 3 from patients with SCC of the oral tongue and/or the floor of mouth who underwent surgery. EGF and TGF-α concentrations were examined by a high throughput antibody bead cytokine assay. Points represent the means of triplicate experiments; bars, standard errors. *, P < 0.05.
Cetuximab inhibits tumor formation and growth and prolongs overall and disease-free survival in an in vivo model of microscopic residual disease
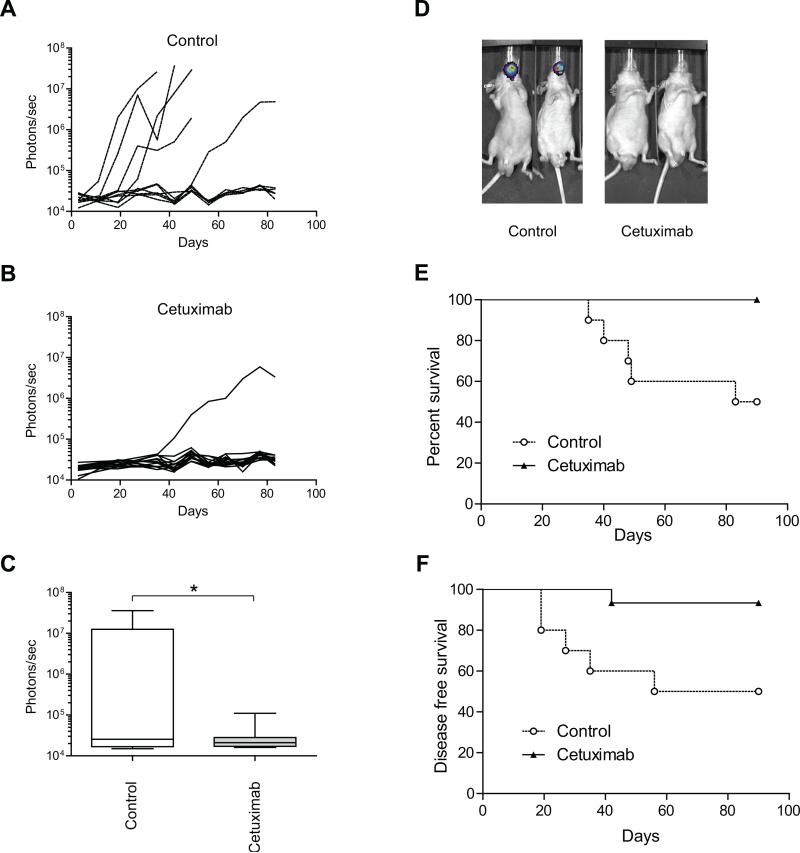
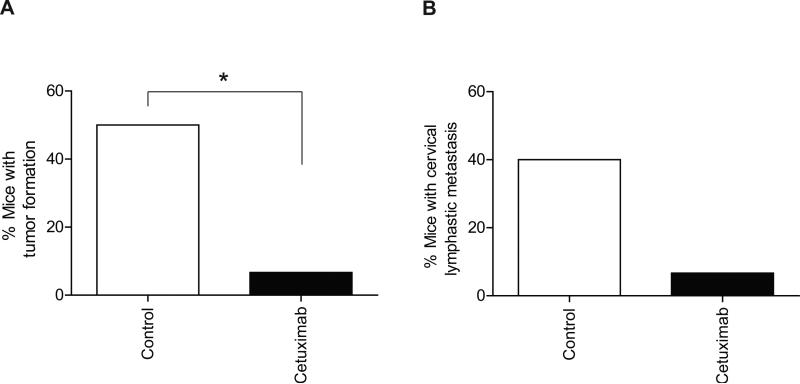
The finding of elevated levels of EGF and TGF-α in the wounds of OSCC patients led us to hypothesize that inhibition of EGF and TGF-α signaling in a minimal residual disease model could inhibit tumor outgrowth and prolong animal survival. Microscopic residual disease is the most likely source of local and/or regional recurrence after surgery in patients treated for OSCC after surgery. Therefore, we established an in vivo model of microscopic residual disease of OSCC. Groups of mice in which OSC-19-luc cells were implanted in the minimal residual model were treated with either cetuximab or placebo and the bioluminescence intensity of OSC-19-luc cells was monitored to measure the effect of cetuximab treatment. A reduction of bioluminescence of OSC-19-luc cells was observed in the cetuximab treatment group compared with the control group (Figure 2A-2D) in our in vivo OSCC model of microscopic residual disease. As shown in Figure 2E, the mean survival durations for the control and cetuximab groups were 86.5 days and 90.0 days, respectively (P=0.0020, log-rank test). Mice with bioluminescence intensities less than 1 × 105 photons/sec were classified as disease-free. As shown in Figure 2F, the mean disease-free survival periods for the control and cetuximab groups were 73.0 days and 90.0 days, respectively, thus, cetuximab treatment also significantly prolonged the disease free period compared with the placebo (P=0.0100, log-rank test). Consistently, tumor formation in mice treated with cetuximab was inhibited significantly in comparison to the control group (50.0% vs. 6.7%, respectively, P=0.0225, Figure 3A). Similarly, fewer regional metastases were observed in the cetuximab treatment group than in the control group (40% vs. 6.7%, respectively, P=0.125, Figure 3B).
Figure 2.
The effects of treatment with cetuximab on tumor growth, tumor formation, overall survival and disease-free survival in an in vivo model of microscopic residual disease. A, Individual mouse data are shown for the control group (10 mice). B, Individual mouse data are shown for the cetuximab treatment group (15 mice). Photon counts were calculated from the imaging data using the IVIS Living Image software. C, Box-and-whisker plots illustrating the effects of treatment on an in vivo model of microscopic residual disease followed by bioluminescence imaging on day 35 after cell inoculation. Horizontal lines in the boxes, mean. Bottom and top boundaries of boxes, 25th and 75th percentiles, respectively. Lower and upper whiskers, 5th and 95th percentiles, respectively. *, P < 0.05. D, Representative bioluminescence images corresponding to OSC-19-luc tumors from each treatment group, 35 days after cell inoculation. Photon counts were calculated from the imaging data using the IVIS Living Image software. E, The in vivo effects of treatments on the overall survival of tumor-bearing OSC-19 mice. F, The in vivo effects of treatment on the disease-free survival of OSC-19 tumor-bearing mice. Animals were euthanized at 90 days after cell inoculation or when they had lost more than 20% of their initial body weight. Survival was analyzed by the Kaplan-Meier method and compared with log-rank tests.
Figure 3.
The effect of treatment with cetuximab on tumor formation and lymph node metastasis in the in vivo model of microscopic residual disease. A, Effects of treatment with cetuximab on tumor formation in the in vivo model of microscopic residual disease. B, Effects of treatment with cetuximab on lymph node metastases in the in vivo model of microscopic residual disease. *, P < 0.05.
Inhibition of murine EGFR results in only minor delay of re-epithelialization and no appreciable difference in the degree of inflammation or fibrosis in the mouse wound-healing model
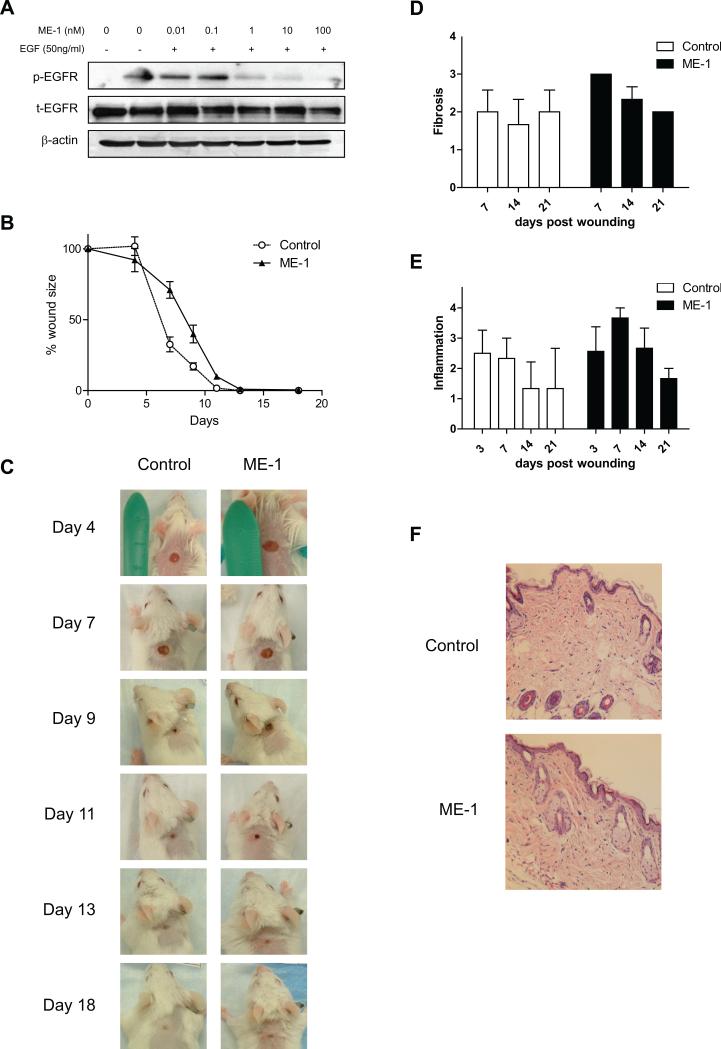
While the presence of EGF and TGF-α in OSCC resection wounds, and the favorable response of OSCC cells to cetuximab treatment in a microscopic residual disease model indicate that early treatment of OSCC patients after surgery could potentially decrease the incidence and severity of local and/or regional recurrence, there is some concern that cetuximab therapy may also inhibit wound healing in the post-surgical patient, since EGF and TGF-α are thought to be involved in post-operative wound healing 30. To address whether cetuximab would adversely affect wound healing, we first determined whether ME-1, a rat-antimouse EGFR monoclonal antibody, inhibits the phosphorylation of EGFR in murine cells. We found that ME-1 suppressed EGF-stimulated phosphorylation of the EGFR in a dose-dependent manner, while the total expression of the non-phosphorylated kinase remained unchanged (Figure 4A).
Figure 4.
The effect of the ME-1 rat-antimouse EGFR antibody in a mouse wound-healing model. A, ME-1 inhibits the phosphorylation of EGFR in the murine oral cancer cell line SCC-7 in vitro. Antibodies to total (unphosphorylated) mouse EGFR and β-actin were used as protein loading controls. B, The effects of ME-1 treatment on wound healing. A mouse wound-healing model was established to determine whether systemic treatment with an EGFR antibody would interfere with wound healing. Wound sizes were measured with microcalipers twice a week. Points represent mean wound size; bars, SE. C, Effect of ME-1 on wound diameter in a mouse wound-healing model. Representative wounds from punch biopsy in each group on days 4, 7, 9, 11, 13, and 18. D-F, The effect of ME-1 on wound histology in a mouse wound-healing model. Mice were randomized to receive treatment with ME-1 (40 mg/kg) or PBS as placebo. The mice were sacrificed on days 3, 7, 14, and 21 of treatment, at which time the wounds were excised and fixed in 10% formalin for histologic evaluation of H&E staining using light microscopy. D, Histological assessment for quality of fibrosis. Columns, mean grade of fibrosis; bars, SE. E, Histological assessment for quality of inflammation. Columns, mean grade of inflammation; bars, SE. F, Representative micrographs of skin wounds from mice treated with vehicle or ME-1 on day 21. Epithelialization is almost complete but slightly irregular. Magnification, ×40.
We next examined the effect of EGFR inhibition with ME-1 on wound-healing; wounded mice were treated with either placebo or ME-1 and then the size of the wound was measured over time. As shown in Figure 4B and 4C, we observed that the decrease in the mean wound sizes of mice in the ME-1 group was delayed slightly compared with that of mice in the control group, but the difference was not statistically significant (P=0.2099).
Histological assessments of wounds from ME-1 and placebo treated mice at different time points were also carried out to determine the effect of EGFR inhibition on fibrosis and inflammation during wound healing (Figure 4D, 4E and 4F). While H&E staining detected a minor increase in inflammation and fibrosis in the ME-1 treatment group compared with the control group, the differences were not significant (P=0.1518 and P=0.1879, respectively).
DISCUSSION
In this study, we confirmed the presence of elevated levels of EGFR ligands in OSCC surgical wound beds but not in flap donor site wounds using human wound drainage fluid samples. We demonstrated that cetuximab inhibited tumor progression in an in vivo model of microscopic residual disease and that the use of a rat-anti mouse EGFR antibody does not significantly delay the process of wound healing in a mouse wound healing model. Taken together, our results demonstrate in a pre-clinical model that intervention in the EGFR pathway with cetuximab treatment soon after surgery could inhibit the repopulation of OSCC cells without impairing wound healing.
The EGFR pathway plays an important role in the regulation of the cellular proliferation, differentiation and survival of HNSCC 16. The EGFR ligands, EGF and TGF-α bind to EGFR, resulting in the stimulation and activation of signaling complexes, leading to cell cycle progression, reduced apoptosis, angiogenesis, and metastatic potential 31. Growth factors such as EGF and TGF-α also play key roles in initiating and sustaining the phases of tissue repair in the wound-healing process 32, 33. It has been reported that early-phase drain fluid contains high concentrations of EGF 34. A recent study also showed that elevated levels of wound drainage catheter fluid from head and neck cancer patients can stimulate the growth of head and neck tumor cells in culture, and that this growth stimulation can be inhibited in vitro by the addition of cetuximab 10. Thus, the release of growth factors such as EGFR ligands induced by surgery to remove the primary tumor and subsequent wound healing might trigger the development and/or progression of microscopic residual HNSCC 9, 35.
In the present study, we observed the elevated levels of EGF and TGF-α in wound DF from head and neck surgeries consistent with the findings of previous reports9, 10. The levels of EGF and TGF-α were higher in wound DF from head and neck surgery sites than from lower-extremity flap donor sites in samples from the same patients. The most likely explanation for this finding is that the source of this cytokine is arising from the microscopic residual tumor cells or a host response to them. A possible alternative explanation is the close proximity of the oral cavity and the neck wound that may be contiguous or communicate during the cancer resection or early recovery interval. This is supported by evidence that elevated levels of human salivary EGF have been found after surgery 36. We also found that the concentration of EGF in oral saliva before the head and neck surgical procedure was similar to that in the head and neck surgical wound drainage fluid (data not shown). However, the level of TGF-α was significantly higher in DFs from the head and neck surgical wound compared with saliva (data not shown) or flap donor site drainage fluid, suggesting that this cytokine most likely arises from the microscopic residual tumor cells or a host response.
Since advanced HNSCC including OSCC tend to recur local-regionally after surgical removal, post-operative adjuvant treatments are frequently needed to attain local-regional disease control. Tumor recurrence has been traditionally thought to occur as the result of the outgrowth of microscopic residual tumor cells, even in patients with negative surgical margins. However, the surgical resection of a cancer itself may contribute to the recurrence of that cancer through stress-induced immunosuppression 37 and/or growth factors and/or cytokines that are expressed during the wound healing process 9. Thus, local-regional failure is a serious issue for patients with OSCC 2, 4, however, there have been no prior animal models that mimic the recurrence of OSCC, even though many animal models of HNSCC have been established so far 4. Therefore, we established a microscopic disease model for OSCC and then tested our hypothesis that intervention with EGFR inhibitors soon after surgery can suppress the accelerated repopulation of OSCC tumor cells in vivo. In the present study, we have shown that cetuximab inhibits tumor growth and tumor formation and prolongs overall survival and disease-free survival in an in vivo model of microscopic residual OSCC. These findings support that early intervention with EGFR targeted treatment can inhibit local oral tumor growth and regional metastatic disease development in a minimal disease model.
Some have voiced concerns that early intervention could contribute to the delayed healing or the development of wound complications such as pharyngocutaneous fistula leading to increased length of stay, resource utilization, and patient anxiety and morbidity 38, 39. Dean et al. have studied human wound healing following combined radiation and cetuximab therapy in head and neck cancer patients in a retrospective analysis in which they found that cetuximab did not significantly increase the risk of post-surgical wound complications 40. Since we did not observe significant inhibition of wound healing in our model in which a monoclonal antibody to murine EGFR, ME-1, was delivered to mice with standardly applied cutaneous wounds, we believe that it is safe to study the treatment of post-resection head and neck cancer patients with EGFR targeted therapy early in the post-operative period within the context of an IRB-approved clinical trial. While we have used cetuximab to inhibit the EGFR pathway in the present study, it is conceivable that EGFR inhibitors such as erlotinib may be also useful for inhibititing postoperative microscopic residual of OSCC.
In conclusion, we are the first to report differential expression of EGFR ligands in OSCC and free flap donor site wound beds as measured in their DF's, that cetuximab inhibits tumor progression in an in vivo model of microscopic residual disease and that post-operative EGFR inhibition does not cause any significant delay of healing in a mouse wound-healing model. We contend that inhibition of the EGFR pathway by cetuximab soon after surgery could delay accelerated repopulation of OSCC cells, without causing wound-healing problems just prior to the initation of external beam radiation therapy. These findings indicate the need for further pre-clinical and clinical evaluation.
ACKNOWLEDGMENTS
We thank Ms. Dawn Chalaire for her critical editorial review of the manuscript.
Grant support: This work was supported by the U.T. MD Anderson Cancer Center PANTHEON program, NIH Specialized Program of Research Excellence Grant P50CA097007, National Research Science Award Institutional Research Training Grant T32CA60374 and NIH Cancer Center Support (Core) Grant CA016672.
Footnotes
Competing interests
We, authors, declare that we have no competing interests.
REFERENCES
- 1.Silverman S., Jr. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 2.Grandi C, Alloisio M, Moglia D, et al. Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg. 1985;8(2):67–73. doi: 10.1002/hed.2890080202. [DOI] [PubMed] [Google Scholar]
- 3.Gath HJ, Brakenhoff RH. Minimal residual disease in head and neck cancer. Cancer Metastasis Rev. 1999;18(1):109–26. doi: 10.1023/a:1006268621730. [DOI] [PubMed] [Google Scholar]
- 4.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26(3-4):645–62. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 5.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48(1):7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal DI, Harris J, Forastiere AA, et al. Early postoperative paclitaxel followed by concurrent paclitaxel and cisplatin with radiation therapy for patients with resected high-risk head and neck squamous cell carcinoma: report of the phase II trial RTOG 0024. J Clin Oncol. 2009;27(28):4727–32. doi: 10.1200/JCO.2008.21.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39(1):137–48. doi: 10.1016/s0360-3016(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Tagliabue E, Agresti R, Carcangiu ML, et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet. 2003;362(9383):527–33. doi: 10.1016/S0140-6736(03)14112-8. [DOI] [PubMed] [Google Scholar]
- 10.Licitra LF, Perrone F, Tamborini E, et al. Effect of antityrosyne kinase agents on in vitro tumor cell proliferation induced by wound drainage fluids (WDFs) of head and neck cancer (HNSCC) patients. J Clin Oncol. 2008;26(15S) Abstr 6077. [Google Scholar]
- 11.O'Reilly MS. The interaction of radiation therapy and antiangiogenic therapy. Cancer J. 2008;14(4):207–13. doi: 10.1097/PPO.0b013e3181836af3. [DOI] [PubMed] [Google Scholar]
- 12.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 14.DeVita VT., Jr. The James Ewing lecture. The relationship between tumor mass and resistance to chemotherapy. Implications for surgical adjuvant treatment of cancer. Cancer. 1983;51(7):1209–20. doi: 10.1002/1097-0142(19830401)51:7<1209::aid-cncr2820510707>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 16.Reuter CW, Morgan MA, Eckardt A. Targeting EGF-receptor-signalling in squamous cell carcinomas of the head and neck. Br J Cancer. 2007;96(3):408–16. doi: 10.1038/sj.bjc.6603566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dassonville O, Formento JL, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol. 1993;11(10):1873–8. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 18.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 19.Xia W, Lau YK, Zhang HZ, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999;5(12):4164–74. [PubMed] [Google Scholar]
- 20.Ulanovski D, Stern Y, Roizman P, Shpitzer T, Popovtzer A, Feinmesser R. Expression of EGFR and Cerb-B2 as prognostic factors in cancer of the tongue. Oral Oncol. 2004;40(5):532–7. doi: 10.1016/j.oraloncology.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 22.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 23.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28(1):8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoi T, Yamaguchi A, Odajima T, Furukawa K. Establishment and characterization of a human cell line derived from a squamous cell carcinoma of the tongue. Tumor Res. 1988;23:43–57. [Google Scholar]
- 25.Sano D, Choi S, Milas ZL, et al. The effect of combination anti-endothelial growth factor receptor and anti-vascular endothelial growth factor receptor 2 targeted therapy on lymph node metastasis: a study in an orthotopic nude mouse model of squamous cell carcinoma of the oral tongue. Arch Otolaryngol Head Neck Surg. 2009;135(4):411–20. doi: 10.1001/archoto.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallman RF, Silini G,M. VPL. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967;39(3):539–49. [PubMed] [Google Scholar]
- 27.Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002;8(1):293–8. [PubMed] [Google Scholar]
- 28.Yigitbasi OG, Younes MN, Doan D, et al. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004;64(21):7977–84. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- 29.Simhon D, Brosh T, Halpern M, et al. Closure of skin incisions in rabbits by laser soldering: I: Wound healing pattern. Lasers Surg Med. 2004;35(1):1–11. doi: 10.1002/lsm.20074. [DOI] [PubMed] [Google Scholar]
- 30.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165(6):728–37. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 31.Burtness B. The role of cetuximab in the treatment of squamous cell cancer of the head and neck. Expert Opin Biol Ther. 2005;5(8):1085–93. doi: 10.1517/14712598.5.8.1085. [DOI] [PubMed] [Google Scholar]
- 32.Brown GL, Nanney LB, Griffen J, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989;321(2):76–9. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- 33.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 34.Aiba-Kojima E, Tsuno NH, Inoue K, et al. Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture. Wound Repair Regen. 2007;15(4):511–20. doi: 10.1111/j.1524-475X.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Wickerham DL, Deutsch M, Anderson S, Redmond C, Fisher ER. Breast tumor recurrence following lumpectomy with and without breast irradiation: an overview of recent NSABP findings. Semin Surg Oncol. 1992;8(3):153–60. [PubMed] [Google Scholar]
- 36.Oxford GE, Jonsson R, Olofsson J, Zelles T, Humphreys-Beher MG. Elevated levels of human salivary epidermal growth factor after oral and juxtaoral surgery. J Oral Maxillofac Surg. 1999;57(2):154–8. doi: 10.1016/s0278-2391(99)90230-6. discussion 158-9. [DOI] [PubMed] [Google Scholar]
- 37.Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996;119(3):316–25. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 38.Panje WR, Namon AJ, Vokes E, Haraf DJ, Weichselbaum RR. Surgical management of the head and neck cancer patient following concomitant multimodality therapy. Laryngoscope. 1995;105(1):97–101. doi: 10.1288/00005537-199501000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Yu P, Robb GL. Pharyngoesophageal reconstruction with the anterolateral thigh flap: a clinical and functional outcomes study. Plast Reconstr Surg. 2005;116(7):1845–55. doi: 10.1097/01.prs.0000191179.58054.80. [DOI] [PubMed] [Google Scholar]
- 40.Dean NR, Sweeny L, Harari PM, et al. Wound healing following combined radiation and cetuximab therapy in head and neck cancer patients. J Wound Care. 2011;20(4):166–70. doi: 10.12968/jowc.2011.20.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]