Abstract
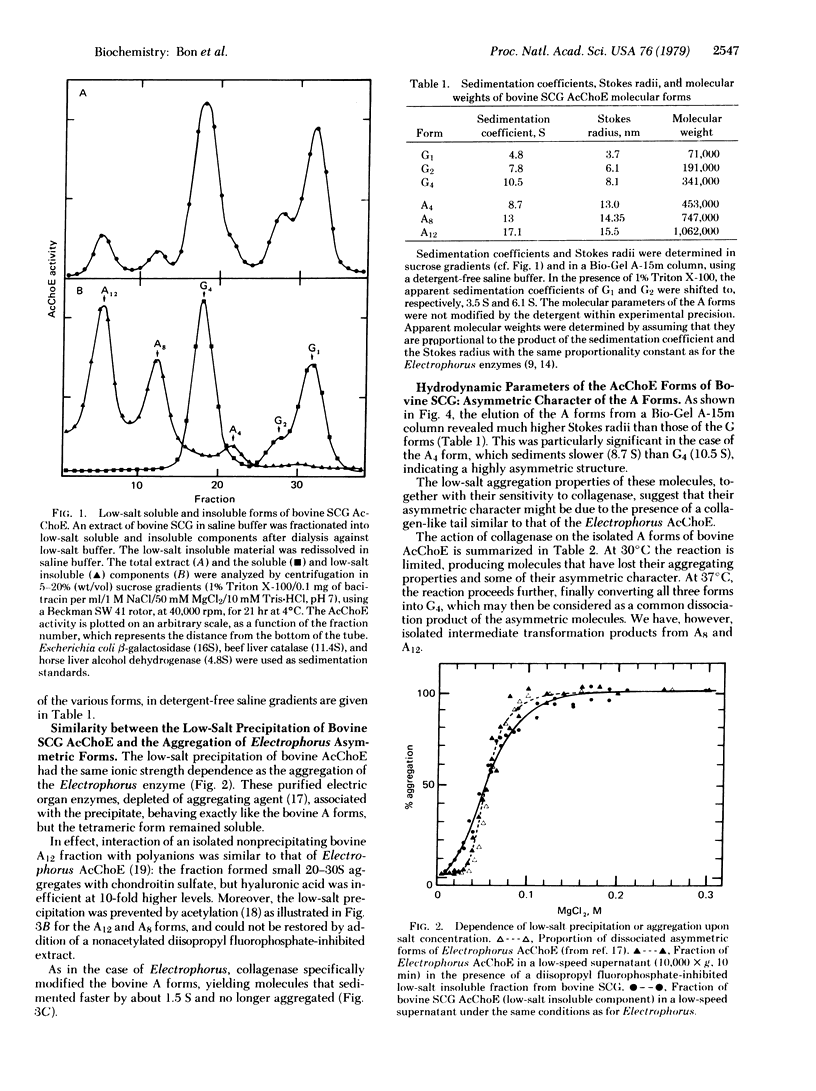
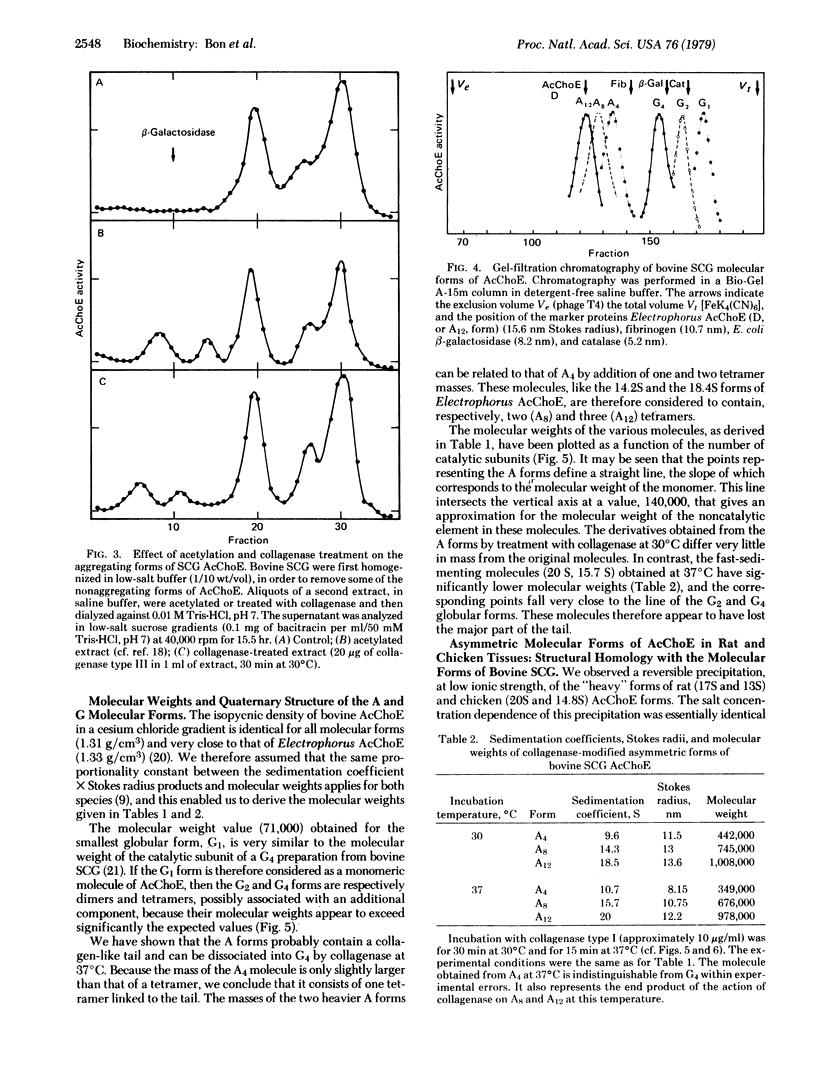
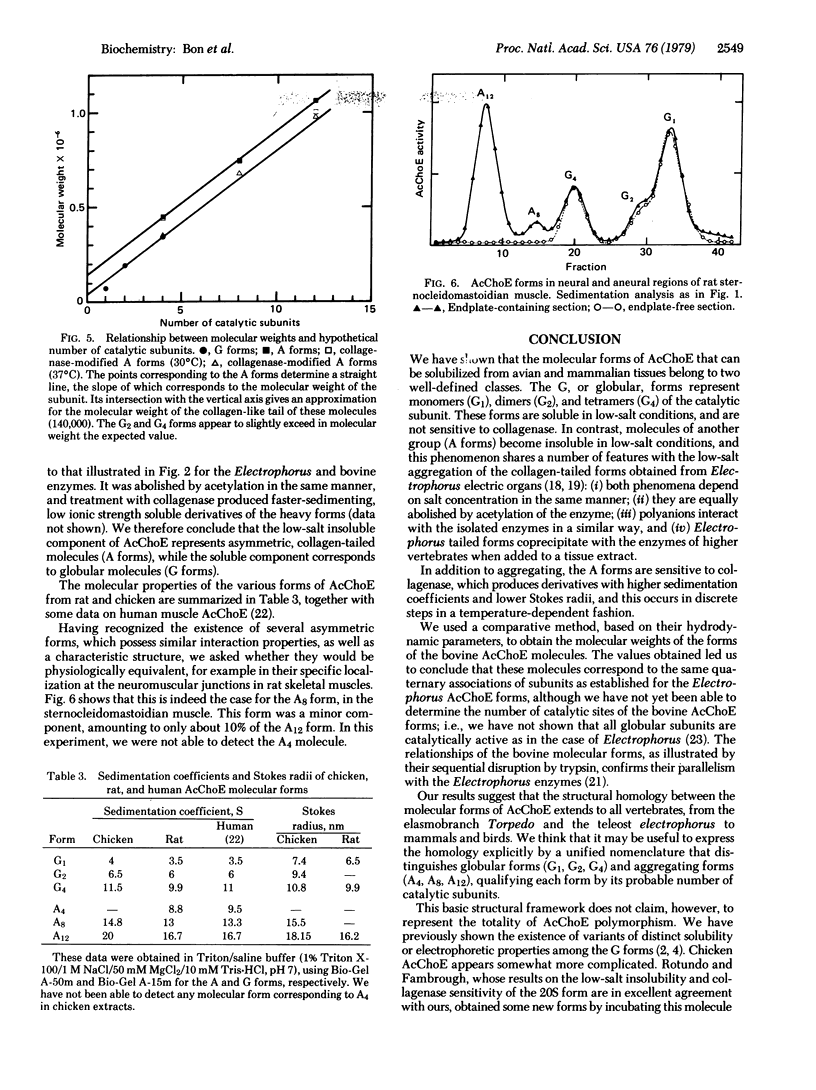
We have identified six molecular forms of acetylcholinesterase (AcChoE: acetylcholine hydrolase, EC 3.1.1.7) in extracts from bovine superior cervical ganglia. We show that three of them resemble the collagen-tailed forms of Electrophorus AcChoE in their hydrodynamic parameters, low-salt aggregation properties, and collagenase sensitivity. The six molecular forms of bovine AcChoE appear structurally homologous to the six forms of electric fish AcChoE that have previously been characterized. They include globular molecules (monomers, dimers, and tetramers) and asymmetric aggregating molecules that possess a collagen-like tail associated with one, two, and three tetramers. We propose to call the globular forms G1, G2, and G4 and the asymmetric forms A4, A8, and A12, the subscripts indicating the number of catalytic subunits. In spite of quantitative differences in their molecular parameters, the AcChoE forms from rat and chicken are clearly homologous to those of bovine AcChoE. Thus the nomenclature we introduce is very probably valid for the main AcChoE molecular forms, at least in vertebrates, and should help to clarify structural relationships and homologies among them. This model, however, does not claim to represent entirely the complex polymorphism of AcChoE, because more or less hydrophobic variants of the G forms have been observed, and because other molecular associations cannot be excluded. We discuss the significance of the globular and collagen-tailed structure for the molecular localization of AcChoE.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Silman I. Molecular structure of elongated forms of electric eel acetylcholinesterase. J Mol Biol. 1978 Nov 5;125(3):293–311. doi: 10.1016/0022-2836(78)90404-7. [DOI] [PubMed] [Google Scholar]
- Bon S., Cartaud J., Massoulié J. The dependence of acetylcholinesterase aggregation at low ionic strength upon a polyanionic component. Eur J Biochem. 1978 Apr;85(1):1–14. doi: 10.1111/j.1432-1033.1978.tb12207.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Huet M., Lemonnier M., Rieger F., Massoulié J. Molecular forms of Electrophorus acetylcholinesterase. Molecular weight and composition. Eur J Biochem. 1976 Sep 15;68(2):523–530. doi: 10.1111/j.1432-1033.1976.tb10840.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagenase sensitivity and aggregation properties of Electrophorus acetylcholinesterase. Eur J Biochem. 1978 Aug 15;89(1):89–94. doi: 10.1111/j.1432-1033.1978.tb20899.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Rieger F., Massoulié J. Propriétés des formes allongées de l'acétylcholinestérase en solution. Rayon de Stokes, densité et masse. Eur J Biochem. 1973 Jun;35(2):372–379. doi: 10.1111/j.1432-1033.1973.tb02849.x. [DOI] [PubMed] [Google Scholar]
- Carson S., Bon S., Vigny M., Massoulié J., Fardeau M. Distribution of acetylcholinesterase molecular forms in neural and non-neural sections of human muscle. FEBS Lett. 1979 Jan 15;97(2):348–352. doi: 10.1016/0014-5793(79)80119-2. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Bon S., Massoulié J. Electrophorus acetylcholinesterase. Biochemical and electron microscope characterization of low ionic strength aggregates. J Cell Biol. 1978 May;77(2):315–322. doi: 10.1083/jcb.77.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giamberardino L., Couraud J. Y. Rapid accumulation of high molecular weight acetylcholinesterase in transected sciatic nerve. Nature. 1978 Jan 12;271(5641):170–172. doi: 10.1038/271170a0. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I. The effects of solubilization procedures on the release and molecular state of acetylcholinesterase from electric organ tissue. J Neurochem. 1974 Dec;23(6):1177–1187. doi: 10.1111/j.1471-4159.1974.tb12215.x. [DOI] [PubMed] [Google Scholar]
- Gisiger V., Vigny M., Gautron J., Rieger F. Acetylcholinesterase of rat sympathetic ganglion: molecular forms, localization and effects of denervation. J Neurochem. 1978 Mar;30(3):501–516. doi: 10.1111/j.1471-4159.1978.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Johnson C. D., Smith S. P., Russell R. L. Electrophorus electricus acetylcholinesterases; separation and selective modification by collagenase. J Neurochem. 1977 Mar;28(3):617–624. doi: 10.1111/j.1471-4159.1977.tb10433.x. [DOI] [PubMed] [Google Scholar]
- Koenig J., Vigny M. Neural induction of the 16S acetylcholinesterase in muscle cell cultures. Nature. 1978 Jan 5;271(5640):75–77. doi: 10.1038/271075a0. [DOI] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Lappi S., Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976 Apr 6;15(7):1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- Marchand A., Chapouthier G., Massoulié J. Developmental aspects of acetylcholinesterase activity in chick brain. FEBS Lett. 1977 Jun 15;78(2):233–236. doi: 10.1016/0014-5793(77)80313-x. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Rieger F. L'acétylcholinestérase des organes électriques de poissons (torpille et gymnote); complexes membranaires. Eur J Biochem. 1969 Dec;11(3):441–455. doi: 10.1111/j.1432-1033.1969.tb00794.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Bosmann H. B. Molecular species of acetylcholinesterase in denervated rat skeletal muscle. Exp Neurol. 1976 Aug;52(2):263–271. doi: 10.1016/0014-4886(76)90170-9. [DOI] [PubMed] [Google Scholar]
- Rieger F., Bon S., Massoulié J., Cartauld J., Picard B., Benda P. Torpedo marmorata acetylcholinesterase; a comparison with the Electrophorus electricus enzyme. Molecular forms, subunits, electron microscopy, immunological relationship. Eur J Biochem. 1976 Sep 15;68(2):513–521. doi: 10.1111/j.1432-1033.1976.tb10839.x. [DOI] [PubMed] [Google Scholar]
- Rieger F., Faivre-Bauman A., Benda P., Vigny M. Molecular forms of acetylcholinesterase: their de novo synthesis in mouse neuroblastoma cells. J Neurochem. 1976 Nov;27(5):1059–1063. doi: 10.1111/j.1471-4159.1976.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Rieger F., Vigny M. Solubilization and physicochemical characterization of rat brain acetylcholinesterase: development and maturation of its molecular forms. J Neurochem. 1976 Jul;27(1):121–129. doi: 10.1111/j.1471-4159.1976.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Sketelj J., McNamee M. G., Wilson B. W. Effect of denervation on the molecular forms of acetylcholinesterase in normal and dystrophic chicken muscles. Exp Neurol. 1978 Jul;60(3):624–629. doi: 10.1016/0014-4886(78)90016-x. [DOI] [PubMed] [Google Scholar]
- Taylor P., Jacobs N. M. Interaction between bisquaternary ammonium ligands and acetylcholinesterase: complex formation studied by fluorescence quenching. Mol Pharmacol. 1974 Jan;10(1):93–107. [PubMed] [Google Scholar]
- Vigny M., Bon S., Massoulié J., Leterrier F. Active-site catalytic efficiency of acetylcholinesterase molecular forms in Electrophorus, torpedo, rat and chicken. Eur J Biochem. 1978 Apr 17;85(2):317–323. doi: 10.1111/j.1432-1033.1978.tb12241.x. [DOI] [PubMed] [Google Scholar]
- Vigny M., Di Giamberardino L., Couraud J. Y., Rieger F., Koenig J. Molecular forms of chicken acetylcholinesterase: effect of denervation. FEBS Lett. 1976 Oct 15;69(1):277–280. doi: 10.1016/0014-5793(76)80703-x. [DOI] [PubMed] [Google Scholar]
- Vigny M., Koenig J., Rieger F. The motor end-plate specific form of acetylcholinesterase: appearance during embryogenesis and re-innervation of rat muscle. J Neurochem. 1976 Dec;27(6):1347–1353. doi: 10.1111/j.1471-4159.1976.tb02614.x. [DOI] [PubMed] [Google Scholar]


