Summary
Background
Sputum smear microscopy is the most widely available diagnostic test for pulmonary tuberculosis in countries with a high burden of the disease. Improving its accuracy is crucial to achievement of case-detection targets established by the Millennium Development Goals. Unfortunately, many patients are unable to submit all of the specimens needed for examination or to return for treatment because standard sputum collection and reporting requires several clinic visits. To inform policy recommendations by a WHO-convened Expert Group, we aimed to assess the accuracy of sputum smear examination with strategies for obtaining sputum on 1 day compared with strategies for obtaining sputum over 2 days.
Methods
We did a systematic review and meta-analysis of research articles comparing the accuracy of front-loaded or same-day microscopy and standard sputum smear microscopy for diagnosis of culture-confirmed pulmonary tuberculosis. We searched Medline, Embase, Biosis, and Web of Science for articles published between Jan 1, 2005, and Feb 14, 2012. Two investigators identified eligible articles and extracted data for individual study sites. We generated pooled summary estimates (95% CIs) for sensitivity and specificity by use of random-effects meta-analysis when four or more studies were available.
Findings
We identified eight relevant studies from five articles enrolling 7771 patients with suspected tuberculosis in low-income countries. Compared with the standard approach of examination of two smears with Ziehl-Neelsen light microscopy over 2 days, examination of two smears taken on the same day had much the same sensitivity (64% [95% CI 60 to 69] for standard microscopy vs 63% [58 to 68] for same-day microscopy) and specificity (98% [97 to 99] vs 98% [97 to 99]). We noted similar results for studies employing light-emitting diode fluorescence microscopy and for studies examining three smears, whether they were compared with two-smear strategies or with one another.
Interpretation
Same-day sputum smear microscopy is as accurate as standard smear microscopy. Data from tuberculosis programmes are needed to document the changes required in the health system to successfully implement the strategy and understand its effects.
Introduction
Strategies to improve the efficiency of sputum smear microscopy, the most widely available method for diagnosis of pulmonary tuberculosis, could improve rates of case detection and treatment initiation in countries with a high incidence of the disease. Until 2007, WHO recommended that individuals with suspected pulmonary tuberculosis provide three sputum specimens for smear microscopy on 2 consecutive days, with one spot specimen collected immediately and one early morning spot and one additional spot specimen collected the next day. At that time, noting that the third specimen increases cumulative sensitivity by only 2–5%,1 WHO began recommending that quality-assured laboratories examine only two sputum specimens.2 This policy sought to decrease laboratory workloads and costs3 and to increase time for smear examination,4,5 but did not address another disadvantage of smear microscopy: the need for the patient to visit a health centre more than once. The high costs of transportation, food, and lost wages associated with diagnostic visits can approach 50% of the household income of patients with suspected tuberculosis, which is a burden that measurably worsens poverty levels for these patients and their families, and leads a large proportion (13–95%) to drop out of the diagnostic pathway before completing sputum examination, receiving results, or starting treatment.6–15
Front-loaded microscopy is a new diagnostic strategy in which two smears are prepared from one or more sputum specimens obtained on the first day a patient is assessed.16–18 When all samples are collected and the results reported on 1 day, the strategy is termed same-day microscopy. In 2009, WHO convened an Expert Group to summarise the evidence supporting same-day smear microscopy for the Strategic and Technical Advisory Group for Tuberculosis (STAG-TB), which subsequently issued a new policy.19 We present the findings of that process, updated with additional evidence from the scientific literature.
Methods
Search strategy and selection criteria
We did our study in accordance with standard guidelines and methods for systematic reviews and meta-analyses of diagnostic tests.20 We formulated three review questions comparing the accuracy of three strategies for diagnosis of pulmonary tuberculosis: first, front-loaded versus standard two-smear microscopy; second, front-loaded versus standard three-smear microscopy; and third, front-loaded two-smear versus front-loaded three-smear microscopy. We assessed these questions in three subgroups of patients: first, patients who had direct sputum smears stained with the Ziehl-Neelsen (ZN) method and assessed by conventional light microscopy; second, patients who had direct sputum smears stained with auramine-O and examined with light-emitting diode (LED) fluorescence microscopy (FM); and third, patients with HIV who were assessed with ZN and LED FM microscopy.
We searched Medline, Embase, Biosis, and Web of Science for primary studies in all languages published from Jan 1, 2005, to Feb 14, 2012 (see appendix for search terms). Additionally, we reviewed studies included in a previous systematic review on the yield of serial sputum acid-fast bacilli examinations,1 hand-searched reference lists of eligible papers and related reviews, and contacted researchers to identify unpublished or ongoing studies.
We included all studies that compared a front-loaded microscopy strategy with a standard microscopy strategy for diagnosis of pulmonary tuberculosis after preparation of sputum smears directly from one or more sputum samples without concentration. We included only studies that used mycobacterial culture on solid or liquid media for one or more specimens from each patient as a reference standard. Two reviewers (JLD and KRS) independently screened the accumulated citations for relevance and reviewed full-text articles with prespecified eligibility criteria; disagreements about study selection were resolved by consensus.
We defined a study as any dataset arising from one country, and extracted data from individual countries separately for multicountry assessments. We defined a diagnostic strategy as an approach to collection of sputum and examination of two or three sputum smears by microscopy. We defined a standard microscopy strategy as one in which one sputum smear was examined on the first day of presentation, with additional specimens obtained and examined the next day (ie, spot-morning or spot-morning-spot sputum-collection schemes). We defined a front-loaded strategy as one in which two sputum smears were examined on the first day the patient presented, and a same-day strategy as a front-loaded strategy in which only two smears were examined overall.
Data extraction
Two reviewers (JLD and KRS) abstracted data and resolved differences by consensus. We appraised study quality with the Quality Assessment Tool for Diagnostic Accuracy Studies—a validated method for diagnostic studies (appendix)—and assessed whether study sites employed external quality-assurance programmes for smear microscopy.21 We displayed the proportion of studies meeting or reporting quality criteria graphically. The appendix lists additional details of the data extraction and quality assessment procedures.
Statistical analysis
We calculated the sensitivity and specificity of each diagnostic strategy with mycobacterial culture as a reference standard. To minimise expected heterogeneity, we decided a priori to analyse data within certain subgroups: studies of ZN light microscopy, studies of FM, and studies enrolling 30 or more patients infected with HIV for ZN microscopy and for LED FM. For every review question and subgroup, we assessed heterogeneity visually with forest plots and summary receiver-operating characteristic (ROC) curves with 95% prediction regions, and statistically with χ² and I² statistics. We generated pooled summary estimates and differences for sensitivity and specificity with 95% CIs by use of hierarchical summary ROC (HSROC) or random-effects meta-analysis when four or more studies were available. We plotted these estimates and 95% confidence regions in ROC space.
We postulated that sensitivity differences of no more than 10% and specificity differences of no more than 2% would qualify two strategies as having similar diagnostic accuracy. We summarised our findings with Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines.22,23
Role of the funding source
WHO commissioned this review and meta-analysis to inform its policy statement, and received an early version of the manuscript in the form of an official report. The funders had no role in data collection or analysis. Three investigators (JLD, AC, and LEC) were also investigators on one or more included studies. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
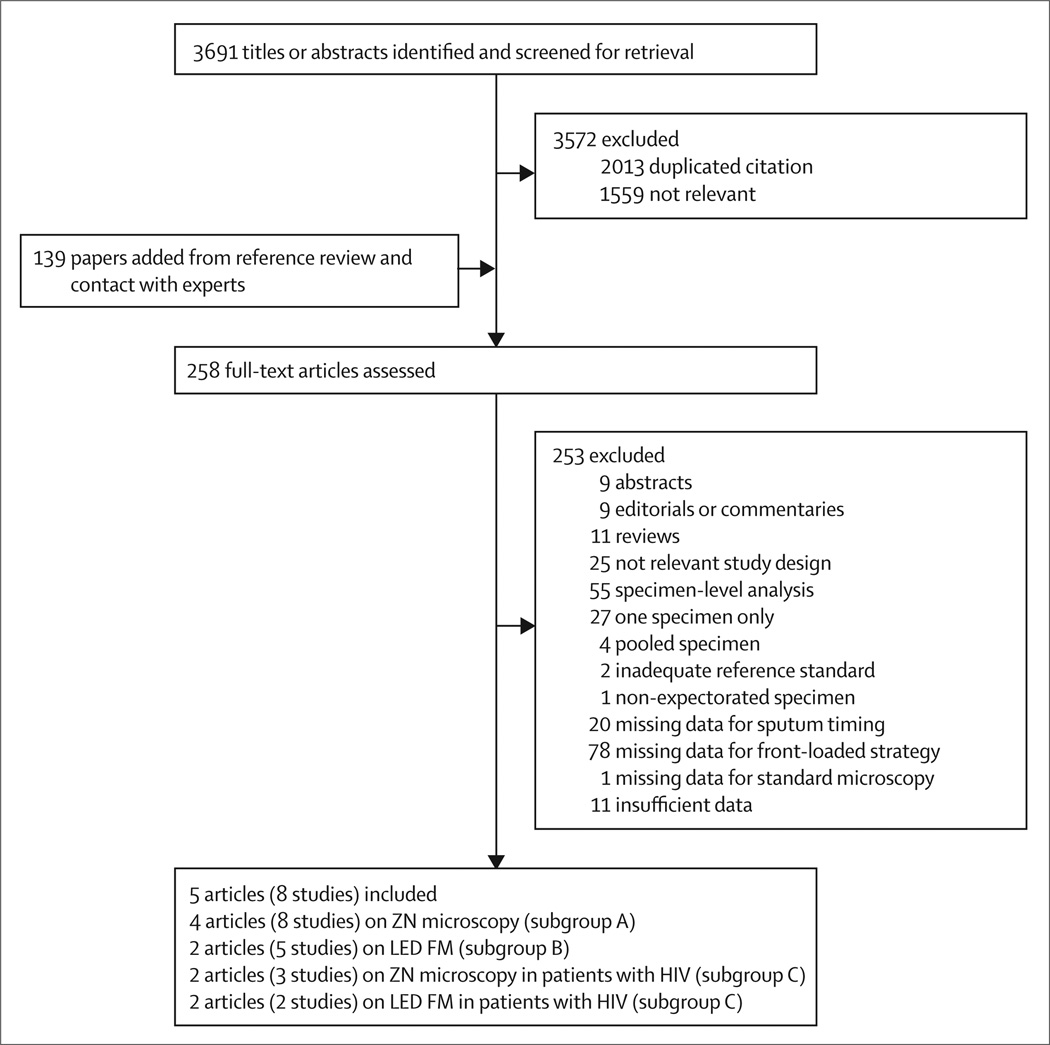
We identified 3691 citations, of which 258 were regarded as potentially relevant (figure 1). After full-text review, five articles17,18,24–26 were included in our analysis, providing data for eight studies from distinct clinical settings. We identified one ongoing study, which was not included, but no completed unpublished studies.
Figure 1. Study selection and stratification.
ZN=Ziehl-Neelsen light microcopy. LED FM=light-emitting diode fluorescence microscopy.
Table 1 shows the characteristics of study design and setting. All studies were done in high tuberculosis prevalence countries and enrolled representative populations (adult outpatients with cough of 2–3 weeks or more; table 2).17,18,24–26 Five of eight studies reported instructing patients on how to produce high-quality sputum.24–27 Six studies did solid culture on Lowenstein-Jensen or Ogawa media,18,25,26 one study did liquid culture on mycobacterial growth-indicator tube media,17 and one study did both solid and liquid cultures.24
Table 1.
Characteristics of included studies
| Setting | Prevalence of tuberculosis |
Number of patients enrolled |
|||
|---|---|---|---|---|---|
| Subgroup A (ZN) | Subgroup B (LED FM) | Subgroup C(HIV) | |||
| Paired observational studies | |||||
| Ramsay et al (2009)18 | |||||
| Nepal | Primary health clinic | 26% | 206 | ·· | ·· |
| Nigeria* | District hospital clinic | 37% | 224 | ·· | ·· |
| Yemen | Subspecialty clinic | 29% | 250 | ·· | ·· |
| Cattamanchi et al (2011)24 | |||||
| Uganda | Referral hospital | 50% | 464 | 464 | ZN 321; LED FM 321 |
| Randomised controlled trial | |||||
| Cuevas et al (2011)25,26† | |||||
| Ethiopia | Primary health clinic | 33% | 1909 | 468 | ZN 101; LED FM 4 |
| Nepal | Subspecialty clinic | 13% | 630 | 526 | ZN 2; LED FM 2 |
| Nigeria* | District hospital clinic | 21% | 1238 | 685 | ZN 485; LED FM 236 |
| Yemen | Subspecialty clinic | 26% | 2850 | 766 | ZN 1; LED FM 0 |
ZN=Ziehl-Neelsen light microscopy. LED FM=light-emitting diode fluorescence microscopy.
Data also reported elsewhere.17
ZN and LED FM results reported separately.
Table 2.
Characteristics of included studies, by subgroup
| Subgroup A (ZN) | Subgroup B (LED FM) | Subgroup C (HIV) | |
|---|---|---|---|
| Number of studies | 8 | 5 | 5 |
| Number of patients enrolled | 7771 | 2909 | 910 |
| Health-care setting | Outpatient | Outpatient | Outpatient |
| Participants | Adults with suspected pulmonary tuberculosis | Adults with suspected pulmonary tuberculosis | HIV-positive adults with suspected pulmonary tuberculosis |
| Front-loaded timing | Spot-spot*(-morning) | Spot-spot*(-morning) | Spot-spot*(-morning) |
| Standard timing | Spot-morning(-spot) | Spot-morning(-spot) | Spot-morning(-spot) |
| Smear preparation | Direct | Direct | Direct |
| Staining | ZN | Auramine-O | ZN |
| Reading | Conventional LM | LED FM | Conventional LM |
ZN=Ziehl-Neelsen light microscopy. LED FM=light-emitting diode fluorescence microscopy. Morning=early morning sputum. Spot=randomly timed specimen. LM=light microscopy.
Apart from Cattamanchi and colleagues,24 in which both same-day smears were prepared from one spot specimen obtained on day 1.
The methodological quality of studies was high for the ZN light microscopy subgroup, with all studies fulfilling all quality criteria (appendix). Study quality was lower for the LED FM (non-random selection in four studies) and HIV (self-selection and convenience sampling in four studies; sparse data) subgroups.
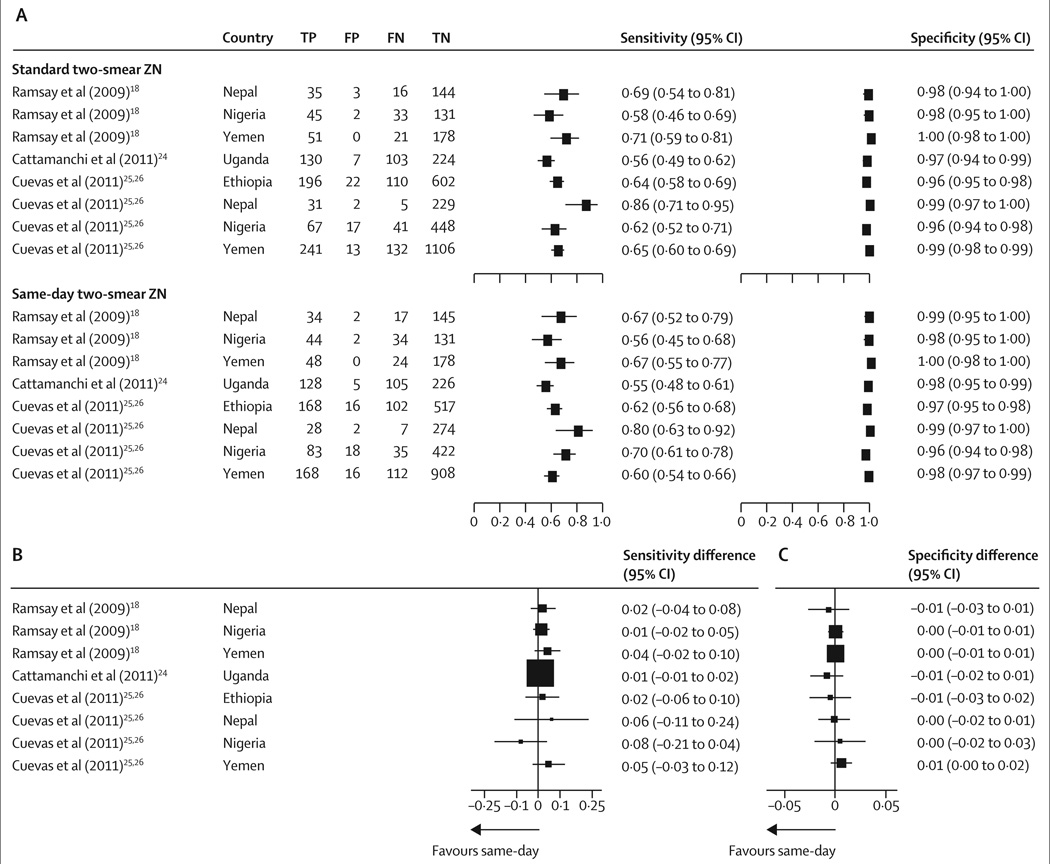
Eight studies compared same-day and standard strategies for two smears with ZN light microscopy. Figure 2 and the appendix show that sensitivity estimates were variable, but specificity estimates were consistent between studies. Pooled summary estimates suggested that sensitivities were much the same for standard microscopy and same-day microscopy; specificities were identical (table 3). Plots of these summary points and their tightly overlapping 95% confidence regions in ROC space confirmed these findings (appendix). Sensitivities and specificities did not differ between standard and same-day microscopy, and both sensitivity differences (p=0·71, I²=0·0%) and specificity differences (p=0·88, I²=0·0%) were consistent between studies (figure 2). Exclusion of one study reporting a single-specimen strategy24 did not change the pooled estimates (sensitivity difference 2·0% [−0·4 to 4·4%]; specificity difference 0·0% [−0·3 to 0·4]). Tables 3 and 4 summarise the results of these and subsequent subgroup analyses.
Figure 2. Diagnostic accuracy of standard two-smear microscopy versus same-day two-smear microscopy.
(A) Sensitivity and specificity. (B) Sensitivity differences, calculated by subtraction of the same-day estimate from the standard estimate. (C) Specificity differences, calculated by subtraction of the same-day estimate from the standard estimate. ZN=Ziehl-Neelsen light microscopy. TP=true positive. FP=false positive. FN=false negative. TN=true negative.
Table 3.
Pooled summary estimates of diagnostic accuracy for two-specimen and three-specimen front-loaded versus standard collection strategies
| Studies (n) | Patients (n) | Sensitivity (95% CI) |
Specificity (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Standard | Front-loaded | Difference* | Standard | Front-loaded | Difference* | |||
| Two smears (spot-morning vs spot-spot) | ||||||||
| ZN | 8 | 7771 | 64% (60 to 69) | 63% (58 to 68) | 1·2% (−0·1 to 2·5) | 98% (97 to 99) | 98% (97 to 99) | 0·0% (−0·4 to 0·4) |
| LED FM | 5 | 2909† | 73% (64 to 81) | 69% (60 to 77) | 2·3% (0·0 to 4·7) | 93% (88 to 97) | 93% (89 to 96) | 0·6% (−0·8 to 1·9) |
| Three smears (spot-morning-spot vs spot-spot-morning) | ||||||||
| ZN | 7 | 7308 | 69% (64 to 75) | 69%‡ (65 to 73) | 01% (−2·2 to 2·3) | 98% (97 to 99) | 98%‡ (97 to 99) | 0·1% (−0·3 to 0·5) |
| LED FM | 4 | 2445† | 79% (69 to 86) | 80% (71 to 86) | −1·6% (−8·4 to 5·1) | 91% (82 to 95) | 89% (80 to 95) | 0·9% (−1·8 to 3·6) |
ZN=Ziehl-Neelsen light microscopy. LED FM=light-emitting diode fluorescence microscopy. Spot=randomly timed specimen. Morning=early morning sputum.
Reported differences are derived from within-study accuracy differences and do not necessarily equal between-strategy differences.
Patients with assessable smear and culture results.
Summary estimates generated with a random effects meta-analysis because the hierarchical summary receiver operating curve model could not be fitted to the data.
Table 4.
Pooled summary estimates of diagnostic accuracy for three-specimen front-loaded versus two-specimen front-loaded collection strategies
| Studies (n) | Patients (n) | Sensitivity (95% CI) |
Specificity (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Three-specimen | Two-specimen | Difference* | Three-specimen | Two-specimen | Difference* | |||
| ZN | 7 | 7308 | 69%†(65 to 73) | 64% (58 to 69) | 3·3% (−0·1 to 6·6) | 98%†(97 to 99) | 98% (97 to 99) | −01% (−0·5 to 0·3) |
| LED FM | 4 | 2445‡ | 80% (71 to 86) | 73% (56 to 85) | 5·2% (−1·6 to 12) | 89% (80 to 95) | 93% (87 to 96) | −3·4% (−7·0 to 0·3) |
ZN=Ziehl-Neelsen light microscopy. LED FM=light-emitting diode fluorescence microscopy.
Reported differences are derived from within-study accuracy differences and do not necessarily equal between-strategy differences.
Summary estimates generated with a random effects meta-analysis because the hierarchical summary receiver operating curve model could not be fitted to the data.
Patients with assessable smear and culture results.
Only one article, a randomised controlled trial25 including 6627 participants in Ethiopia, Nepal, Nigeria, and Yemen, reported on differential losses to follow-up for the two diagnostic strategies. Patients assigned to the same-day strategy were half as likely to fail to submit a second specimen as were patients assigned to the standard strategy, but follow-up rates were high in both groups (97·6% for the same-day strategy vs 94·2% for the standard strategy; difference 3·4% [95% CI 2·3 to 4·6]; p<0·001).25
Seven studies compared front-loaded and standard strategies with ZN light microscopy when three specimens were collected. Sensitivity estimates were variable, but specificity estimates were consistent across studies (appendix). Pooled summary estimates showed much the same sensitivities for standard microscopy and same-day microscopy; specificities were identical (table 3). Sensitivities and specificities did not differ between standard and front-loaded microscopy strategies (table 3), and these differences were consistent across studies for both sensitivity (p=0·77, I²=0·0%) and specificity (p=0·96, I²=0·0%; appendix). In the one study reporting data for loss to follow-up, patients assigned to the front-loaded strategy were no more likely to submit three specimens than were those assigned to the standard strategy (94·1% for the front-loaded vs 92·8% for the standard approach; difference 1·2% [95% CI −0·4 to 2·8]).25
Seven studies compared three-specimen and two-specimen front-loaded strategies with ZN light microscopy, with sensitivities and specificities as previously noted (appendix). Pooled sensitivity and specificity differences showed that the three-specimen front-loaded strategy was no more sensitive (difference 3·3% [95% CI −0·1 to 6·6]) or specific (difference −0·1% [−0·5 to 0·3]) than the two-specimen front-loaded strategy. Specificity did not differ between three-specimen and two-specimen strategies (table 4). Although sensitivity differences varied (p=0·12, I²=41%), specificity differences were consistent (p=0·98, I²=0·0%) across studies (appendix).
Five studies from two reports24,26 compared front-loaded and standard strategies with LED FM. Pooled estimates of sensitivity and specificity were heterogeneous between studies (appendix), but no heterogeneity was noted in terms of sensitivity differences (p=0·60, I²=0·0%) or specificity differences (p=0·74, I²=0·0%; appendix). For the two-specimen LED FM strategies, the sensitivities of standard and front-loaded strategies were much the same (table 3). The summary points and their broad but overlapping 95% confidence regions plotted in ROC space supported these findings (appendix). Specificities of standard LED FM and front-loaded LED FM were much the same (table 3). Findings were much the same for the three-specimen collection strategy (appendix). The sensitivities of standard LED FM and front-loaded LED FM did not differ significantly (table 3). Equally, specificities of standard LED FM and front-loaded LED FM did not differ with collection of three specimens (table 3). Three-specimen front-loaded LED FM was not significantly more sensitive than was two-specimen front-loaded LED FM, and was similarly specific (table 4, appendix).
Two articles assessed front-loaded procedures versus standard collection with direct ZN light microscopy and LED FM in patients infected with HIV. In one article,25 which included 589 HIV-positive patients with suspected tuberculosis predominantly from one study in Ethiopia and one study in Nigeria, a same-day ZN light microscopy strategy had better sensitivity than did a standard two-specimen strategy (67% for the same-day strategy vs 49% for the standard strategy; difference −18% [95% CI −35 to 0]) and similar specificity (95% vs 97%; difference 2% [−1 to 5]), with much the same results for the three-specimen strategies (two studies) and for two-specimen and three-specimen strategies employing LED FM (one study). For 321 HIV-positive patients reported in a second article from Uganda,24 same-day, single-specimen, two-smear microscopy and standard two-specimen, two-smear ZN light microscopy had much the same sensitivities (49% for the same-day strategy vs 51% for the standard approach, difference 2% [−3 to 5%]) and specificities (97% vs 99%; difference 2% [−1 to 4]). A comparison of single-specimen and standard strategies with LED FM in the same population produced much the same sensitivities (57% vs 60%; difference 3% [−2 to 8]) and specificities (97% vs 97%; difference 0 [−1 to 1]).
We rated the quality of evidence for all outcomes in the subgroup analyses of ZN light microscopy and LED FM as moderate. We downgraded one point for indirectness because test results (ie, true positives, false positives, false negatives, and true negatives) were used as surrogates for patient-related outcomes (eg, numbers of patients who started treatment or dropped out; appendix) in the paired studies, and because of heterogeneity and imprecision in the randomised studies. We rated the quality of the LED FM studies as moderate, after deduction of points for indirectness in the paired studies and heterogeneity and imprecision in the randomised studies. We did not deduct additional points for non-random sampling of patients in the randomised studies because of the overall strength of the study design. The quality of evidence among patients infected with HIV was rated as low, with an additional point subtracted for convenience sampling and sparse data.
Discussion
In our systematic review of eight studies of direct ZN light microscopy enrolling 7771 patients, front-loaded and standard microscopy strategies had much the same diagnostic accuracies for two-sputum and three-sputum smear strategies. Moreover, all estimates of sensitivity differences were precise and smaller than the difference prespecified as important. These findings suggest that one of the key limitations of sputum smear microscopy as a tuberculosis case-finding technique, the requirement for repeated clinic visits, could be eliminated with no loss in diagnostic accuracy. If widely implemented, same-day microscopy could provide important benefits to several groups, including patients with tuberculosis (reduced costs and earlier diagnosis), patients suspected of but not having tuberculosis (reduced costs and earlier exclusion of tuberculosis), and tuberculosis programmes (fewer visits for patients suspected of tuberculosis and fewer smear-positive tuberculosis patients lost into the community).
We also addressed several additional questions important for implementation of same-day microscopy. First, we identified no important sensitivity differences between the two-smear and three-smear front-loaded strategies. Second, front-loaded LED FM was as sensitive as standard LED FM when either two or three specimens were examined. Finally, for patients infected with HIV, frontloaded and standard microscopy seemed much the same in terms of diagnostic accuracy for ZN (three studies of two smears, two studies of three smears) and LED FM (two studies of two smears, one study of three smears); however, because there were few data, additional high-quality studies enrolling more patients infected with HIV at more sites than were identified in our review would provide greater precision and more generalisable evidence.
In 2009, findings from our systematic review were assessed by a WHO Expert Group and subsequently by STAG-TB, an independent group that reviews tuberculosis policy drafts and supporting documentation. In 2010, WHO issued a new policy stating that, “countries that have successfully implemented current WHO policy for a two-specimen case-finding strategy consider a switch to the same-day diagnosis approach, especially in settings where patients are likely to default from the diagnostic process. Countries that are still using the three specimen case-finding strategy should consider a gradual change to the same-day diagnosis approach, once WHO-recommended external microscopy quality assurance systems are in place and good quality microscopy results have been documented. It is essential that implementation of a same-day-diagnosis approach consider the programmatic, logistic, and operational implications at country level.”19
Our systematic review has several strengths, including a standardised protocol that accords with published guidelines for systematic reviews and meta-analyses. We obtained equivalent results through use of several approaches to data analysis (standard random-effects meta-analysis, generalised estimating-equations modelling, and HSROC analysis), and the overall methodological quality of included studies was high.
Although we did not systematically search for studies reporting patient-related outcomes, our comprehensive search strategy, including contact with the authors of all primary studies, make it likely that we would have identified such studies, and we did extract data for patient-related and programme-related outcomes when present. Unfortunately, we identified only one report25 that mentioned patient-related outcomes, in which patients assessed with front-loaded microscopy were half as likely to drop out of the diagnostic process as were those assessed with standard microscopy. Although the absolute decrease in dropout in that randomised controlled trial was small, greater reductions are likely in routine settings where dropout rates from standard microscopy are high. Although we did not identify any information about the timing of results reporting or treatment initiation, the WHO same-day diagnosis policy statement recommends that smear examination and results reporting occur before the patient leaves the health centre, to allow same-day treatment initiation among smear-positives. Same-day reporting might also have significant benefits for patients with negative smears, by reducing the economic burden of an additional clinic visit to collect results. Data from routine settings are needed to document the health-system changes required to successfully put the strategy into practice and understand its effect, comparing time-to-treatment initiation in patients suspected of pulmonary tuberculosis, costs to the patient, and satisfaction of the patient before and after implementation of same-day microscopy. Demonstration of the benefits for these patient-related and health-system outcomes would further strengthen the case for same-day microscopy.
Some observers have raised concerns about the potential effects of same-day microscopy on nosocomial tuberculosis transmission, because patients could spend additional time at the clinic on the day of initial assessment while submitting a second specimen and waiting for results. WHO guidelines on tuberculosis infection control recommend “speeding up management of [patients with suspected tuberculosis] so that they spend as little time as possible at the [health-care] facility”,28 and future studies should assess strategies to achieve this. Such measures include examination of two smears from one sputum collection and collection of specimens from patients with cough and other tuberculosis symptoms immediately after arrival in the clinic so that results are available while the clinician is assessing the patient. The former strategy in particular merits further assessment, because it might be simpler to implement in programmatic settings than would be collection of two specimens 1 h apart. Adjunctive measures including provision of face masks to patients suspected of tuberculosis and separating them from other clinic patients could also be employed to compensate for the additional clinic waiting time needed by same-day microscopy.29 Ultimately, if same-day microscopy can ensure that all smear-positive patients receive immediate tuberculosis treatment, the benefits in reduced tuberculosis transmission30 in the community would probably outweigh the nosocomial transmission risk arising from having patients waiting for an additional 1 h in the clinic.
Finally, in high-income settings where sputum culture is done in addition to microscopy, the effect of elimination of the morning sputum culture in those assessed with same-day collection is unknown. The incremental yield of culture of morning sputum (9%)31 is similar to that reported for culture of a second spot specimen (15%),32 but direct comparisons of how different specimen collection strategies affect the accuracy and usefulness of sputum culture are needed.
Same-day and front-loaded diagnostic strategies are a relatively new idea. All identified studies were done recently, with the earliest publication from 2007. Our search strategy might have missed relevant earlier studies, although we carefully reviewed bibliographies of related reviews and contacted any author who we thought might have relevant data. Statistical tests and graphical methods used to detect potential publication bias in meta-analyses of randomised trials are not accurate when applied to diagnostic data.33 Therefore, we cannot exclude the possibility of publication bias.
Same-day and front-loaded strategies are much the same in terms of diagnostic accuracy as are standard strategies for diagnosis of pulmonary tuberculosis by direct ZN light microscopy, irrespective of whether two or three specimens are collected. Overall, the moderate quality of evidence available supports introducing same-day microscopy into routine practice. The proportion of patients completing diagnostic evaluation was slightly greater with same-day than with standard microscopy in a large randomised controlled trial. Additional data for other patient-related outcomes are needed from routine settings. Finally, to realise the potential benefits of same-day strategies in sparing poor patients the costs of making several visits to health facilities, in increasing tuberculosis case detection, and in decreasing tuberculosis transmission in the community, sputum specimens ought not to just be collected on the first day of assessment—they should also be examined on that day so that results can be reported to clinicians and treatment started promptly.
Supplementary Material
Acknowledgments
JLD was supported by the WHO (P0025508) and US National Institutes of Health (NIH; K23AI080147). AC was supported by the NIH (K23HL094141). We thank Lydia Kivihya-Ndugga, Maarten van Cleeff, and Mohammed Yassin for helping obtain additional data, and for providing helpful suggestions on the analysis; Eric Vittinghoff for assistance with building hierarchical statistical models; Lelia Chaisson for assistance with preparation of the report; and Gloria Won for assistance in searching for and obtaining relevant articles.
Funding
WHO and US National Institutes of Health.
Footnotes
Contributors
JLD, AC, LEC, and KRS designed the study. JLD and KRS screened studies for inclusion and wrote the report. JLD, AC, LEC, and KRS analysed and interpreted the data. JLD, AC, LEC, PCH, and KRS critically revised the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
J Lucian Davis, Pulmonary and Critical Care Medicine Division, San Francisco General Hospital, and Curry International Tuberculosis Center, University of California, San Francisco, San Francisco, CA, USA.
Adithya Cattamanchi, Pulmonary and Critical Care Medicine Division, San Francisco General Hospital, and Curry International Tuberculosis Center, University of California, San Francisco, San Francisco, CA, USA.
Luis E Cuevas, Child and Reproductive Health Group, Liverpool School of Tropical Medicine, Liverpool, UK.
Philip C Hopewell, Pulmonary and Critical Care Medicine Division, San Francisco General Hospital, and Curry International Tuberculosis Center, University of California, San Francisco, San Francisco, CA, USA.
Karen R Steingart, Department of Health Services, University of Washington School of Public Health, Seattle, WA, USA.
References
- 1.Mase SR, Ramsay A, Ng V, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2007;11:485–495. [PubMed] [Google Scholar]
- 2.WHO. Reduction of number of smears for the diagnosis of pulmonary TB. Geneva: World Health Organization; 2008. [Google Scholar]
- 3.Katamba A, Laticevschi D, Rieder HL. Efficiency of a third serial sputum smear examination in the diagnosis of tuberculosis in Moldova and Uganda. Int J Tuberc Lung Dis. 2007;11:659–664. [PubMed] [Google Scholar]
- 4.Cambanis A, Ramsay A, Wirkom V, Tata E, Cuevas LE. Investing time in microscopy: an opportunity to optimise smear-based case detection of tuberculosis. Int J Tuberc Lung Dis. 2007;11:40–45. [PubMed] [Google Scholar]
- 5.Walker D, McNerney R, Mwembo MK, Foster S, Tihon V, Godfrey-Faussett P. An incremental cost-effectiveness analysis of the first, second and third sputum examination in the diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:246–251. [PubMed] [Google Scholar]
- 6.Kemp J, Squire SB, Nyirenda IK, FML S. Is tuberculosis diagnosis a barrier to care? Trans R Soc Trop Med Hyg. 1996;90:472. [Google Scholar]
- 7.Edginton ME, Wong ML, Phofa R, Mahlaba D, Hodkinson HJ. Tuberculosis at Chris Hani Baragwanath Hospital: numbers of patients diagnosed and outcomes of referrals to district clinics. Int J Tuberc Lung Dis. 1997;1:326–332. [PubMed] [Google Scholar]
- 8.Chandrasekaran V, Ramachandran R, Cunningham J, et al. Factors leading to tuberculosis diagnostic drop-out and delayed treatment initiation in Chennai, India. Int J Tuberc Lung Dis. 2005;9:172. [Google Scholar]
- 9.Nota A, Ayles H, Perkins M, Cunningham JA. Factors leading to tuberculosis diagnostic drop-out and delayed treatment initiation in urban Lusaka. Int J Tuberc Lung Dis. 2005;9:305. [Google Scholar]
- 10.Ouyang H, Chepote F, Gilman RH, Moore DA. Failure to complete the TB diagnostic algorithm in urban Peru: a study of contributing factors. Trop Doct. 2005;35:120–121. doi: 10.1258/0049475054037002. [DOI] [PubMed] [Google Scholar]
- 11.Squire SB, Belaye AK, Kashoti A, et al. ‘Lost’ smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis. 2005;9:25–31. [PubMed] [Google Scholar]
- 12.Zhang T, Tang S, Jun G, Whitehead M. Persistent problems of access to appropriate, affordable TB services in rural China: experiences of different socio-economic groups. BMC Public Health. 2007;7:19. doi: 10.1186/1471-2458-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp JR, Mann G, Simwaka BN, Salaniponi FM, Squire SB. Can Malawi’s poor afford free tuberculosis services? Patient and household costs associated with a tuberculosis diagnosis in Lilongwe. Bull World Health Organ. 2007;85:580–585. doi: 10.2471/BLT.06.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thongraung W, Chongsuvivatwong V, Pungrassamee P. Multilevel factors affecting tuberculosis diagnosis and initial treatment. J Eval Clin Pract. 2008;14:378–384. doi: 10.1111/j.1365-2753.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 15.Konde-Lule J, den Brave P, Coblens F. Third INTERACT scientific workshop. Entebbe, Uganda: 2009. Evaluation of tuberculosis suspects at Kampala city clinics. [Google Scholar]
- 16.Cambanis A, Yassin MA, Ramsay A, Squire SB, Arbide I, Cuevas LE. A one-day method for the diagnosis of pulmonary tuberculosis in rural Ethiopia. Int J Tuberc Lung Dis. 2006;10:230–232. [PubMed] [Google Scholar]
- 17.Hirao S, Yassin MA, Khamofu HG, et al. Same-day smears in the diagnosis of tuberculosis. Trop Med Int Health. 2007;12:1459–1463. doi: 10.1111/j.1365-3156.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay A, Yassin MA, Cambanis A, et al. Front-loading sputum microscopy services: an opportunity to optimise smear-based case detection of tuberculosis in high prevalence countries. J Trop Med. 2009;2009:1–6. doi: 10.1155/2009/398767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Same-day diagnosis of tuberculosis: policy statement. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 20.Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. In: Analysing and presenting results Cochrane handbook for systematic reviews of diagnostic test accuracy. Deeks J, Bossuyt P, Gatsonis C, editors. London: The Cochrane Collaboration; 2010. Version 0.9.0. [Google Scholar]
- 21.Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol. 2006;6:9. doi: 10.1186/1471-2288-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattamanchi A, Huang L, Worodria W, et al. Integrated strategies to optimize sputum smear microscopy: a prospective observational study. Am J Respir Crit Care Med. 2011;183:547–551. doi: 10.1164/rccm.201008-1207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuevas LE, Yassin MA, Al-Sonboli N, et al. A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med. 2011;8:e1000443. doi: 10.1371/journal.pmed.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuevas LE, Al-Sonboli N, Lawson L, et al. LED fluorescence microscopy for the diagnosis of pulmonary tuberculosis: a multi-country cross-sectional evaluation. PLoS Med. 2011;8:e1001057. doi: 10.1371/journal.pmed.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007;369:1955–1960. doi: 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Addendum to WHO guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings, 1999. Geneva: World Health Organization; 2006. Tuberculosis infection control in the era of expanding HIV care and treatment. [Google Scholar]
- 29.Dharmadhikari AS, Mphahlele M, Stoltz A, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 2012;185:1104–1109. doi: 10.1164/rccm.201107-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley RL, Mills CC, O’Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 31.Schoch OD, Rieder P, Tueller C, et al. Diagnostic yield of sputum, induced sputum, and bronchoscopy after radiologic tuberculosis screening. Am J Respir Crit Care Med. 2007;175:80–86. doi: 10.1164/rccm.200608-1092OC. [DOI] [PubMed] [Google Scholar]
- 32.Chan W, Chia M, Lee LK, Macfadyen DM. Bacteriological measures for the detection of cases of pulmonary tuberculosis. Bull World Health Organ. 1971;45:551–558. [PMC free article] [PubMed] [Google Scholar]
- 33.Tatsioni A, Zarin DA, Aronson N, et al. Challenges in systematic reviews of diagnostic technologies. Ann Intern Med. 2005;142:1048–1055. doi: 10.7326/0003-4819-142-12_part_2-200506211-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.