Abstract
An enzymatically activatable prodrug of doxorubicin was covalently coupled, using click-chemistry, to the hydrophobic core of poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl)-methacrylamide-lactate] micelles. The release and cytotoxic activity of the prodrug was evaluated in vitro in A549 non-small-cell lung cancer cells after adding β-glucuronidase, an enzyme which is present intracellularly in lysosomes and extracellularly in necrotic areas of tumor lesions. The prodrug-containing micelles alone and in combination with standard and β-glucuronidase-producing oncolytic vaccinia viruses were also evaluated in vivo, in mice bearing A549 xenograft tumors. When combined with the oncolytic viruses, the micelles completely blocked tumor growth. Moreover, a significantly better antitumor efficacy as compared to virus treatment alone was observed when β-glucuronidase virus treated tumor-bearing mice received the prodrug-containing micelles. These findings show that combining tumor-targeted drug delivery systems with oncolytic vaccinia viruses holds potential for improving anticancer therapy.
Introduction
The administration of anticancer agents is generally associated with dose-limiting side effects. Many different drug delivery systems have been proposed to enhance the biodistribution and the tumor accumulation of chemotherapeutic drugs, and to thereby improve the balance between their efficacy and their toxicity 1-8. In recent years, polymeric micelles based on poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl)-methacrylamide-lactate] (mPEG-b-pHPMAmLacn) have been investigated as biodegradable carriers of hydrophobic drugs, and have shown significant potential 9-14.
Doxorubicin (DOX) is a potent anticancer drug and commonly used as a model chemotherapeutic. However, its use is limited by severe cardiotoxicity 15. For this reason, prodrug therapies have been proposed to increase the therapeutic index of DOX. We recently reported the covalent coupling of a synthetic derivative, DOX-propGA3, to the azide-modified core of mPEG-b-pHPMAmLac2 (mPEG-b-p(HPMAmLac2-co-AzEMA) micelles 16. This prodrug showed much less toxicity as compared to the parent drug before two-step activation by 1) chemical hydrolysis of the propargylic ester and 2) conversion to DOX by the enzyme β-glucuronidase. This enzyme is normally present in the lysosomes of (cancer) cells and is released into the extracellular compartment of tumors upon necrosis 17-20.
Oncolytic virotherapy is a re-emerging therapeutic concept that takes advantage of the tumor-specific replication of viruses. Among the different viruses tested thus far, genetically engineered vaccinia virus strains are among the most promising candidates 21, 22. These viruses display high infectivity and a fast replication cycle, and lead to cell lysis, immune-mediated cell death and vascular collapse within the tumor 23. In a number of studies, it was shown that recombinant vaccinia viruses (rVACV) derived from the Lister-strain are effective in treating different solid tumor types (reviewed in 21). Monitoring of the successful tumor colonization and the therapeutic effects can be performed by different imaging modalities, including optical 24, optoacoustic 25, PET-26, 27, and by MR-imaging 25. Reporter gene-encoding rVACV can therefore be considered theranostic agents 28-30.
In addition, vaccinia virus-mediated oncolytic therapy has been combined with radiotherapy 31, 32 as well as with chemotherapy 33. Moreover, the therapeutic effect can be enhanced by vaccinia virus-mediated expression of e.g. anti-angiogenic single-chain antibodies 34 or of prodrug-converting enzymes for so-called gene-directed enzyme prodrug therapy (GDEPT) 35. GDEPT and ADEPT (antibody-directed enzyme prodrug therapy) have shown enhanced antitumor efficacy in experimental tumor models in combination with glucuronide prodrugs 36.
Here, we evaluated the performance of doxorubicin-prodrug-loaded mPEG-b-p(HPMAmLac2-co-AzEMA) micelles (pg-micelles) against A549 human non-small-cell lung adeno-carcinoma cells (NSCLC) in vitro and in vivo. The antitumor efficacy of pg-micelles versus free DOX was investigated in combination with oncolytic vaccinia virotherapy. Additionally, the influence of virus-encoded overexpression of bacterial β-glucuronidase on the conversion of the prodrug was assessed.
Materials and Methods
Synthesis of DOX-propGA3-containing mPEG-b-p(HPMAmLac2-co-AzEMA) copolymers
The synthesis of the azide-modified copolymer and its covalent linkage to DOX-propGA3 via click-chemistry were performed as in 16. In the present study, 80 mol % HPMAmLac2 and 20 mol % AzEMA were polymerized using (mPEG5000)2-ABCPA as macroinitiator. For the coupling of the prodrug, DOX-propGA3 and the polymer (5% w/w ratio of DOX-propGA3 to polymer) were dissolved in dimethylformamide. Next, CuSO4 (1 eq to DOX-propGA3) and sodium ascorbate (1 eq to DOX-propGA3) dissolved in H2O were added, and the mixture was stirred for 48 h under a nitrogen atmosphere. The resulting prodrug conjugate was isolated by precipitation in diethyl ether, dissolved in H2O, dialyzed (membrane cut-off 12-14 kDa) against water for 24 h, and finally recovered by freeze-drying.
Preparation and characterization of pg-micelles
The lyophilized prodrug copolymer conjugate (29 μg DOX-equivalents/mg) was dissolved in PBS (25 mg/ml) overnight at 0°C on an orbital shaker. The solution was then incubated in pre-heated (50°C) water for 60 s to induce the formation of micelles 37, and subsequently cooled down to room temperature.
Dynamic light scattering (DLS) measurements were performed using a Malvern CGS-3 multiangle goniometer (Malvern Ltd., Malvern, U.K., with a JDS Uniphase 22 mW He-Ne laser operating at 632 nm, an optical fiber-based detector and a digital LV/LSE-5003 generator, measurement angle 90°). The autocorrelation function was analyzed by the cumulants method (fitting a single exponential to the correlation function to obtain the mean size and PDI) and the CONTIN routine (fitting a multiple exponential to the correlation function to obtain the distribution of particle size). Transmission electron microscopy (TEM) was carried out on the prodrug-loaded micelles using a Philips Tecnai 12 microscope equipped with a Biotwin-lens and a LaB6 filament, operated at 120 kV acceleration voltage. Negative uranyl acetate staining (2%) and Glow Discharged grids (copper 200 mesh grid with a carbon-coated thin polymer film, Formvar on top) were used for sample preparation. Images were captured with a SIS Megaview II CCD-camera and processed with AnalySIS software.
DOX release from pg-micelles (in PBS, pH 7.4, also containing 0.1% w/v bovine serum albumin, at 37°C) was monitored in the presence and absence of 20 μg/mL β-glucuronidase (from bovine liver, type B-10, 10,000 units/mg, Sigma-Aldrich Co., Germany). The concentration of DOX-GA3 and DOX in the release medium was measured over time with gradient HPLC chromatography, using a C18 Sunfire column and potassium phosphate buffer pH 3 (20 mM) with 5% acetonitrile as eluent A (75-60% in 12 minutes) and 100% acetonitrile as eluent B (excitation wavelength 480 nm, emission wavelength 560 nm).
In vitro cytotoxicity assay
A549 NSCLC were seeded at 5,000 cells/cm2 in DMEM (4.5 g/L glucose), 100 U/mL penicillin G, 100 U/mL streptomycin, 10% fetal bovine serum (FBS) and incubated at 37°C, 5% CO2. Cells were exposed to DOX, liposomal DOX (DPPC:Cholesterol:DSPE-PEG2000 molar composition 1.85:1:0.15, with comparable characteristics to commercial formulation Doxil®), pg-micelles or empty micelles at serial dilutions ranging from 0.001 to 250 μg/ml (DOX-equivalents). Cells were incubated with the micellar dispersions with or without the addition of 20 μg/mL β-glucuronidase. For the in vitro cytotoxicity assays, cells were harvested after 3 days and cell viability was determined by flow cytometry with 7AAD (Biolegends, San Diego, CA) using the FACSCanto II flow cytometer and the FACSDiva software v6.1.2 (BD Biosciencies, San Jose, CA).
Recombinant vaccinia virus strains
The glucuronidase encoding rVACV GLV-1h68 has been described previously 38 as well as the glucuronidase-negative control rVACV GLV-1h190 39 which encodes TurboFP635 instead of the glucuronidase gene.
Animal experiments
Male athymic nude mice (Harlan, Livermore, CA, USA) were subcutaneously injected on their right flank with 5×106 A549 cells 30 days (day -30) before DOX or pg-micelles were administered. Fifteen days later (day -15), mice were injected into the retroorbital sinus vein with PBS or 5×106 pfu of GLV-1h190 and GLV-1h68, respectively. On day 0, mice received injections of PBS (controls), DOX or pg-micelles as treatment, which were repeated on days 5, 10, 16 and 23. For each treatment, 3 mg/kg DOX-equivalents (free DOX or pg-micelles) were intravenously injected into the lateral tail vein, up to DOX maximum tolerated dose (5 × 3 mg/kg) 10. Tumor volumes were calculated from digital caliper measurements (0.5 × length × width2) and normalized to those on the last day prior to DOX injection. All animal experiments were carried out in compliance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Explora BIOLABS (protocol number EB11-025).
Glucuronidase assay
Determination of glucuronidase activity was performed with minor variations according to Hess et al. 40. Briefly, tumors were isolated 26 days after the first DOX treatment, weighted, snap-frozen in liquid nitrogen and stored at −80°C. For the glucuronidase assay, tumors were thawed and lysed in saline (2 ml/g tumor tissue) using gentleMACS M Tubes (Miltenyi Biotec, Bergisch-Gladbach, Germany) in a gentleMACS dissociator (Miltenyi Biotec). In black 384-well plates with a clear bottom, 5 μl of the lysate was analyzed in a total assay volume of 80 μl containing 2.5 μg Fluorescein Di-β-D-glucuronide (FDGlcU). The analysis was performed in PBS (pH 7.4) and in acetate buffer (1 M ammonium acetate and 1.2 M acetic acid in dH2O; pH 4.5) respectively, each supplemented with 2% FBS. After 1 h incubation at 37°C, the fluorescence signal was read using an Infinite 200 Pro Microplate Reader (Tecan, Crailsheim, Germany; excitation wavelength: 489 nm with 9 nm bandwidth, emission wavelength: 520 nm with 20 nm bandwidth, gain: 75). Results are shown as relative fluorescent units (RFU).
Statistical analysis
Results are presented as average ± standard deviation. Statistical analysis was performed using GraphPad Prism 5.01, employing the two-tailed student’s t-test. A p-value < 0.05 was considered to represent statistical significance.
Results and discussion
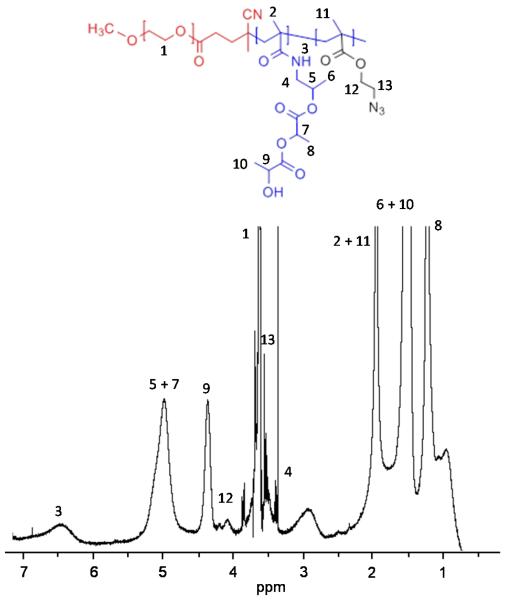
The block copolymer mPEG-b-p(HPMAmLac2-co-AzEMA) was synthesized by free radical polymerization of the HPMAmLac2 and AzEMA monomers with a PEG-based macroinitiator 16. The molar feed ratio of HPMAmLac2 and AzEMA was 80:20, and in the obtained copolymer the ratio was 92:8, as calculated from the 1H-NMR analysis shown in Fig. 1. Thus, 40 mol % of the AzEMA monomer in the feed was built in the polymer.
Figure 1.
1H-NMR spectrum of mPEG-b-p(80%HPMAmLac2-co-20%AzEMA) in CDCl3.
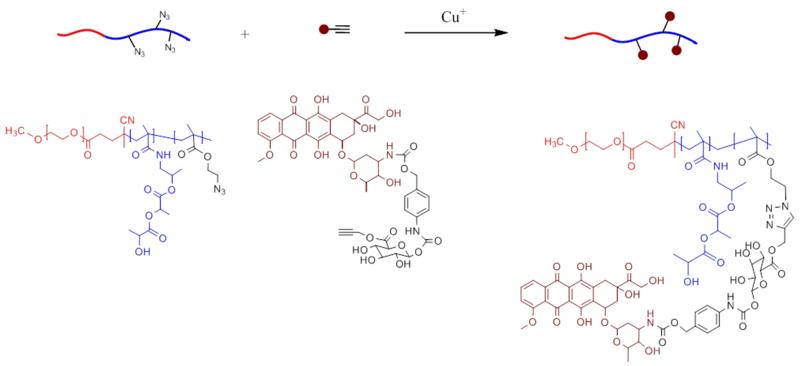
The azide-modified polymer was reacted with DOX-propGA3 41 by click-chemistry following a Cu(I)-catalyzed azide-alkyne cycloaddition 42, as schematized in Fig. 2. The molecular weights, Mw (27.0 kDa) and Mn (12.5 kDa), were determined by GPC 43, giving rise to a polydispersity index (Mw/Mn) of 2.2. A high percentage of prodrug conjugation (90%) was obtained for an azide/DOX-propGA3 molar ratio of 3.9:1.
Figure 2.
Schematic representation of the azide-alkyne click-coupling of mPEG-b-p(HPMAmLac2-co-AzEMA) polymer with DOX-propGA3.
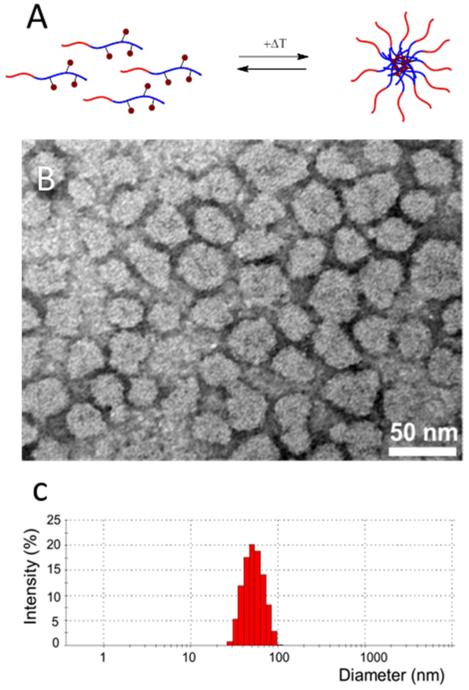
The prodrug-conjugated polymer was soluble in water at 0 °C and had a critical micelle temperature (CMT) of 2 °C 43. Above the CMT, the thermosensitive block of the polymer, p(HPMAmLac2-co-AzEMA), becomes hydrophobic and consequently micelles are formed upon rapid heating to 50 °C. Fig. 3A depicts the reversible formation of micelles with the prodrug localized within the hydrophobic core. The spherical morphology of the micelles was visualized by TEM (Fig. 3B), and their hydrodynamic diameter (Zavg: 51 nm) and polydispersity (PDI: 0.064) were determined using DLS (Fig. 3C).
Figure 3.
Schematic representation of the formation of micelles upon rapid heating of prodrug-containing block copolymers (A). TEM observation (B) and hydrodynamic size distribution of DOX-GA3-containing PEG-pHPMAm-based polymeric micelles (C).
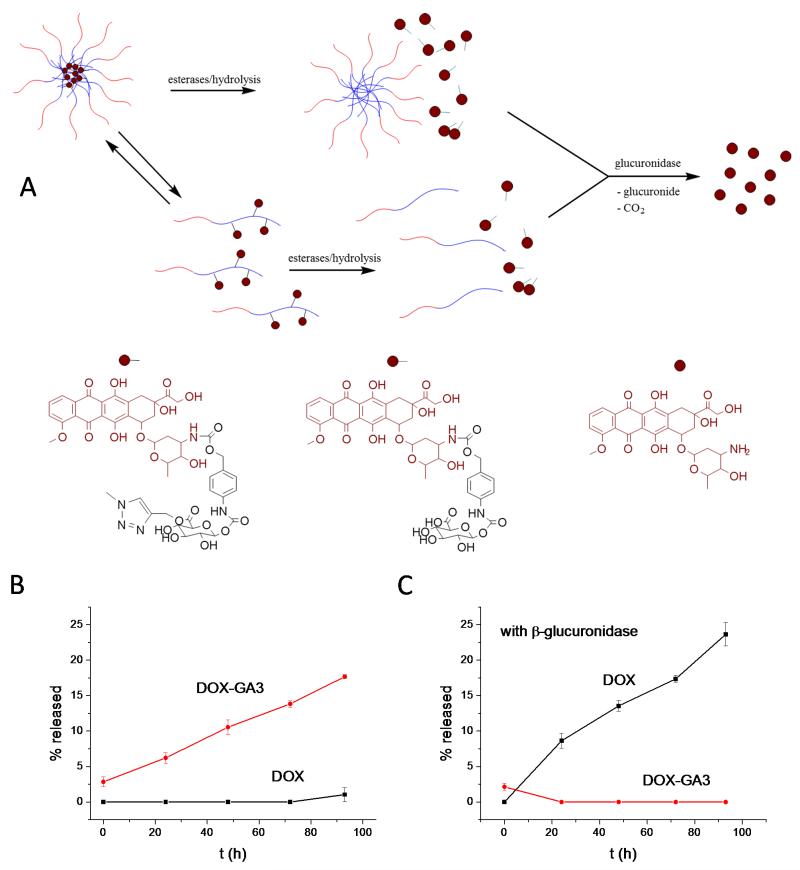
According to the scheme depicted in Fig. 4A, DOX-propGA3 is firstly hydrolyzed and released from the core of the micelles or from (free) polymer chains as an intermediate prodrug of DOX, DOX-GA3. This compound is a substrate for β-glucuronidase, which converts it into free DOX after cleavage of the glucuronide spacer 44. Consequently, in the absence of enzyme, only DOX-GA3 is progressively released from the micelles (as detected by HPLC; Fig. 4B), and hardly any conversion of DOX-GA3 to free DOX takes place. When the same experiment is performed in the presence of glucuronidase (20 μg/mL), DOX-GA3 is rapidly and efficiently converted into free DOX (Fig. 4C). The very similar prodrug release (Fig. 4B) and conversion kinetics (Fig. 4C) show that DOX-GA3 release from the micelles via hydrolysis, rather than DOX generation via enzymatic cleavage, is the rate-limiting step in drug activation in the presence of enzyme.
Figure 4.
Schematic representation of the two-step activation of DOX by 1) the hydrolysis of DOX-propGA3 and concomitant release of DOX-GA3 from micelles or free polymer-prodrug conjugates (which exist in a dynamic equilibrium), and 2) enzymatic conversion of DOX-GA3 into free DOX (A). Release profiles of DOX-GA3 and DOX from PEG-pHPMAm-based polymeric micelles in the absence (B) and presence (C) of β-glucuronidase.
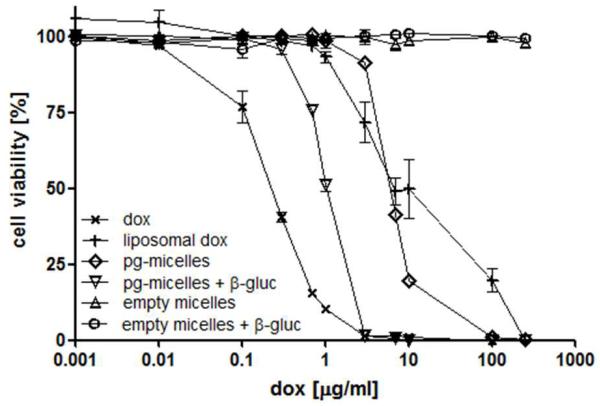
The cytotoxicity of pg-micelles was tested in A549 cells (Fig. 5). Empty micelles, with and without the addition of β-glucuronidase in the culture medium, did not affect cell viability in the concentration range tested (0.034-8.600 μg polymer/mL). The prodrug-loaded micelles (0.001 to 250 μg/ml DOX-equivalents), on the other hand, displayed significant cytotoxicity, in particular in the presence of β-glucuronidase (6-fold higher cytotoxicity as compared to absence of enzyme: IC50 6.5 vs. 1.1 μg DOX-equivalents/mL, respectively; versus 0.2 μg/mL for free DOX). The IC50 of liposomal DOX (6.7 μg/mL) was comparable to that of prodrug-loaded micelles in the absence of β-glucuronidase, which is in line with the notion that DOX release from liposomes is relatively slow 45.
Figure 5.
In vitro efficacy of free doxorubicin (dox), liposomal doxorubicin, empty micelles and doxorubicin-prodrug-containing micelles (pg-micelles) in the presence and absence of β-glucuronidase in A549 human NSCLC cells. Concentrations are plotted as doxorubicin-equivalent concentrations. Empty micelles were used at the same polymer concentrations as those of pg-micelles, ranging from 0.034 to 8,600 μg/mL.
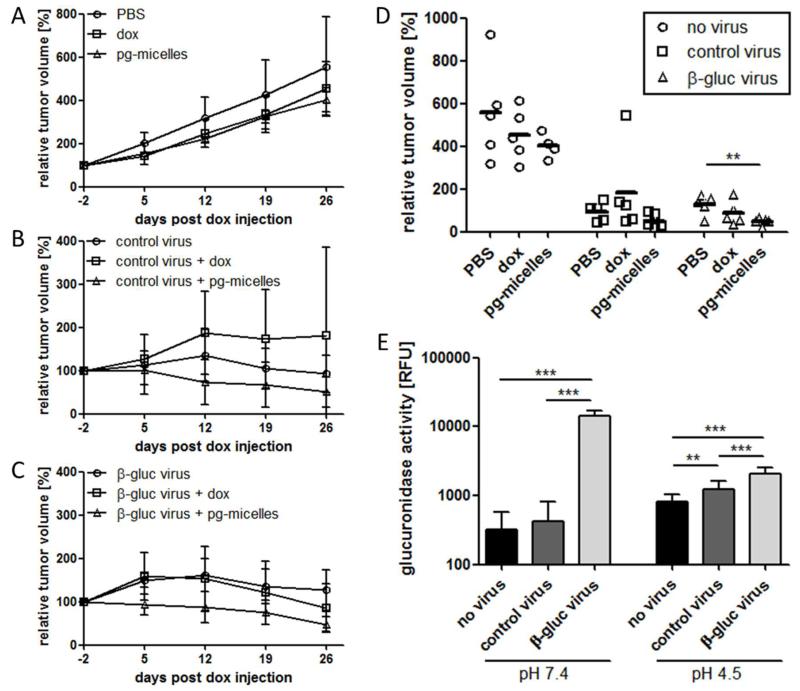
As expected from previous studies 46, the injection of free DOX into A549 tumor-bearing mice slightly slowed down tumor growth (Fig. 6A and 6D). Without virus co-treatment, similar antitumor efficacy was observed for pg-micelles, which indicates that the micelles accumulate to some extent in tumors via EPR (Enhanced Permeation and Retention) 47, 48, and are partially activated by endogenous levels of β-glucuronidase within tumors and tumor cells (lysosomes). When combined with control (GLV-1h190) and β-glucuronidase-producing (GLV-1h68) vaccinia viruses, the pg-micelles turned out to be more efficient than the free drug. It should be taken into account in this regard, however, that at the viral doses used (5×106 pfu), both the control and the enzyme-expressing virus were already highly effective in inhibiting tumor growth: as exemplified by Fig. 6B-D, even in the absence of DOX- or pg-micelle-cotreatment, tumor volumes never exceeded 200%, confirming the strong oncolytic potential of vaccinia viruses 21-27. Interestingly, when combined with free DOX, the oncolytic activity of GLV-1h190 was somewhat reduced (Fig. 6B), but this seems largely due to one non-responder in this group (Fig. 6D). Conversely, when combined with pg-micelles, there was a tendency toward improved efficacy as compared to viral treatment alone (Fig. 6B and 6D), which manifested in a complete inhibition of tumor growth (i.e. never exceeding 100%; Fig. 6B). As expected, this effect is somewhat more pronounced upon combining the pg-micelles with the β-glucuronidase-producing virus (Fig. 6C and 6D), resulting in significantly smaller tumor volumes as compared to virus treatment alone (p<0.01; Fig. 6D).
Figure 6.
In vivo efficacy of doxorubicin-prodrug-loaded PEG-pHPMAm-based micelles and free doxorubicin alone (A), and in combination with control (B) and β-glucuronidase-producing oncolytic vaccinia viruses (C), in nude mice bearing A549 NSCLC xenograft tumors. Individual relative tumor volumes at the last day of follow-up (day 26), showing significantly improved efficacy upon combining pg-micelles with β-glucuronidase-producing viruses (D). β-glucuronidase activity levels determined at pH 7.4 and pH 4.5 in lysates from tumors treated with PBS, control virus, and β-glucuronidase-producing virus (E). ** indicates p<0.01 and *** indicates p<0.005.
To verify that this enhanced efficacy is due to virus-mediated expression of the enzyme, we also assessed the glucuronidase activity levels in the tumors. As shown in the left panel in Fig. 6E, analysis in PBS (pH 7.4) revealed that the glucuronidase activity in GLV-1h68-treated tumors was more than 30-fold higher than in GLV-1h190- and PBS-treated tumors. This confirms successful tumor colonization by GLV-1h68 and efficient production of the enzyme. To discriminate between the GLV-1h68-mediated production of bacterial β-glucuronidase and the release of human β-glucuronidase from A549 cells upon (virus-induced) tumor necrosis, enzyme activity levels were also evaluated at pH 4.5. The right panel in Fig. 6E clearly demonstrates in this regard that the high enzyme activity observed at pH 7.4 is caused by virus-mediated enzyme production (since bacterial β-glucuronidase is more active at neutral than at acidic pH 49). Under control conditions, on the other hand, i.e. upon PBS and GLV-1h190 treatment, the levels of enzyme activity were higher at acidic than at neutral pH (note that human β-glucuronidase has a pH-optimum at 4.8 49). The fact that enzyme activity at pH 4.5 was higher in GLV-1h190-treated than in PBS-treated animals (right panel in Fig. 6E) explains, at least partially, why pg-micelles were also effective upon treatment with control viruses (Fig. 6B), likely causing tumor necrosis and thereby resulting in the release of human β-glucuronidase from A549 cells. The observation that the overall levels of enzyme activity, both at pH 7.4 and 4.5, are higher upon GLV-1h68-treatment than upon GLV-1h190- and PBS-treatment is in line with the notion that the combination of GLV-1h68 with pg-micelles appeared to be the most efficient treatment for inhibiting tumor growth (Fig. 6D). Regarding the higher activity of bacterial β-glucuronidase at pH 7.4, it should be noted that since micelles are poorly internalized 50 and virus-encoded β-glucuronidase is released from the tumor cells upon tumor cell lysis 40, it is expected that part of the prodrug is activated in the extracellular space having a pH close to neutral.
In summary, we here provide proof-of-principle for combining DOX-prodrug-loaded polymeric micelles with standard and β-glucuronidase-producing oncolytic vaccinia viruses, resulting in significantly improved tumor growth inhibition as compared to virotherapy alone. Even though further optimization of the timing and dosing of virotherapy and tumor-targeted drug delivery is necessary, these findings demonstrate the potential of combined viro-nano-therapy.
Conclusions
A doxorubicin-glucuronide (DOX-propGA3) prodrug has been covalently coupled, via click chemistry, to mPEG-b-p(HPMAmLac2-co-AzEMA) thermosensitive block copolymers, which upon rapid heating in aqueous solution form micelles with a size of ~50 nm and with low polydispersity. The enzyme-responsive activation of DOX was demonstrated in vitro in A549 cells in the presence of β-glucuronidase, which is expressed in lysosomes and in necrotic tumor areas. As compared to the free drug, in combination with oncolytic vaccinia viruses, prodrug-loaded micelles led to a complete inhibition of tumor growth, and were significantly more effective than treatment with the viruses alone. These proof-of-principle findings indicate that the combination of tumor-targeted drug delivery with oncolytic virotherapy holds significant potential.
Acknowledgements
This work was financially supported by the European Union (FP7 Marie Curie Intra-European Fellowship for Career Development, project NanoSmart, to ERH; and FP6 MediTrans, to GS), by the European Research Council (Starting Grant 309495, NeoNaNo, to TL) and by a graduate stipend from the University of Würzburg via a Research Service Grant awarded by Genelux Corporation (to MH). Teva Pharmachemie has financially supported the synthesis of the prodrug DOX-propGA3. The authors furthermore thank Terry Trevino for assistance in cell culture. JS and AAS are employees and shareholders of Genelux Corporation.
References
- 1.Davis ME, Chen Z, Shin DM. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 2.Duncan R. Nat. Rev. Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 3.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Pharm. Res. 2010;27:2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lammers T, Kiessling F, Hennink WE, Storm G. J. Control. Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 5.Deng C, Jiang Y, Cheng R, Meng F, Zhong Z. Nano Today. 2012;7:467–480. [Google Scholar]
- 6.Devadasu VR, Bhardwaj V, Kumar M. Chem. Rev. 2013;113:1686–1735. doi: 10.1021/cr300047q. [DOI] [PubMed] [Google Scholar]
- 7.Kopecek J, Kopeckova P. Adv. Drug Deliv. Rev. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svenson S. Curr. Opin. Solid State Mater. Sci. 2012;16:287–294. [Google Scholar]
- 9.Shi Y, van Steenbergen MJ, Teunissen EA, Novo L, Gradmann S, Baldus M, van Nostrum CF, Hennink WE. Biomacromolecules. 2013;14:1826–1837. doi: 10.1021/bm400234c. [DOI] [PubMed] [Google Scholar]
- 10.Talelli M, Oliveira S, Rijcken CJF, Pieters EHE, Etrych T, Ulbrich K, van Nostrum RCF, Storm G, Hennink WE, Lammers T. Biomaterials. 2013;34:1255–1260. doi: 10.1016/j.biomaterials.2012.09.064. [DOI] [PubMed] [Google Scholar]
- 11.Talelli M, Iman M, Varkouhi AK, Rijcken CJF, Schiffelers RM, Etrych T, Ulbrich K, van Nostrum CF, Lammers T, Storm G, Hennink WE. Biomaterials. 2010;31:7797–7804. doi: 10.1016/j.biomaterials.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Coimbra M, Rijcken CJF, Stigter M, Hennink WE, Storm G, Schiffelers RM. J. Control. Release. 2012;163:361–367. doi: 10.1016/j.jconrel.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Crielaard BJ, Rijcken CJF, Quan L, van der Wal S, Altintas I, van der Pot M, Kruijtzer JAW, Liskamp RMJ, Schiffelers RM, van Nostrum CF, Hennink WE, Wang D, Lammers T, Storm G. Angew. Chem. Int. Edit. 2012;51:7254–7258. doi: 10.1002/anie.201202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talelli M, Rijcken CJF, Hennink WE, Lammers T. Curr. Opin. Solid State Mater. Sci. 2012;16:302–309. [Google Scholar]
- 15.Schimmel KJM, Richel DJ, van den Brink RBA, Guchelaar HJ. Cancer Treat. Rev. 2004;30:181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Talelli M, Morita K, Rijcken CJF, Aben RWM, Lammers T, Scheeren HW, van Nostrum CF, Storm G, Hennink WE. Bioconjugate Chem. 2011;22:2519–2530. doi: 10.1021/bc2003499. [DOI] [PubMed] [Google Scholar]
- 17.Beratis NG, Kaperonis A, Eliopoulou MI, Kourounis G, Tzingounis VA. J. Cancer Res. Clin. Oncol. 2005;131:371–376. doi: 10.1007/s00432-004-0649-5. [DOI] [PubMed] [Google Scholar]
- 18.Dodds HM, Tobin PJ, Stewart CF, Cheshire P, Hanna S, Houghton P, Rivory LP. J. Pharmacol. Exp. Ther. 2002;303:649–655. doi: 10.1124/jpet.102.039040. [DOI] [PubMed] [Google Scholar]
- 19.Sperker B, Werner U, Murdter TE, Tekkaya C, Fritz P, Wacke R, Adam U, Gerken M, Drewelow B, Kroemer HK. Naunyn-Schmiedebergs Arch. Pharmacol. 2000;362:110–115. doi: 10.1007/s002100000260. [DOI] [PubMed] [Google Scholar]
- 20.Bosslet K, Straub R, Blumrich M, Czech J, Gerken M, Sperker B, Kroemer HK, Gesson JP, Koch M, Monneret C. Cancer Res. 1998;58:1195–1201. [PubMed] [Google Scholar]
- 21.Chen NG, Szalay AA. Future Virol. 2010;5:763–784. [Google Scholar]
- 22.Kirn DH, Thorne SH. Nat. Rev. Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 23.Guse K, Cerullo V, Hemminki A. Expert Opin. Biol. Ther. 2011;11:595–608. doi: 10.1517/14712598.2011.558838. [DOI] [PubMed] [Google Scholar]
- 24.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, Goebel W, Szalay AA. Nat. Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 25.Stritzker J, Kirscher L, Scadeng M, Deliolanis NC, Morscher S, Symvoulidis P, Schaefer K, Zhang Q, Buckel L, Hess M, Donat U, Bradley WG, Ntziachristos V, Szalay AA. Proc. Natl. Acad. Sci. USA. 2013;110:3316–3320. doi: 10.1073/pnas.1216916110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N, Zhang Q, Yu YA, Stritzker J, Brader P, Schirbel A, Samnick S, Serganova I, Blasberg R, Fong Y, Szalay AA. Mol. Med. 2009;15:144–151. doi: 10.2119/molmed.2009.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddad D, Chen NG, Zhang Q, Chen CH, Yu YA, Gonzalez L, Carpenter SG, Carson J, Au J, Mittra A, Gonen M, Zanzonico PB, Fong Y, Szalay AA. J. Transl. Med. 2011;9:36. doi: 10.1186/1479-5876-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelkar SS, Reineke TM. Bioconjugate Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 29.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Accounts Chem. Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 30.Rojas JJ, Thorne SH. Theranostics. 2012;2:363–373. doi: 10.7150/thno.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckel L, Advani SJ, Frentzen A, Zhang Q, Yu YA, Chen NG, Ehrig K, Stritzker J, Mundt AJ, Szalay AA. Int. J. Cancer. 2013 doi: 10.1002/ijc.28296. doi: 10.1002/ijc.28296. [DOI] [PubMed] [Google Scholar]
- 32.Advani SJ, Buckel L, Chen NG, Scanderbeg DJ, Geissinger U, Zhang Q, Yu YA, Aguilar RJ, Mundt AJ, Szalay AA. Clin. Cancer Res. 2012;18:2579–2590. doi: 10.1158/1078-0432.CCR-11-2394. [DOI] [PubMed] [Google Scholar]
- 33.Yu YA, Galanis C, Woo Y, Chen N, Zhang Q, Fong Y, Szalay AA. Mol. Cancer Ther. 2009;8:141–151. doi: 10.1158/1535-7163.MCT-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frentzen A, Yu YA, Chen N, Zhang Q, Weibel S, Raab V, Szalay AA. Proc. Natl. Acad. Sci. USA. 2009;106:12915–12920. doi: 10.1073/pnas.0900660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seubert CM, Stritzker J, Hess M, Donat U, Sturm JB, Chen N, von Hof JM, Krewer B, Tietze LF, Gentschev I, Szalay AA. Cancer Gene Ther. 2011;18:42–52. doi: 10.1038/cgt.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Graaf M, Boven E, Scheeren HW, Haisma HJ, Pinedo HM. Curr. Pharm. Design. 2002;8:1391–1403. doi: 10.2174/1381612023394485. [DOI] [PubMed] [Google Scholar]
- 37.Neradovic D, Soga O, Van Nostrum CF, Hennink WE. Biomaterials. 2004;25:2409–2418. doi: 10.1016/j.biomaterials.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, Marincola FM, Szalay AA. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Chen NG, Minev BR, Szalay AA. J. Transl. Med. 2012;10:167. doi: 10.1186/1479-5876-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess M, Stritzker J, Hartl B, Sturm JB, Gentschev I, Szalay AA. J. Transl. Med. 2011;9:172. doi: 10.1186/1479-5876-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aben RWM, Scheeren HW, Cornelissen JJ, Lamberuts M, de Vos D, Haisma HJ. EP Application, EP2098533 A1. 2009
- 42.van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, Hennink WE. Bioconjugate Chem. 2009;20:2001–2016. doi: 10.1021/bc900087a. [DOI] [PubMed] [Google Scholar]
- 43.Rijcken CJ, Snel CJ, Schiffelers RM, van Nostrum CF, Hennink WE. Biomaterials. 2007;28:5581–5593. doi: 10.1016/j.biomaterials.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 44.Houba PHJ, Boven E, van der Meulen-Muileman IH, Leenders RGG, Scheeren JW, Pinedo HM, Haisma HJ. Int. J. Cancer. 2001;91:550–554. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1075>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 45.Barenholz Y. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Kraus-Berthier L, Jan M, Guilbaud N, Naze M, Pierre A, Atassi G. Clin. Cancer Res. 2000;6:297–304. [PubMed] [Google Scholar]
- 47.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin V. Adv. Drug Deliv. Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Zenser TV, Lakshmi VM, Davis BB. Drug Metab. Dispos. 1999;27:1064–1067. [PubMed] [Google Scholar]
- 50.Talelli M, Iman M, Rijcken CJF, van Nostrum CF, Hennink WE. J. Control. Release. 2010;148:e121–e122. doi: 10.1016/j.jconrel.2010.07.092. [DOI] [PubMed] [Google Scholar]