Abstract
Mouse models have been widely utilized to elucidate the basic principles and regulatory mechanisms of primordial follicle activation. Outside their natural environment, the growth of follicles might be affected by unknown factors in vitro and the elimination of regulation in vivo. Currently, in vitro culture and transplantation of ovaries under the kidney capsule are two commonly used incubation methods. However, the limited number of studies that have been published compare various incubation systems and reveal differences between ovaries that are incubated and grown in vivo. We compare the number of primordial, primary and secondary follicles in cultured, transplanted and in-vivo-grown ovaries. We investigate the expression levels of four genes, including zona pellucida 3 (ZP3), growth and differentiation factor-9 (GDF-9), proliferating cell nuclear antigen (PCNA) and anti-Müllerian hormone (AMH). Our results suggest that in vitro culture accelerates follicle activation, delays the transition from primary to secondary follicles and affects the expression patterns of ZP3, GDF-9, PCNA and AMH. A larger number of secondary follicles in ovaries cultured in alpha-minimal essential medium (α-MEM) had intact zona pellucida compared with those grown in Dulbecco’s modified Eagle medium containing Ham’s F-12 nutrient mixture (D/F12), suggesting that α-MEM is a better basal medium. The transplanted ovaries demonstrated the most similar characteristics to the in-vivo-grown ovaries, indicating that transplantation provided an optimal environment for ovarian incubation. This study has thus established the similarities and differences between in-vivo-grown and incubated ovaries, demonstrated that transplantation can mostly mimic the environment of ovarian growth in vivo and determined the optimal basal culture medium between α-MEM and D/F12.
Electronic supplementary material
The online version of this article (doi:10.1007/s00441-013-1678-7) contains supplementary material, which is available to authorized users.
Keywords: Culture system, Transplantation, Ovary, Follicle development, Spontaneous activation, Mouse
Introduction
The ovarian primordial follicle pool is established during embryonic development (e.g., in cows and humans) or at birth (e.g., in rodents). This pool of primordial follicles constitutes the complete supply of oocytes that have the potential to ovulate. After primordial follicle development is initiated, the follicles are destined either to ovulate or to undergo atresia. The factors that control the initiation of primordial follicle development are crucial for female fertility. Mechanisms for the initiation of follicular growth (activation) have only been partially elucidated and additional information concerning this process might be obtained by using a rodent model.
The fetal ovarian tissue of the mouse can be grown in vitro for more than 50 days (Blandau et al. 1965). The addition of substances such as chemicals, metabolic substrates, nutrients and hormones into the culture medium has been used to identify extra-ovarian factors that affect the regulation of ovarian function (Devine et al. 2002). Three-dimensional (3D) culture systems are now routinely used in many studies, ranging from cancer biology to tissue modeling and remodeling. Placement of the ovarian tissue onto a physical support in culture wells or dishes has been widely utilized. The use of a physical support provides a biological scaffold onto which the cells and organoids can grow. Synthetic matrices (Jackson et al. 2009; King et al. 2011) and man-made membranes or filters (Devine et al. 2002) are commonly used for support. However, early follicular growth cannot be accomplished in alginate (the most commonly used matrix) culture systems and mature secondary follicles fail to survive in this type of culture system (King et al. 2011). Thus, porous (0.4 μm) membranes are used in most laboratories that study the mechanisms of primordial follicular growth activation and the maintenance of quiescence (Parrott and Skinner 1999; Kezele et al. 2002b; Nilsson et al. 2002; Trombly et al. 2009). By this membrane culture technique, ovarian tissues can absorb sufficient nutrients from the medium below the support but are minimally submerged. In the earliest studies, the culture medium consisted in equal parts of chicken embryo extract prepared in Pannett and Compton’s solution and chicken plasma (Martinovitch 1938). The methods of Parrott and Skinner (1999) have been employed in most studies and depending upon the species, some slight modifications have been made. In contrast, the basal culture media appears to be randomly chosen upon personal preference. Alpha-minimal essential medium (α-MEM) and Dulbecco’s modified Eagle medium containing Ham’s F-12 nutrient mixture (D/F12) have been widely used for in vitro ovarian cultures. However, few original research studies have been performed to compare the different culture media.
Ovarian tissue transplantation is another noteworthy procedure that is used for follicular maturation. Orthotopic and heterotopic transplantation of fresh/cryopreserved whole ovaries/ovarian cortices has been performed in various animal species (Brannstrom and Diaz-Garcia 2011). The main application of this surgical technique is to develop human ovarian tissue, with the aim of to re-establishing fertility in cancer patients after fertility-threatening treatments (Smitz et al. 2010). Because of immunological rejection, the best site for these grafts has been identified to be under the kidney capsule in studies of castrated mice and rats (Aubard 2003). The recipient animals have been shown to serve as “incubators” for ovaries obtained from lethal genetic-deficient mice (Moniruzzaman et al. 2007) and ovaries obtained after short-term treatment in vitro (Li et al. 2010; Moniruzzaman et al. 2010). However, the drawback of this technique is clear. The transplanted ovaries are exposed to unidentified or unquantifiable biological components from the recipient animals. Thus, in vitro culture in a medium with defined components is still an irreplaceable method and is therefore utilized in most studies.
In vitro and in vivo environments differ significantly from each other and differences in the gene expression profiles of in-vivo- and in-vitro-cultured ovarian follicles have been demonstrated (Parrish et al. 2011). In some cases, results differ depending on whether the experiment is performed in vivo or in vitro. For example, one in vivo study indicated that progesterone acts to prevent nest breakdown via the inhibition of cellular apoptosis, whereas no evidence was found in vitro (Kezele and Skinner 2003), suggesting that the loss of an in vivo environment was sufficient to affect the experimental outcome. More importantly, the mechanism used in vitro might not exist in vivo. These results of in vitro experiments might be highly dependent on the presence of various factors, such as in co-treatments and the precise culture conditions. Thus, transplantation might provide a more stable ovarian growth microenvironment that could generate more reliable results.
In this study, ovaries obtained from 3-day-old C57BL/6J mice were cultured with various basal media or transplanted under the kidney capsule of castrated mice for 6 days. The development of follicles was examined in cultured, transplanted and freshly isolated ovaries from age-matched mice. We further profiled the protein expression patterns of four developmental genes, including zona pellucida 3 (ZP3), growth and differentiation factor-9 (GDF-9), proliferating cell nuclear antigen (PCNA) and anti-Müllerian hormone (AMH). In addition, we examined the mRNA expression of oocyte-derived Zp3, Gdf9 and granulosa-cell-derived Amh. The current study was designed to characterize cultured and transplanted ovaries and to evaluate the culture and transplantation methods by comparing the resultant ovaries with in-vivo-grown ovaries.
Materials and methods
Animals
Healthy female C57BL/6J mice were purchased from the Center for Laboratory Animal Administration of the Center for Disease Control and Prevention of Hubei Province (Wuhan, PR China) and bred at the Center for Laboratory Animal Administration of Tongji Medical College (Wuhan, PR China). The mice received humane care according to the Guide for the Care and Use of Laboratory Animals of the Chinese Academy of Sciences. The mice were provided with commercial pellet feed and had access to water ad libitum. They were allowed to give birth and were housed in a room with controlled light cycles (14 h light and 10 h dark) under specific pathogen-free conditions. For the culture and transplantation experiments, ovaries obtained from 3-day-old female mice were used because these ovaries predominantly contained primordial follicles. The ovaries were examined after 6 days of culture or transplant and thus, 9-day-old females were chosen as age-matched control mice. Day 1 was designated as the first 24 h after birth.
Ethics statement
The experiments were approved by the Animal Care Committee of the Tongji Medical College at the Huazhong University of Science and Technology in China.
Reagents
D/F12, α-MEM, penicillin/streptomycin (5,000 U/ml and 5,000 μg/ml, respectively), AlbuMAX, TRIzol, custom-designed primers and the Superscript III One-Step RT-PCR system were purchased from Invitrogen (Carlsbad, CA, USA). Leibovitz’s L-15 medium (L15) was purchased from Life Technologies (Grand Island, N.Y., USA). Bovine serum albumin (BSA), L-ascorbic acid, insulin, transferrin and sodium pyruvate (PNa) were obtained from Sigma-Aldrich (St. Louis, Mo., USA). Millicell-CM filter inserts were purchased from Millipore (Billerica, Mass., USA). The SYBR Green PCR master mix was purchased from Applied Biosystems (Foster City, Calif., USA). ZP3 polyclonal rabbit antibody and GDF-9 polyclonal goat antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif., USA). The AMH goat antibody was purchased from R&D Systems (Minneapolis, USA). The PCNA antibody, biotinylated secondary antibody and streptavidin biotin-peroxidase complex (SABC) were obtained from Boster Biological Technology (Boster, China). Peroxidase with 3,3′-diaminobenzidine tetrahydrochloride (DAB) was purchased from Zhongshan Goldenbridge Biotechnology (Zhongshan Goldenbridge, China).
In vitro ovarian culture
C57BL/6J female mice (3 days old) were deeply anesthetized with sodium pentobarbital (3 %, 2.5 ml/kg). The uterus, oviduct, fat and excess connective tissue were separated and the ovaries were placed on a piece of 0.4-μm Millicell-CM filter membrane, which was floating on 400 μl D/F12 or α-MEM supplemented with 0.1 % AlbuMAX, 0.1 % BSA, 0.05 mg/ml L-ascorbic acid, 0.23 mmol/l PNa, 50 μg/ml insulin, 27.5 μg/ml transferrin, 5 U/ml penicillin and 5 μg/ml streptomycin. Fine forceps were used to place a drop of medium over the top of the ovary to prevent its drying. The ovaries were cultured at 37 °C in a humidified atmosphere containing 5 % CO2 for 6 days. The media were removed and replaced with fresh media every 2 days. A fresh drop of medium was placed on top of each ovary with each media change. At the end of the culture period, the ovaries were either fixed in 4 % paraformaldehyde or stored at −80 °C for RNA isolation.
Transplantation
C57BL/6J female mice (3 days old) were deeply anesthetized with sodium pentobarbital (3 %, 2.5 ml/kg). The ovaries were removed, separated from the uterus, oviduct, fat and excess connective tissue and placed in sterile L15 medium containing 1 % penicillin/streptomycin at room temperature. Simultaneously, an ovariectomy was performed on the 3-month-old recipient mice. Under deep sodium pentobarbital anesthesia, the ovaries were exposed by using a paralumbar incision, the ovarian bursa was incised opposite of the ovarian hilum and the ovary was gently removed from the ovarian bursa. The ovary was excised by clamping at the ovarian hilum to prevent bleeding. After one kidney was exposed, sterile micro-tweezers were used to tear a small hole in the kidney capsule and one 3-day-old ovary was placed between the kidney tissue and its capsule. The muscle and skin were then sutured and the mice were regularly observed throughout convalescence for signs of pain and discomfort. After 6 days of individual housing, the recipient mice were killed by CO2 asphyxiation followed by decapitation and the ovaries without the attached kidney tissues were removed for histological analysis or stored at −80 °C for RNA isolation. All of the procedures were performed by using sterile instruments and aseptic techniques.
Morphological analysis and follicle counting
All of the paraffin sections were either used for immunohistochemical analysis or stained with hematoxylin and eosin (H&E). The number of follicles at each developmental stage were quantified in two serial sections from the largest cross-section through the center of the ovary (Parrott and Skinner 1999).
Primordial follicles are non-growing follicles and consist in an oocyte that is partially or completely encapsulated by flattened squamous pregranulosa cells; primary follicles contain an oocyte surrounded by a single layer of cuboidal-shaped granulosa cells; secondary follicles contain an oocyte surrounded by multiple layers of granulosa cells. A more accurate classification defines a type 2 follicle as a small oocyte with a few cells attached to its cell surface but without a complete ring of cells. Type 3a is an oocyte surrounded by a complete ring of follicle cells but with no more than 20 follicle cells on the largest cross-section. Type 3b is defined as a growing oocyte surrounded by one complete ring of follicle cells and with 21 to 60 cells present on the largest cross-section. Type 4 is defined as a growing oocyte surrounded by two layers of follicle cells and with 61 to 100 cells on the largest cross-section (Pedersen and Peters 1968).
The diameters of the oocytes were measured under a microscope. In the largest cross-section, only oocytes with visible nuclei were quantified. Both the largest and smallest diameters of each oocyte were measured and these measurements were then averaged. The data are expressed as the means ± SEM from at least 20 ovaries in each group.
Apoptosis assays
Apoptosis was assessed with a fluorescence-based FragEL DNA fragmentation kit (QIA39, Merck, Darmstadt, Germany). Paraffin sections were deparaffinized, rehydrated and permeabilized with 20 μg/ml proteinase K at 37 °C for 10 min in a humid chamber. After being washed, the sections were equilibrated with fresh equilibration buffer at 37 °C for 30 min. The sections were then incubated with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) reaction mixture at 37 °C for 1 h in a dark humid chamber. A reaction lacking enzyme was performed in parallel as a negative control (Fig. S1b). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Apoptotic cells carrying DNA labeled with fluorescein isothiocyanate (FITC)-dUTP were observed under a fluorescence microscope (Leica, Germany). The number of cells that stained positively (above the same threshold intensity) was determined in each image. Microscope settings and threshold values were kept constant for all images for a particular stain.
Immunohistochemistry
The localizations of ZP3, GDF-9, AMH and PCNA were determined by immunohistochemical analysis. The ovaries were fixed in 4 % paraformaldehyde for 24 h, transferred to 70 % ethanol, embedded in paraffin and serially sectioned (5 μm thick). Every 5th section was mounted onto a slide. Paraffin sections were heated at 60 °C for 2 h. Following deparaffinization and serial rehydration, antigen retrieval was performed on the sections by microwaving the tissues in sodium citrate buffer (0.01 M, pH 6.0) for 10 min. These sections were left to cool at room temperature and then washed three times for 5 min in phosphate-buffered saline (PBS), incubated in 3 % H2O2 for 15 min at 37 °C, followed by three additional 5-min washes. Subsequently, the tissues were blocked with 20 % normal goat serum or 5 % BSA for 1 h at 37 °C. After removal of the blocking reagent, the tissues were incubated with the appropriately diluted primary antibodies overnight at 4 °C. The sections were then washed in PBS and incubated with biotinylated secondary antibody and SABC. Antigen expression in the sections was detected with DAB. Negative controls (Fig. S1c, d) were incubated with PBS instead of the primary antibody. Images were captured by using confocal microscopy (DM4000B; Leica, Germany).
The immunostained slides were analyzed by using the commercially available ImageJ software (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nihimage/). Digital images were converted to 8-bit grayscale prior to the determination of the mean staining intensity per unit area of the selected structures. The polygonal selection tool was used to select the respective structures as described below. For GDF-9 (n = 20), the mean staining intensity per unit area for the cytoplasm of the oocytes at each developmental stage was determined. For AMH (n = 21), the mean staining intensity was determined for the granulosa cell layers of each follicle, which were outlined from the oocyte and theca cells with sufficient margins to avoid contamination of the untargeted cells. An average of 10 images from each ovary were obtained. All of the data were calculated by using the pixel brightness value. A higher pixel value represents stronger immunoreactivity.
RNA isolation and real-time polymerase chain reaction
Following 6 days of in vitro culture or transplantation, the ovaries were snap-frozen in liquid nitrogen and stored at −80 °C. For real-time polymerase chain reaction (PCR) analysis, RNA was isolated from seven ovaries per group. Total RNA was isolated by using TRIzol according to the manufacturer’s instructions. The RNA concentration was determined by using a NanoDrop spectrophotometer (λ = 260/280 nm; ND 1000; NanoDrop Technologies, Wilmington, Del., USA). Up to 2 μg total or amplified RNA was used for each reverse transcription (RT) reaction and 100 ng of cDNA served as the template for PCR. Real-time PCR assays were performed by using the SYBR Green PCR master mix and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh) served as the endogenous control. Gene amplification was relatively quantified by using the comparative cycle threshold (Ct) method (ΔΔCt; Livak and Schmittgen 2001). All of the reactions were run in triplicate with the following cycling program: 10 min holding at 95 °C and 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 20 s and extension at 72 °C for 30 s at which point the data were acquired. The melting conditions of the products were determined by using a temperature gradient from 60 °C to 95 °C with a 0.5 °C step increase. The PCR primers used were: Gapdh, 5′-TGT GTC CGT CGT GGA TCT GA-3′/5′-TTG CTG TTG AAG TCG CAG GAG-3′; Amh, 5′-TCC TAC ATC TGG CTG AAG TGA TAT G-3′/5′-CAG GTG GAG GCT CTT GGA ACT-3′; Gdf9, 5′-CGG GTG ACT GCC ATG GA-3′/5′-GGC AGA GTT GTT CAG AGT GTA TAG CA-3′; and Zp3, 5′-GAC TTC CAC GGT TGC CTT G-3′/5′-GCA GTC CAG CCT TCC ACA G-3′.
Statistical analysis
All of the results are expressed as the means ± SEM and were analyzed with SPSS 18.0 computer software (SPSS, Chicago, Ill., USA). Comparisons of multiple treatment groups or means were analyzed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) post hoc tests. Kruskal-Wallis nonparametric tests were used to assess the PCNA-positive granulosa cells and the proportion of developing follicles with zona pellucida filaments. Post hoc comparisons were performed by using Bonferroni-corrected and Mann–Whitney U tests. The assigned level of significance for all of the tests was P < 0.05.
Results
Observations of cultured and transplanted ovaries
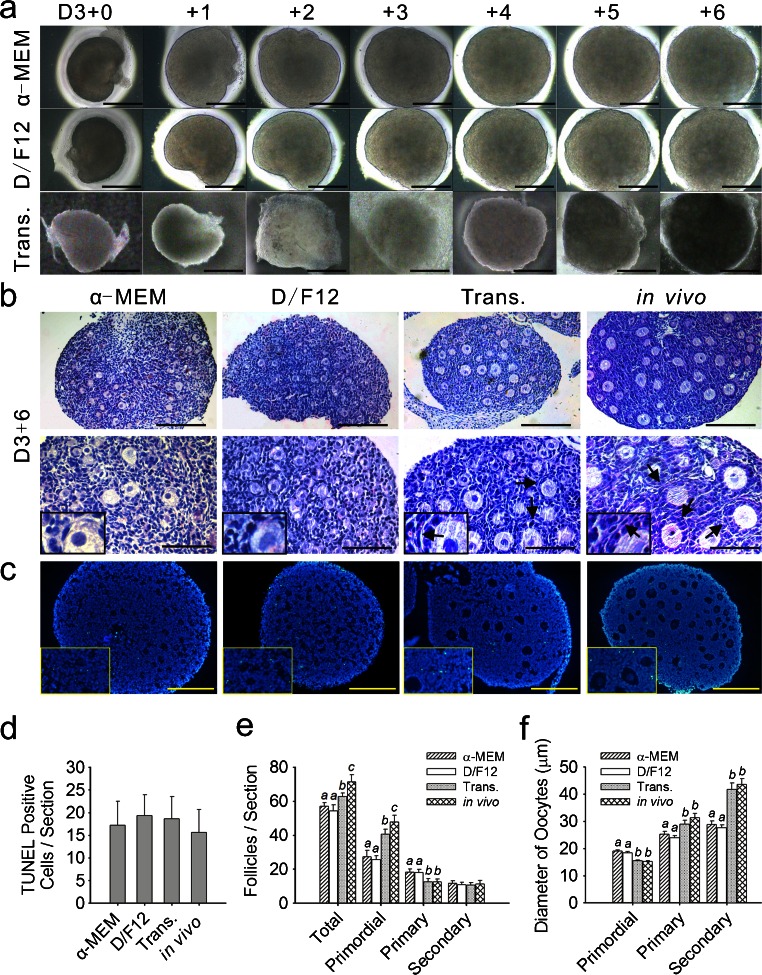
Whole ovaries were dissected from 3-day-old mice and cultured on floating filters or transplanted under the kidney capsule as previously described in the Materials and methods. The 3-day-old mice ovaries predominantly contained primordial follicles and a small number of early primary follicles (Fig. S2). The term D3+0 ovaries refers to the ovaries obtained from 3-day-old mice within 24 h after culture or transplantation. Slight damage caused by mechanical dissection was observed in the D3+0 ovaries. On day 0 of culture, some cells lay outside of the ovarian outline and the number of these diffused cells increased during in vitro culture (Fig. 1a). We stained the culture membrane with H&E (Fig. S3) and cells of two different sizes (arrows and arrowheads) were observed under and outside of the cultured ovaries (Fig. S3e, f). After 6 days of incubation, the ovaries appeared healthy under stereomicroscopy, as no obvious dark areas were detected (Fig. 1a).
Fig. 1.
Morphology of incubated ovaries and the development of follicles. a Observations of in-vitro-cultured and transplanted (Trans.) ovaries by stereomicroscopy (D3+0 time at which the ovaries were dissected from the 3-day-old mice). Images were captured every 24 h. Ovaries appeared healthy, without any obvious dark areas. b Ovaries were cultured for 6 days in enriched α-MEM or enriched D/F12, transplanted for 6 days under the kidney capsule in castrated mice and then freshly isolated from 9-day-old mice. Hematoxylin/eosin (H&E) staining (arrows thecal layer). Bars 160 μm (top row), 80 μm (bottom row). Insets High-magnification views. c TUNEL assays with no observable differences in cellular apoptosis among the four groups. Bars 80 μm. Insets High-magnification views. d Numbers of positively stained cells for TUNEL were counted in each stained section. Values are expressed as the means ± SEM; n = 11–15. e Following culture and transplantation, follicles were classified and quantified. Values are expressed as the means ± SEM; n = 20–22. Values with different letters were significantly different in the same stage follicles from the four groups (P < 0.05). f Mean diameters (μm ± SEM) of the oocytes from follicles at various stages in the cultured, transplanted (Trans.) and in-vivo-grown ovaries. Values with different letters were significantly different in the same stage follicles taken from the four groups (P < 0.05); n = 20–22
After 6 days of incubation, the ovaries contained some primordial follicles and many primary and secondary follicles of different sizes (Fig. 1b). Most of the primordial follicles were located in the cortical part of the ovary and the growing follicles were mostly found in the central part. Compared with the cultured ovaries, the growing follicles in the transplanted and in-vivo-grown ovaries had a better structure in the thecal layer (Fig. 1b, arrows) in which the stromal cells near the basal lamina were aligned parallel to each other and constituted the thecal layer. TUNEL assays were performed on the ovarian sections. Similar levels of apoptotic cell staining for incubated and in-vivo-grown ovaries were observed (Fig. 1c, d). The low-level TUNEL labeling was not indicative of necrotic tissue, thereby confirming the healthy status of the incubated ovaries.
Effects of the culture systems and transplantation on follicular development and oocyte growth
To determine the effects of the in-vitro-culture systems and transplantation on the growth of the follicles, the number of primordial, primary and secondary follicles was determined in the cultured, transplanted and in-vivo-grown ovaries (Fig. 1e). Both culture and transplantation caused follicle loss (P < 0.05) but the reduction in the total number of follicles was smaller in the transplanted ovaries compared with the cultured ovaries. After in vitro culture, the number of primordial follicles was lower compared with the in vivo and transplanted groups (P < 0.05), with a smaller but significant reduction being observed in the transplanted ovaries compared with the ovaries grown in vivo (P < 0.05). Uncoupled from the decreased number of primordial follicles, the number of primary follicles in the cultured ovaries was higher compared with the in vivo-grown ovaries and relatively fewer primary follicles had further developed into secondary follicles, as no significant differences were found in the number of secondary follicles (P > 0.05). These results indicated that the culture systems accelerated the initiation of primordial follicular growth but delayed the transition from primary to secondary follicles. Transplantation reduced the number of primordial follicles but did not affect the developing follicles.
We next measured the oocytes at all developmental stages to determine the effects of culture and transplantation on oocyte growth. In the cultured ovaries, the mean diameter of the oocytes of the primordial follicles was considerably (P < 0.05) larger than that of the transplanted and in-vivo-grown ovaries (Fig. 1f). Conversely, the mean oocyte diameter of the primary and secondary follicles was smaller (P < 0.05) in the cultured ovaries. The oocytes from the transplanted ovaries presented similar (P > 0.05) diameters to the ovaries grown in vivo (Fig. 1f). These results suggested that the culture systems promoted the growth of the oocytes in the primordial follicles but inhibited the growth of the oocytes in the primary and secondary follicles. Moreover, transplantation did not considerably affect the oocyte growth at any stage.
Effects of the culture systems and transplantation on oocyte quality
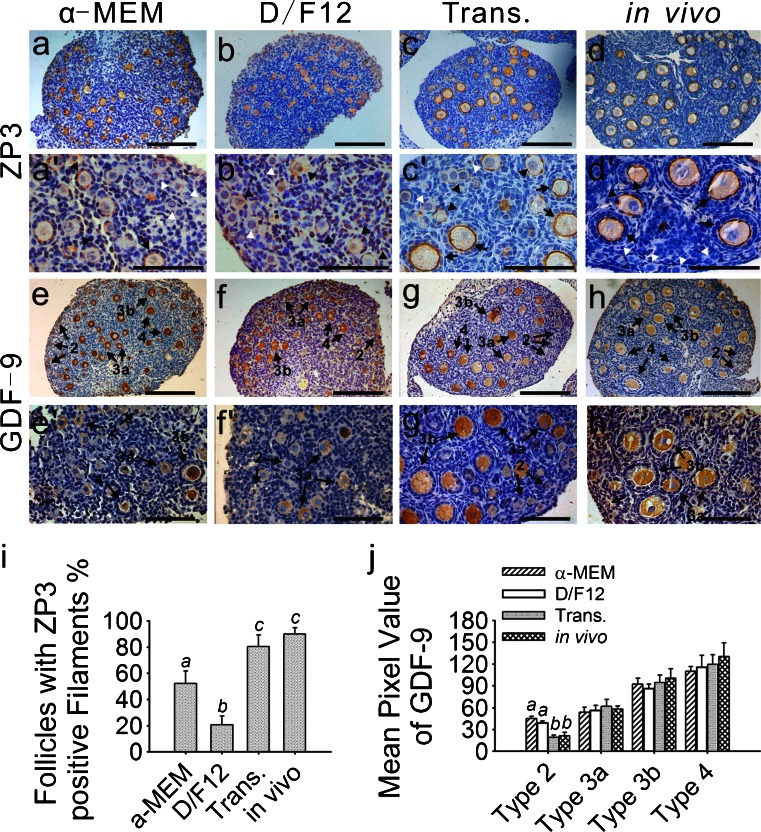
To evaluate the culture systems and transplantation further, an immunohistochemical study was performed on each experimental group. The immunoreactivity of ZP3 was examined to evaluate oocyte quality and zona pellucida structure. All of the developing follicles in the four groups were ZP3-positive and no staining was observed in the primordial follicles (Fig. 2a–d, a’–d’, white arrowheads). The transplanted ovaries had the most similar staining intensity and pattern to the in-vivo-grown ovaries. The highest intensity of ZP3 staining was present in the secondary follicles in the transplanted and in-vivo-grown ovaries (Fig. 2c, d, c’, d’). In these two groups, the zona pellucida filaments were intact within most of the developing follicles (80.3 % and 90.0 %, respectively; Fig. 2i). The same stage follicles in the ovaries cultured in enriched α-MEM were moderately stained (Fig. 2a, a’) and 52.1 % of the developing follicles had intact but thinner zona pellucida filaments (Fig. 2i). In contrast with the other groups, only 20.7 % of the developing follicles in the ovaries cultured in enriched D/F12 contained ZP3-positive filamentous structures (Fig. 2i) and most of the growing follicles were devoid of positive filaments but the cytoplasm of the growing oocytes was moderately stained (Fig. 2b, b’).
Fig. 2.
Immunohistochemical localization of zona pellucida 3 (ZP3) and growth and differentiation factor-9 (GDF-9). Ovaries that were freshly isolated from 9-day-old mice, cultured or transplanted for 6 days were collected. Immunohistochemistry was performed as described. a-d, a’-d’ ZP3 expression was present in all of the developing follicles in the four groups, whereas no staining was observed in the primordial follicles (white arrowhead). Compared with the transplanted (c, c’) and in-vivo-grown ovaries (d, d’), the developing follicles from the ovaries cultured in enriched α-MEM (a, a’) contained intact but thinner ZP3-positive filaments (arrows). Staining in the D/F12-medium-cultured ovaries was predominantly located in the cytoplasm of the oocytes (arrowheads follicles lacking intact filaments) and lacked an intact filamentous structure surrounding the oocytes (b, b’). Strongly ZP3-positive filaments were observed in most of the developing follicles (arrows) from the transplanted (c, c’) and in-vivo-grown ovaries (d, d’). e, f, e’, f’ GDF-9 staining was specific to the cytoplasm of the oocytes. In the cultured ovaries (e, f, e’, f’), the oocytes from the type 2 follicles began to demonstrate weak staining in the cytoplasm and more intense staining was detected in the more developed follicles. However, no immunoreactivity was present in the type 2 follicles in the transplanted (g, g’) or in-vivo-grown ovaries (h, h’). Bars 160 μm (a–h), 80 μm (a’–h’). i Percentages of follicles with intact zona pellucida filaments are represented as the percentage of total developing follicles from each group. In the ovaries cultured in enriched D/F12, only 20.7 % of the developing follicles contained ZP3-positive filamentous structures; this was significantly lower than the value in the other groups. For example, the ovaries cultured in enriched α-MEM contained 52.1 % developing follicles with ZP3-positive filamentous structures. No significant difference was observed between the transplanted (80.3 %) and in-vivo-grown ovaries (90.0 %). Different letters indicate significant differences among the four groups (P < 0.05); n = 19. j Intensity of the GDF-9 immunoreaction was measured as the mean value of pixel brightness in the cytoplasm of the oocytes. Follicles were classified as described. Higher pixel value reflects higher immunoreactivity. In cultured ovaries, the mean pixel value of the type 2 (primordial) follicles was significantly high. No significant difference was seen in the other stages of follicles from the four groups. Different letters indicate significant differences among the follicles at the same stage taken from the four different groups (P < 0.05); n = 20
Oocyte quality was also assessed by analyzing the expression of GDF-9. GDF-9 staining is specific to the cytoplasm of the oocytes. In the transplanted and in-vivo-grown ovaries, the oocytes within the primordial follicles (type 2) failed to stain for GDF-9 (Fig. 2g, h, g’, h’). In contrast, weak immunoreactivity was found in the type 2 follicles in the cultured ovaries (Fig. 2e, f, e’, f’), consistent with the quantification results (P < 0.05; Fig. 2j). In all of the ovaries, the oocytes from the type 3a primary follicles demonstrated moderate staining in the cytoplasm and a higher level staining was detected in the further developing follicles (Fig. 2e–h, e’–h’). No clear differences in the pixel values for the developing follicles at each stage could be observed among the four groups (Fig. 2j).
Effects of the culture systems and transplantation on granulosa cell growth
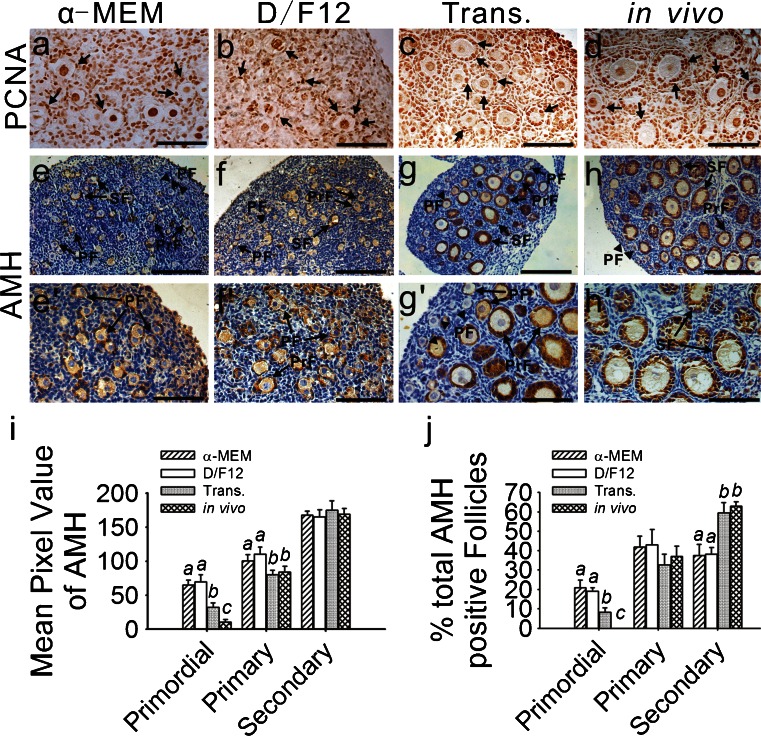
Granulosa cell proliferation was examined by PCNA staining in the cultured, transplanted and in-vivo-grown ovaries (Fig. 3a–d). No significant difference (P > 0.05) was observed in the transplanted and in-vivo-grown ovaries. In these two groups, approximately 9.0 % of the granulosa cells within the primordial follicles and more than 70.0 % within the developing follicles were PCNA-positive (Table 1). Compared with the transplanted and in-vivo-grown ovaries, the proportion of PCNA-positive granulosa cells within the primordial follicles was significantly higher in the cultured ovaries, although the proportion in the developing follicles was predominantly lower (P < 0.05; Table 1).
Fig. 3.
Immunohistochemical localization of proliferating cell nuclear antigen (PCNA) and anti-Müllerian hormone (AMH). a–d PCNA expression was specific to the nuclei of both the oocytes and granulosa cells. To reveal clear PCNA-positive staining, the nuclei were not counterstained with hematoxylin. Positive immunoreactivity (arrows) is represented by dark brown staining. e-h, e’–h’ AMH immunoreactivity was present in the cytoplasm of the granulosa cells. The theca cells and interstitium in all of the ovaries failed to stain for AMH. In the cultured (e, f, e’, f’) and transplanted ovaries (g, g’), AMH expression was found in the granulosa cells from some of the primordial, primary and secondary follicles (PF primordial follicle, PrF primary follicle, SF secondary follicles, arrow positive follicles, arrowhead negative follicles). No AMH-positive primordial follicles were found in the in-vivo-grown ovaries (h, h’). Bars 160 μm (a–h), 80 μm (e’–h’).i Intensity of the AMH immunoreaction was measured as the mean value of pixel brightness in the granulosa cells of the follicles at various stages. Higher pixel value represents higher immunoreactivity. Different letters indicate significant differences among the mean values of pixel brightness in the granulosa cells of the same stage follicles from the four different groups (p < 0.05); n = 21. j Percentages of AMH-positive follicles at different stages are represented as the % of total AMH-positive follicles from each group. Different letters indicate significant differences among the percentages of positive follicles at the same stage taken from the four different groups (P < 0.05); n = 21
Table 1.
Percentages of proliferating cell nuclear antigen (PCNA)-positive granulosa cells (GCs) within the primordial and developing follicles in ovaries that were grown in vivo, transplanted, or cultured for 6 days
| Treatment | Percentage of PCNA-positive GCs within primordial follicles | Percentage of PCNA-positive GCs within developing follicles | |
|---|---|---|---|
| In vivo | 9.92 ± 1.34 a | 78.67 ± 5.82 a | |
| Transplanted | 9.14 ± 2.19 a | 73.60 ± 7.03 a | |
| Cultured | α-MEM | 28.82 ± 1.99 b | 42.55 ± 2.37 b |
| D/F12 | 29.69 ± 1.85 b | 44.61 ± 2.38 b | |
Data are presented as the means ± SEM; n = 23 per group
Values with different letters denote significant differences among the four groups (P < 0.05)
To assess the quality of the granulosa cells, somatic-cell-derived AMH was examined. AMH protein expression was found in the cytoplasm of the granulosa cells in the ovaries obtained from all of the experimental groups. However, no AMH staining was observed in the thecal cells or interstitium (Fig. 3e–h, e’–h’). In the in-vivo-grown ovaries, the granulosa cells in the secondary follicles had a high intensity of AMH staining and moderate staining was observed in the primary follicles. Moreover, no staining occurred in the primordial follicles (Fig. 3h, h’). However, some primordial follicles from the cultured (Fig. 3e, f, e’, f’) and transplanted (Fig. 3g, g’) ovaries exhibited weak immunoreactivity, an observation supported by our quantification results (Fig. 3i). These findings suggested that the granulosa cells from the positive primordial follicles had acquired the ability to synthesize AMH. The staining density of the primordial follicles in the cultured ovaries was significantly (P < 0.05) higher than in the transplanted ovaries (Fig. 3i). Further categorical analysis revealed that the primordial follicles contributed to approximately 20.0 % and 8.3 % of the total AMH-positive follicles in the cultured and transplanted ovaries, respectively (Fig. 3j). The primary follicles in the cultured ovaries had a relatively higher expression compared with the same stage follicles from the other two groups and the secondary follicles exhibited a similar expression level in all of the groups (P > 0.05; Fig. 3i). Furthermore, 59.4 % and 63.0 % of the total AMH-positive follicles in the transplanted and in-vivo-grown ovaries were secondary follicles, whereas this percentage decreased to 37.4 % and 38.0 % in the ovaries cultured in enriched α-MEM and enriched D/F12, respectively (Fig. 3j).
Effects of the culture systems and transplantation on mRNA expression during ovarian growth
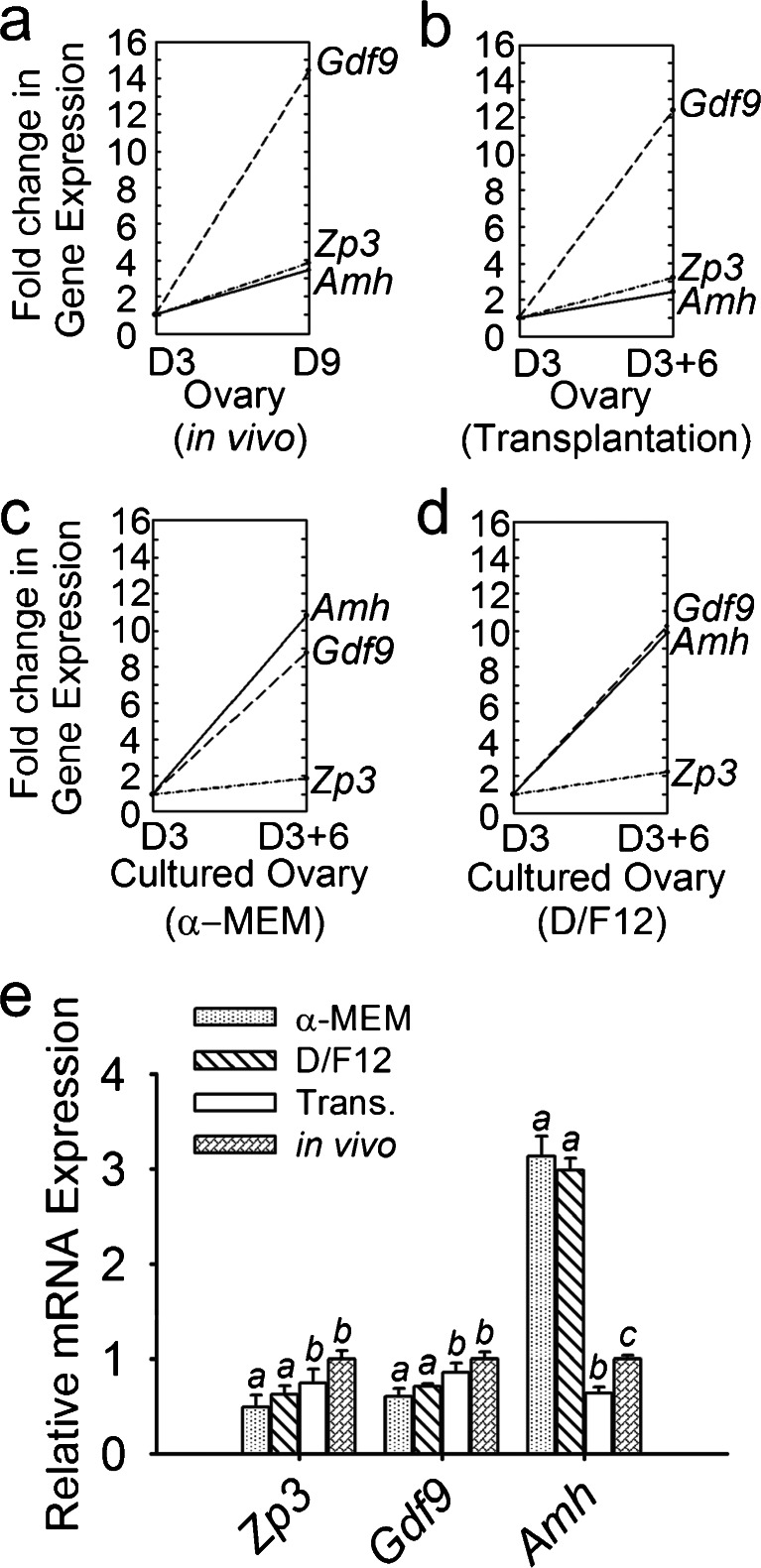
To investigate whether the change in protein expression in the cultured ovaries was reflected in the levels of mRNA expression, the mRNA expression of oocyte-specific markers (Gdf9 and Zp3) and somatic-cell-derived Amh was examined by using semi-quantitative real-time PCR (Fig. 4). In the in-vivo-grown ovaries, the expression of Amh, Gdf9 and Zp3 in freshly isolated 9-day-old ovaries increased with fold changes of 3.43, 14.47 and 3.70, respectively, compared with 3-day-old ovaries (Fig. 4a). In the transplanted ovaries, the fold change of each gene was lower than in vivo but a significant difference was observed only in the expression of Amh (P < 0.05; Fig. 4e). Gdf9 was the gene that increased the most dramatically in both the transplanted and in-vivo-grown ovaries (Fig. 4a, b). However, the expression level of the genes increased in a different mode in the cultured ovaries. Amh increased approximately 11-fold after 6 days of culture, whereas Gdf9 and Zp3 exhibited a moderate increase (Fig. 4c, d). Significant differences in the Amh expression levels were observed among the cultured, transplanted and in-vivo-grown ovaries (P < 0.05; Fig. 4e). In addition, no changes were seen in the levels of mRNA for Gdf9 and Zp3 between the transplanted and in-vivo-grown ovaries (P > 0.05; Fig. 4c).
Fig. 4.
Relative gene expression patterns and levels in the four groups. Gene expression levels of the ovaries that were freshly isolated from 3-day-old mice were defined as the baselines. Compared with each baseline, the fold changes are presented for the freshly isolated ovaries from 9-day-old mice (a), ovaries incubated under the kidney capsule of castrated mice for 6 days (b), ovaries cultured for 6 days in enriched α-MEM (c) and ovaries cultured for 6 days in enriched D/F12 (d). The fold changes in the expression of Zp3, Gdf9 and Amh among these four groups are shown in e. In the in-vivo-grown ovaries, the expression of Zp3, Gdf9 and Amh in freshly isolated 9-day-old ovaries increased, with fold changes of 3.43, 14.47 and 3.70, respectively, compared with 3-day-old ovaries. Amh significantly increased approximately 11-fold after 6 days in culture and decreased after transplantation (Trans.; P < 0.05). Compared with the in-vivo-grown ovaries, the fold changes in the expression of Zp3 and Gdf9 were lower in the cultured ovaries (P < 0.05), whereas they were similar in the transplanted ovaries (P > 0.05). Data are presented as the means ± SEM from five separate experiments; n = 7 per group. Values with different letters denote significant differences (P < 0.05)
Discussion
In vitro culture and transplantation of ovaries are two commonly used incubation methods. However, few studies have been published that compare different incubation systems and reveal differences between ovaries incubated or grown in vivo. The aim of this study has been to evaluate the effects of culture system and transplantation on the initiation of primordial follicle development and the transition from primary to secondary follicles. Our results showed that both cultured and transplanted ovaries had a generally healthy status. However, in vitro culture promoted primordial follicle loss, accelerated primordial follicle activation and delayed the transition from primary to secondary follicles. Moreover, the culture systems affected the quality of oocytes and granulosa cells in growing follicles, as the expressions of oocyte-derived ZP3, GDF-9 and granulosa-cell-derived AMH were altered in the cultured ovaries. In addition to the low quality, the proliferation of granulosa cells was decreased in the culture system. Compared with cultured ovaries, the transplanted ovaries presented characteristics more similar to those seen in in-vivo-grown ovaries, indicating that transplantation can mostly mimic the environment of ovary growth in vivo.
Our results further showed that an increasing number of cells diffused away from the ovaries during culture. Previous studies have reported that cortical follicles can travel toward the surface and these follicles have been identified as subepithelial or intra-epithelial follicles. Further transitions result in the oocytes or follicles lying completely outside of the ovary (Tingen et al. 2009). These diffused cells exhibited two different sizes in our study, indicating two different cell types. We assumed that the larger cells were oocytes and that the smaller cells were ovarian somatic cells. These diffused cells might also be present because of damage during isolation and primordial follicle shedding during culture. Somatic cell proliferation might have also partially contributed to the increasing number of diffused cells. More importantly, in vitro culture, which is a diffused method, might enhance this cortical follicle shedding, thereby promoting cell diffusion and an increase in primordial follicle loss.
In our study, in vitro culture significantly decreased the number of primordial follicles of the 3-day-old mice ovaries over 6 days. In addition to primordial follicle shedding as previously described, spontaneous activation and the development of these follicles might account for these subsequent stages. The spontaneous activation of primordial follicles has also been observed in the culture of whole rodent ovaries (Eppig and O’Brien 1996; Parrott and Skinner 1999; Nilsson et al. 2001). This may arise because culture medium is richer in nutrients (amino acids, carbohydrates and vitamins) than physiological conditions (Hartshorne 1997), which promote follicular development. In addition, the endogenous production of Kit ligand (KITL) and other potential exogenous growth factors, to which follicles are not exposed in vivo, might contribute to spontaneous activation (Parrott and Skinner 1999). Another hypothesis is that the absence of several important inhibitory factors, such as steroid hormones (Kezele and Skinner 2003) and AMH (Durlinger et al. 2002; Carlsson et al. 2006), might also trigger spontaneous activation. We have demonstrated that, although both AMH mRNA (Fig. 4c, d, e) and protein (Fig. 3i, j) expression are higher in the cultured ovaries compared with the transplanted and in-vivo-grown ovaries, the high expression of AMH is not sufficient to inhibit spontaneous activation.
In contrast with in vitro culture, transplantation did not cause clearly observed spontaneous activation in this study. These results were similar to in ovo experiments, in which the spontaneous activation of primordial follicles, which occurred in vitro, could be completely inhibited in ovo (Fortune et al. 2000; Cushman et al. 2002; Gigli et al. 2005). Grafted ovarian tissue is rapidly vascularized in ovo (Martinez-Madrid et al. 2009). AMH in the chick circulation inhibits follicle activation (Gigli et al. 2005). In our study, AMH and/or other factors of systemic origin might have regulated primordial follicle recruitment and prevented the spontaneous activation in the transplanted ovaries.
The loss of primordial follicles in the transplanted ovaries was mainly attributed to ischemic injuries. The kidney is rich in angiogenic factors, provides an ideal site for rapid revascularization and enhances the survival of grafts (Cox et al. 1996). Despite these natural advantages, ischemic injuries prior to revascularization are still a significant problem for implants. In mice, the presence of revascularization in autografts is observed 3 days after transplantation (Nugent et al. 1998). A previous study has reported that approximately 50 % of the primordial follicles survive in heterotopically grafted newborn mouse ovaries (Liu et al. 2002). Our results show a considerably lower rate of primordial follicle loss. This may be attributable to our use of 3-day-old mouse ovaries, in which follicular assembly is nearly completed and primordial follicles have formed (Rajah et al. 1992). The non-growing population of primordial follicles is located in a relatively avascular layer in the ovarian cortex and has lower metabolic requirements, suggesting that primordial follicles are less sensitive to ischemia. However, in newborn mouse ovaries, selected oocytes undergo a wave of apoptosis and surviving oocytes further form primordial follicles (Kezele et al. 2002a). During the process of assembly, ischemia might promote the apoptosis of oocytes and inhibit the formation of primordial follicles, resulting in a massive loss of primordial follicles in grafted newborn ovaries.
ZP3 is one of three sulfated glucoproteins in the mouse zona pellucida matrix and can be detected only in growing oocytes. As the diameter of the oocyte increases, the mRNA expression level of ZP3 also increases continuously prior to ovulation (Epifano et al. 1995). In our study, the ZP3 mRNA (Fig. 4c–e) and protein (Fig. 2a–d, a’–d’, i) expression levels were lower in the cultured ovaries, suggesting that the growth of the oocytes in the developing follicles was delayed. This growth delay was also indicated by smaller oocyte diameters in the developing follicles in vitro (Fig. 1f). The absence of FSH (follicle-stimulating hormone) in the culture system might delay the growth of the oocyte, as FSH receptors are expressed in the oocytes of intermediate and primary follicles (Meduri et al. 2002), indicating a potential effect of FSH on oocyte growth in primary follicles. Although similar Zp3 mRNA was found in the ovaries cultured in both media (Fig. 4e), a remarkably different pattern of ZP3 protein expression was observed in our immunohistochemical study in which ZP3 was expressed predominantly in the cytoplasm of the growing oocytes in the ovaries cultured in D/F12 medium (Fig. 2b, b’). These results can be explained by the delayed secretion and accumulation of the ZP3 protein in the cytoplasm. An additional possibility might be that ZPs extensively self-assemble within either the endoplasmic reticulum or the Golgi apparatus and the polymerized matrix might not be packaged and exported (Epifano et al. 1995; Jovine et al. 2004; Jovine et al. 2007).
GDF-9 is expressed in an oocyte-specific manner from an early stage (Dong et al. 1996). Low levels of GDF-9 expression are correlated with decreased oocyte quality (Rahman et al. 2012). In our study, although the staining density in growing follicles was similar among the four groups (Fig. 2j), the cultured ovaries exhibited a lower level of GDF-9 mRNA expression (Fig. 4e), indicating that the quality of oocytes was lower in the cultured ovaries compared with that of the transplanted and in-vivo-grown ovaries. Previous studies have reported that GDF-9 immunoreactivity is first observed at low (and variable) levels in the oocytes of type 3a follicles and is higher in the oocytes of type 3b follicles (Elvin et al. 1999). However, in our study, some type 2 follicles in the cultured ovaries were GDF-9-positive (Fig. 2e, f, e’, f’) and combined with the larger mean diameters of the oocytes (Fig. 1f), these results indicate the asynchronous development of primordial follicles in the culture system. Prior to the growth of pre-granulosa cells, the oocytes of the primordial follicles were activated and acquired the ability to synthesize GDF-9. The weaker proliferation of granulosa cells within the developing follicles (Table 1) could cause an imprecise analysis of follicular category in the cultured ovaries. This might be the reason that the pixel values for the same stage follicles were similar in all of the groups. Furthermore, the lower level of GDF-9 in the cultured ovaries might delay the transition of primary to secondary follicles. For example, GDF-9-deficient animals show an arrest in follicular development beyond the primary stage (Dong et al. 1996).
AMH is produced by the granulosa cells of early primary follicles as soon as primordial follicular growth is initiated. Thus, AMH is a marker of early follicular development (Durlinger et al. 2002). The significantly increased Amh mRNA expression in the cultured ovaries (Fig. 4c–e) indicated that the activation of the primordial follicles was accelerated and more primary follicles were formed, a finding that was also consistent with the results of our follicle quantification (Fig. 1e). This over-activation might stimulate granulosa cells to synthesize more AMH and the excess AMH might then function as a feedback regulator, acting to slow down the initiation of primordial follicle growth. Recent studies have reported that AMH expression is primarily found in the stromal cells surrounding oocyte nests and primordial follicles in rats (Nilsson et al. 2011). Consistent with a previous study (Durlinger et al. 2002), AMH expression in ovarian stromal cells from neonatal and 3-day-old mice was not detected by using immunohistochemistry in our study (data not shown). This can be attributed to the differences in the species used between studies. Surprisingly, AMH expression was found in some primordial follicles from the cultured and transplanted ovaries indicating that these AMH-positive granulosa cells were premature.
PCNA is considered the most reliable and versatile marker for cell proliferation (Iatropoulos and Williams 1996). A low percentage of PCNA-positive granulosa cells within the developing follicles was observed in the cultured ovaries, suggesting that the in-vitro-culture system decreased granulosa cell proliferation in the developing follicles. This might also the explain the lack of a pronounced theca layer in the growing follicles in the cultured ovaries, as granulosa-cell-derived factors, such as KITL (Parrott and Skinner 2000) and epidermal growth factor (Erickson and Case 1983), can attract stroma cells around the follicle and subsequently stimulate their proliferation and differentiation to form a theca layer. A previous study reported that PCNA expression marked the initiation of follicular growth, coinciding with and, in some cases, preceding the first sign of granulosa cell growth and preceding oocyte growth (Oktay et al. 1995). The cultured ovaries exhibited more PCNA-positive granulosa cells within their primordial follicles, indicating that the primordial follicle pool was over-activated in this system. As reported previously, PCNA expression can be upregulated in some quiescent cells by growth factors (Jaskulski et al. 1988). Thus, prematurely activated oocytes in the primordial follicle might secrete a large amount of growth factors and promote PCNA expression in the granulosa cells.
In conclusion, we have assessed the follicular content, oocyte growth and the expression of ZP3, GDF-9 and AMH in in-vitro-cultured, transplanted and in-vivo-grown ovaries. We have demonstrated that transplantation can mostly mimic the environment of ovary growth in vivo and have shown that α-MEM is a better basal medium than D/F12. Genes that are important for follicular development and that are differentially expressed in the culture system could be potential targets to improve culture conditions. This study provides a basis for further research studies into the creation of an optimal environment for follicular development with the aim of investigating the mechanisms of follicular initiation and of obtaining high-quality secondary follicles to produce mature oocytes that are competent for fertilization (Jin et al. 2010).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Negative controls for TUNEL assays and immunohistochemical staining. TUNEL assays (a) and a negative control (b) were performed in two serial sections. Bars 40 μm. Negative controls for the immunohistochemical staining of ZP3, GDF-9, AMH (c) and PCNA (d). Bars 160 μm, 80 μm. (JPEG 3 kb)
Morphology of ovaries obtained from 3-day-old mice. a Low-magnification view. Bar 100 μm. b High-magnification view. Bar 50 μm. c Primordial follicles. d Primary follicles. Bars 25 μm (c, d). (JPEG 3 kb)
Diffuse cells obtained from ovaries were cultured on membranes. Ovaries were placed on the membranes and cultured for 6 days (left, top). H&E staining of the membrane was performed after removal of the ovaries (right, top). Same letters mark the same ovaries. a-c Low-magnification view of the H&E-stained membrane. d-f High-magnification view of the H&E-stained membrane. e, f Large cells (arrows) and small cells (arrowheads). a1-a3 High-magnification view of the ovary from the tetragon top seen under a stereomicroscope (a1), after H&E staining (a2) and merged image (a3). (JPEG 10 kb)
Footnotes
Shuo Wang and Shuhong Yang contributed equally to this manuscript.
The authors have nothing to disclose.
This work was supported by grants from the Research Fund for the Public Service Section of the National Health Ministry of China (no. 200802159), the National Natural Science Foundation of China (no. 30973148) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 200804870009).
Contributor Information
Aiyue Luo, Phone: +86-15927174850, FAX: +86-27-83662681, Email: luoaiyue@aliyun.com.
Shixuan Wang, Phone: +86-27-83663180, FAX: +86-27-83662681, Email: sxwang@tjh.tjmu.edu.cn.
References
- Aubard Y. Ovarian tissue xenografting. Eur J Obstet Gynecol Reprod Biol. 2003;108:14–18. doi: 10.1016/S0301-2115(02)00424-4. [DOI] [PubMed] [Google Scholar]
- Blandau R, Warrick E, Rumery RE. In vitro cultivation of fetal mouse ovaries. Fertil Steril. 1965;16:705–715. doi: 10.1016/s0015-0282(16)35761-2. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Diaz-Garcia C. Transplantation of female genital organs. J Obstet Gynaecol Res. 2011;37:271–291. doi: 10.1111/j.1447-0756.2010.01416.x. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- Cox SL, Shaw J, Jenkin G. Transplantation of cryopreserved fetal ovarian tissue to adult recipients in mice. J Reprod Fertil. 1996;107:315–322. doi: 10.1530/jrf.0.1070315. [DOI] [PubMed] [Google Scholar]
- Cushman RA, Wahl CM, Fortune JE. Bovine ovarian cortical pieces grafted to chick embryonic membranes: a model for studies on the activation of primordial follicles. Hum Reprod. 2002;17:48–54. doi: 10.1093/humrep/17.1.48. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Rajapaksa KS, Hoyer PB. In vitro ovarian tissue and organ culture: a review. Front Biosci. 2002;7:d1979–d1989. doi: 10.2741/devine. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/en.143.3.1076. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/me.13.6.1035. [DOI] [PubMed] [Google Scholar]
- Epifano O, Liang LF, Familari M, Moos MC, Jr, Dean J. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development. 1995;121:1947–1956. doi: 10.1242/dev.121.7.1947. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Case E. Epidermal growth factor antagonizes ovarian theca-interstitial cytodifferentiation. Mol Cell Endocrinol. 1983;31:71–76. doi: 10.1016/0303-7207(83)90031-X. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Mol Cell Endocrinol. 2000;163:53–60. doi: 10.1016/S0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71:480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod. 1997;2:94–104. doi: 10.1530/ror.0.0020094. [DOI] [PubMed] [Google Scholar]
- Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol. 1996;48:175–181. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]
- Jackson KS, Inoue K, Davis DA, Hilliard TS, Burdette JE. Three-dimensional ovarian organ culture as a tool to study normal ovarian surface epithelial wound repair. Endocrinology. 2009;150:3921–3926. doi: 10.1210/en.2008-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskulski D, Gatti C, Travali S, Calabretta B, Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1988;263:10175–10179. [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. A duplicated motif controls assembly of zona pellucida domain proteins. Proc Natl Acad Sci U S A. 2004;101:5922–5927. doi: 10.1073/pnas.0401600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. Features that affect secretion and assembly of zona pellucida glycoproteins during mammalian oogenesis. Soc Reprod Fertil Suppl. 2007;63:187–201. [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Kezele P, Nilsson E, Skinner MK. Cell-cell interactions in primordial follicle assembly and development. Front Biosci. 2002;7:d1990–d1996. doi: 10.2741/kezele. [DOI] [PubMed] [Google Scholar]
- Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/S0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- King SM, Quartuccio S, Hilliard TS, Inoue K, Burdette JE. Alginate hydrogels for three-dimensional organ culture of ovaries and oviducts. J Vis Exp. 2011;52 pii:2804. doi: 10.3791/2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kawamura K, Cheng Y, Liu S, Klein C, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Van der Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17:605–611. doi: 10.1093/humrep/17.3.605. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Donnez J, Van Eyck AS, Veiga-Lopez A, Dolmans MM, Van Langendonckt A. Chick embryo chorioallantoic membrane (CAM) model: a useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil Steril. 2009;91:285–292. doi: 10.1016/j.fertnstert.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Martinovitch P. The development in vitro of the mammalian gonad. Ovary and ovogenesis. Proc R Soc Lond B Biol Sci. 1938;125:232–249. doi: 10.1098/rspb.1938.0024. [DOI] [Google Scholar]
- Meduri G, Charnaux N, Driancourt MA, Combettes L, Granet P, Vannier B, Loosfelt H, Milgrom E. Follicle-stimulating hormone receptors in oocytes? J Clin Endocrinol Metab. 2002;87:2266–2276. doi: 10.1210/jc.87.5.2266. [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M, Sakamaki K, Akazawa Y, Miyano T. Oocyte growth and follicular development in KIT-deficient Fas-knockout mice. Reproduction. 2007;133:117–125. doi: 10.1530/REP-06-0161. [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M, Lee J, Zengyo M, Miyano T. Knockdown of FOXO3 induces primordial oocyte activation in pigs. Reproduction. 2010;139:337–348. doi: 10.1530/REP-09-0207. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. doi: 10.1016/S0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65–73. doi: 10.1016/S0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of anti-Mullerian hormone (AMH) on ovarian primordial follicle assembly. PLoS One. 2011;6:e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil. 1998;114:341–346. doi: 10.1530/jrf.0.1140341. [DOI] [PubMed] [Google Scholar]
- Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53:295–301. doi: 10.1095/biolreprod53.2.295. [DOI] [PubMed] [Google Scholar]
- Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction. 2011;142:309–318. doi: 10.1530/REP-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/en.140.9.4262. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit ligand actions on ovarian stromal cells: effects on theca cell recruitment and steroid production. Mol Reprod Dev. 2000;55:55–64. doi: 10.1002/(SICI)1098-2795(200001)55:1<55::AID-MRD8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Mazzilli M, Pennarossa G, Brevini TA, Zecconi A, Gandolfi F. Chronic mastitis is associated with altered ovarian follicle development in dairy cattle. J Dairy Sci. 2012;95:1885–1893. doi: 10.3168/jds.2011-4815. [DOI] [PubMed] [Google Scholar]
- Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn. 1992;194:177–192. doi: 10.1002/aja.1001940303. [DOI] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol Reprod. 2009;81:16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology. 2009;150:1014–1024. doi: 10.1210/en.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Negative controls for TUNEL assays and immunohistochemical staining. TUNEL assays (a) and a negative control (b) were performed in two serial sections. Bars 40 μm. Negative controls for the immunohistochemical staining of ZP3, GDF-9, AMH (c) and PCNA (d). Bars 160 μm, 80 μm. (JPEG 3 kb)
Morphology of ovaries obtained from 3-day-old mice. a Low-magnification view. Bar 100 μm. b High-magnification view. Bar 50 μm. c Primordial follicles. d Primary follicles. Bars 25 μm (c, d). (JPEG 3 kb)
Diffuse cells obtained from ovaries were cultured on membranes. Ovaries were placed on the membranes and cultured for 6 days (left, top). H&E staining of the membrane was performed after removal of the ovaries (right, top). Same letters mark the same ovaries. a-c Low-magnification view of the H&E-stained membrane. d-f High-magnification view of the H&E-stained membrane. e, f Large cells (arrows) and small cells (arrowheads). a1-a3 High-magnification view of the ovary from the tetragon top seen under a stereomicroscope (a1), after H&E staining (a2) and merged image (a3). (JPEG 10 kb)