Abstract
Objective
Determine whether auditory cortex (AC) organization changed following eighth cranial nerve surgery in adults with vestibular-cochlear nerve pathologies. We examined whether hearing thresholds pre- and post-op correlated with increased ipsilateral activation of AC from the intact ear.
Study Design
During magnetic resonance imaging sessions pre- and 3 and 6 months postoperation, subjects listened with the intact ear to noise-like random spectrogram sounds.
Setting
Departments of Radiology and Otolaryngology of Washington University School of Medicine.
Subjects and Methods
Three with acoustic neuromas received Gamma Knife radiosurgery (GK); one with Meniere’s disease and five with acoustic neuromas had surgical resections (SR); two of the latter also had GK. Hearing thresholds in each ear were for pure tone stimuli from 250 to 8000 Hz before and after surgery (3 and 6 months). At the same intervals, we imaged blood oxygen level-dependent responses to auditory stimulation of the intact ear using an interrupted single-event design.
Results
Hearing thresholds in two of three individuals treated with GK did not change. Five of 6 individuals became unilaterally deaf after SRs. Ipsilateral AC activity was present pre-op in 6/9 individuals with ipsilateral spatial extents greater than contralateral in 3 of 9. Greater contralateral predominance was significant especially in left compared to right ear affected individuals, including those treated by GK.
Conclusion
Lateralization of auditory evoked responses in AC did not change significantly post-op possibly due to pre-existing sensory loss before surgery, indicating that less than profound loss may prompt cortical reorganization.
Keywords: Auditory cortex, Acoustic Neuroma, Unilateral Deafness, Neuroplasticity, Gamma Knife Radiosurgery, functional imaging
INTRODUCTION
Monaural acoustic stimulation evokes larger responses contralateral to the stimulated ear with normal hearing. This results in hemispheric asymmetry in auditory cortex (AC), characterized by greater contralateral compared to ipsilateral response magnitudes and spatial extents to the stimulated ear. According to one study, contralateral dominant responses only occur with left ear stimulation,1 but others reported contralateral asymmetry with auditory inputs to either ear.2–4 In contrast, with unilateral hearing loss, stimulation of the intact ear evokes greater ipsilateral than contralateral activity, especially notable in core and adjacent belt AC fields.3–10 Neural changes occur immediately, from periphery to cortex, following deafferentation in many sensory systems.11–17 For example, in adult guinea pigs enhanced ipsilateral auditory evoked responses occurred in AC within 2–3 weeks after unilateral cochlea hair cell damage.18 Sudden unilateral hearing loss in humans similarly reduced asymmetrical AC activity within days.19–22 After surgical resection (SR) of a left acoustic neuroma, one study reported symmetrical bilateral responses to 1 kHz tone bursts at 5 weeks and expanded ipsilateral activation at 1 year post SR.9 Comparably, auditory evoked field potentials (AEF) were larger and had shorter latencies for ipsilateral compared to contralateral ear inputs at 1 month after SRs.10,23 However, one study reported near normal contralateral asymmetrical AEF response magnitudes and shorter latencies to hearing speech and non-speech sounds with an intact left ear and right ear deafness. For those with an intact right ear and left ear deafness, significant ipsilateral activation occurred.8 These differences among studies warrant additional investigation of altered asymmetrical activation to monaural stimulation in auditory cortex.
We evoked responses in AC with random spectrogram sounds (RSS) before and at 3 and months after surgical treatment for unilateral eighth cranial nerve pathology. Only one of 6 individuals treated with a SR, had preserved hearing on the affected side. In three individuals treated with Gamma Knife radiosurgery (GK), pre-op hearing persisted in 2; in 1, hearing decreased but did not reach a profound level. These results are consistent with individuals receiving GK treatments in multi-institution meta-analyses24,25 where >60% had hearing preservation. Given hearing differences with treatment modalities, we also determined whether individuals treated with GK showed less lateralization reorganization in AC.
METHODS
We measured blood oxygen level-dependent (BOLD) responses using echo-planar imaging (EPI) sequences in 9 individuals before and after surgery. The study was reviewed and approved by the Human Studies Institutional Review Board of Washington University and was in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All enrolled individuals gave informed consent.
Eight individuals had unilateral acoustic neuromas and one had Ménière's disease (Table 1A). The acoustic neuromas mostly were at the cerebellopontine angle but with varied sizes including several extending intracanalicular (Table 1B). Cases 1, 3, 5, and 6 had surgical resections (SR); cases 2 and 4 had SR followed by GK; and cases 7, 8, 9 had GK exclusively (Table 1B).
Table 1.
Patient demographics and hearing thresholds in the affected ear.
| Affected Ear Thresholds | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Affected Ear |
Gender | Agea | 3fPTA Pre |
FFPTA Pre |
3fPTA 3 mob |
FFPTA 3 mo |
3fPTA 6 mo |
FFPTA 6 mo |
| 1c | R | F | 28 | 70.0 | 73.1 | 120.0 | 120.0 | 120.0 | 120.0 |
| 2 | R | M | 50 | 50.0 | 55.7 | 120.0 | 120.0 | 120.0 | 120.0 |
| 3 | R | F | 43 | 46.7 | 54.4 | 120.0 | 120.0 | NAd | NA |
| 4 | R | M | 45 | 15.0 | 33.1 | 120.0 | 120.0 | 120.0 | 120.0 |
| 5 | R | F | 47 | 30.0 | 48.1 | 33.3 | 48.8 | 35.0 | 49.4 |
| 6 | L | M | 39 | 48.3 | 46.7 | 120.0 | 120.0 | 120.0 | 120.0 |
| 7 | L | M | 52 | 43.3 | 61.3 | 50.0 | 63.8 | NA | NA |
| 8 | L | F | 56 | 35.0 | 38.3 | 36.7 | 50.0 | 38.3 | 40.8 |
| 9 | L | F | 53 | 18.3 | 28.1 | 61.7 | 65.8 | 61.7 | 61.1 |
| Mean | 45.9 | 39.6 | 48.8 | 86.9 | 92.0 | 87.9 | 90.2 | ||
| SEM | 2.9 | 5.7 | 4.7 | 13.4 | 11.2 | 15.5 | 14.2 | ||
| Tumor size and location per case. | ||||
|---|---|---|---|---|
| Case | Size (cm) | Volume | Location | Treatmente |
| 1 | n/af | n/a | n/a | SR vestibular nerve |
| 2 | 3.4 × 3.2 | CPA, residual tumor | Radical subtotal SR followed with GK | |
| 3 | 2 | CPA, extending in | SR | |
| 4 | 2.7 × 2.2 × | CPA, extending in | SR followed with GK | |
| 5 | 0.5 × 1 | IAC | Middle fossa craniotomy | |
| 6 | 1.6 × 2.1 × | CPA | Sub-occipital craniotomy with SR | |
| 7 | 1.2 × 1 | 683.4 | CPA, extending in | GK |
| 8 | 0.6 | 217 | IAC mid canal | GK |
| 9 | 1700 | CPA, extending in | GK | |
Age at time of study enrollment.
One hundred twenty assigned to all thresholds with no response to test equipment limits.
Case 1 had Meniere’s syndrome.
Patient not available for threshold testing or imaging scans, NA.
Cerebellopontine angle (CPA); intracanalicular (IAC).
Treatments included Gamma Knife radiosurgery, GK and surgical resection, SR.
Not applicable (n/a); other dimensions as noted by surgeons in the patient records.
We measured monaural audiometric hearing thresholds for each pre- and post-op session with insert phones and pure tone stimuli from 250 to 8000 Hz, presented in a double walled sound booth while using a standard Hughson-Westlake procedure.26
During imaging, individuals heard noise-like random spectrogram sounds (RSS) presented monaurally to the intact ear through magnetic resonance compatible circumaural, cushion sealed headphones.3 These previously described stimuli3,27,28 result from manipulation and combination of temporal and spectral parameters for 1638 pure tones spanning a 6-octave bandwidth (250–16000 Hz). RSS stimuli have matching average intensities across spectral regions and temporal ranges, thereby avoiding confounds of differing bandwidth, intensity, or duration as specifying variables common to speech. Additionally, there is independent control of spectral and temporal sound complexity. The complexity of RSS was low or high, based on temporal rates (8 for low and 30 Hz for high) or number of spectral bands (3 for low and 16 for high). Participants pressed an optical key to signal detection of an oddball trial in which the complexity of the RSS differed from that during most other trials in an imaging run.3 Stimulus intensity in the intact ear was 70 dB SPL. The stimuli were predictably below audibility for the opposite ear, which was plugged and muffed with an expected mean interaural bone conduction attenuation of 64 dB for the RSS bandwidth.29 The sound system bandwidth was approximately 160 to 5 kHz with a 10dB/Octave falloff at >5 kHz.
RSS presentations of 2 second durations occurred during 9 s silent intervals in 11 s volume acquisitions (TRs) of an interrupted single event design.30 EPI at the beginning of an immediately following TR had delays of 2–9 s from the onset of a RSS during silence in the preceding TR, which allowed reconstruction of a hemodynamic response.30 Image acquisition, preprocessing procedures, and analyses of BOLD responses were as previously described.3 Briefly, following a GLM analysis, an F-test per voxel assessed whether the BOLD response variance associated with presentation of a RSS stimulus was greater than that due to baseline noise. This test of significance involved no assumptions regarding the hemodynamic response function. Additionally, we transformed F-statistics to equally probable z-scores (F-Zstats) that were multiple comparisons corrected based on Monte Carlo simulations.31 The correction threshold for p=0.05 was z-scores of z=4.0 over 12 face-connected voxels.
Each individual’s brain was rendered into the PALS-B12 CARET surface-based atlas32,33 by using Surefit software. The vertex mesh approximated the mid-cortical thickness of each hemisphere in the native brain. We registered volume-based data (VBD) of corrected F-Zstats to vertices based on nearest coordinate neighbors. Next, deformation of each native brain surface to the vertices for the left and right hemispheres of the PALS-B12 atlas normalized the brains.32 These procedures also registered VBD to the atlas coordinate surface space. The deformation maps created for each brain when applied to the native anatomy retained original brain structure but in the atlas coordinate space of vertices. Thus, surface maps for registered F-Zstats were viewable with respect to participant brain anatomy, but with all distribution distinctions between individuals based on a standard number of vertices. The analyses focused on AC areas Te1, Te2, and Te3 as previously described.3 The combined Te areas occupied the posterior superior temporal plane (Figure 1). Te1 encompasses Heschl’s gyrus and adjoining caudal rostral areas as part of a core primary auditory cortical field; Te2 is caudal to Te1 within planum temporale and is within the caudal belt cortical field; and Te3 is lateral to Te1 along the superior temporal gyral crown within planum polare and is a component of the lateral belt cortical field.34–38
Figure 1.
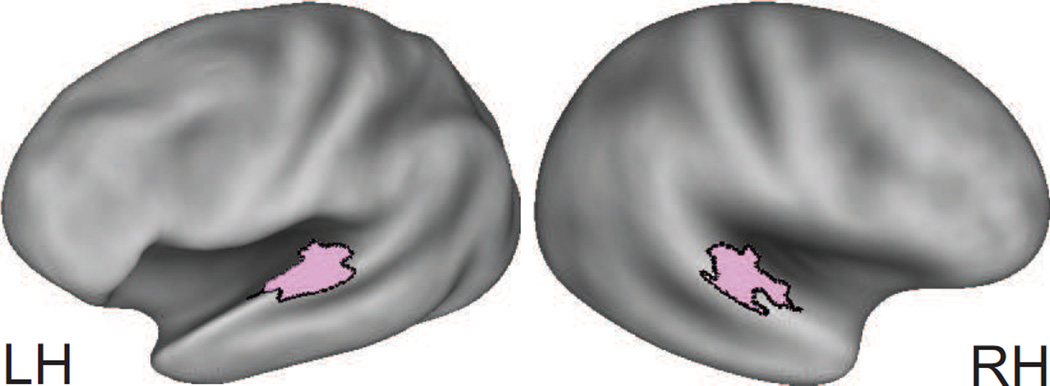
Combined surface areas for Te1, Te2, and Te33 (painted pink and within black borders) shown on very inflated PALS-B12 Caret Atlas. The view into the sulcus is tilted and rotated.
Spatial extents reflected area measurement within the combined surface of the three Te areas whose uncorrected F-Zstats had a threshold value of > 2.57 (i.e., p < 0.005). The boundaries of these areas reflected brain anatomy in each individual brain. We computed a lateralization index (LI) across the Te combined surface area for pre- and each post-op imaging session and for the measurements of surface area that was ipsilateral and contralateral to stimulation of the intact ear.2,39,40 The LI was in percent: LI = 100 × [measure (contralateral) − measure (ipsilateral)] / [measure (contralateral) + measure (ipsilateral)]. Positive LIs indicated greater spatial extents contralateral to the monaurally stimulated ear.
RESULTS
Hearing Loss
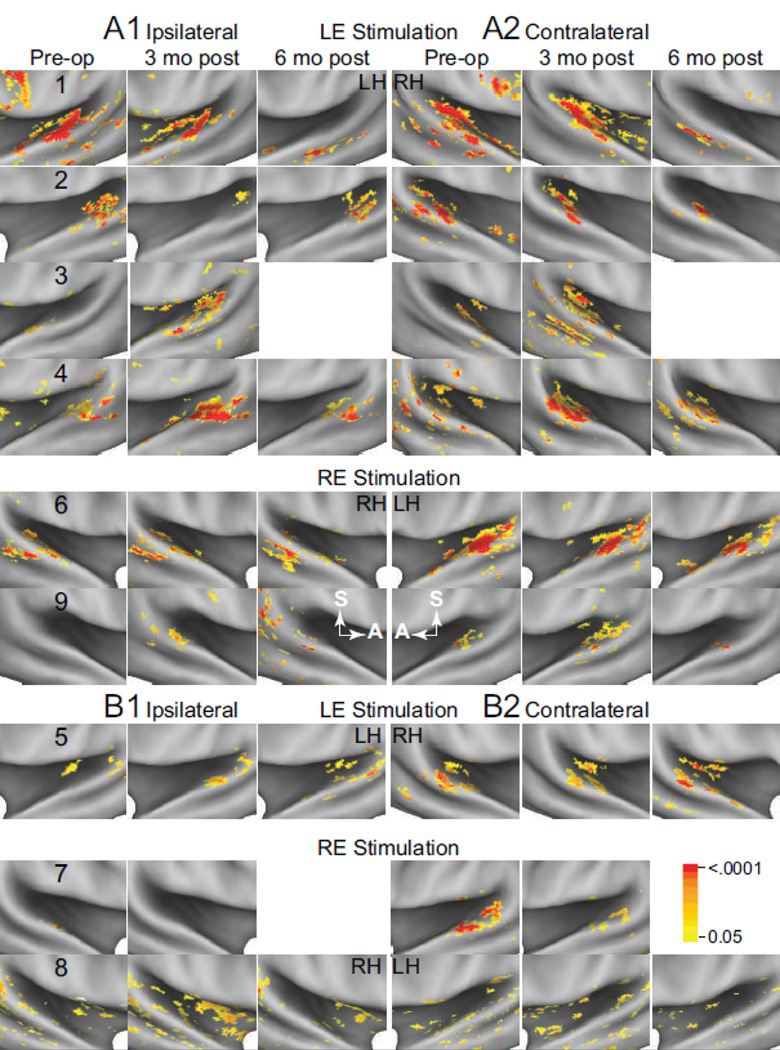
The audiograms in 5 individuals showed profound hearing loss across all frequencies at 3 months post-op (Figure 2, #1–4, 6). These losses at 120 dB hearing level were in the right ear for #1–4 and left ear in #6. Four other individuals had a moderate to severe sloping high frequency loss. Three had losses pre-op and thresholds were unchanged at 3 months postop (Figure 2, #5, 7, and 8). One individual showed a hearing loss at 3 months post op (Figure 2, #9). Three of the four with minimal or no change in hearing threshold following surgery had GK treatments (Table 1B, Figure 2, #7–9). The group pre-op pure tone average (PTA) hearing threshold was ~40 dB hearing level for 3 frequencies (0.5, 1, and 2 kHz) and ~49 dB hearing level for the full frequency range (Table 1A). Hearing levels in the intact ear were mostly normal or showed a mild sloping high frequency hearing loss that did not change after treatment. Laterality of Activity in AC: Significant pre-op activation in the combined Te areas was ipsilateral to the intact ear in 7 of 9 individuals (Figure 3A1 and B1) and was contralateral to stimulation in all individuals (Figure 3A2 and B2), indicating bilateral activation of AC to monaural stimulation in most individuals. Patches of contralateral activity with p values of <0.0001 occurred in ipsilateral AC in 4 (Figure 3A1, B1, Pre-op: red painted patches) and in contralateral AC in 5 individuals (Figure 3A2 and B2). Bilateral activity was present in all but individual #7 at 3 months post-op. All 7 available individuals at 6 months post-op showed some bilateral activity (Figure 3).
Figure 2.
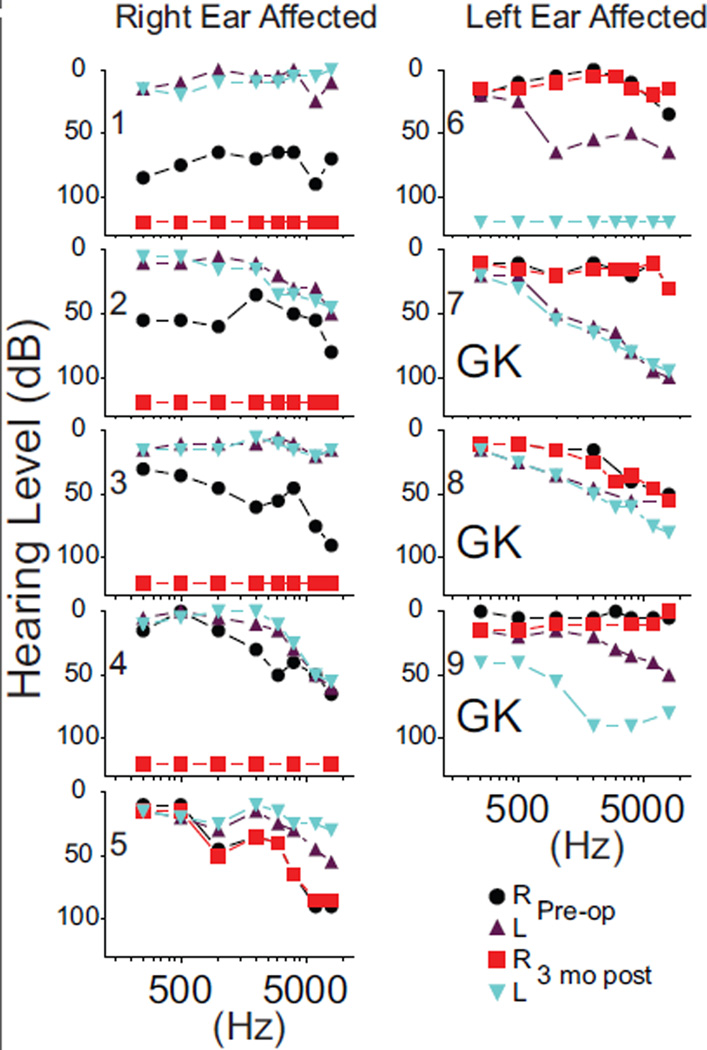
Pure-tone semilog audiometry plots prior to (preoperative) and 3 months after (3 months postoperative) eighth nerve resections in cases with right or left affected ears. GK indicates, Gamma Knife radiosurgery.
Figure 3.
Z score maps for individuals with (A) and without (B) hearing loss. View as shown in Figure 1. White arrows A and S indicate anterior and superior directions in each hemisphere. LH indicates left hemisphere; RH, right hemisphere.
Surface Areas
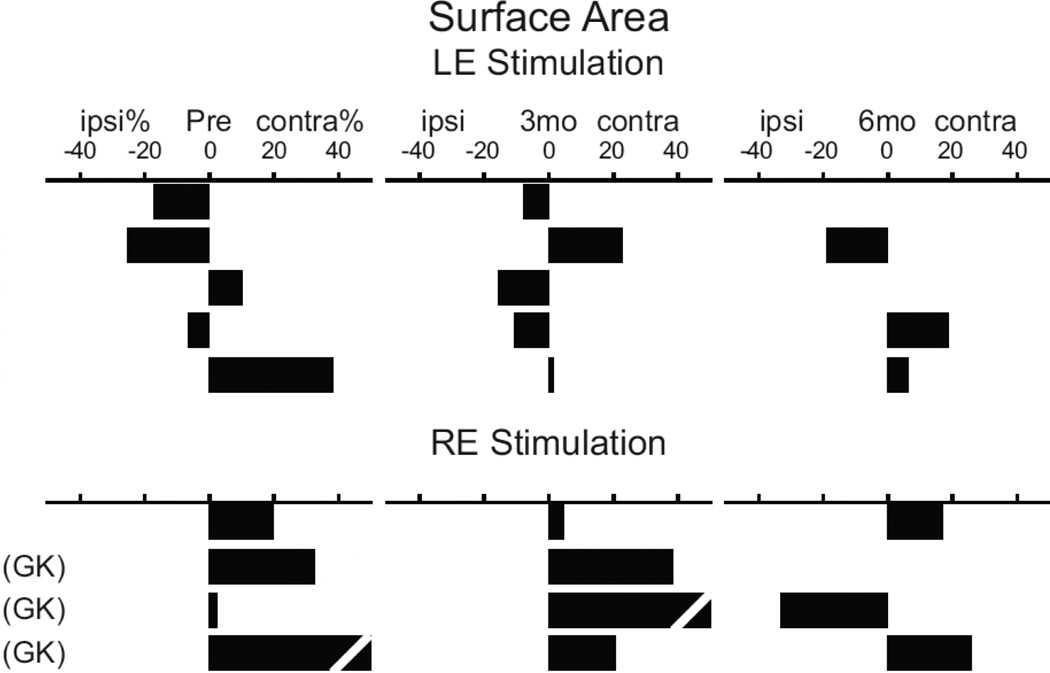
The lateralization index (LI) used surface areas to quantify the balance between ipsilateral compared to contralateral activation distributions (Figure 4). Prior to surgery, 3 individuals with an intact left ear (#1, 2, and 4) showed a higher ipsilateral LI percent. Two others with intact left ears and all with good right ears had a higher contralateral LI percent (Figure 4). At 3 months post-op, 4 individuals with intact left ears had higher ipsilateral LIs (Figure 4, #1, 3–5). Two of these 4 had LIs that reversed from a pre-op contralateral LI (Figure 4, #3 and 5). At 6 months post-op, individuals with intact left or right ears showed reversals from contralateral LIs at 3 months post-op to ipsilateral LIs.
Figure 4.
Laterality index for surface areas within auditory cortex for preoperative and postoperative imaging sessions. Diagonal gaps in bars for 8 and 9 indicate LI% that exceeded scale maximum. LE indicates left ear; RE, right ear.
The right ear affected individuals, who had increased ipsilateral activated surface areas prior to surgery (Figure 4, #1, 2, and 4), possibly showed this lateralization due to prior small elevations in hearing thresholds (Figure 2). Of the 4 individuals with affected right ears and total hearing loss after surgery, two had persistent ipsilateral predominance (#1 and 4), one gained ipsilateral activation (#3), and one switched to contralateral (#2). The right ear affected individual #5 with no post-op change in the hearing threshold showed a switch from contralateral to nearly symmetrical lateralization.
All left ear affected individuals (#6–9) showed LIs favoring contralateral AC extents through all imaging sessions even though some ipsilateral activity was present in all but individual #7. Only individual #6 sustained total hearing loss whereas the other three received GK treatment and showed no alteration in pre-op hearing thresholds.
In summary, prior to any treatments, 8 individuals showed some ipsilateral activation but only three with intact left ears showed a higher ipsilateral than contralateral lateralization index. LIs indicating a contralateral bias occurred in 6 individuals and 3 of them had GK treatments. Lateralization distinctions for significant activity in AC did not vary with the stimulated ear.
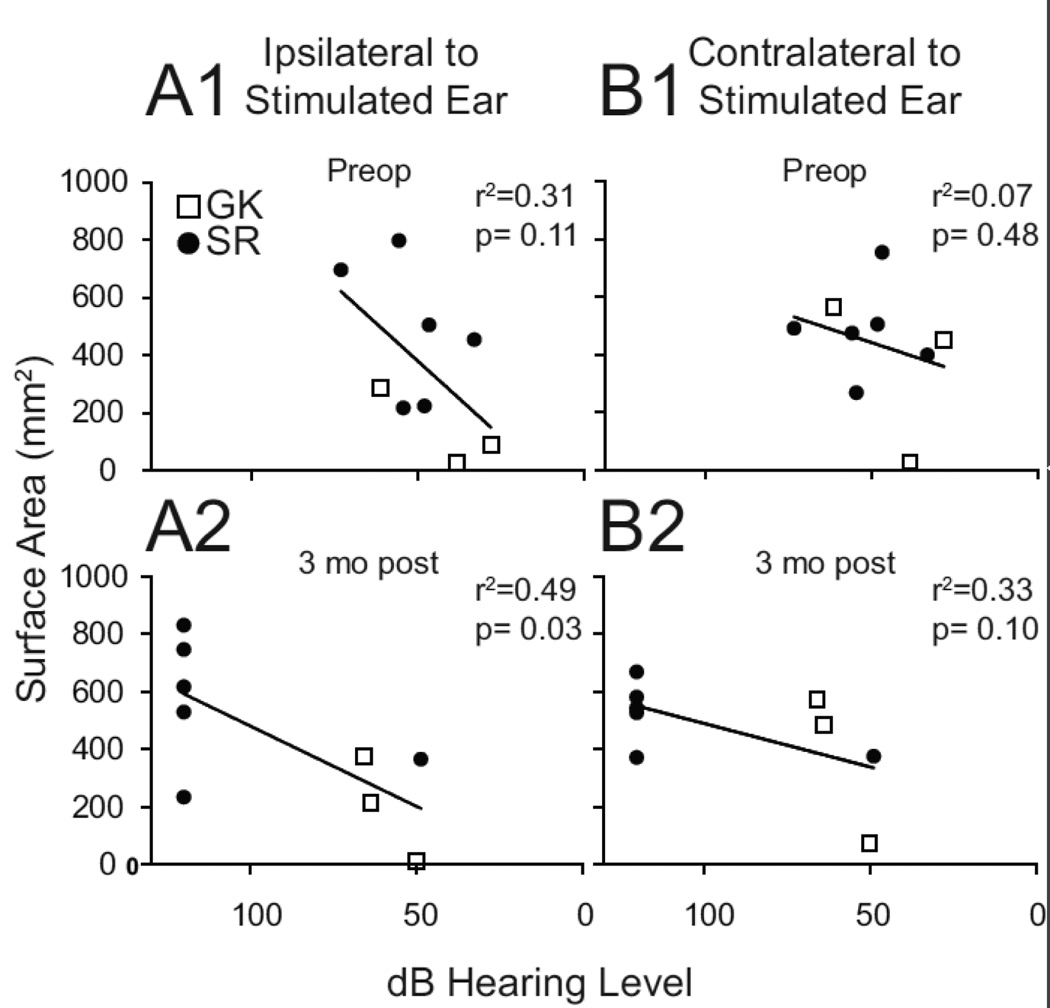
The regression of hearing thresholds with surface areas ipsilateral to the intact ear was not significant prior to surgery but reached significance at the 3 months post-op (Figure 5 A1 and A2). The lowest spatial extents ipsilateral to stimulating the intact ear occurred in individuals treated with GK and whose hearing thresholds in the affected ear changed the least. There was no significant regression for contralateral surface areas (Figure 5 B1 and B2).
Figure 5.
Regression analyses of surface area relative to hearing levels in auditory cortex ipsilateral (A1, 2) and contralateral (B1, 2) to the stimulated intact ear. GK indicates Gamma Knife radiosurgery; SR, surgical resection.
DISCUSSION
Most individuals showed bilateral activation of auditory cortex before and after eighth cranial nerve surgery, indicating little evidence for treatment induced reorganization of lateralization in auditory cortex. These results are at variance with a study of one individual with an affected right ear who had predominant contralateral activation before and a shift towards more symmetrical activation following a surgical resection of an acoustic neuroma.9 This individual had matching normal binaural hearing thresholds before and total right hearing loss after surgical resection of the neuroma. In contrast, hearing thresholds before surgery of 9 individuals in the current study were above the 15–25 dB hearing level of age-matched normal hearing individuals tested by us3 and the pre-op hearing threshold reported previously.9 Elevated hearing thresholds in the affected ear before surgery were prevalent in those with acoustic neuromas even without a hearing loss perceived by the individual.25,41 Consequently, the single individual described by Bilecen and colleagues might have been exceptional rather than representative.
Auditory cortex lateralization varies in different auditory cortical fields and also differs with unilateral deafness in left and right ears. In chronic left ear deafness, right ear stimulation evoked greater left hemisphere (contralateral) response magnitudes only in primary auditory cortex, greater right hemisphere (ipsilateral) responses in belt area Te3, and equal magnitudes in bilateral parabelt areas in comparison to activation from monaural right ear stimulation in normal hearing individuals.3 In the current study, a contralateral spatial activation asymmetry occurred in the left hemisphere (contralateral) of the three left ear affected cases receiving GK treatments. However, this asymmetry was present even before surgery. Auditory cortex lateralization in right ear affected individuals did not confirm prior findings that right ear deafness leads to fewer examples of changes in contralateral (right hemisphere) asymmetry according to AEF measures.6,8 As noted previously in comparing individuals with chronic right ear deafness to those with normal hearing, left ear stimulation evoked larger left hemisphere (ipsilateral) response magnitudes in primary-core auditory cortex, larger responses in right hemisphere (contralateral) belt auditory fields (Te2 and Te3), and equivalent response magnitudes in bilateral parabelt areas.3 These prior findings were consistent with the current finding of larger left hemisphere (ipsilateral) spatial activation extents even prior to surgery in 3 of 5 right ear affected patients. Thus, contrary to prior speculations, there was no evidence that functional plastic changes were more prevalent in the right than in the left auditory cortex. More important, the presence of auditory cortex lateralization changes in the studied cases prior to surgery and the larger sample data set of previously studied chronic cases did not support the speculation that the right temporal lobe has a greater potential for structural re-organization possibly involving re-myelination.6,8 However, our data relied on activation evoked by RSS stimulation and might not reflect auditory cortex lateralization evoked by speech inputs in different intact ears of individuals with unilateral deafness.
A clinically important and unexpected finding was minimal, non-significant changes in auditory cortex lateralization from pre- to post-op imaging sessions with monaural stimulation. A practical implication of this finding is that the studied individuals already sustained some deafferentation prior to surgery. Others have previously found audiograms with elevated thresholds in some patients who were unaware of hearing loss.24,25 Despite preservation of preop hearing levels after gamma knife surgeries, the current findings realistically indicate little likelihood of reversing altered auditory cortex lateralization changes that resulted from pre-op hearing loses. These observations suggest that neuroplasticity in pre-op auditory cortex reflected the effects of possibly less than complete unilateral hearing loss. Reversible lateralization shifts can also occur without material deafferentation as shown by such changes following sudden short-term functional yet reversible deafness.19,21,22 Several studies in animals have shown that partial damage to isolated portions of the cochlea can provoke auditory cortex reorganization of tonotopic maps.13,15,42 Similarly, cortical reorganization with minimal sensory deficits is not an exclusive property of the auditory cortex as shown by changes in the somatosensory system of adult animals and humans experiencing sensory deafferentation.11,43,44 Another clinically significant finding was confirmation of prior reports that gamma knife surgeries better maintained pre-op hearing levels.24,25 Additionally, however, GK, in better preserving the eighth nerve, functionally supported the lateralization pattern in auditory cortex, especially a more normal contralateral asymmetry despite enhanced ipsilateral activity not normally present in most normal hearing individuals. Thus, lateralization patterns found before surgery persisted in post-op imaging sessions because GK possibly did no or minimal further nerve damage. The observed lateralization reflected what pre-existing nerve injury had already instigated. An important notion, however, was that even optimal tumor excision did not reestablish a normal auditory cortex organization because the pre-existing tumor already induced nerve pathology.
Alterations in crossed inhibitory connections normally present with ipsilateral inputs possibly provide the underlying mechanism responsible for the effects of partial unilateral deafferentation before surgery. Altered inhibition probably arises from changes in the auditory brainstem45 and also interhemispheric cortical connections that influence local inhibitory synapses.14,16 The observed increase in ipsilateral spatial extents to auditory stimulation of an intact ear might have indicated prior deafferentation and reduced inhibition of crossed inhibition even without severely affecting hearing levels. A relevant future clinical objective might involve direct attempts to effect crossed inhibition through micro-stimulation of interhemispheric auditory connections or the auditory brainstem, above the damaged eighth nerve.
ACKNOWLEDGEMENTS
Support for the research reported in this publication came from the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) and from the National Institute of Deafness and Other Communication Disorders R01DC009010, both of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
We thank Alvin Agato for his assistance in analyzing the imaging data.
Footnotes
Conflicts of Interest: There are no conflicts of interest declared by any of the authors.
REFERENCES
- 1.Hine J, Thornton R, Davis A, et al. Does long-term unilateral deafness change auditory evoked potential asymmetries? Clin Neurophysiol. 2008;119:576–586. doi: 10.1016/j.clinph.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Schönwiesner M, Krumbholz K, Rübsamen R, et al. Hemispheric asymmetry for auditory processing in the human auditory brain stem, thalamus, and cortex. Cereb Cortex. 2007;17:492–499. doi: 10.1093/cercor/bhj165. [DOI] [PubMed] [Google Scholar]
- 3.Burton H, Firszt JB, Holden T, et al. Activation lateralization in human core, belt, and parabelt auditory fields with unilateral deafness compared to normal hearing. Brain Res. 2012;1454:33–47. doi: 10.1016/j.brainres.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheffler K, Bilecen D, Schmid N, et al. Auditory cortical responses in hearing subjects and unilateral deaf patients as detected by functional magnetic resonance imaging. Cereb Cortex. 1998;8:156–163. doi: 10.1093/cercor/8.2.156. [DOI] [PubMed] [Google Scholar]
- 5.Ponton CW, Vasama J-P, Tremblay K, et al. Plasticity in the adult human central auditory system: evidence from late-onset profound unilateral deafness. Hearing Res. 2001;154:32–44. doi: 10.1016/s0378-5955(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 6.Khosla D, Ponton CW, Eggermont JJ, et al. Differential ear effects of profound unilateral deafness on the adult human central auditory system. JARO. 2003;4:235–249. doi: 10.1007/s10162-002-3014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firszt JB, Ulmer JL, Gaggl W. Differential representation of speech sounds in the human cerebral hemispheres. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:345–357. doi: 10.1002/ar.a.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanss J, Veuillet E, Adjout K, et al. The effect of long-term unilateral deafness on the activation pattern in the auditory cortices of French-native speakers: Influence of deafness side. BMC Neurosci. 2009;10:23. doi: 10.1186/1471-2202-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilecen D, Seifritz E, Radü EW, et al. Cortical reorganization after acute unilateral hearing loss traced by fMRI. Neurology. 2000;54:765–767. doi: 10.1212/wnl.54.3.765. [DOI] [PubMed] [Google Scholar]
- 10.Vasama J-PCA, Makela JP, Pyykko I, et al. Abrupt unilateral deafness modifies function of human auditory pathways. Neuroreport. 1995;6:961–964. doi: 10.1097/00001756-199505090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 12.Hendry SHC, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 13.Rajan R, Irvine DRF, Wise LZ, et al. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- 14.Razak KA, Fuzessery ZM. GABA shapes a systematic map of binaural sensitivity in the auditory cortex. J Neurophysiol. 2010;104:517–528. doi: 10.1152/jn.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson D, Irvine DRF. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 16.Sanes DH, Kotak VC. Developmental plasticity of auditory cortical inhibitory synapses. Hearing Res. 2011;279:140–148. doi: 10.1016/j.heares.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- 18.Popelar J, Erre J, Aran J, et al. Plastic changes in ipsi-contralateral differences of auditory cortex and inferior colliculus evoked potentials after injury to one ear in the adult guinea pig. Hearing Res. 1994;72(1–2):125–134. doi: 10.1016/0378-5955(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Kouzaki H, Nishida Y, et al. Cortical representation of hearing restoration in patients with sudden deafness. Neuroreport. 2002;13:1829–1832. doi: 10.1097/00001756-200210070-00029. [DOI] [PubMed] [Google Scholar]
- 20.Fujiki N, Naito Y, Nagamine T, et al. Influence of unilateral deafness on auditory evoked magnetic field. Neuroreport. 1998;9:3129–3133. doi: 10.1097/00001756-199810050-00002. [DOI] [PubMed] [Google Scholar]
- 21.Morita T, Hiraumi H, Fujiki N, et al. A recovery from enhancement of activation in auditory cortex of patients with idiopathic sudden sensorineural hearing loss. Neurosci Res. 2007;58:6–11. doi: 10.1016/j.neures.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Po-Hung Li L, Shiao A-S, Lin Y-Y, et al. Healthy-side dominance of cortical neuromagnetic responses in sudden hearing loss. Ann Neurol. 2003;53:810–815. doi: 10.1002/ana.10599. [DOI] [PubMed] [Google Scholar]
- 23.Vasama J-P, Marttila T, Lahin T, et al. Auditory pathway function after vestibular schwannoma surgery. Acta Otolaryngol. 2001;121:378–383. doi: 10.1080/000164801300102833. [DOI] [PubMed] [Google Scholar]
- 24.Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010;112:851–859. doi: 10.3171/2009.8.JNS0985. [DOI] [PubMed] [Google Scholar]
- 25.Arthurs B, Fairbanks R, Demakas J, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev. 2011;34:265–279. doi: 10.1007/s10143-011-0307-8. [DOI] [PubMed] [Google Scholar]
- 26.Carhart R, Jerger J. Preferred method for clinical determination of pure tone thresholds. J Speech Hear Disord. 1959;24:330–345. [Google Scholar]
- 27.Warrier C, Wong P, Penhune V, et al. Relating structure to function: Heschl’s gyrus and acoustic processing. J Neurosci. 2009;29:61–69. doi: 10.1523/JNEUROSCI.3489-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schönwiesner M, Rübsamen R, von Cramon DY. Hemispheric asymmetry for spectral and temporal processing in the human antero-lateral auditory belt cortex. Eur J Neurosci. 2005;22:1521–1528. doi: 10.1111/j.1460-9568.2005.04315.x. [DOI] [PubMed] [Google Scholar]
- 29.Brännström K, Lantz J. Interaural attenuation for Sennheiser HDA 200 circumaural earphones. Int J Audiol. 2010;49:467–471. doi: 10.3109/14992021003663111. [DOI] [PubMed] [Google Scholar]
- 30.Belin P, Zilbovicius M, Crozier S, et al. Lateralization of speech and auditory temporal processing. J Cogn Neurosci. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- 31.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 32.Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 33.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morosan P, Rademacher J, Schleicher A, et al. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- 36.Rademacher J, Morosan P, Schormann T, et al. Probabilistic mapping and volume measurement of human primary auditory cortex. Neuroimage. 2001;13:669–683. doi: 10.1006/nimg.2000.0714. [DOI] [PubMed] [Google Scholar]
- 37.Woods DL, Alain C. Functional imaging of human auditory cortex. Curr Opin Otolaryngol Head Neck Surg. 2009;17:407–411. doi: 10.1097/MOO.0b013e3283303330. [DOI] [PubMed] [Google Scholar]
- 38.Woods DL, Herron TJ, Cate AD, et al. Functional properties of human auditory cortical fields. Front Syst Neurosci. 2010;4:1–13. doi: 10.3389/fnsys.2010.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obleser J, Eisner F, Kotz SA. Bilateral speech comprehension reflects differential sensitivity to spectral and temporal features. J Neurosci. 2008;28:8116–8123. doi: 10.1523/JNEUROSCI.1290-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schönwiesner M, Rübsamen R, von Cramon DY. Spectral and temporal processing in the human auditory cortex—revisited. Ann NY Acad Sci. 2005;1060:89–92. doi: 10.1196/annals.1360.051. [DOI] [PubMed] [Google Scholar]
- 41.Wind JJ, Leonetti JP, Raffin MJM, et al. Hearing preservation in the resection of vestibular schwannomas: patterns of hearing preservation and patient-assessed hearing function. J Neurosurg. 2011;114:1232–1240. doi: 10.3171/2010.11.JNS091752. [DOI] [PubMed] [Google Scholar]
- 42.Schwaber MKMD, Garraghty PEPD, Kaas JHPD. Neuroplasticity of the adult primate auditory cortex following cochlear gearing loss. Am J Otol. 1993;14:252–258. [PubMed] [Google Scholar]
- 43.Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proc Natl Acad Sci U S A. 1993;90:10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones EG, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 45.Langers DRM, Van Dijk P, Backes WH. Lateralization, connectivity and plasticity in the human central auditory system. Neuroimage. 2005;28:490–499. doi: 10.1016/j.neuroimage.2005.06.024. [DOI] [PubMed] [Google Scholar]