Abstract
Antiphospholipid syndrome (APS) is a multisystem autoimmune condition characterized by vascular thromboses and/or pregnancy loss associated with persistently positive antiphospholipid antibodies (aPL). Catastrophic APS (CAPS) is the most severe form of APS with multiple organ involvement developing over a short period of time, usually associated with microthrombosis. ‘Definite’ and ‘probable’ CAPS have been defined based on the preliminary classification criteria; however, in a real-world setting, aPL-positive patients with multiple organ thromboses and/or thrombotic microangiopathies exist who do not fulfill these criteria. Previous APS diagnosis and/or persistent clinically significant aPL positivity is of great importance for the CAPS diagnosis; however, almost half of the patients who develop CAPS do not have a history of aPL positivity. The purpose of this paper is to summarize the diagnostic challenges and the recently updated diagnostic algorithms for CAPS providing a ‘step-by-step’ approach for clinicians (and researchers) in the assessment of patients with multiple organ thromboses.
Keywords: anti-β2-glycoprotein-1 antibody, antiphospholipid syndrome, anticardiolipin antibody, catastrophic antiphospholipid syndrome, lupus anticoagulant, thrombosis
Introduction
Antiphospholipid syndrome (APS) is a multisystem autoimmune condition characterized by vascular thromboses and/or pregnancy loss associated with persistently positive antiphospholipid antibodies (aPL; measured with lupus anticoagulant [LA] test, anticardiolipin antibody [aCL] enzyme-linked immunosorbent assay [ELISA], and/or anti-β2-glycoprotein-I antibody [aβ2GPI] ELISA) [Miyakis et al. 2006] (Table 1).
Table 1.
Updated antiphospholipid syndrome classification criteria [Miyakis et al. 2006].
|
Clinical criteria
1. Vascular thrombosis: ≥ 1 clinical episodes of arterial, venous, or small vessel thrombosis, in any tissue or organ 2. Pregnancy morbidity: (a) ≥ 1 unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation, or (b) ≥ 1 premature births of a morphologically normal neonate before the 34th week of gestation because of: eclampsia, severe preeclampsia, or recognized features of placental insufficiency, or (c) ≥ 3 unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded. |
|
Laboratory criteria
1. Lupus anticoagulant present in plasma, on ≥ 2 occasions at least 12 weeks apart 2. Anticardiolipin antibody of IgG and/or IgM isotype, in medium or high titer (>40 GPL or MPL, or > the 99th percentile), on ≥ 2 occasions, at least 12 weeks apart. 3. Anti-β2-glycoprotein-I antibody of IgG and/or IgM isotype, in medium or high titer (> the 99th percentile), on ≥ 2 occasions, at least 12 weeks apart. |
| Definite APS is present if at least one of the clinical criteria and one of the laboratory criteria are met. |
In its most severe form, a minority of patients develop life-threatening multiple organ thromboses, usually associated with microthrombosis, recognized as catastrophic APS (CAPS) (Table 2) [Asherson et al. 2003; Vora et al. 2006]. The unique characteristics of CAPS are: (a) rapid onset thromboses resulting in multiple organ dysfunction syndrome; (b) common association with other thrombotic microangiopathies (TMAs); (c) evidence of systemic inflammatory response syndrome; (d) high risk of unusual organ involvement; and (e) relatively high mortality rate despite optimal therapy [Cervera and Asherson, 2004].
Table 2.
Preliminary classification criteria for catastrophic antiphospholipid syndrome [Asherson et al. 2003].
| 1. Evidence of involvement of three or more organs, systems and/or tissues |
| 2. Development of manifestations simultaneously or in less than a week |
| 3. Confirmation by histopathology of small-vessel occlusion* |
| 4. Laboratory confirmation of the presence of antiphospholipid antibodies† |
| Definite catastrophic antiphospholipid syndrome |
| • All four criteria present |
| Probable catastrophic antiphospholipid syndrome |
| • All four criteria, except only two organs, systems, and/or tissues involved |
| • All four criteria, except for the absence of laboratory confirmation of antiphospholipid antibodies |
| • Criteria 1, 2, and 4 |
| • Criteria 1, 3, and 4, with the development of a third event more than 1 week but within 1 month of presentation, despite anticoagulation |
Vasculitis may coexist, but significant thrombosis must be present as well.
“Positive aPL” twice 12 weeks apart (of note, the original Sapporo APS classification criteria required two positive aPL tests 6 weeks apart [Wilson et al. 1999], which has been changed to 12 weeks as part of the updated Sapporo APS classification criteria [Miyakis et al. 2006].
The purpose of this review article is to summarize the diagnostic challenges and the recently updated diagnostic algorithms for CAPS. A detailed description of the clinical manifestations [Cervera, 2010b, 2012; Cervera et al. 2009] and treatment [Asherson et al. 2003; Cervera, 2010a; Erkan, 2006] of CAPS can be found elsewhere.
APS: how to diagnose a relatively common disease?
Given that multiple well-established reversible (acquired) and/or irreversible (genetic) thrombotic risk factors exist, the Updated Sapporo APS Classification Criteria [Miyakis et al. 2006] (Table 1) were formulated to facilitate APS clinical research. However, also for clinical practice purposes, aPL-positive patients should be evaluated based on these criteria in order to determine whether they have ‘clinically significant’ aPL profiles, which is critical in preventing the overdiagnosis of the syndrome.
There are several important practice points that will help physicians determine whether a patient has a ‘clinically significant’ aPL profile: (a) transient aPL positivity is common during infections and thus documentation of the persistent (at least 12 weeks apart) autoimmune aPL is crucial for diagnostic purposes [Miyakis et al. 2006]; (b) a positive LA test is a better predictor of aPL-related thrombotic events compared with other aPL tests [Galli et al. 2003]; (c) whenever possible, LA test should be tested off anticoagulation as both false-negative and false-positive results can occur in anticoagulated patients; (d) the specificity of aCL and aβ2GPI ELISA tests for aPL-related clinical events increases with higher titers; (e) the risk of thrombosis in aPL-positive patients rises with increasing number of thrombosis risk factors [Hudson et al. 2003; Hansen et al. 2001; Rosendaal et al. 1997]; (f) approximately half of the APS patients with thrombosis have at least one non-aPL thrombosis risk factor at the time of their vascular event [Erkan et al. 2002a; Kaul et al. 2007; Giron-Gonzalez et al. 2004]; (g) IgG isotype is generally more commonly associated with clinical events compared with IgM isotype; (h) even if IgA aCL and IgA aβ2GPI are not part of the updated Sapporo APS Classification Criteria, there have been recent reports of isolated IgA aCL or aβ2GPI positivity in patients with aPL-related clinical events and no other thrombosis risk factors [Kumar et al. 2009; Samarkos et al. 2006]; and (i) triple aPL positivity (LA, aCL, and aβ2GPI) can be clinically more significant than double or single aPL positivity [Pengo et al. 2011] although this remains controversial [Erkan and Lockshin, 2012].
In addition, physicians should keep in mind that clinical manifestations related to aPL represent a spectrum: (a) aPL positivity without clinical events; (b) aPL positivity solely with non-criteria manifestations (e.g. thrombocytopenia, hemolytic anemia, cardiac valve disease, aPL nephropathy); (c) APS based on arterial/venous thrombosis and/or pregnancy morbidity; and (d) CAPS.
In summary, demonstration of a ‘clinically significant’ aPL profile (persistent LA test and/or moderate- to high-titer aPL ELISA) is critical while evaluating aPL-positive patients, including those with multiple organ thromboses.
CAPS spectrum
Definite or probable CAPS
During the 10th International Congress on aPL in 2002, preliminary classification criteria for CAPS were proposed (Table 2) [Asherson et al. 2003] and validated in 2005 [Cervera et al. 2005]. Definite CAPS is defined as thromboses in three or more organs developing in less than a week, microthrombosis in at least one organ and persistent aPL positivity. However, if a patient has only three out of these four requirements, then the patient is classified as probable CAPS. The purpose of the probable CAPS definition is to keep clinicians on high alert for the rapidly progressive nature of CAPS leading to early diagnosis and aggressive treatment.
‘CAPS-like’ disease
In clinical practice, aPL-positive patients exist that do not fulfill the definite or probable CAPS criteria; however, they still create a significant management challenge for physicians. The authors like to define these patients as ‘CAPS-like’, as these patients require close monitoring for the development of CAPS and many times they require aggressive management similar to CAPS. aPL-positive patients with medium- to large-vessel thromboses in two organs with or without concurrent bleeding, isolated microthrombosis with bleeding (pulmonary or adrenal hemorrhage), severe thrombocytopenia with or without bleeding, and severe HELLP syndrome with single organ thrombosis can be included in this group [Erkan et al. 2002a]. Although controversial, the authors would consider patients with a deep vein thrombosis leading to a pulmonary embolus (or similarly a left ventricle thrombus leading to a stroke) to be a single event; depending on the development of other aPL-related manifestations, these patients may or may not be included in this CAPS-like category as well.
Microangiopathic APS
Whereas microangiopathy is defined as the disease of small blood vessels, TMA describes microangiopathy with ischemia due to fibrin formation and/or platelet aggregation resulting in occlusion of arterioles and capillaries. If TMA patients develop nonimmune hemolytic anemia with schistocytes, then the term thrombotic microangiopathic hemolytic anemia (TMHA) is used.
Based on the International Web-based CAPS Registry (see http://infmed.fcrb.es/en/web/caps) analysis, patients with thrombocytopenia, when compared with those without thrombocytopenia, are more likely to develop hemolysis, schistocytes, disseminated intravascular coagulation (DIC), and high fibrin degradation products [Bayraktar et al. 2007]. Thus, a group of CAPS patients exist with predominant hematologic manifestations, sometimes overlapping with other TMAs such as malignant hypertension [Shah et al. 2007], thrombotic thrombocytopenic purpura (TTP), hemolytic-uremic syndrome (HUS), HELLP syndrome, and heparin-induced thrombocytopenia (HIT). Thus, the term ‘microangiopathic APS’ has been proposed to encompass aPL-positive patients with predominant TMA features [Asherson, 2006; Asherson et al. 2007; Asherson and Cervera, 2008]. Unfortunately, the true prevalence of aPL in these TMAs is unknown and, without comparison studies between CAPS and other TMAs, it is difficult to know whether aPL are bystanders or pathogenic in these patients.
Regardless of the possible casual association between aPL and TMAs, aPL is a poor prognostic factor for TMA patients. For instance, if an aPL patient develops HELLP syndrome, there is an earlier onset (<24 weeks), more severe clinical course with hepatic infarcts, and lack of improvement after delivery [George et al. 2007]. Our personal experience supports the poor prognostic role of aPL in TMA patients; however, further systematic data from aPL-positive TMA patients are needed.
Thrombotic storm
Thrombotic storm is defined on the basis of a clinical phenotype, not by laboratory tests or aPL positivity (Table 3). In the absence of a major trigger, patients present with severe thrombotic events affecting multiple vascular structures [Kitchens et al. 2011]. Approximately half of the thrombotic storm patients are reported to have aPL, but a detailed analysis of these patients’ aPL profiles, i.e. whether they have clinically significant aPL profiles, is missing [Ortel et al. 2012; Manco-Johnson et al. 2012]. Compared with CAPS, macrovascular thrombosis appears to be more characteristic of thrombotic storm, although microvascular occlusions may be underestimated, given the extent of large vessel involvement in this prothrombotic phenotype [Ortel et al. 2012].
Table 3.
Clinical characteristics of thrombotic storm (adapted from Kitchens et al. [2011])*.
|
Characteristics of thrombotic storm usually not encountered: cancer (excluding minor skin cancers); myocardial infarction in the setting of advanced coronary artery disease; cocaine use associated with symptom onset; expected thrombotic complications associated with intravascular devices; known paroxysmal nocturnal hemoglobinuria or myeloproliferative disorder; multitrauma/severe trauma (e.g. multiple limb injury); premorbid clinical status before development of thrombotic complications.
Based on the thrombotic storm concept, several comparable disorders (including CAPS) with an extreme prothrombotic presentation may possess a similar underlying pathophysiologic process representing an extreme response to an initial prothrombotic stimulus. Thus, the genetics of thrombotic storm are currently being investigated with the hypothesis that prothrombotic genetic risk factor(s) trigger an accelerated form of thrombosis following an initial event (see http://www.thromboticstorm.com).
CAPS: diagnostic challenges
False-positive aPL results
A positive aPL test can be associated with infections (usually low-titer aPL ELISA) [Kim et al. 2009; Avcin and Toplak, 2007; Wenzel et al. 2002] and/or anticoagulation (positive LA test) [Wenzel et al. 2002; Pengo et al. 2007] thus, at times, aPL can occur as bystanders, not necessarily as contributors to thrombosis.
False-negative aPL results
In APS or CAPS patients, rarely aPL become transiently negative at the time of thrombosis [Miret et al. 1997], possibly because of its consumption [Drenkard et al. 1989].
Overlapping features of thrombotic microangiopathies
As discussed above, a continuum of thrombotic microangiopathic conditions exists, e.g. TTP, HUS, HELLP syndrome, and CAPS; at times it may be difficult to differentiate between these conditions due to significant overlapping features. Thus, in some aPL-positive patients with multiple organ thromboses, aPL positivity might not correlate with pathogenesis [Asherson et al. 2007].
Sepsis and CAPS share similarities
Sepsis is the systemic response to infection marked by systemic inflammatory response syndrome (SIRS), which is marked by the two or more of the following: (a) temperature >38ºC or < 36ºC; (b) heart rate >90 beats/min; (c) respiratory rate >20 breaths/min or PaCO2 <32 mmHg; and (d) white blood cell count >12,000 cells/mm3, <4000 cells/mm3, or with >10% immature (band) forms. When sepsis is associated with DIC, potential complications include bleeding, thrombocytopenia, and microthrombosis; all are also common in CAPS patients. Thus, both the pathophysiology and clinical manifestations of CAPS resemble sepsis with the ultimate development of multiple organ dysfunction syndrome [Levy et al. 2003].
HIT and CAPS share similarities
HIT usually occurs within 4–10 days of heparin treatment. The severe form (type II) is an immune-mediated disorder due to the heparin/platelet factor 4 (PF4) complex antibodies [Alpert et al. 2008]. Given that both arterial and venous thromboses can occur in HIT patients, heparin receiving aPL-positive patients with thrombocytopenia and multiple organ thromboses can be a diagnostic challenge for clinicians. In addition, anti-heparin/PF4 antibody ELISA can be positive in up to 10% of heparin-naïve aPL-positive patients [Alpert et al. 2008] due to autoantibodies against PF4. These antibodies are distinct from the antibodies seen in HIT patients (against heparin/PF4 complex, rather than PF4 alone), which can lead to an incorrect diagnosis of HIT [Pauzner et al. 2009]. In these patients, functional platelet aggregation assays such as heparin-induced platelet activation and aggregation assays can be helpful for the correct diagnosis.
CAPS: diagnostic algorithms and considerations
For the reasons discussed above, the diagnosis of catastrophic APS can be challenging, and sometimes the differential diagnosis cannot be narrowed to a single disease during the acute period. Thus, continuous assessment of patients is warranted. The updated algorithms were created to provide a ‘step-by-step’ approach for clinicians to assess patients with multi-organ thromboses [Erkan et al. 2010] (Algorithm A–C).
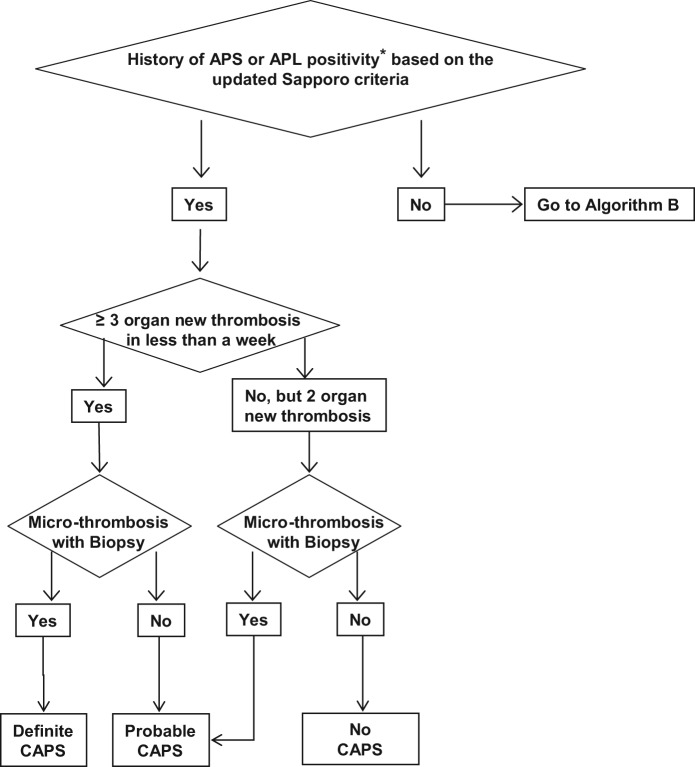
Algorithm A.
Catastrophic antiphospholipid syndrome (CAPS) diagnosis in patients with history of antiphospholipid syndrome (APS) or persistent antiphospholipid antibody (aPL) positivity [Erkan et al. 2010]. Reprinted from [Erkan et al. 2010] with permissions from Elsevier.
*Our recommendation for the definition of ‘positive aPL’ is: lupus anticoagulant (LA) test positive based on the guidelines of International Society of Thrombosis and Haemostasis [Pengo et al. 2009]; anticardiolipin antibody (aCL) IgG/M ≥40 U, and/or anti-β2-glycoprotein-I antibody (aβ2GPI) IgG/M ≥40 U. Caution and further assessment(s) are required if: (a) LA test is performed in anticoagulated patients; (b) aCL or aβ2GPI IgG/M titers are in the range of 20–39 U; and/or (c) aCL or aβ2GPI IgA is the only positive aPL enzyme-linked immunosorbent assay (ELISA) test.
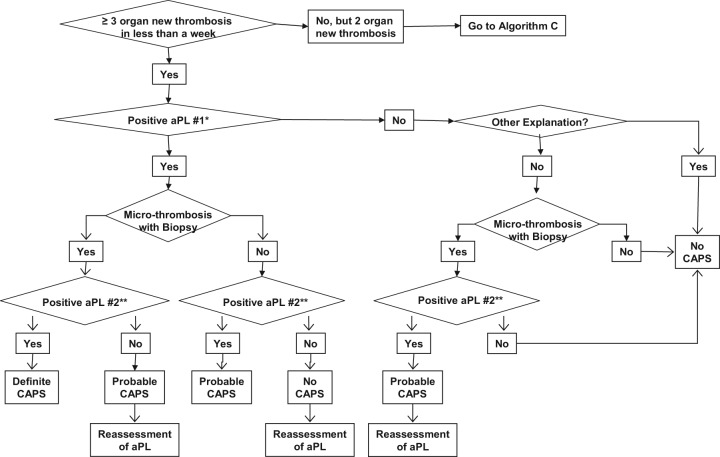
Algorithm B.
Catastrophic antiphospholipid syndrome (CAPS) diagnosis in patients without history of antiphospholipid syndrome (APS) or persistent antiphospholipid antibody (aPL) positivity [Erkan et al. 2010]. Reprinted from [Erkan et al. 2010] with permissions from Elsevier.
*Our recommendation for the definition of ‘positive aPL’ is: lupus anticoagulant (LA) test positive based on the guidelines of International Society of Thrombosis and Haemostasis [Pengo et al. 2009]; anticardiolipin antibody (aCL) IgG/M ≥40 U, and/or anti-β2-glycoprotein-I antibody (aβ2GPI) IgG/M ≥40 U. Caution and further assessment(s) are required if: (a) LA test is performed in anticoagulated patients; (b) aCL or aβ2GPI IgG/M titers are in the range of 20–39 U; and/or (c) aCL or aβ2GPI IgA is the only positive aPL enzyme-linked immunosorbent assay (ELISA) test.
**‘Positive aPL’ twice 12 weeks apart (of note, the original Sapporo APS classification criteria required two positive aPL tests 6 weeks apart [Wilson et al. 1999], which has been changed to 12 weeks as part of the updated Sapporo APS classification criteria [Miyakis et al. 2006].
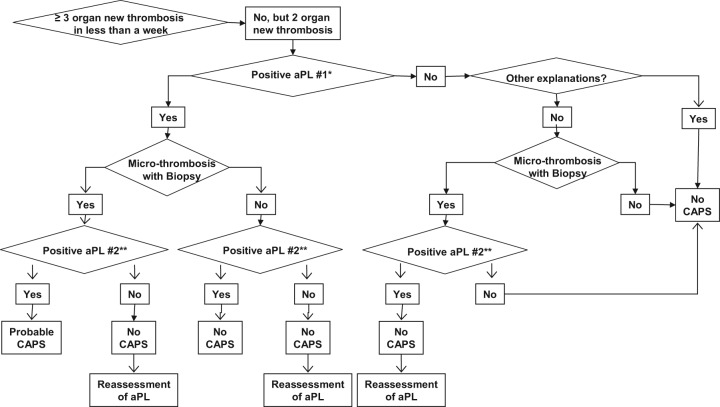
Algorithm C.
Catastrophic antiphospholipid syndrome (CAPS) diagnosis in patients without history of antiphospholipid syndrome (APS) or persistent antiphospholipid antibody (aPL) positivity [Erkan et al. 2010]. Reprinted from [Erkan et al. 2010] with permissions from Elsevier.
*Our recommendation for the definition of ‘positive aPL’ is: lupus anticoagulant (LA) test positive based on the guidelines of International Society of Thrombosis and Haemostasis [Pengo et al. 2009]; anticardiolipin antibody (aCL) IgG/M ≥40 U, and/or anti-β2-glycoprotein-I antibody (aβ2GPI) IgG/M ≥40 U. Caution and further assessment(s) are required if: (a) LA test is performed in anticoagulated patients; (b) aCL or aβ2GPI IgG/M titers are in the range of 20–39 U; and/or (c) aCL or aβ2GPI IgA is the only positive aPL enzyme-linked immunosorbent assay (ELISA) test.
**‘Positive aPL’ twice 12 weeks apart (of note, the original Sapporo APS classification criteria required two positive aPL tests 6 weeks apart [Wilson et al. 1999], which has been changed to 12 weeks as part of the updated Sapporo APS classification criteria [Miyakis et al. 2006].
Does the patient have history of APS or persistent aPL positivity?
Almost half of the patients develop CAPS without history of aPL positivity [Cervera, 2010a]. Thus, previous APS diagnosis based on the updated Sapporo APS Classification Criteria [Miyakis et al. 2006] or persistent clinically significant aPL positivity (LA test positivity and/or moderate- to high-titer aPL ELISA) without history of aPL-related clinical events is of great importance for diagnosis [Erkan et al. 2010].
Does the patient have three or more new organ thromboses developing in less than a week?
Three or more organ thromboses developing in less than a week is the cornerstone of CAPS. However, only two organ thromboses developing in less than a week (with or without the development of a third thrombotic event in more than a week but less than a month, despite anticoagulation) can lead to the diagnosis of ‘probable’ CAPS. Hematological manifestations should not be counted separately as organ system involvement [Erkan et al. 2010].
Does the patient have microthrombosis?
Although pathological confirmation of microthrombosis is one of the requirements for ‘definite’ CAPS, biopsy may not be possible during an acute CAPS episode due to severe thrombocytopenia and/or unstable clinical course. Thus, the diagnosis should be continuously reassessed when a biopsy or autopsy provides new information [Erkan et al. 2010]. However, the risks versus benefits of performing any procedure, including biopsies, need to be carefully weighed in aPL-positive patients.
Does the patient have ‘other explanations’ for multiple organ thromboses and/or microthrombosis?
The most challenging aspect of the diagnosis is when a patient with multiple organ thromboses is found to have a positive aPL for the first time, and the patient also has other non-aPL thrombosis risk factors (e.g. postoperative period, features of other thrombotic microangiopathies, infection with or without sepsis, DIC, and HIT). In a patient such as the one described above, a common challenging scenario is the first detection of a positive LA test while on anticoagulation and/or the presence of a low-titer aPL ELISA test. Given that a continuum of conditions may exist with overlapping clinical and laboratory features, catastrophic APS diagnosis requires a careful and continuous assessment in patients who may have other explanations for multiple organ thromboses [Erkan et al. 2010].
CAPS: team approach to diagnosis
CAPS is a challenging systemic disease. In addition to multiple organ thromboses, noncriteria manifestations of aPL can commonly occur. Bleeding and infections frequently complicate the disease course, which directly affect the prognosis. Thus, both diagnosis and management require a team approach including but not limited to rheumatology, hematology, intensive care, infectious disease, nephrology and a plasma exchange team, and obstetrics when relevant. The multidisciplinary team should meet at least once a day as the clinical course can change quickly in these patients.
Conclusion
APS is a systemic autoimmune disease with both thrombotic and nonthrombotic manifestations in which non-aPL thrombosis risk factors as well as the importance of the ‘clinically significant’ aPL profile should be kept in mind for diagnosis. CAPS is the most severe form of APS with multiple organ thromboses, usually accompanied by microthrombosis and hematologic manifestations. The clinical manifestations of CAPS may evolve gradually, commonly overlapping with other thrombotic microangiopathies, requiring a high index of clinical suspicion. Although the discussion about the treatment of CAPS is beyond the scope of this review article, it is critical to initiate the treatment urgently if the diagnosis of CAPS is clinically suspected, even without the confirmatory aPL tests. We hope that our review paper will help physicians better assess aPL-positive patients with multiple organ thromboses, with the ultimate goal of preventing both ‘underdiagnosis’ and ‘overdiagnosis’ of this complex and highly fatal disease.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors have no conflicts of interest to declare.
Contributor Information
Cassyanne L. Aguiar, Hospital for Special Surgery, Weill Medical College of Cornell University, 535 East 70th Street, New York, NY 10021, USA
Doruk Erkan, Barbara Volcker Center for Women and Rheumatic Diseases, Hospital for Special Surgery, Weill Medical College of Cornell University, New York, NY, USA.
References
- Alpert D., Mandl L., Erkan D., Yin W., Peerschke E., Salmon J. (2008) Anti-heparin platelet factor 4 antibodies in systemic lupus erythematosus are associated with IgM antiphospholipid antibodies and the antiphospholipid syndrome. Ann Rheum Dis 67: 395–401 [DOI] [PubMed] [Google Scholar]
- Asherson R. (2006) New subsets of the antiphospholipid syndrome in 2006: “PRE-APS” (probable APS) and microangiopathic antiphospholipid syndromes (“MAPS”). Autoimmun Rev 6: 76–80 [DOI] [PubMed] [Google Scholar]
- Asherson R., Cervera R. (2008) Microvascular and microangiopathic antiphospholipid-associated syndromes (“MAPS”) semantic or antisemantic? Autoimmun Rev 7: 164–167 [DOI] [PubMed] [Google Scholar]
- Asherson R., Cervera R., de Groot P., Erkan D., Boffa M., Piette J., et al. (2003) Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus 12: 530–534 [DOI] [PubMed] [Google Scholar]
- Asherson R., Pierangeli S., Cervera R. (2007) Microangiopathic antiphospholipid associated syndromes revisited new concepts relating to antiphospholipid antibodies and syndromes. J Rheumatol 34: 1793–1795 [PubMed] [Google Scholar]
- Avcin T., Toplak N. (2007) Antiphospholipid antibodies in response to infection. Curr Rheumatol Rep 9: 212–218 [DOI] [PubMed] [Google Scholar]
- Bayraktar U., Erkan D., Bucciarelli S., Espinosa G., Cervera R., Asherson R. (2007) Catastrophic antiphospholipid syndrome (CAPS): comparison between CAPS patients with and without thrombocytopenia. Clin Exp Rheumatol 25: 1418021501 [Google Scholar]
- Cervera R. (2010a) Update on the diagnosis, treatment, and prognosis of the catastrophic antiphospholipid syndrome. Curr Rheumatol Rep 12: 70–76 [DOI] [PubMed] [Google Scholar]
- Cervera R. on behalf of CAPS Registry Project Group (2010b) Catastrophic antiphospholipid syndrome (CAPS): update from the ‘CAPS Registry’. Lupus 19: 412–418 [DOI] [PubMed] [Google Scholar]
- Cervera R. (2012) CAPS Registry. Lupus 21: 755–757 [DOI] [PubMed] [Google Scholar]
- Cervera R., Asherson R. (2004) Multiorgan failure due to rapid occlusive vascular disease in antiphospholipid syndrome: the ‘catastrophic’ antiphospholipid syndrome. APLAR J Rheumatol 7: 254–262 [Google Scholar]
- Cervera R., Bucciarelli S., Plasin M., Gomez-Puerta J., Plaza J., Guillermo P., et al. for the CAPS Registry Project Group (2009) Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the “CAPS Registry”. J Autoimmunity 32: 240–245 [DOI] [PubMed] [Google Scholar]
- Cervera R., Font J., Gomez-Puerta A., Espinosa G., Cucho M., Bucciarelli S., et al. for CAPS Registry Project Group (2005) Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann Rheum Dis 64: 1205–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenkard C., Sanchez-Guerrero J., Alarcon-Segovia D. (1989) Fall in antiphospholipid antibody at time of thromboocclusive episodes in systemic lupus erythematosus. J Rheumatol 16: 614–617 [PubMed] [Google Scholar]
- Erkan D. (2006) Therapeutic and prognostic considerations in catastrophic antiphospholipid syndrome. Autoimmun Rev 6: 98–103 [DOI] [PubMed] [Google Scholar]
- Erkan D., Espinosa G., Cervera R. (2010) Catastrophic antiphospholipid syndrome: updated diagnostic algorithms. Autoimmun Rev 10: 74–79 [DOI] [PubMed] [Google Scholar]
- Erkan D., Lockshin M. (2012) High risk antiphospholipid antibody profile: matter of the number or titer of tests? E-letter to the editor (re: Incidence of first thrombembolic event in asymptomatic carries of high-risk aPL profile: a multicenter prospective study, Pengo et al. Blood 2011;17:4714), Published online on 24 January 2012, available at: http://bloodjournal.hematologylibrary.org/letters [DOI] [PubMed] [Google Scholar]
- Erkan D., Yazici Y., Lockshin M. (2002a) Catastrophic antiphospholipid syndrome (CAPS) or antiphospholipid syndrome (APS) with a catastrophic event? Lupus 11: 617 [Google Scholar]
- Erkan D., Yazici Y., Peterson M., Sammaritano L., Lockshin M. (2002b) A crosssectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology 41: 924–929 [DOI] [PubMed] [Google Scholar]
- Galli M., Luciani D., Bertolini G., Barbui T. (2003) Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 101: 1827–1832 [DOI] [PubMed] [Google Scholar]
- George D., Vasanth L., Erkan D., Bass A., Salmon J., Lockshin M. (2007) Primary antiphospholipid syndrome presenting as HELLP syndrome. Hosp Special Surg J 3: 216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Gonzalez J., Garcia del Rio E., Rodriguez C., Rodriguez-Martorell J., Serrano A. (2004) Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: prospective analysis of 404 individuals. J Rheumatol 31: 1560–1567 [PubMed] [Google Scholar]
- Hansen K., Kong D., Moore K., Ortel T. (2001) Risk factors associated with thrombosis in patients with antiphospholipid antibodies. J Rheumatol 28: 2018–2024 [PubMed] [Google Scholar]
- Hudson M., Herr A.L., Rauch J., Neville C., Chang E., Ibrahim R., et al. (2003) The presence of multiple prothrombotic risk factors is associated with a higher risk of thrombosis in individuals with anticardiolipin antibodies. J Rheumatol 30: 2385–2391 [PMC free article] [PubMed] [Google Scholar]
- Kaul M., Erkan D., Sammaritano L., Lockshin M. (2007) Assessment of the 2006 revised antiphospholipid syndrome classification criteria. Ann Rheumat Dis 66: 927–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Moskowitz N., Dicarlo E., Bass A., Erkan D., Lockshin M. (2009) Catastrophic antiphospholipid syndrome triggered by sepsis. Hosp Special Surg J 5: 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens C., Erkan D., Brandao L., Hahn S., James A., Kulkarni R., et al. (2011) Thrombotic storm revisited: preliminary diagnostic criteria suggested by the Thrombotic Storm Study Group. Am J Med 124: 290–296 [DOI] [PubMed] [Google Scholar]
- Kumar S., Papalardo E., Sunkureddi P., Najam S., Gonazlez E., Pierangeli S. (2009) Isolated elevation of IgA anti-Beta2glycoprotein I antibodies with manifestations of antiphospholipid syndrome: a case series of five patients. Lupus 18: 1011–1014 [DOI] [PubMed] [Google Scholar]
- Levy M., Fink M., Marshall J., Abraham E., Angus D., Cook D., et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256 [DOI] [PubMed] [Google Scholar]
- Manco-Johnson M., Wang M., Goldenberg N., Soep J., Gibson E., Knoll C., et al. (2012) Treatment, survival, and thromboembolic outcomes of thrombotic storm in children. J Pediatrics 161: 682–688 [DOI] [PubMed] [Google Scholar]
- Miret C., Cervera R., Reverter J., Garcia-Carrasco M., Ramos M., Molla M., et al. (1997) Antiphospholipid syndrome without antiphospholipid antibodies at the time of the thrombotic event: transient ‘seronegative’ antiphospholipid syndrome? Clin Exp Rheumatol 15: 541–544 [PubMed] [Google Scholar]
- Miyakis S., Lockshin M., Atsumi T., Branch D., Brey R., Cervera R., et al. (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4: 295–306 [DOI] [PubMed] [Google Scholar]
- Ortel T., Kitchens C., Erkan D., Brandao L., Hahn S., James A., et al. (2012) Clinical causes and treatment of the thrombotic storm. Exp Rev Hematol 5: 653–659 [DOI] [PubMed] [Google Scholar]
- Pauzner R., Greinacher A., Selleng K., Althaus K., Shenkman B., Seligsohn U. (2009) False-positive tests for heparin-induced thrombocytopenia in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Thromb Haemost 7: 1070–1074 [DOI] [PubMed] [Google Scholar]
- Pengo V., Biasiolo A., Gresele P., Marongiu F., Erba N., Veschi F., et al. (2007) Survey of lupus anticoagulant diagnosis by central evaluation of positive plasma samples. J Thromb Haemost 5: 925–930 [DOI] [PubMed] [Google Scholar]
- Pengo V., Ruffatti A., Legnani C., Testa S., Fierro T., Marongiu F., et al. (2011) Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 118: 4714–4718 [DOI] [PubMed] [Google Scholar]
- Pengo V., Tripodi A., Reber G., Rand J., Ortel T., Galli M., et al. (2009) Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 7: 1737–1740 [DOI] [PubMed] [Google Scholar]
- Rosendaal F., Siscovick D., Schwartz S., Beverly R., Psaty B., Longstreth W., Jr, et al. (1997) Factor V Leiden (resistance to activated protein C) increases the risk of myocardial infarction in young women. Blood 89: 2817–2821 [PubMed] [Google Scholar]
- Samarkos M., Davies K., Gordon C., Loizou S. (2006) Clinical significance of IgA anticardiolipin and anti-beta2-GP1 antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Clin Rheumatol 25: 199–204 [DOI] [PubMed] [Google Scholar]
- Shah A., Higgins J., Chakravarty E. (2007) Thrombotic microangiopathy hemolytic anemia in a patient with SLE: diagnostic difficulties. Nat Clin Pract Rheumatol 3: 357–362 [DOI] [PubMed] [Google Scholar]
- Vora S., Asherson R., Erkan D. (2006) Catastrophic antiphospholipid syndrome. J Intensive Care Med 21: 144–159 [DOI] [PubMed] [Google Scholar]
- Wenzel C., Stoiser B., Locker G., Laczika K., Quehenberger P., Kapiotis S., et al. (2002) Frequent development of lupus anticoagulants in critically ill patients treated under intensive care conditions. Crit Care Med 30: 763–770 [DOI] [PubMed] [Google Scholar]
- Wilson W., Gharavi A., Koike T., Lockshin M., Branch D., Piette J., et al. (1999) International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 42: 1309–1311 [DOI] [PubMed] [Google Scholar]