Abstract
Plant phenological events are influenced by climate factors such as temperature and rainfall. To evaluate phenological responses to water availability in a Spring Heath-Pine wood (Erico-Pinetum typicum), the focus of this study was to determine intra-annual dynamics of apical and lateral growth of co-occurring early successional Larix decidua and Pinus sylvestris and late successional Picea abies exposed to drought. The effect of reduced plant water availability on growth phenology was investigated by conducting a rainfall exclusion experiment. Timing of key phenological dates (onset, maximum rate, end, duration) of growth processes were compared among species at the rain-sheltered and control plot during 2011 and 2012. Shoot and needle elongation were monitored on lateral branches in the canopy at c. 16 m height and radial growth was recorded by automatic dendrometers at c. 1.3 m height of > 120 yr old trees. Different sequences in aboveground growth phenology were detected among the three species under the same growing conditions. While onset of radial growth in April through early May was considerably preceded by onset of needle growth in Larix decidua (5 - 6 weeks) and shoot growth in Pinus sylvestris (c. 3 weeks), it occurred quite simultaneously with onset of shoot growth in Picea abies. Low water availability had a minor impact on onset of aboveground growth, which is related to utilization of stored water, but caused premature cessation of aboveground growth. At the control plot mean growing season length was 130 days in Pinus sylvestris, 95 days in Larix decidua and 73 days in Picea abies supporting the hypothesis that early successional species are resource expenders, while late successional species are more efficient in utilizing resources and develop safer life strategies. High synchronicity found in culmination of radial growth in late spring (mid-May through early June) prior to occurrence of more favourable environmental conditions in summer might indicate sink competition for carbohydrates to belowground organs. This is supported by completion of apical growth in mid June in all species, except for needle growth of Pinus sylvestris, which lasted until early August. Phenological observations of conifers exposed to drought revealed that tree water status early during the growing season determines total annual aboveground growth and besides temperature, species-specific endogenous and/or environmental factors (most likely photoperiod and/or different threshold temperatures) are involved in controlling apical and lateral growth resumption after winter dormancy.
Keywords: aboveground growth, drought, intra-annual growth, mixed conifer forest, phenology, tree growth
Introduction
Plants are finely tuned to the seasonality of their environment and coordinate the timing of activity in different organs, i.e., root, stem, shoot and foliage (for a review see Polgar and Primack, 2011). To evaluate impact of climate extremes on tree growth, environmental signals that trigger phenological events like growth onset and end, and maximum growth rate need to be elucidated. Differences in plant phenology among species indicate different responses to abiotic factors (temperature, photoperiod, drought) or endogenous control (e.g, carbohydrate availability, hormonal regulation). It is well established that in temperate and boreal trees the timing of growth resumption after winter dormancy is temperature driven (Linkosalo et al., 2006; Hänninen and Tanino, 2011; Begum et al., 2013). As a result of climate warming the spring phenology of trees has advanced during recent decades (Menzel et al., 2006; Cleland et al., 2007), although photoperiodic constraints restrict response of some boreal and temperate tree species to increasing temperatures (Wareing, 1956; Körner and Basler, 2010). The duration of growth also defines the period where damage from extreme weather events like late or early frost, drought or heat waves may occur (Cannell and Smith, 1986; Hänninen, 1991). That phenology is also responsive to rainfall and water availability was reported for Mediterranean regions and tropical dry forests (e.g., Borchert, 1994; Peñuelas et al., 2004; Bernal et al., 2011). Hence, monitoring growth phenology of different organs and their coordination is a useful approach for characterizing the tree growth sensitivity to occurrence of climate extremes expected to occur more often in a warmer climate (IPCC, 2007).
The growth phenology of different tree organs was compared within and among species of cold environments, i.e., of boreal (Zhai et al., 2012) or alpine treeline species (Rossi et al., 2009), and temperate forest trees (Cuny et al., 2012; Michelot et al., 2012). Moser et al. (2010) found that needles in Larix decidua appeared several weeks before onset of radial stem growth occurred, which is in agreement with the hypothesis that resumption of cambial growth after winter dormancy is influenced by auxin coming from buds, developing leaves and shoots (Savidge et al., 1988; Sundberg and Uggla, 1998). On the other hand, Rossi et al. (2009) and Zhai et al. (2012) detected earlier reactivation of radial stem growth with respect to shoot growth in coniferous species including Larix decidua corroborating the absence of a relationship between timing of cambial reactivation and auxin levels (e.g., Funada et al., 2002). These contrasting results need further clarification and we are also not aware of any study comparing growth phenology of aboveground tree organs (i.e, shoot, foliage and stem) from boreal-montane conifer species in response to drought, although drought is considered one of the main climatic constraints for tree growth (Allen et al., 2010) and water is required for extension of foliage, shoot and water conducting cells in the stem.
In dry inner Alpine valleys soil water availability represents a significant environmental constraint to tree growth (e.g., Eilmann and Rigling 2012; Schuster and Oberhuber 2013). Swidrak et al. (2011) reported that in Pinus sylvestris exposed to early season drought in an inner Alpine environment, temperature controls onset of radial growth after winter dormancy. On the other hand, drought occurring during summer was found to induce premature growth cessation in conifers (e.g., Pichler and Oberhuber, 2007; Gonzalez-Benecke et al., 2010). Because phenological assessment in these studies is restricted to radial growth, a comparative assessment of intra-annual dynamics of apical and lateral growth among co-occurring conifers exposed to drought is considered as necessary to evaluate growth response of different organs and tree species to drought.
Therefore, the objectives of this study were to compare (i) seasonal phenology of needle, shoot and stem growth within and among trees in a dry-mesic mixed-conifer forest where early successional Larix decidua and Pinus sylvestris and late successional Picea abies co-occur and (ii) drought sensitivity of main phenological events, i.e., onset, time of maximum growth, growth duration and end. Based on the hypothesis stated by Körner and Basler (2010) that early-successional species adopt riskier life strategies than late-successional species, we expected that growth resumption after winter dormancy occurs earlier and growth ceases later in early successional Larix decidua and Pinus sylvestris than in late successional Picea abies. Furthermore, we hypothesized that growth resumption is controlled by temperature and unresponsive to drought while growth cessation is sensitive to water availability and that lateral growth onset at breast height occurs after shoot and needle growth in the crown has begun.
Material and Methods
Study area
The study site is part of a postglacial rock-slide area situated in the montane belt (c. 750 m a.s.l.) within the inner Alpine dry valley of the Inn River (Tyrol, Austria, 47°13′53″ N, 10°50′51″ E) and has a relatively continental climate with mean annual precipitation and temperature of 716 mm and 7.3 °C, respectively (long-term mean during 1911-2010 at Ötz, 812 m a.s.l., 5 km from the study area). The dominating plant community is an open Spring Heath-Pine wood (Erico-Pinetum typicum). On scattered dry-mesic sites mixed stands composed of Pinus sylvestris 60 %, Picea abies 20 % and Larix decidua 20 % are developed. Shallow soils of protorendzina type, i.e., rendzic leptosols according to the FAO classification system (FAO, 2006), are developed and consist of unconsolidated, coarse-textured materials with low water holding capacity (soil depth 10 – 20 cm). A thick moss layer dominates the understorey, indicating slightly moist conditions.
Two nearby sites (c. 50 m in linear distance) where Larix decidua, Pinus sylvestris and Picea abies grow side by side were selected (control and rain-sheltered plot) and scaffolds of 16 m height were constructed to reach the upper crowns of all three species at both plots. Stand height and canopy coverage were 15 to 18 m and c. 70 %, respectively. The study site was slightly facing north (slope angle 5 °). At one plot a transparent rain-proof sheet was installed below the canopy at 1.0 to 1.3 m aboveground covering an area of 240 m2 to induce artificial drought during the growing season (March - September). However, stretching of roots outside roofing could not be prevented, because restrictions imposed by nature conservation prohibited trenching at the border of the rain shield. All measurements were carried out on dominant trees with age ranging from 122 to 207 yr. Pinus sylvestris and Picea abies trees selected at the control and rain-sheltered plot had quite similar age, diameter and tree height (Table 1). Increment cores were taken to determine cambial age of selected trees at breast height.
Table 1.
Age and size of trees selected for phenological analyses at the control and rain-sheltered (D) plot.
| Species | Age1 (years) | Diameter1 (cm) | Height (m) | |||
|---|---|---|---|---|---|---|
| Control | D | Control | D | Control | D | |
| Pinus sylvestris | 170 | 186 | 30.1 | 31.4 | 18 | 17.5 |
| Picea abies | 141 | 122 | 22.3 | 22.3 | 16 | 17.5 |
| Larix decidua | 152 | 207 | 32.8 | 47.5 | 18 | 20 |
Cambial age and diameter recorded at breast height
Monitoring growth phenology of apical and lateral meristems
Phenological observations of shoot, needle and stem growth were carried out to define the beginning and the end of the growing season. Shoot elongation and needle enlargement were monitored on lateral branches in the sun crown of all species (one tree per species and plot) during two consecutive years (2011 and 2012). Several apical shoots were marked at the beginning of the growing season in early March, whereby shoots representing average growth in the crown were selected. Phenology of shoot and needle growth was recorded in weekly to 14 day intervals from mid March through October (shorter intervals after budburst at the start of the growing season; ten developing needles and three shoots per species and plot). Shoot lengthening and needle growth were measured with a precision manual calibre to the nearest 1 mm. Swollen and slightly elongated buds of Pinus sylvestris and Picea abies marked onset of shoot growth, whereby at this time bud scales still covered the bud. New needles broke free 2 - 3 weeks and within one week after first detection of bud swelling in Pinus sylvestris and Picea abies, respectively. Needles enclosed in bud scales showed pre-developed length of about 2.5 mm in Pinus sylvestris and 5 mm in Picea abies. In Larix decidua needle development was recorded on short-shoots, while shoot elongation was determined on long-shoots. Bud break of short-shoots occurred several weeks before onset of shoot lengthening. Radial growth was considered to have begun when one horizontal row of tracheids was detected in the enlarging phase (see below). End of needle, shoot and radial growth was defined when modelled growth reached 90 % of final growth (see Data analyses below). Growing season length was defined as the period from bud-break (i.e., green needle tissue visible) or bud-swelling (i.e., onset of shoot elongation) until end of aboveground growth (i.e., when modelled growth reached 90 % of final growth).
Electronic band dendrometers with automatic temperature compensation (dendrometer type DC2, Ecomatik, Munich, Germany) were installed about 1.30 m aboveground in late October 2010 to record intra-annual radial growth. The measuring cable consisted of Invar-steel, which shows a thermal expansion coefficient < 1.4 * 10−6 / K. The precision of the dendrometers was < 0.01 mm. Dead outermost layers (periderm) of the bark were slightly removed to reduce the influence of hygroscopic swelling and shrinkage of the bark on dendrometer records and to ensure close contact with the stem (cf. Zweifel and Häsler, 2001). Data were recorded with data loggers programmed to record measurements taken every 30 minutes and daily increment of stem radius was calculated by averaging all daily measurements (48 values/day).
Because cell enlargement is regarded the major driving force for radial stem increase (cf. Deslauriers et al., 2003; Gruber et al., 2009), dendrometer traces were set to zero at the day of the year, when first row of enlarging cells was detected. To determine onset of radial stem increment of the three species during the growing seasons 2011 and 2012, we took wood micro-cores (2.5 mm in diameter and ca. 2 cm in length) from 8 trees/species at a nearby plot. Microcores were sampled in weekly intervals in a spiral up the stem using the Trephor tool (Rossi et al., 2006). A distance of 2 – 3 cm in tangential and longitudinal direction was kept to avoid lateral influences of wound reactions on adjacent sampling positions. Immediately after extraction, cores were stored in a microtube with 10 % aqueous ethanol and stored at 5 °C. Cores were embedded in glycolmethacrylate (Technovit 7100) and polymerised after adding an accelerator. Tracheid development was observed in ca. 12 μm thick transverse sections cut with a rotary microtome and stained with a water solution of 0.05 % cresyl fast violet. Because we recently found that onset of radial stem growth within the study area is controlled by air temperature rather than soil moisture content (Swidrak et al., 2011), the determination of radial growth onset by taking microcores from nearby trees is justified. Furthermore, by applying this approach we avoided effects of repeated wounding on dendrometer traces.
Data analyses
Intra-annual dynamics of needle, shoot and radial growth (dendrometer records) were modelled with a Gompertz function using the nonlinear regression procedure included in the Origin software package (OriginLab Corporation, Northampton, MA, USA). The Gompertz equation proved its versatility in describing growth-limiting processes (e.g., Zeide, 1993; Rossi et al., 2003) and enabled determination of critical points of intra-annual growth such as timing of maximum growth and growth cessation (i.e., 90 % of final growth completed) and growth rate.
Microclimate records
During the study period daily precipitation, relative air humidity and air temperature were collected automatically (ONSET, Pocasset, MA, USA) within the stand at c. 2 m height and above canopy at top of a scaffold at c. 18 m height. Measuring intervals for all sensors were 30 min and mean daily air temperatures were calculated by averaging all measurements (48 values per day). Volumetric soil water content in 5 – 10 cm soil depth was recorded at both plots (ThetaProbes Type ML2x, Delta-T, Cambridge, England). Three sensors were placed at each site and measuring intervals were set to 60 min. Mean daily water content (Vol. %) was calculated by averaging all measurements from three sensors.
Results
Environmental variables during growing seasons 2011 and 2012
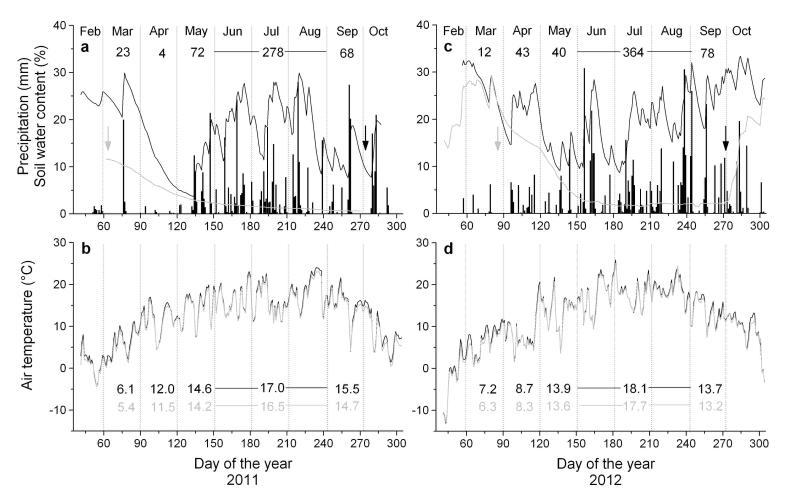
Climate in 2011 and 2012 distinctly deviated at the start of the growing season in spring. Mean daily air temperatures above canopy in April and May were 3.3 and 0.7 °C higher in 2011 compared to 2012. Furthermore, an almost continuous drought period lasted from 19 March to 13 May 2011 (Fig. 1a, b), which caused soil water content to drop to c. 5 Vol. % in May at both plots. In 2012 frequent rainfall events in March and April caused high soil moisture content (c. 25 Vol. %) until May, when soil moisture content temporarily dropped to 10 Vol. % due to low rainfall and strongly increasing temperature (Fig. 1 c, d). Air temperature and rainfall during summer 2012 exceeded records in 2011 by c. 1 °C and 86 mm, respectively. Mean soil water content at the rain-sheltered site dropped below 5 Vol. % in early and late May 2011 and 2012, respectively (Fig. 1a, c).
Fig. 1.
a-d Climate variables and soil water content (Vol. %) recorded during the growing seasons 2011 and 2012 within the study area. (a, c) Daily precipitation sum (bars) and soil water content at the control (black line) and rain-sheltered site (grey line). (b, d) Mean daily air temperature recorded in the canopy and below canopy at 2 m height (black and grey line, respectively). Mean monthly air temperature in and below canopy in March through May, during summer (June – August) and in September are indicated in black and grey, respectively. Date of installation and removal of rain-proof sheet are indicated by grey and black arrows, respectively.
Temporal dynamics of aboveground growth
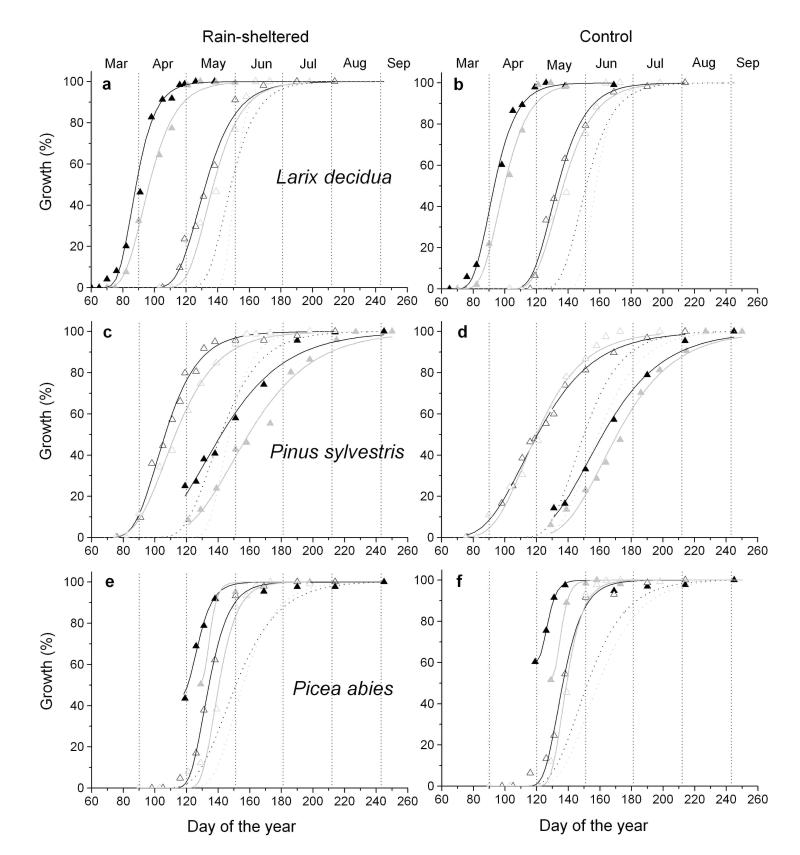
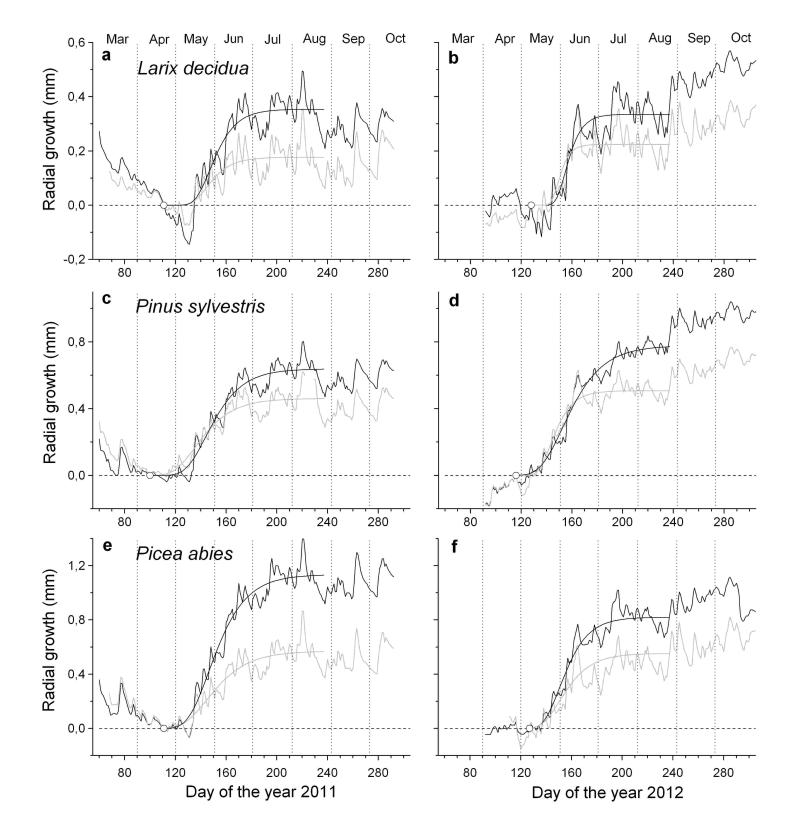
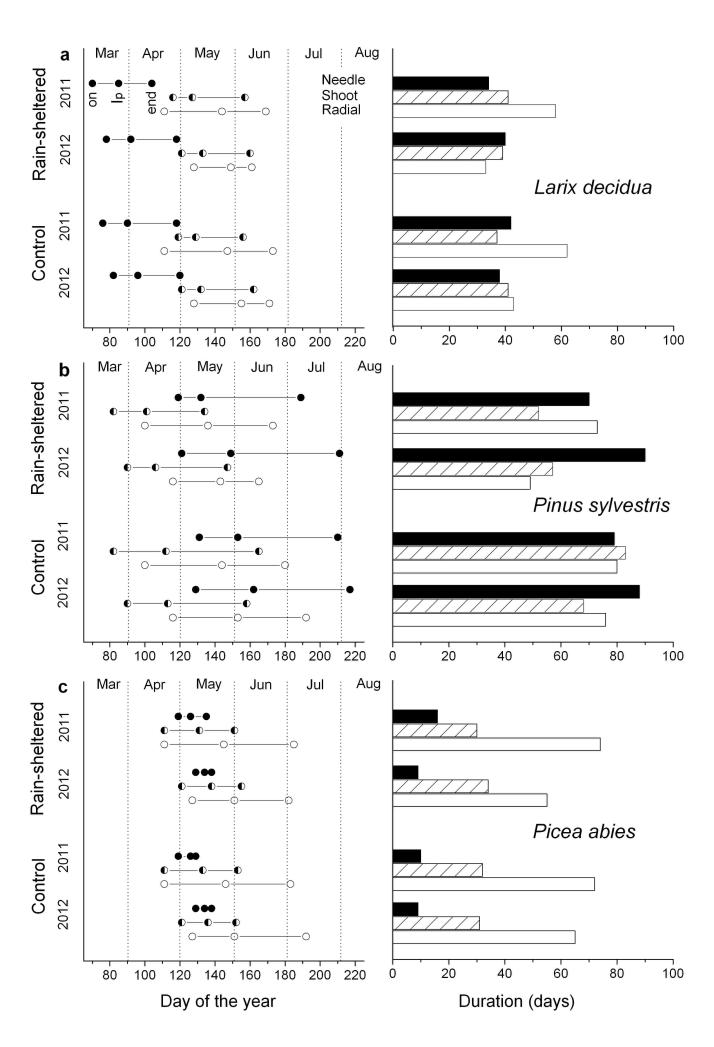
Quite different sequences in aboveground growth phenology were detected among species (Fig. 2a-f). The first phenological event recorded during the year was bud-burst of short-shoots in Larix decidua in early to mid-March. Bud-burst was followed after 5 – 6 weeks by onset of shoot and radial growth. At this time needle growth on short-shoots was nearly completed (Fig. 2a-b). Bud-swelling and shoot elongation in Pinus sylvestris occurred between mid- and end of March and started c. 3 weeks before radial growth commenced and 4 – 6 weeks before onset of needle growth was detected (Fig. 2c-d). Winter dormancy was terminated at the latest in Picea abies. Shoot and radial growth started end of April to early May, which was followed by onset of needle unfolding within a few days (Fig. 2e-f). Radial growth was consistently narrower at the rain-sheltered compared to control plot in all species and in both study years (Fig. 3). Radial growth onset in Larix decidua and Pinus sylvestris occurred 5 - 6 weeks and c. 3 weeks after bud-break or bud-swelling was detected in the canopy, respectively (Fig. 4a-b). In Picea abies bud-swelling in the canopy occurred simultaneously and about one week ahead to onset of radial growth in 2011 and 2012, respectively (Fig. 4c). Comparing growth resumption after winter dormancy among years, it is obvious that radial growth started about 2 weeks earlier in 2011 than 2012 in all species and at both study plots (Fig. 4a-c). Gompertz function fitted growth of different organs in all species and both study years quite well (Table 2).
Fig. 2.
a-f Intra-annual dynamics of needle (closed triangles), shoot (open triangles) and radial growth (dotted lines) in 2011 (black) and 2012 (grey) of Larix decidua (a, b), Pinus sylvestris (c, d) and Picea abies (e, f) at the rain-sheltered and control plot given in percent of final growth and modelled by applying the Gompertz function (for parameters see Table 2).
Fig. 3.
a-f Mean daily radial increment curves of Larix decidua (a, b), Pinus sylvestris (c, d) and Picea abies (e, f) in 2011 and 2012 modelled by applying the Gompertz function. Dendrometer records from the rain-sheltered and control plot are indicated in black and grey lines, respectively. Open circles indicate development of first horizontal row of enlarging tracheids.
Fig. 4.
a-c Critical phenological dates (onset = on; maximum daily growth = Ip; 90 % of final growth = end) and duration of needle, shoot and radial growth in Larix decidua (a), Pinus sylvestris (b) and Picea abies (c) in 2011 and 2012 at the rain-sheltered and control plot.
Table 2.
Parameters of the Gompertz-function for needle, shoot and diameter growth in 2011 and 2012 (C = control; D = rain-sheltered; N = needle growth, S = shoot growth, R = radial growth; Ip = inflection point, κ = rate of change parameter, doy = day of the year).
| Larix decidua | Pinus sylvestris | Picea abies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | Ip (doy) | κ | R2 | Ip (doy) | κ | R2 | Ip (doy) | κ | |
| D_N2011 | 0.998 | 85±0.5 | 0.120±0.006 | 0.982 | 132±1.6 | 0.036±0.004 | 0.986 | 126±0.7 | 0.160±0.022 |
| D_N2012 | 0.994 | 92±0.8 | 0.086±0.006 | 0.983 | 149±1.7 | 0.036±0.003 | 0.992 | 134±1.7 | 0.317±0.104 |
| D_S2011 | 0.990 | 127±0.8 | 0.075±0.007 | 0.991 | 101±0.8 | 0.070±0.004 | 0.998 | 131±0.3 | 0.115±0.006 |
| D_S2012 | 0.986 | 133±1.4 | 0.082±0.011 | 0.995 | 106±0.8 | 0.055±0.003 | 0.991 | 138±0.9 | 0.127±0.016 |
| D_R2011 | 0.661 | 144±2.3 | 0.091±0.025 | 0.887 | 136±1.2 | 0.060±0.006 | 0.850 | 145±1.5 | 0.055±0.007 |
| D_R2012 | 0.745 | 149±0.8 | 0.190±0.050 | 0.927 | 143±0.8 | 0.104±0.011 | 0.866 | 151±1.2 | 0.072±0.009 |
| C_N2011 | 0.996 | 90±0.7 | 0.103±0.007 | 0.994 | 153±1.3 | 0.034±0.003 | 0.980 | 126±0.6 | 0.264±0.063 |
| C_N2012 | 0.992 | 96±1.0 | 0.094±0.009 | 0.987 | 162±1.3 | 0.041±0.003 | 0.998 | 134±1.1 | 0.297±0.070 |
| C_S2011 | 0.993 | 129±0.7 | 0.082±0.007 | 0.996 | 112±0.6 | 0.041±0.002 | 0.994 | 133±0.6 | 0.113±0.011 |
| C_S2012 | 0.984 | 132±1.57 | 0.075±0.010 | 0.991 | 113±1.1 | 0.050±0.003 | 0.995 | 136±0.6 | 0.140±0.014 |
| C_R2011 | 0.865 | 147±1.3 | 0.087±0.013 | 0.947 | 144±0.9 | 0.062±0.005 | 0.958 | 146±0.7 | 0.060±0.004 |
| C_R2012 | 0.853 | 155±1.1 | 0.135±0.025 | 0.976 | 153±0.6 | 0.056±0.003 | 0.935 | 151±0.7 | 0.079±0.006 |
The maximum rate of radial growth in all trees peaked between 16 May and 26 May in 2011, and 22 May and 3 June in 2012, respectively, indicating low variability in temporal dynamics of wood formation among studied species and environmental conditions (Table 2). On the other hand, maximum needle elongation differed by c. 7 weeks between Larix decidua and Pinus sylvestris in both study years. Among study plots maximum needle growth differed mostly in Pinus sylvestris (2 – 3 weeks), whereby earlier maximum occurred at the rain-sheltered plot. In Larix decidua autumn needle senescence, i.e., widespread discoloration from green to yellow on the whole crown, was recorded in mid October in both study years, whereby at the rain-sheltered plot chlorophyll bleaching at this time was more pronounced. Date of maximum shoot elongation in Larix decidua and Picea abies varied between 7 May, in 2011, and 17 May, in 2012, respectively, at both study plots. In comparison, shoot growth in Pinus sylvestris peaked 2 – 3 and 3 – 4 weeks earlier at the control and rain-sheltered plot, respectively, in both study years.
Growth rate and growth duration of different organs distinctly differed among the three species (Fig. 4a-c, Table 2). With regard to needle and shoot growth Picea abies presented shortest time periods of growth and highest growth rates, while Pinus sylvestris showed longest durations and lowest growth rates. Time period of radial stem growth of Larix decidua was somewhat shorter, but occurred at higher growth rates as compared with the evergreen species. Mean growing season during study years lasted (i) from early March through mid-June in Larix decidua, i.e., c. 90 and 95 days at the rain-sheltered and control plot, respectively, (ii) from mid-March through early August in Pinus sylvestris, i.e., c. 115 and 130 days at the rain-sheltered and control plot, respectively, and (iii) from mid-April through mid July in Picea abies, i.e., c. 67 and 73 days at the rain-sheltered and control plot, respectively.
Discussion
It is well established that temperature strongly effects growth resumption after winter dormancy in plants (Körner, 2006; Menzel and Sparks, 2006; Lüttge and Hertel, 2009). Consistently, earlier onset of aboveground growth in 2011 compared to 2012 is related to exceptionally warm temperatures prevailing in spring 2011. On the other hand, soil water content dropped continuously from late March through early May 2011 on both plots, it is reasonable to assume that at the start of the growing season conifers consume water reserves in the root, stem and branches allowing onset of aboveground growth to occur irrespective of soil water content (cf. Holbrook, 1995; Grip and Hällgren, 2005). Swidrak et al. (2011) also reported that air temperature sum rather than precipitation triggers start of radial growth in Pinus sylvestris under drought, suggesting that water stored in sapwood is used for growth resumption in spring. Furthermore, the existence of a temperature threshold in the range of 5 – 7 °C above which significant tree growth occurs has been frequently reported (Rossi et al., 2008; Swidrak et al., 2011). Hence, if onset of meristem activity after winter dormancy is solely regulated by exceeding a specific temperature threshold, a simultaneous resumption of apical and radial growth in all species may be expected.
However, in our study growth resumption after winter dormancy differed by several weeks between early successional Larix decidua and Pinus sylvestris and late successional Picea abies. Our results suggest that strikingly delayed growth onset of late successional Picea abies compared to co-occurring species in both years and at both plots is due to constraints upon growth by the photoperiod, while growth resumption in early successional Larix decidua and Pinus sylvestris might be regulated by temperature only. This interpretation is consistent with studies by Partanen et al. (1998, 2001) and Mølmann et al. (2006), who found that rest break in Picea abies is affected by photoperiod and light quality. Körner and Basler (2010) also reported that growth onset of late successional tree species is commonly constrained by photoperiod threshold. Earlier onset of aboveground growth in Larix decidua and Pinus sylvestris also supports the hypothesis that pioneer species adopt riskier life strategies compared to late successional Picea abies (Körner, 2006). Furthermore, early successional species presented longer duration of apical growth and lower growth rates compared to late successional Picea abies. This different behaviour supports the hypothesis that early successional species are expending resources, while late successional species are more efficient in utilizing resources and develop safer life strategies (Körner, 2006; Boyden et al., 2009). Contrasting growth strategies adopted by co-occurring species were also detected in studies by Cuny et al. (2012) in a temperate coniferous forest in France and by Zhai et al. (2012) in a boreal forest in Canada. It has to be considered that deciduous leaf habit of Larix decidua requires early needle development to recommence carbon assimilation. On the other hand, it is a well-known phenomenon that needle elongation in pines is a long-lasting, slow process and considerable shoot extension occurs before onset of needle growth (Dougherty et al., 1994).
In this study we also found that in Larix decidua and Pinus sylvestris apical growth in the canopy recommenced several weeks earlier than lateral stem growth at breast height. On the other hand, quite synchronous onset of apical and lateral growth in Picea abies indicates that species-specific differences in regulation of growth onset within a tree exist. Additionally, site-specific environmental factors might be involved in influencing growth resumption of different organs, because Rossi et al. (2009) detected earlier reactivation of radial stem growth with respect to shoot growth in three conifers including Larix decidua at the Alpine timberline (2080 m a.s.l.), where temperature rather than precipitation is limiting tree growth (Körner, 2012). Similarly, in a boreal forest earlier onset of stem growth than shoot and needle growth was observed in Pinus banksiana by Zhai et al. (2012). On the other hand, similar to our observations Moser et al. (2010) found that along altitudinal gradients in the Swiss Alps needles in Larix decidua appeared 3-4 weeks before onset of radial stem growth occurred. Experimental studies are required to determine whether (i) different threshold temperatures for growth resumption in apical and lateral meristems exist (cf. Begum et al., 2013) or (ii) low water availability at the start of the growing season impairs auxin production, basipetal transport and/or stimulation of cambium activity, to explain delayed onset of radial stem growth compared to shoot growth found within the study area.
The synchronicity observed in the maximum rate of radial growth among species, which occurred between mid May through early June, indicates that timing of wood formation in co-occurring deciduous and evergreen conifers is controlled by the same external and/or endogenous factors. Rathgeber et al. (2011) and Cuny et al. (2012) reported similar results for Abies alba belonging to different crown classes within a close monospecific stand and for three conifer species (Pinus sylvestris, Picea abies, Abies alba) grown intermixed in a temperate forest, respectively. Results suggest that timing of maximum radial growth is influenced by temporal dynamics of needle and/or shoot growth, most likely because almost 50 % of the annual carbon gain is allocated to wood (Grote, 1998). That maximum radial growth in Larix decidua and Picea abies occurred several weeks after needle lengthening was completed is in accordance with findings of Hansen and Beck (1990) and Oribe et al. (2003) that wood formation mainly depends on current-year assimilates. In Pinus sylvestris, however, stored assimilates and/or new assimilates of needles from previous year(s) provided carbon for radial growth, because onset of radial growth preceded needle development. Temporary depletion of soluble carbohydrates after onset of radial growth in Pinus sylvestris corroborates that stored carbon is used in this species for earlywood formation within the study area (Oberhuber et al., 2011). Although dynamics of intra-annual apical and lateral growth differed strongly among the three conifers, maximum daily growth of all organs peaked in March through June in all species at both study plots. Because more favourable environmental conditions, i.e., increase in soil water content, was recorded at the control plot during summer, a pronounced internal competition in carbon allocation between above- and belowground sinks might exist. That in forests carbon partitioning to belowground is high at low resource availability was stated by Litton et al. (2007). Furthermore, it is a well-known phenomenon that increased carbon allocation to roots and mycorrhiza occur in response to drought (McDowell et al., 2008). Because long-term climate/tree growth relationships investigated in the study area also revealed drought sensitivity of all three species (Schuster and Oberhuber, 2013 and references therein), we suggest that the early decrease in aboveground growth is an adaptation to cope with extreme environmental conditions, i.e., low water availability prevailing within the study area, which requires increased carbon allocation to belowground organs. Low priority of aboveground growth is also indicated in our study by a distinct reduction of radial growth in all species and in both study years in response to artificial induced drought. However, we are aware of shortcomings of our study, i.e., a comparison of shoot, needle and radial growth among the rain-sheltered and control plot is precluded due to missing sample depth. On the other hand, results revealed earlier maximum rate of needle, shoot and radial growth in Larix decidua and Pinus sylvestris and earlier cessation of aboveground growth in Pinus sylvestris at the rain-sheltered vs. control plot in both study years. Because there are several reports that duration of wood formation and shoot extension in conifers is adversely affected by water deficits in summer (Dougherty, 1994; Thabeet et al., 2009; Pichler and Oberhuber, 2007; Eilmann et al., 2011), it can be deduced that early successional species are less efficient in water uptake in mixed stands where Picea abies co-occurs. We suggest that the shallow-rooting Picea abies is highly adapted to absorb scattered low rainfall events prevailing within the study area (Schmid and Kazda, 2002). However, if evaporative demand increases with predicted climate warming, Pinus sylvestris will benefit from declining water availability due to high adaptability to drought-prone conditions and outcompete Picea abies at dry-mesic sites. Minimal radial growth rates detected in Larix decidua at both plots corroborate findings of Eilmann and Rigling (2012) that Larix decidua is maladjusted to dry conditions, which is possibly related to its deciduous habit and/or anisohydric strategy, i.e., high transpiration rates are maintained under drought (Schuster et al., unpublished) causing finally impairment of tree water status (Breda et al., 2006).
The consistently moderate response of apical and lateral growth and absence of significant lag effects to artificial induced drought is most likely due to water absorption from fine roots which reached beyond the rain shield, which may have increasingly spread to better soil water availability outside the rain exclusion area. On the other hand, considering (i) the utilization of water stored in the stem prior to construction of the rain shield for growth resumption, and that (ii) a low amount of precipitation early during the growing season is the primary climatic factor limiting radial growth of all three conifer species within the study area (Schuster and Oberhuber, 2013) and (iii) maximum growth rates peaked before more favourable, i.e., moister conditions prevailed during summer, results indicate that tree water status early during the growing season determines total annual aboveground growth to a large extent. This reasoning is supported by Rossi et al. (2012), who found that climatic variables, which affect the onset of xylem differentiation in spring influence tree-ring formation and Leo et al. (2013), who reported that roofing within the study area significantly reduced plant water availability throughout the growing season as indicated in lower predawn needle water potentials and reduction in sap flow density in roofed as compared to control trees.
Taken together our results suggest that different interactions of endogenous (phytohormonal regulation, carbon allocation) and environmental factors (temperature, photoperiod, drought) are involved in controlling seasonal dynamics of apical and lateral meristems in conifers exposed to drought. Functionally oriented studies are needed as the next step, to disentangle in detail the influence of these factors on temporal growth dynamics and the relationship among apical and lateral growth in trees.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF), project number P22280-B16 “Conifer radial stem growth in response to drought”. We thank Sylvia Farbmacher for help with recording growth phenology in 2011. We also thank the editor-in-chief Prof. (retired) Dr. Rainer Lösch and two anonymous reviewers for their valuable suggestions and comments to improve the manuscript.
References
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg E.H. (Ted), Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim J-H, Allard G, Running SW, Semerci A, Cobb N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 2010;259:660–684. [Google Scholar]
- Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R. Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013;147:46–54. doi: 10.1111/j.1399-3054.2012.01663.x. [DOI] [PubMed] [Google Scholar]
- Bernal M, Estiarte M, Peñuelas J. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biol. 2011;13:252–257. doi: 10.1111/j.1438-8677.2010.00358.x. [DOI] [PubMed] [Google Scholar]
- Borchert R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology. 1994;75:1437–1449. [Google Scholar]
- Boyden SB, Reich PB, Puettmann KJ, Baker TR. Effects of density and ontogeny on size and growth ranks of three competing tree species. J. Ecol. 2009;97:277–288. [Google Scholar]
- Breda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adapation processes and long-term consequences. Ann. For. Sci. 2006;63:625–644. [Google Scholar]
- Cannell MGR, Smith RI. Climatic warming, spring budburst and frost damage on trees. J. Appl. Ecol. 1986;23:177–191. [Google Scholar]
- Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007;22:357–365. doi: 10.1016/j.tree.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Cuny HE, Rathgeber CBK, Lebourgeois F, Fortin M, Fournier M. Life strategies in intra-annual dynamics of wood formation: example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012;32:612–625. doi: 10.1093/treephys/tps039. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Can. J. For. Res. 2003;33:190–200. [Google Scholar]
- Dougherty PM, Whitehead D, Vose JM. Environmental influences on the phenology of pine. Ecol. Bull. 1994;43:64–75. [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Pannatier EG, Rigling A. Drought alters timing, quantity and quality of wood formation in Scots pine. J. Exp. Bot. 2011;62:2763–2771. doi: 10.1093/jxb/erq443. [DOI] [PubMed] [Google Scholar]
- Eilmann B, Rigling A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012;32:178–187. doi: 10.1093/treephys/tps004. [DOI] [PubMed] [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 2006. (World Soil Resources Reports, Vol. 103). [Google Scholar]
- Funada R, Kubo T, Sugiyama T, Fushitani M. Changes in levels of endogenous plant hormones in cambial regions of stems of Larix kaempferi at the onset of cambial activity in springtime. J. Wood Sci. 2002;48:75–80. [Google Scholar]
- Gonzalez-Benecke CA, Martin TA, Clark A, III, Peter GF. Water availability and genetic effects on wood properties of loblolly pine (Pinus taeda) Can. J. For. Res. 2010;40:2265–2277. doi: 10.1093/treephys/tpp118. [DOI] [PubMed] [Google Scholar]
- Grip H, Hällgren J-E. Water cycling in coniferous forest ecosystems. In: Andersson F, editor. Ecosystems of the World. Vol. 6, Coniferous forests. Elsevier; Amsterdam: 2005. pp. 385–426. [Google Scholar]
- Grote R. Integrating dynamic morphological properties into forest growth modelling-II. Allocation and mortality. For. Ecol. Manage. 1998;111:193–210. [Google Scholar]
- Gruber A, Zimmermann J, Wieser G, Oberhuber W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 2009;66:503. doi: 10.1051/forest/2009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen H. Does climate warming increase the risk of frost damage in northern trees? Plant Cell Environ. 1991;14:449–454. [Google Scholar]
- Hänninen H, Tanino K. Tree seasonality in a warming climate. Trends Plant Sci. 2011;16:412–416. doi: 10.1016/j.tplants.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Hansen J, Beck E. The fate and path of assimilation products in the stem of 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees. 1990;4:16–21. [Google Scholar]
- Holbrook NM. Stem water storage. In: Gartner BL, editor. Plant stems: Physiology and functional morphology. Academic Press; San Diego: 1995. pp. 151–175. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the IPCC. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Körner C. Significance of temperature in plant life. In: Morison JIL, Morecroft MD, editors. Plant growth and climate change. Blackwell Publishing Ltd; Oxford: 2006. pp. 48–69. [Google Scholar]
- Körner C, Basler D. Phenology under global warming. Science. 2010;327:1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine treelines. Functional ecology of the global high elevation tree limits. Springer; Basel: 2012. [Google Scholar]
- Leo M, Oberhuber W, Schuster R, Grams TEE, Matyssek R, Wieser G. Evaluating the effect of plant water availability on inner-alpine coniferous trees based on sap flow measurements. Eur. J. For. Res. 2013 in press. [Google Scholar]
- Linkosalo T, Hakkinen R, Hanninen H. Models of the spring phenology of boreal and temperate trees: is there something missing? Tree Physiol. 2006;26:1165–1172. doi: 10.1093/treephys/26.9.1165. [DOI] [PubMed] [Google Scholar]
- Litton CM, Raich JW, Ryan MG. Carbon allocation in forest ecosystems. Glob. Chang. Biol. 2007;13:2089–2109. [Google Scholar]
- Lüttge U, Hertel B. Diurnal and annual rhythms in trees. Trees. 2009;23:683–700. [Google Scholar]
- McDowell NG, Pockman W, Allen C, Breshears D, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams D, Yepez EA. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb? New Phytol. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Menzel A, Sparks T. Temperature and plant development: phenology and seasonality. In: Morison JIL, Morecroft MD, editors. Plant growth and climate change. Blackwell Publishing Ltd; Oxford: 2006. pp. 70–95. [Google Scholar]
- Michelot A, Simard S, Rathgeber C, Dufrêne E, Damesin C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012;32:1033–1043. doi: 10.1093/treephys/tps052. [DOI] [PubMed] [Google Scholar]
- Mølmann JA, Juntilla O, Johnsen Ø, Olsen JE. Effects of red, far-red and blue light in maintaining growth in latitudinal populations of Norway spruce. Plant Cell Environ. 2006;29:166–172. doi: 10.1111/j.1365-3040.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- Moser L, Fonti P, Büntgen U, Esper J, Luterbacher J, Franzen J, Frank D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010;30:225–233. doi: 10.1093/treephys/tpp108. [DOI] [PubMed] [Google Scholar]
- Oberhuber W, Swidrak I, Pirkebner D, Gruber A. Temporal dynamics of non-structural carbohydrates and xylem growth in Pinus sylvestris exposed to drought. Can. J. For. Res. 2011;41:1590–1597. doi: 10.1139/x11-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localisation of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees. 2003;17:185–192. [Google Scholar]
- Partanen J, Koski V, Hänninen H. Effects of photoperiod and temperature on the timing of bud burst in Norway spruce (Picea abies) Tree Physiol. 1998;18:811–816. doi: 10.1093/treephys/18.12.811. [DOI] [PubMed] [Google Scholar]
- Partanen J, Leinonen I, Repo T. Effect of accumulated duration of the light period on bud burst in Norway spruce (Picea abies) of varying ages. Silva Fenn. 2001;35:111–117. [Google Scholar]
- Peñuelas J, Filella I, Zhang X, Llorens L, Ogaya R, Lloret F, Comas P, Estiarte M, Terradas J. Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytol. 2004;161:837–846. doi: 10.1111/j.1469-8137.2004.01003.x. [DOI] [PubMed] [Google Scholar]
- Pichler P, Oberhuber W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manage. 2007;242:688–699. [Google Scholar]
- Polgar CA, Primack RB. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytol. 2011;191:926–941. doi: 10.1111/j.1469-8137.2011.03803.x. [DOI] [PubMed] [Google Scholar]
- Rathgeber CBK, Rossi S, Bontemps J-D. Cambial activity related to tree size in a mature silver-fir plantation. Ann. Bot. 2011;108:429–438. doi: 10.1093/aob/mcr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Morin H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 2003;21:33–39. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 2006;27:89–97. [Google Scholar]
- Rossi S, Deslauriers A, Gričar J, Seo J-W, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008;17:696–707. [Google Scholar]
- Rossi S, Rathgeber CBK, Deslauriers A. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann. For. Sci. 2009;66:206. [Google Scholar]
- Rossi S, Morin H, Deslauriers A. Causes and correlations in cambium phenology: towards an integrated framework of xylogenesis. J. Exp. Bot. 2012;63:2117–2126. doi: 10.1093/jxb/err423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge RA. Auxin and ethylene regulation of diameter growth in trees. Tree Physiol. 1988;4:401–414. doi: 10.1093/treephys/4.4.401. [DOI] [PubMed] [Google Scholar]
- Schmid I, Kazda M. Root distribution of Norway spruce in monospecific and mixed stands on different soils. For. Ecol. Manage. 2002;159:37–47. [Google Scholar]
- Schuster R, Oberhuber W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees. 2013;27:61–69. doi: 10.1007/s00468-012-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg B, Uggla C. Origin and dynamics of indoleacetic acid under polar transport in Pinus sylvestris. Physiol. Plant. 1998;104:22–29. [Google Scholar]
- Swidrak I, Gruber A, Kofler W, Oberhuber W. Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiol. 2011;31:483–493. doi: 10.1093/treephys/tpr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabeet A, Vennetier M, Gadbin-Henry C, Denelle N, Roux M, Caraglio Y, Vila B. Response of Pinus sylvestris to recent climatic events in the French Mediterranean region. Trees. 2009;23:843–853. [Google Scholar]
- Wareing PF. Photoperiodism in woody plants. Annu. Rev. Plant Physiol. 1956;7:191–214. [Google Scholar]
- Zeide B. Analysis of growth equations. For. Sci. 1993;39:594–616. [Google Scholar]
- Zhai L, Bergeron Y, Huang J-G, Berninger F. Variation in intra-annual wood formation, and foliage and shoot development of three major Canadian boreal tree species. Am. J. Bot. 2012;99:827–837. doi: 10.3732/ajb.1100235. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Häsler R. Dynamics of water storage in mature subalpine Picea abies: temporal and spatial patterns of change in stem radius. Tree Physiol. 2001;21:561–569. doi: 10.1093/treephys/21.9.561. [DOI] [PubMed] [Google Scholar]