Abstract
Paracatalytic enzyme modifications result from the oxidation of enzyme—substrate carbanions by extrinsic oxidants. During the oxidation of enzyme-activated substrates, transiently reactive intermediates are generated which, without being released from the enzyme, modify groups at the active site. For enzymes producing carbanion intermediates, the combination of the normal substrate with a suitable electron acceptor has thus been proposed as a highly specific binary system for their active site-directed modification. In this study, the structural features of paracatalytically modified fructose-1,6-bisphosphate aldolase (D-fructose-1,6-bisphosphate D-glyceraldehyde-3-phosphate lyase, EC 4.1.2.13) from rabbit muscle have been elucidated. This enzyme is completely inactivated within 60 min in the presence of fructose 1,6-bisphosphate in saturating concentration and 0.5 mM hexacyanoferrate(III) (pH 7.6, 25°C). The inactivation is caused by covalent incorporation of one triosephosphate derivative per subunit. Peptide analysis showed that the triosephosphate derivative forms an intrachain crosslink between lysine-146 and lysine-227. According to previous independent experimental evidence, both lysyl residues are located at the active site: the ε-amino group of lysine-227 forms a Schiff base intermediate with the carbonyl group of the substrate [Lai, C. Y., Nakai, N. & Chang, D. (1974) Science 183, 1204-1206] and alkylation of lysine-146 by the affinity labeling reagent N-bromoacetylethanolamine phosphate inactivates the enzyme [Hartman, F. C. & Brown, J. P. (1976) J. Biol. Chem. 251, 3057-3062]. The present data thus establish paracatalytic modification as a mode of active site-directed enzyme modification.
Keywords: active site-directed enzyme modification, self-inactivation of enzymes, intrachain crosslink, carbanion oxidation, mechanism of enzyme action
Full text
PDF

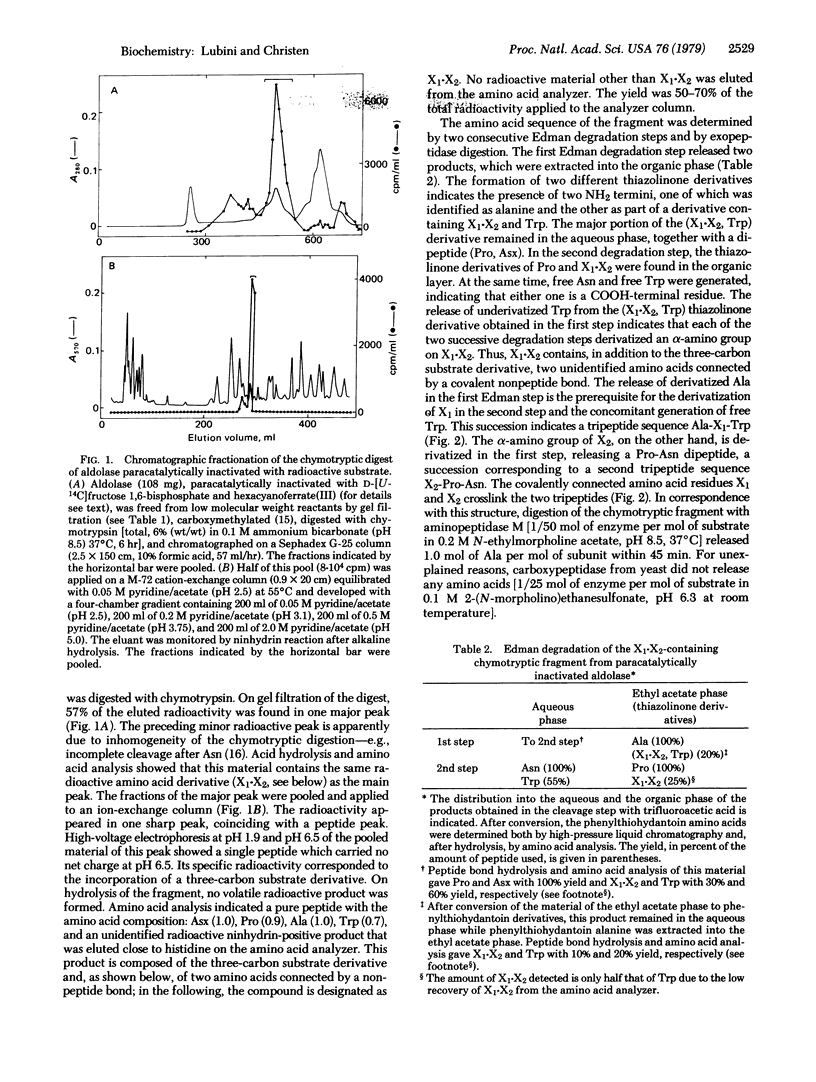
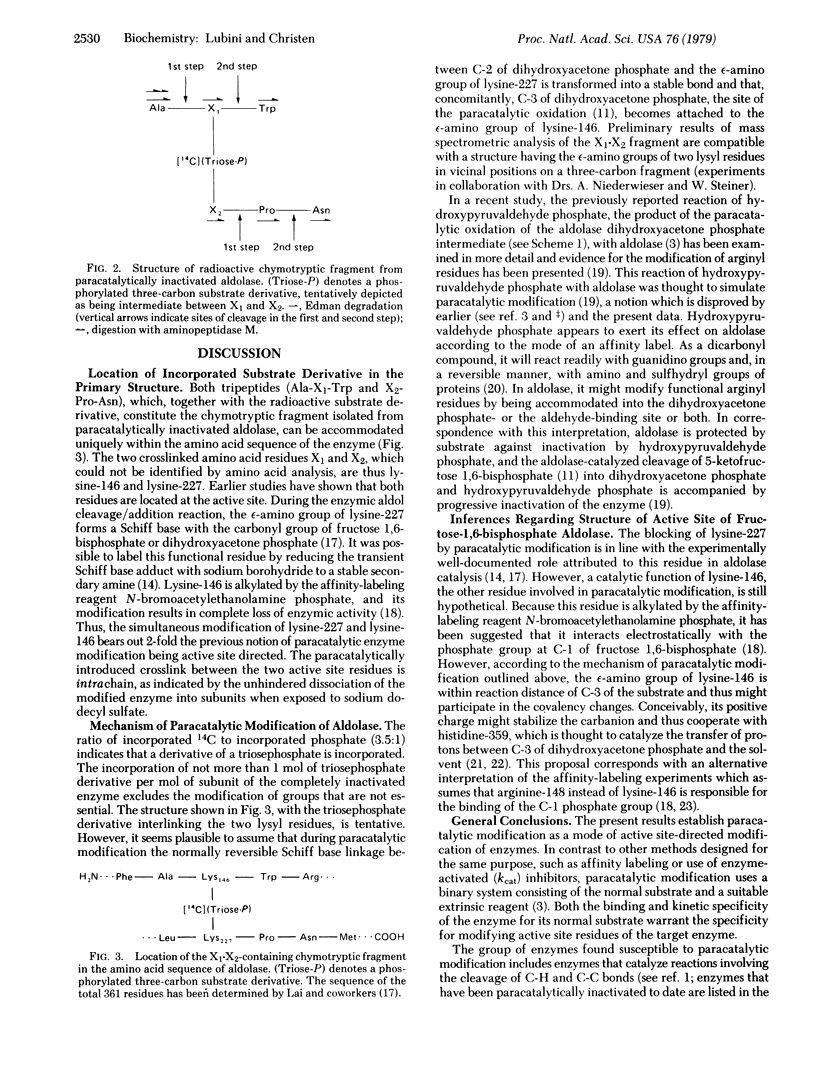

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christen P., Anderson T. K., Healy M. J. H2O2 oxidizes an aldolase dihydroxyacetone phosphate intermediate to hydroxymethylglyoxal phosphate. Experientia. 1974 Jun 15;30(6):603–605. doi: 10.1007/BF01921497. [DOI] [PubMed] [Google Scholar]
- Christen P., Cogoli-Greuter M., Healy M. J., Lubini D. Specific irreversible inhibition of enzymes concomitant to the oxidation of carbanionic enzyme-substrate intermediates by hexacyanoferrate (III). Eur J Biochem. 1976 Mar 16;63(1):223–231. doi: 10.1111/j.1432-1033.1976.tb10224.x. [DOI] [PubMed] [Google Scholar]
- Christen P. Paracatalytic enzyme modification by oxidation of enzyme-substrate carbanion intermediates. Methods Enzymol. 1977;46:48–54. doi: 10.1016/s0076-6879(77)46009-9. [DOI] [PubMed] [Google Scholar]
- Hartman F. C., Brown J. P. Affinity labeling of a previously undetected essential lysyl residue in class I fructose bisphosphate aldolase. J Biol Chem. 1976 May 25;251(10):3057–3062. [PubMed] [Google Scholar]
- Hartman F. C., Suh B., Welch M. H., Barker R. Inactivation of class I fructose diphosphate aldolases by the substrate analog N-bromoacetylethanolamine phosphate. J Biol Chem. 1973 Dec 10;248(23):8233–8239. [PubMed] [Google Scholar]
- Hartman F. C., Welch M. H. Identification of the histidyl residue of rabbit muscle aldolase alkylated by N-bromoacetylethanolamine phosphate. Biochem Biophys Res Commun. 1974 Mar 15;57(1):85–92. doi: 10.1016/s0006-291x(74)80360-8. [DOI] [PubMed] [Google Scholar]
- Healy M. J., Christen P. Mechanistic probes for enzymatic reactions. Oxidation-reduction indicators as oxidants of intermediary carbanions (studies with aldolase, aspartate aminotransferase, pyruvate decarboxylase, and 6-phosphogluconate dehydrogenase). Biochemistry. 1973 Jan 2;12(1):35–41. doi: 10.1021/bi00725a006. [DOI] [PubMed] [Google Scholar]
- Healy M. J., Christen P. Reaction of the carbanionic aldolase-substrate intermediate with tetranitromethane. Identification of the products, hydroxypyruvaldehyde phosphate and D-5-ketofructose 1,6-diphosphate. J Am Chem Soc. 1972 Nov 1;94(22):7911–7916. doi: 10.1021/ja00777a039. [DOI] [PubMed] [Google Scholar]
- Kobashi K., Lai C. Y., Horecker B. L. Organic phosphate groups in native and borohydride-reduced aldolase. Arch Biochem Biophys. 1966 Nov;117(2):437–444. doi: 10.1016/0003-9861(66)90433-4. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Nakai N., Chang D. Amino acid sequence of rabbit muscle aldolase and the structure of the active center. Science. 1974 Mar;183(130):1204–1206. doi: 10.1126/science.183.4130.1204. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Stokes A. M., Hill H. A., Riordan J. F. Arginine as the C-1 phosphate binding site in rabbit muscle aldolase. FEBS Lett. 1975 Jun 1;54(1):70–72. doi: 10.1016/0014-5793(75)81070-2. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Patthy L. Role of nascent alpha-ketoaldehyde in substrate-dependent oxidative inactivation of aldolase. Eur J Biochem. 1978 Jul 17;88(1):191–196. doi: 10.1111/j.1432-1033.1978.tb12437.x. [DOI] [PubMed] [Google Scholar]


