Abstract
Repeated bouts of acute and chronic lung infections are responsible for progressive pulmonary function decline in individuals with cystic fibrosis (CF), ultimately leading to respiratory failure and death. Pseudomonas aeruginosa is the archetypical CF pathogen, causes chronic infection in 70% of individuals, and is associated with an accelerated clinical decline. The management of P. aeruginosa in CF has been revolutionized with the development and widespread use of inhaled antibiotics. Aerosol delivery of antimicrobial compounds in CF enables extremely high concentrations of antibiotics to be reached directly at the site of infection potentially overcoming adaptive resistance and avoiding the potential for cumulative systemic toxicities. Tobramycin inhalation powder (TIP) represents the first dry powder inhaled (DPI) antibiotic available for use in CF. DPIs are notable for a markedly reduced time for administration, ease of portability, and increased compliance. TIP has been developed as a therapeutic alternative to tobramycin inhalation solution (TIS), the standard of care for the past 20 years within CF. Relative to TIS 300 mg nebulized twice daily in on-and-off cycles of 28 days duration, TIP 112 mg twice daily via the T-326 inhaler administered on the same schedule is associated with marked time savings, increased patient satisfaction, and comparable clinical end points. TIP represents an innovative treatment strategy for those individuals with CF and holds the promise of increased patient compliance and thus the potential for improved clinical outcomes.
Keywords: aerosolized, nebulized, antibiotics, tobramycin inhalation solution, TOBI, TIS, dry powder inhaler, DPI
Introduction
Cystic fibrosis (CF) is the most common lethal genetic disease seen in individuals of Northern European decent.1 It is diagnosed at a rate of 1:3400 live births in populations living in North America and Europe. The disease is manifested by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), a chloride transport channel resulting in impaired electrolyte transport across epithelial surfaces.2 The disease results in multisystem complications that may include sinopulmonary disease, exocrine and endocrine pancreatic dysfunction, impaired gastrointestinal transit, nutritional impairment, electrolyte disturbances, accelerated bone loss, and altered fertility potentials.1,3 In particular, CF is known as a disease of the respiratory tract, as the bulk of the morbidity and mortality attributable to this condition is associated with chronic pulmonary disease.
CF lung disease is notable for remarkably thick respiratory secretions resulting in impairment of mucociliary clearance.4 Accordingly, inhaled bacteria and particulate matter are ineffectively cleared from the respiratory tract. The viscous environment of the retained secretions in the respiratory tract promotes the development of chronic lower airway infections with environmental and pathogenic bacteria and fungi. Repeated episodes of infection and subsequent inflammation result in progressive lung injury, eventually manifesting as airways thickening, bronchiectasis, and end-stage lung disease manifesting as respiratory failure and ultimately death.1,3,5 Although CF is typified by a number of particular bacterial infections, Pseudomonas aeruginosa represents the archetypal CF pathogen.
Ultimately, 60% to 80% of CF patients will become chronically infected with P. aeruginosa.6,7 Chronic infection with P. aeruginosa and its conversion to an alginate hyper-producing, mucoid phenotype is associated with progressive decline in lung function, worsening radiographic appearance, increased risk of hospitalization, and reduced survival.8–11P. aeruginosa is notorious for its high rates of intrinsic antimicrobial resistance owing to a wide repertoire of mechanisms including reduced outer membrane permeability, multiple redundant broad spectrum drug efflux pumps, antimicrobial modifying enzymes, and target site changes.12 Furthermore, the emergence of de novo resistance is readily observed upon exposure to antimicrobial therapy.
Antibiotics have long been a standard treatment regimen in CF.3 However, the armamentarium available for clinicians to treat both acute and chronic infectious complications of CF is tremendously limited.13,14 Further compounding these difficulties is the limited availability of oral antibiotics with sufficient bioavailability to treat infections, with only 3 drugs possessing even modest activity: ciprofloxacin, levofloxacin, and chloramphenicol. The use of routine regular parenteral antipseudomonal antibiotics has been associated with reduced rates of pulmonary function decline and increased survival.15 Unsurprisingly, this strategy is associated with significant strain on limited health care infrastructure and staffing resources. Furthermore, recent studies have suggested that there is a cumulative nephrotoxic potential associated with parenteral antibiotics such as aminoglycosides (gentamicin demonstrating a greater risk then tobramycin) and colistin.16–18 Certain patient comorbidities, especially CF-related diabetes, further enhance this risk. Elborn et al conducted a prospective multicenter study comparing elective quarterly parenteral antibiotic treatment courses versus pulmonary exacerbation driven delivery of parenteral antibiotics.19 After 3 years of follow up, not only was there no difference in the rates of lung function decline in those individuals treated with symptom-driven prescriptions of parenteral antibiotics, fewer courses of treatments were required. Accordingly, the practice of regular “tune up” antibiotic treatments for asymptomatic chronic P. aeruginosa infection CF has largely been abandoned.20
With limited oral and parenteral therapies available, CF clinicians sought alternate antimicrobial treatment strategies. The concept of aerosolized antibiotics as a means of delivering potent therapy to the lower airways in CF was first proposed by Paul di Sant’Agnese and Dorothy Andersen in 1946.21 The theory of this approach is that aerosol delivery of antibiotics allows for the local deposition of supratherapeutic levels of drugs directly to the infected airways overcoming many intrinsic or adaptive resistance mechanisms while avoiding the potential for high levels of systemic exposure to the drug and resultant toxicity.22,23 Since this time, clinicians have used antimicrobials “off label” (including drugs from a multitude of classes such as aminoglycosides, beta-lactams [including carbapenems, cephalosporins, monobactams, and ureidopenicillins], fluoroquinolones, fosfomycin, glycopeptides, polymixins, and even antiseptics. However, as these drugs have traditionally been formulated and stabilized for parenteral delivery, there is often a high degree of intolerance. In particular, cough and chest tightness are thought to arise from airway irritation due to the chemical composition of these drugs including low pH, hypertonicity, and the presence of preservative such as bisulfites and phenols.22,24
Critically, in vitro predicted susceptibility to antibacterial agents appears to have little to do with the derived clinical benefit of aerosolized antibiotics in CF for the management of chronic P. aeruginosa infection. Firstly, it has been well established that chronic P. aeruginosa infection is typified by tremendous heterogeneity and phenotypic diversity.25–27 The antibiotic susceptibility profiles of multiple identical appearing isolates can be tremendously varied, and, as such, susceptible populations of organisms are likely always to persist at some level. More importantly, however, is the recognition that tremendous quantities of active drug can be safely delivered to the airways through aerosolization.22,23,28 One must recall that the break points used in defining in vitro predicted antibiotic susceptibility are determined by physiologically achievable levels of drugs delivered parenterally.28 As aerosol administration of drugs allows for supratherapeutic levels of the drug to be deposited directly at the site of infection while avoiding potential systemic toxicities, most resistance mechanisms can be overcome. Indeed, the clinical benefit derived from aerosolized antibiotics is of the same magnitude in patients infected by drug resistant P. aeruginosa as those with other susceptible isolates. Furthermore, evidence suggests that although concentrations of antibiotics may not be sufficient to be bactericidal, they may have the potential to downregulate virulence factor production, thereby reducing overall pathogenic potential.29,30
Antibiotics available for aerosol delivery in CF
There are now 3 commonly used aerosolized drugs, from 3 separate classes of antibiotics, to treat chronic P. aeruginosa infection within CF airways (Table 1). A full description of the benefits and detractors for each drugs use is beyond the scope of this limited manuscript. Colistin (colistin-methate sodium) is a polymixin antibiotic that was the first drug to be commonly used in chronic maintenance therapy.31 This represents the only parenteral formulation of drug that remains commonly used. Colistin is commonly used as a chronic medication in Europe but remains uncommon in North America. A recent dry powder inhaler version of colistin has been developed and is licensed for use in Europe alone at this point.32 Aztreonam lysine for inhalation (AZLI, Cayston, Gilead) is the most recently licensed drug. This drug has been developed and marketed using a new high efficiency nebulization system that uses vibrating mesh technology to generate precise particle sizes in a very short time period.33–37 For a recent review on this agent, readers are referred to the manuscript by Parkins and Elborn.38
Table 1.
Common inhaled therapies for management of chronic P. aerugionsa infection within CF.
| Product | Drug | Formulation | Administarion | Administration time (min) | Dose (mg) or MU | Storage | Common A/E |
|---|---|---|---|---|---|---|---|
| Aminoglycosides | |||||||
| Preservative free Tobramycin109 | TOB | IV | J-Neb | N/a (estimated <12 min) | 80 | Refrig. | Cough, chest tightness |
| TOBI40,98 | TOB | Aer | J-Neb | 20 | 300 | Refrig. | Cough, dysgeusia |
| BramiTob83 | TOB | Aer | J-Neb/ (HEN) | 13 (5) | 300 | Refrig. | Cough, dysgeusia |
| TIP98 | TOB | Aer | DPI | 5 | 112 | Room temp | Cough, dysgeusia |
| Colistin | |||||||
| Colomycin110 | COL | IV | Neb | 15 | 1–2 MU | Refrig. | Cough, |
| Colobreathe32 | COL | Aer | DPI | ~1 | 1.662 MU | Room temp | Cough, pharyngeal pain, dysgeusia |
| Beta-lactams | |||||||
| Aztreonam33,34 | AZT | Aer | HEN | 3 | 75 | Refrig. | Cough |
Abbreviations: TOB, tobramycin; COL, colistin; AZT, aztreonam; IV, IV formulation; Aer, aerosol specific formulation; J-Neb, jet nebulizer; HEN, high efficiency nebulizer; Refrig, must be refrigerated.
Inhaled antibiotics constitute one of the key disease modifying groups of therapies available for the management of individuals with CF and chronic P. aeruginosa infection. According to data from the Epidemiologic Study of Cystic Fibrosis (ESCF), a multicenter observational cohort of >18,000 individuals with CF, almost 60% of patients with chronic P. aeruginosa infection are receiving at least 1 inhaled antipseudomonal drug.39 Regular administration of aerosolized antibiotics has been demonstrated to improve quality of life and nutritional status and reduce the risk of pulmonary exacerbation, the need for rescue antibacterial therapy and hospitalization, rates of pulmonary function decline, and health care associated expenditures.33,35,40,41 Observational data suggest that use of inhaled antibiotics may even be associated with a lower risk of CF related mortality.42
Tobramycin is the most commonly used inhaled antipseudomonal drug in North America. Parenteral tobramycin preparations used for aerosolization were first described in 1983 by Stephens et al.43 The CF community has used tobramycin in various formulations for over 30 years. Presently, multiple different tobramycin products are available and commonly used in CF. These include the preservative-free parenteral preparations, solutions for nebulization, and dry powder inhaler preparations (Table 1).
Tobramycin was the first agent selected for the development of a commercial preparation for the management of chronic P. aeruginosa infection in CF, designed specifically for aerosol delivery. The rationale for the selection of this drug over others was many fold: low levels of resistance; higher intrinsic activity relative to comparable aminoglycosides such as gentamicin; a prolonged postantibiotic effect; stability under a variety of conditions; being assayable relatively easily using conventional technologies; and being relatively palatable.23 The unique challenges associated with the delivery of an aerosolized antibiotic to treat chronic lower airways infection relate to the mechanism of administration. For the drug to target the lower airways, it must first pass through the oropharynx and upper airways without impaction.22 Factors that influence the distribution of drug particles include the particle size and heterogeneity determined predominately by combination of nebulizer and compressor used, flow rate, as well as patient factors such as inspiratory capacity, respiratory rate, and degree of structural lung disease.22,23 Furthermore, drug exhaust back into the natural environment must be minimized to avoid wastage and contamination. Accordingly, drug regulatory agencies have mandated that drugs developed, studied, and marketed for aerosol delivery must be associated with a single defined device in which drug aerosol kinetics have been determined.
Tobramycin is an aminoglycoside antibiotic derivative of kanamycin from Streptomyces tenebrarius that has been in clinical use since 1974.44–47 Tobramycin has potent activity against aerobic Gram-negative bacilli and, in particular, the archetypical CF pathogen P. aeruginosa. Its spectrum of activity in Gram-positive organisms is limited to staphylococci. Aminoglycosides bind with high avidity to a highly conserved region in the mRNA decoding region of the bacterial 30S ribosomal subunit.48 Their binding to this region, while reversible, causes misreading of mRNA and aberrant protein synthesis. In Gram-negative organisms, the positive charge associated with aminoglycosides further results in an attraction to the negatively charged lipopolysaccharides (LPS) thereby displacing positively charged divalent cations.49 Disruption of LPS integrity results in increased permeability. However, take up of aminoglycosides requires an active electron transport chain to generate a sufficient electrical potential difference.50 This mandate explains the lack of activity against anaerobic organisms. Ultimately, aminoglycosides are bactericidal owing to the toxic effects of altered protein synthesis, cellular membrane dysregulation, and drug accumulation. Like all aminoglycosides, tobramycin exposure in susceptible organisms results in a disproportionate suppression of bacterial cell growth well after exposure to the drug (up to 10–12 hours in vivo), a phenomenon known as “a prolonged postantibiotic effect.”51
Specific CF pathogens are notable for their intrinsic resistance to tobramycin, including members of the Burkholderia cepacia complex (Bcc), Stenotrophomonas maltophilia, and Achromobacter xylosoxidans.52 While tobramycin acts as a potent antipseudomonal drug, resistance can develop through a myriad of mechanisms including reduced uptake, increased efflux, rRNA methylases targeting site modification, and the production of aminoglycoside modifying enzymes.12 In particular, tobramycin resistant P. aeruginosa isolates from CF lower airways are most commonly associated with the hyperexpression of the MexXY-OprM multidrug efflux pumps.53–55 For a full review of the acquired resistance mechanisms for tobramycin in P. aeruginosa, readers are referred to one of several excellent sources.12,56 A critical distinction about the use of aerosol delivery of tobramycin is the extraordinarily high levels that are achievable, with the ability to overcome most adaptive resistance mechanisms. Accordingly, the derived clinical benefit of chronic aerosolized tobramycin is of the same magnitude in individuals infected with tobramycin resistant isolates as those with susceptible isolates.28,57
One critical point unique to aerosol administration of aminoglycoside antibiotics is that CF sputum has inherent inhibitory activity that reduces the efficacy of aminoglycosides.58,59 In particular, sputum constituents such as mucin glycoprotein and free eukaryotic and bacterial DNA bind aminoglycosides, owing to their highly cationic state. Moreover, the chemical properties of sputum including pH and tonicity, further reduce their potency. The cumulative effect that is observed is reduction of drug efficacy by a factor of 10. For example, inhibiting the growth of a homogenous population of P. aeruginosa with a tobramycin minimum inhibitory concentration (MIC) of 8 μg/mL grown in purified sputum requires tobramycin supplemented to achieve a level of at least 80 μg/mL in sputum. This is not unexpected, as data from pyogenic infections have long demonstrated that aminoglycosides have limited activity in purulent collections that share many of the same physical characteristics.60
Tobramycin inhalation solution: The first aerosol ready formulation of antipseudomonal therapy in CF
The development of a specific tobramycin inhalation solution (TIS) for use in chronic CF infection involved multiple investigator led clinical trials evaluating different doses, dosing intervals and delivery devices.61–64 For a full account of this process, the reader is referred to the excellent review by Smith.23 In their landmark publication, Ramsey et al evaluated TIS (TOBI) 300 mg nebulized twice daily via the PARI LC PLUS jet nebulizer (PARI, Richmond, VA) and the Pulmo-Aide compressor (DeVilbiss, Somerset, PA) to placebo through 3 drug cycles over a 24-week time period.40 Each cycle included a 4-week “on” drug period followed by a 4-week “off” period during which the patients did not take aerosolized antibiotics. Patients included in this study were ≥6 years of age with an FEV1 percent predicted between 25% and 75%. The summative data from these 2 simultaneously run multicenter trials involving a total of 520 patients demonstrated that TIS resulted in a 12% relative improvement in FEV1, a 26% reduction in pulmonary exacerbation, reduced hospitalizations, and a reduced sputum P. aeruginosa density by 1.1 log10.
TOBI received regulatory approval for license and use by the FDA in 1997 for use with the PARI LC PLUS nebulizer. Though originally developed by Patho- Genesis Corporation (Seattle, WA), through a series of corporate merges and acquisitions, TOBI is now a product of Novartis International AG (Basel, Switerland). In the newest consensus guidelines endorsed by the Cystic Fibrosis Foundation, TIS received an “A” recommendation (defined as “the committee strongly recommends that clinicians routinely provide this therapy”) for the use of individuals ≥6 years of age with moderate to severe lung disease chronically infected with P. aeruginosa where the net certainty of benefit is high and the net benefit estimate is substantial.65
To evaluate TIS in individuals with mild CF lung disease and chronic P. aeruginosa infection, Murphy et al initiated an independent study.66 This multicenter trial evaluating the effects of TIS in pediatric patients was terminated early owing to poor recruitment. Recruitment proved difficult as clinicians were reluctant to expose their patients to potentially receiving placebo therapy rather than active drug in which earlier trials had established clinical benefit in individuals with moderate lung disease. Though extremely underpowered, as only 20% of the intended patients completed the study, a >2.4 fold reduction in risk for hospitalization due to pulmonary exacerbation was observed although no overall improvement in the primary endpoint of FEV1 was noted in those receving TIS. In those individuals ≥6 years of age with mild lung disease chronically infected with P. aeruginosa, the product was given a “B” recommendation (defined as “the committee recommends that clinicians routinely provide this therapy”) where the net certainty of benefit is moderate and the net benefit estimate is moderate.65
Multiple studies have assessed the economic impact of TIS on both outpatient drug costs and total cost of care for individuals living with CF.67,68 Under the UK model of CF care, Iles et al followed 41 individuals with CF prescribed TIS for 24 weeks and compared them with age- and sex-matched controls. Baseline data suggested that the TIS treated group in the prior year was considerably sicker, with greater requirements for inhaled and IV antibiotics, and were hospitalized more frequently and for longer periods. However, when prescribed TIS, these indices fell considerably, suggesting that while total costs may increase due to outpatient use of TIS, considerable cost savings (in particular, hospitalization associated costs of care and parenteral drugs) markedly offset the cost equating to an approximate 17.24 GBP/day. As all hospital costs were not captured, it is likely that this is an overestimate of residual cost. Similar findings were observed by a Canadian group who modeled TIS associated costs and savings under the Canadian model of health care using data extrapolated from the original TIS trial.40,68
Unintended consequences: Increasing burden of care associated with aerosolized therapy to maintain respiratory health
In the last 3 decades, the rate of disease progression in CF has been dramatically reduced owing to many new therapeutic interventions.1,3,5 In fact national data registries demonstrate median predicted survival of individuals with CF extending into the fourth or even fifth decade of life.69,70 Much of the focus of the CF therapeutic drug development pipeline that has ultimately enabled this impressive progress has been on the development of antibacterial agents to treat chronic P. aeruginosa respiratory infection.71,72 Unfortunately, the same therapies that have improved longevity and respiratory function while reducing symptom burden, have imposed a considerable therapeutic burden on those afflicted. In a study by Sawicki et al, the average time dedicated to treatment was a mean of 108 (± 58) minutes per day.73 In particular, perception of treatment burden was most associated with the use of more than 1 nebulized medication (in particular TIS and DNase) and the need of airway clearance for a total of ≥30 minutes daily.
This tremendous treatment burden poses considerable challenges to individuals living with CF who must incorporate their CF care while trying to balance a life of family, career, and education. In reality, patients are forced to choose between time dedicated to treatment and their other responsibilities. Of patients who meet CF criteria for TIS, only 67% were prescribed the drug in 2008.74 Many patients remain hesitant to initiate this drug owing to its excessive treatment burden and, as such, are deprived of its clinical benefit. In those patients prescribed TIS, compliance estimates for patients taking TIS 300 mg twice daily range from 27% to 85%75–78 depending on the study design, duration, and means of monitoring compliance. When reviewing prescription fill data from national databases for those patients prescribed TIS for more than 1 year, Briesacher et al observed that only 7% of CF patients filled more than 4 (of an ideal 6) 28-day treatment courses in a year.78,79 The most common reported reason for noncompliance with TIS administration is lack of time required for its prolonged nebulization.76 Additionally, individuals more likely to be noncompliant with nebulized therapies are those with advancing lung disease and the greatest treatment burden and most in need of the treatment.80,81 As one would expect, lower than recommended use of TIS is associated with lower then desired clinical benefit. Briesacher et al observed that in those individuals filling more then 4 courses of TIS in a calendar year period were less likely to be hospitalized (odds ratio, 0.4) than those with less frequent medication refills.78
Recognizing this emerging schism within CF, technological and pharmaceutical companies have looked to develop more efficient means of antibiotic delivery. High efficiency nebulizers using vibrating mesh technologies have been developed by several different companies, some partnered with a particular drug (such as the Altera from PARI) and others to be used in a more general fashion (such as the PARI Rapid and the i-neb Adaptive Aerosol Delivery System [Phillips Respironics (Andover, MA, USA)]).82–84 Others have looked to dry powder technologies.32,85–89
Dry powder inhaled tobramycin: The development of TIP and PulmoSphere technologies
Despite almost a half-century of experience with inhaled antibacterial therapies for the management of lower airways infection, dry powder inhalers (DPI) have only recently entered the market. The reason for this delay relates to the copious complexities involved in delivering the very high doses of drugs to the lower airways required for antimicrobials to be effective.86 Improvements in particle manufacturing processes have now enabled 10s of milligrams of drug to be delivered efficiently with DPIs at a time, whereas traditional DPI devices have only had to deliver 1/1000 of this amount. The PulmoSphere technology ( Novartis International AG, Basel, Switzerland) used for the manufacturing of TIP uses an emulsion-based spray drying process to generate particles of precisely defined size, porosity, and density. To do this, submicron oil in water emulsion droplets are created using a high-pressure homogenization of perfluorooctyl bromide (perflubron) using distearoylphosphatidylcholine (DPSC), a natural pulmonary surfactant component, to stabilize the created droplets. The milled particles dry in milliseconds and the evaporation of water and perfluorooctyl bromide leave behind highly porous spherical compounds of DSPC-coated tobramycin (Fig. 1). In this manner, intraparticle cohesive forces are minimized. The PulmoSphere particles of tobramycin used in TIP have a geometric mean diameter of 1–2.7 μm and a median mass diameter of <4 μm, ideal for delivery of drug to the lower airways. The drug is packaged into a hypromellose capsules, each containing 28 mg of active drug. Capsules are individually stored in aluminum blister packs of 4 (for ease of dosing) in order to protect them from moisture in the environment.
Figure 1.
High resolution scanning electron microscopy (SEM) of a PulmoSphere particle of tobramycin generated with TIP.41 Copyright © 2011 Novartis Pharmaceuticals Canada Inc.
TIP is delivered using the custom designed T-326 inhaler (or TOBI podhaler) (Novartis Pharmaceuticals, San Carlos, CA) (Fig. 2). The T-326 has 2 specific components: a removable mouthpiece as well as a body that contains the capsule chamber and spring activated button used to puncture the TIP capsule, which are housed in a secure carrying case enabling ease of drying. Use of the device requires no power source, and the stability of the packaged capsules enables extreme portability. Counseling patients on optimal administration technique is critical for the proper delivery of drug to the lower airways. Importantly, the T-326 device is meant to be inhaled through only; patients are meant to remove the device from the mouth before exhaling. Exhalation through the device can result in moisture accumulation that adversely impacts device performance and thus drug delivery.
Figure 2.
A day’s supply of TIP. This picture demonstrates the T-326 Inhaler for use with TIP (TOBI podhaler) depicted with 2 separate blister packs of 4 × 28 mg capsules of TIP. The T-326 device has a removable mouthpiece where individual capsules of TIP are placed. When depressed, the activator button releases a spring mechanism to puncture the hypromellose capsules releasing the drug during patient inspiration. Images used with permission from Novartis. Copyright © 2011 Novartis Pharmaceuticals Canada Inc.
When the blue button is depressed, the spring-activated needles puncture the hypromellose capsule creating an opening through which the drug can be released. While not intended for consumption, the hypromellose capsule is safe and nontoxic. If a portion of the capsule is either inhaled and or swallowed, it will quickly be absorbed.90 The T-326 inhaler has been engineered to have low air flow resistance (R = 0.08 [cmH20]1/2/LPM). Provided patients can generate an inhaled volume of greater then 1 litre, 90% or more of the content of each 28 mg capsule can be emptied with the first inhalation. When studied, this seems to be near universal amongst CF patients.91–93 When assessing patients with the full spectrum of CF airways disease (from mild to advanced), investigators have determined that children aged 6 to 10 have a mean peak flow of 68.7 L/min (± 13.1) and inhaled volumes of 1.20 L (± 0.39), those 11 to 18 years have a mean peak flow of 79.3 L/min (± 15.0) and volumes of 1.63 L (± 0.6), and adults have a mean peak flow of 81.1 L/min (± 14.4) and inhaled volumes of 2.06 L (± 0.68).94,95 This study has been replicated and similar results reported suggesting broad generalizability.92,93 However, to ensure that the contents of every capsule have been fully administered, the manufacturer recommends 2 inhalations be performed from each punctured capsule.96
Critical to the ease of function of TIP is its low maintenance. The T-326 inhaler is designed to not require cleaning after each use as would be common practice for traditional reusable nebulizers used to deliver antibiotics to the lower airways in CF. Instead, the T-326 is to be wiped dry with a cloth immediately following each use and stored in the protective container. A new T-326 inhaler should be used each week to ensure performance of TIP over time. A single box contains a month’s supply of TIP, which includes 4 smaller individuals boxes each containing 1 week’s supply of drug and a T-326 inhaler, as well as an extra inhaler in case one is misplaced or damaged.
Pharmacokinetics of TIP
TIP was developed as a therapeutic alternative to conventional TIS. To achieve this, investigators sought to identify the dose of TIP 28 mg capsules required to replicate the pharmacokinetics of TIS with respect to pulmonary drug delivery and limited systemic absorption. Geller et al conducted a multicenter phase 1/2, open-label, single dose escalation cohort study of individuals with CF who were ≥6 years of age with a FEV1 predicted >40% during periods of clinical stability, provided they expectorated sputum regularly.86 Participants were randomized in a 3:1 ratio (TIP to TIS) into 1 of the 5 arms of the study. The 4 arms included the new drug TIP delivered in 1 of 2 capsule amounts: 28 mg (in 2 × 14 mg capsules), 56 mg (in 4 × 14 mg capsules), 56 mg (in 2 × 28 mg capsules), and 112 mg (in 4 × 28 mg capsules). The fifth group, the control group, received conventional TIS 300 mg nebulized using the associated PARI LC Plus Nebulizer (Richmond, VA, USA) and a DeVilbiss Pulmo-Aide compressor (Summerset, PA, USA). Eighty-six patients received at least 1 study drug, and all but 1 patient completed the study protocol.
Expectorated sputum concentrations were measured using a validated reverse- phase, high performance liquid chromatography (HPLC) method with ultraviolet detection to determine lower airway delivery of tobramycin. Patients rinsed their mouths and gargled with normal saline prior to expectorating to eliminate the possibility of oropharyngeal tobramycin deposits from contaminating the sputum samples. An observed dose-response increase in deposition of tobramycin was appreciable (Table 2). Sputum concentrations of tobramycin peaked at 30 minutes following administration for all doses and declined subsequently thereafter. A high degree of intersubject variability was observed as is common for aerosol antibiotic studies. Systemic absorption of aerosolized tobramycin was minimal and peaked at 1 hour following administration. TIP dosed at 112 mg (4 × 28 mg capsules) best replicated sputum pharmacokinetics of TIS 300 mg. In clinical studies using TIP 112 mg, no increase in sputum tobramycin levels at the end of 28-day cycles of treatment were observed41,97 during repeated cycles, and TIP consistently achieved higher sputum concentrations then TIS: 1979 ± 2770 μg/g versus 1074 ± 1182 μg/g.98
Table 2.
Sputum and serum pharmacokinetics of tobramycin during dose escalation studies of TIP relative to conventional TIS.
| Drug | TIS | TIP | ||
|---|---|---|---|---|
|
|
|
|
||
| Dosing regimen (mg) | 300 | 56 (2 caps) | 84 (3 caps) | *112 (4 caps) |
| Administration time (min) | 15.8 ± 4 | 2.5 ± 1.1 | 4.5 ± 1.1 | 4.9 ± 1.8 |
| Sputum | ||||
| Cmax (μg/g) | 737 ± 1028 | 574 ± 527 | 1092 ± 1052 | 1048 ± 1080 |
| AUC (0–∞) (μg · hr/mL) | 1302 ± 1127 | 855 ± 469 | 2044 ± 1334 | 1740 ± 809 |
| Tmax (hours) | 0.5 (0.5–2) | 0.5 (0.5–4) | 0.5 (0.5–2) | 0.5 (0.5–1) |
| T1/2 (hours) | 1.7 ± 1.6 | 1.3 ± 1.5 | 0.8 ± 0.8 | 2.2 ± 1.7 |
| Serum | ||||
| Cmax (μg/mL) | 1.04 ± 0.58 | 0.5 ± 0.21 | 0.7 ± 0.33 | 1.02 ± 0.53 |
| AUC (0–∞) (μg · hr/mL) | 5.3 ± 2.6 | 2.9 ± 1.2 | 4.1 ± 1.5 | 5.1 ± 2 |
| Tmax (hours) | 1 (0.5–2) | 1 (0.5–2) | 1 (1–2) | 1 (0.5–2) |
| T1/2 (hours) | 3.0 ± 0.8 | 3.3 ± 0.8 | 3.4 ± 1.0 | 3.1 ± 0.4 |
Notes:
Licensed dose of TIP. Values expressed as mean ± standard deviation, except Tmax expressed as mean (range).
Adapted from Geller et al.86
Abbreviations: Cmax, Maximum concentration; AUC, area under the curve; Tmax, time to maximum concentration; T1/2, half-life.
Limited systemic absorption of tobramycin is evident for TIP at all initial studied doses, although a dose response is observed (Table 2).86 In clinical studies of 112 mg of TIP, there was no evidence of accumulation of peak serum tobramycin through successive cycles (cycle 1: 1.99 ± 0.59 μg/mL and cycle 2: 1.64 ± 0.96 μg/mL).98 Similarly, trough tobramycin levels did not increase with successive cycles (cycle 1: 0.29 ± 0.27 μg/mL and cycle 2: 0.38 ± 0.44 μg/mL).
Clinically evaluable end points
TIP has been evaluated in 2 placebo-controlled studies41,97 in relatively treatment naïve patients as well as in a comparison noninferiority trial relative to conventional TIS.98 The EVOLVE [NCT00918957]41 and EDIT(Establish Dry powder effIcacy in cysTic fibrosis)97 trials represent similarly designed phase 3 trials comparing TIP with placebo through 3 cycles of 28 days on and off drug— cycle 1 as double blind placebo-controlled and cycle 2 and 3 as open-label crossover extensions. The EDIT trial, performed more than 4 years later was only conducted as a result of requirements for Food and Drug Administration licensing in the United States. Both trials were significantly hampered owing to the necessity of recruitment of prospective patients by clinicians in a placebo-controlled trial, whereby a known effective therapy was forced to be withheld. As such, slow patient recruitment forced sponsors to expand their enrollment into developing countries where CF service programs were in their infancy lacking established protocols and extremely limited access to medications. This effect was especially evident in the later study EDIT. Furthermore, centers with little clinical trial experience were included, and multiple issues of major protocol violations including aberrant pulmonary function testing, drug errors, and so on were noted.
The EVOLVE trial41 was powered to identify a therapeutic efficacy for TIP of a predicted 11% improvement in mean relative FEV1 change at the end of cycle 1. Accordingly, it was intended to enroll 140 patients chronically infected with P. aeruginosa. Thirty-eight centers in Eastern Europe, Latin America, and the United States were enrolled to recruit patients aged 6 to 21 years of age with FEV1 percent predicted from ≥25% to ≤80% to receive TIP or placebo in a randomized 1:1 fashion. Participants were excluded if they had been treated with any inhaled antipseudomonal antibiotic in the prior 4 months, had a history of prior Bcc infection, renal toxicity, aminoglycoside intolerance, or a recent episode of submassive hemoptysis. Participants were essentially TIS treatment naïve, where only 4% been exposed to TIS in the preceding year. EVOLVE was discontinued by the external data safety monitoring committee at the first interim safety analysis when 80 patients who had completed cycle 1 had met predefined stop criteria. At this point, 102 patients had already been enrolled and 95 patients treated. Accordingly, only 79 patients completed the study.
The final analysis of EVOLVE was complicated by the eventual necessary exclusion of 18 patients by a blinded panel of experts. These patients were followed in specific centers where inconsistent pulmonary function testing was reported. The modified intent-to-treat population consisted of 29 patients who received TIP and 32 patients treated with placebo. After 1 full cycle of treatment (28 days), significant improvement in the primary end point of improvement in baseline FEV1 was identified to exist in the TIP group, with a 13.3% difference between the groups (95% confidence interval [CI], 5.3%–21.3%), P = 0.0016. Furthermore, when the placebo group crossed over and received active treatment during the open-label extension in cycles 2 and 3, a similar improvement was observed. Not only was the proportion of patients requiring rescue therapy with antimicrobials during cycle one lower in the TIP group (13% vs. 18%), the duration of rescue antimicrobial required use was shorter in the TIP group (13.3 ± 3.39 days vs. 19.3 ± 14.54 days). Furthermore, no patients were hospitalized for pulmonary exacerbations during cycle 1 in the TIP treatment arm, whereas 12.2% of the placebo group required hospitalization (P = 0.07).
The EDIT97 trial used similar inclusion and exclusion criteria, with the only significant difference being that the duration for which patients had to be free of inhaled anti-pseudomonal antibiotics was reduced to four months. EDIT also used a marginally different manufacturing process to generate the same Pulmo- Sphere particles used in EVOLVE, which was necessary for the production of TIP on a commercial scale. Like EVOLVE, EDIT was also powered to detect a relative improvement in FEV1 of 11%, hence requiring 100 patients to be enrolled in a 1:1 fashion to receive either TIP or placebo. Despite extensive recruitment efforts involving inclusion of more then 17 centers from Eastern Europe, North Africa, and South Asia, only a limited number of eligible participants could be identified. Recruitment was closed after a mere 62 patients were randomized owing to futility of further enrollment. Data analysis was marred in several instances by improper spirometry data and 3 patients who were dispensed the wrong randomized treatment. While underpowered, the study identified a relative difference in FEV1 improvement of 5.9% (P = 0.15) in the ITT population and 7.9% (P = 0.09) in the prespecified observed case population. The data were further confounded by an extreme outlier data point from a 7-year-old boy with a BMI of 9 kg/m2. With the exclusion of this data, a post hoc analysis based on the actual treatments received quantified the difference observed between TIP and placebo to be 6.7% (P = 0.1) for the ITT and 8.8% (P = 0.06) for the observed case population. Although the need for rescue antimicrobial therapy was no different between groups, the duration of therapy was lower in those receiving TIP (8.3 ± 7.5 days vs. 13 ± 2.7 days).
The EAGER study98 (Establish A new Gold standard Efficacy and safety with tobramycin in cystic fibRosis) (NCT00388505) was a comparator trial between TIS and TIP. EAGER was designed as a noninferiority study to assess the safety and efficacy of TIP during 24 weeks (3 cycles of 4 weeks on/off) of therapy relative to the established gold standard of TIS. A total of 553 patients chronically infected with P. aeruginosa (≥6 years of age with a percent predicted FEV1 ≥25 and ≤75%) were enrolled, and 517 received at least 1 dose of active drug in an open-label randomized trial comparing TIP 112 mg twice daily (4 × 28 mg capsules) via the T-326 Inhaler to TOBI 300 mg/5 mL twice daily (Novartis Pharmaceuticals) nebulized via a PARI LC Plus jet nebulizer (PARI, Richmond, VA, USA) using the DeVilbiss PulmoAide compressor at a ratio of 3:2.76 Patients who had received any systemic or inhaled antipseudomonal antibiotic in the prior 28 days, had a history of prior Bcc infection, ototoxicity, renal toxicity, or aminoglycoside intolerance, or had had a recent bout of submassive hemoptysis were excluded. Unlike both EVOLVE and EDIT, EAGER included patients that were extensively pretreated with inhaled antipseudomonals. In particular, 82.1% of patients receiving TIP and 82.3% of patients receiving TOBI had prior TIS exposure. The final a priori analysis was restricted to the per protocol (PP) population, that is, those patients who had completed the study without major protocol deviations including noncompliance with >80% of study drug. The PP population included 60.8% of patients randomized to TIP and 66.5% of patients randomized to TIS.
The primary outcome assessed was relative changes in FEV1 from baseline in each group. Comparable increases in FEV1 with each “on cycle” treatment were observed, and, during “off cycles,” the subsequent declines were similar (Fig. 3A). The mean relative improvement on day 28 of the third cycle was 5.8% in the TIP treatment arm and 4.7% in the TIS arm (NS), with a mean treatment difference (TIP-TIS) of 1.1% favoring TIP. However, the magnitude of the treatment difference was greater in children relative to adults, that is, patients 6 to 13 years of age (4.7%), those 13 to 19 years of age (3.7%), and adults ≥20 years of age (−0.8%).57 Secondary end points were similarly compared. Despite a greater overall proportion of the TIP arm receiving additional antibiotics relative to TIS arm (64.9% vs. 54.5%, P = 0.15), the number of patients hospitalized for respiratory-related events was comparable (24.4% vs. 22.0%). Investigators determined the use of oral ciprofloxacin accounted for the increase in additional antipseudomonal treatment (47.7% vs. 34.0%). Such additional use was likely driven by an increase in observed drug cough frequency due to the inhaled powder.
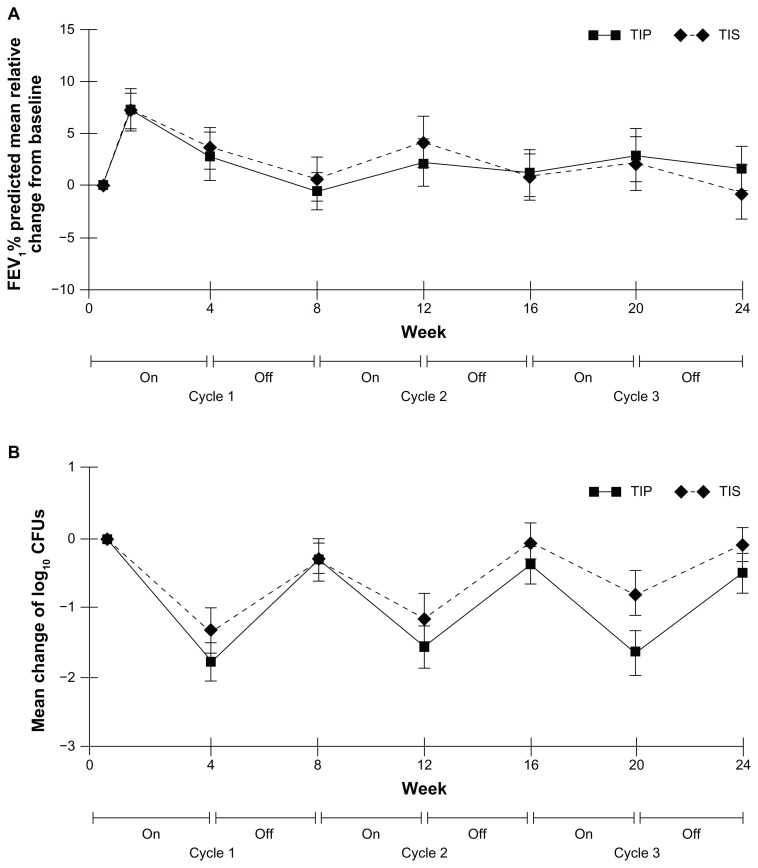
Figure 3.
Comparative efficacy of TIP versus TIS from the EAGER trial.98 (A) No difference in the primary end point of relative change from baseline FEV1 through 24 weeks and, in particular, on day 28 of cycle 3. (B) Relative change in P. aeruginosa sputum density through 24 weeks of treatment. Reproduced with permission from Elsevier.41
Patient satisfaction and time for administration
Unlike other CF trials involving inhaled antipseudomonals, EAGER did not utilize specific patient reported outcomes in assessing efficacy. However, patient satisfaction was evaluated using a version of the treatment satisfaction questionnaire for medication (TSQM).99,100 The 14 TSQM items are answered on a Likert type scale and encompass 4 domains (effectiveness, side effects, convenience, and global satisfaction) corresponding to distinct aspects related to patient perception and satisfaction with treatment. The score is obtained for each domain by summing the corresponding items transformed on a 0 to 100 scale where higher values indicate an increased level of satisfaction, better perceived effectiveness and convenience, and reduced burden of side effects.99
Importantly, TIP was observed to have a statistically significant increase in patient perception of effectiveness relative to TIS (74.8 vs. 65.4, difference of 9.36 ± 1.46, P < 0.0001). Global satisfaction was also greater with TIP (76.2 vs. 71.0, difference of 5.20 ± 1.66, P = 0.002).98 In multivariate regression, higher patient satisfaction and a lower perceived impact of drug associated side effects are affiliated with superior treatment compliance,100 thus, suggestive of TIP’s ability to better patient enhance compliance and potentially improve clinical outcomes under real world conditions.
The dosing time for TIP was significantly shorter than for TIS, 5.6 minutes versus 19.7 minutes (P < 0.0001), excluding the time to set up and clean and sterilize the devices, where relevant.98 The marked time saving as well as the lack of requirements for nebulizer maintenance was recognized. Using the TSQM, TIP was perceived to be more convenient then TIS, with a mean score of 82.7 versus 58.4 (P < 0.0001).
Microbiologic end points
Microbiologic end points are included as endpoints to measure efficacy in studies of all inhaled antipseudomonal drugs in CF because it has traditionally been presumed that the bactericidal effect of the drug is responsible for the derived clinical response. P. aeruginosa sputum density fell at a sharper rate in the placebo-controlled trials of EVOLVE and EDIT where patients populations were relatively naïve to inhaled antibiotics when compared with the treatment-experienced patients of the EAGER trial. EVOLVE reported more specific microbiologic outcomes then EDIT, where sputum P. aeruginosa density was reduced 2.61 log10 CFU/g (± 2.53) in the TIP group versus 0.43 log10 CFU/g (± 1.05) in placebo for mucoid isolates and 1.91 log10 CFU/g (± 2.54) versus 0.15 log10 CFU/g (± 0.68) for nonmucoid isolates.41 Clearance of P. aeruginosa cultures were significantly higher with TIP than placebo (41.4% vs. 0% at day 29).97
Sputum density of P. aeruginosa was followed through 3 cycles of use in EAGER and resulted in data similar to those of the original TIS trial40 (Fig. 3B). During “on” cycles, sputum P. aeruginosa density fell and rose during “off” cycles. On day 28 of the third cycle, sputum P. aeruginosa density was further reduced from baseline in the TIP arm than in the TIS group (−1.6 log10 CFU/g vs. −0.92 log10 CFU/g for mucoid isolates and −1.77 log10 CFU/g vs. −0.73 log10 for nonmucoid isolates).
Data on the emergence of resistance to tobramycin were collected and reported in both EVOLVE and EAGER. Through 3 consecutive cycles in the EVOLVE trial, a trend toward increasing frequency of tobramycin resistant isolates was observed relative to baseline in the TIP treated arm (as determined using the parenteral breakpoint of 8 μg/mL) (18.5% vs. 9%, P = 0.44 for mucoid isolates and 28.6% vs. 6.7%, P = 0.07 for nonmucoid isolates).41 However, similar trends were noted in the placebo treated group. No difference in tobramycin MIC of P. aeruginosa isolates following 3 cycles of treatment were observed in EAGER between the treatment arms. The direct comparison of an isolate’s MIC before and after starting therapy is confounded by marked antibiogram heterogeneity in chronic CF infections.25–27 For what it is worth, the proportion of patients where isolates had an MIC increase ≥2 fold for TIP and TOBI were 48.7% versus 39.6% and ≥4 fold increase 33.7% versus 27.3%, respectively. Emergence of P. aeruginosa isolates that were previously susceptible to tobramycin at baseline (<8 ug/mL) but currently to nonsusceptible (≥8 ug/mL) at the completion of 3 cycles did not differ between groups: 19.1% versus 14.3%. None of the TIP trials reported rates of nonpseudomonal CF pathogen emergence during therapy (such as S. maltophilia, A. xylosoxidans, or Bcc), which were observed in the past trials using TIS.40
Safety and adverse events associated with TIP
While parenteral tobramycin preparations are notorious for the potential to cause nephrotoxicity,101,102 vestibular/ototoxicity,103,104 and, rarely, neuromuscular blockades,105 aerosol preparations are extremely safe. Despite decades of use, only isolated reports of nephrotoxicity attributable to aerosolized tobramycin products have been documented in CF.106 Similarly, vestibular toxicity and ototoxicity are both extraordinarily rare and involve case reports of a CF patient with genetic predispositions107 or usage of doses well above those routinely recommended, further compounded by impaired drug clearance.108
In the 2 placebo-controlled trials, TIP was compared with a placebo consisting of the excipients used in the spray drying process to manufacture TIP: DSPC and calcium chloride.41,97 The incidence and types of adverse events (AE) between these 2 trials were quite different but were in keeping with expectations of AE occurring in a population with chronic lung disease. In the first trial, EVOLVE, AE occurred in the placebo treated group (75.5%) more often than then in the TIP group (50%) (P = 0.01). AE in EDIT occurred much less frequently: 34% of placebo recipients and 27% of TIP recipients. In EVOLVE, the commonest complaint in the TIP treated group was cough (13%), which occurred more regularly in the placebo group (26%). Conversely, cough was observed in 10% of TIP treated patients and not in placebo treated patients in EDIT. Acute bronchospasm was unusual in either arm, although twice as common with placebo in EVOLVE. Many of the side effects attributed to TIP may be due to carrier molecules of the excipient as suggested by the higher frequency of AE observed in the placebo treated arm of EVOLVE. Other less frequent AE included exacerbations of chronic lung disease and pharyngeal pain. Hypoacusis was reported to occur at a rate of 6% in EDIT, observed at equal frequency in both groups, and not observed at all in EVOLVE.
In the comparative trial EAGER, AE were higher in TIP treated patients than in TIS (90.3% vs. 84.2%, P < 0.05).98 The most common AE reported in the TIP treatment arm were cough (48.4%), lung disorder (33.8%), dyspnea (15.6%), and pyrexia (15.6%). Importantly, TIP was associated with increased cough (48.4% vs. 31.1), dysphonia (13.6% vs. 3.8%), and dysgeusia (3.9% vs. 0.5%). However, the frequency of patients experiencing AE decreased with subsequent cycles, suggesting an attenuation or adaptation to local irritation. Discontinuations were greater in the TIP treated arm then TIS. However, discontinuation declined after the first cycle, suggesting that patients who tolerated the drug after 1 cycle would continue to tolerate it long term. Interestingly, a subgroup analysis demonstrated that discontinuations were greatest in those individuals >20 years of age, a group likely more familiar with the more traditional TIS method of tobramycin dosing.57 The higher rates of local AE events such as cough, dysphonia, and dysgeusia associated with TIP may reflect a larger deposition of tobramycin to the posterior pharynx causing irritation which lessens with time.98 Nonetheless, subjective assessment of AE did not differ between groups using the TSQM scale (TIP 92.1 vs. TOBI 92.6, P = 0.68). Serious adverse events (SAE) were similar between groups (27.4% vs. 29.2%). The most common SAE was related to pulmonary exacerbations, and these did not differ between groups: 19.5% versus 18.7%. Notably, rates of bronchospasm (a priori defined as an acute relative drop in FEV1 of >20%) did not differ between groups: 5.2% versus 5.3%. Audiology was performed in a small subgroup of patients (25.3% TIP and 21.5% TIS), suggesting that while a slightly higher number of TIP treated patients (25.6% vs. 15.6%) experienced a decrease from baseline at any point during the trial, no consistent trend was observed and these reductions were generally transient.
Four deaths occurred in TIP related clinical trials. One death in EVOLVE occurred in the placebo group. Although 3 deaths were reported in the TIP arm of EAGER, none were related to the study drug.
TIP in the real world
Despite the theoretical potential advantages associated with the use of TIP as an easy, efficient, and intrinsically portable means of delivering aerosolized tobramycin to the lower airways, very little data exist on its use outside of industry-led clinical studies. The first group to present data has been from the West Midlands Regional Adult CF Centre in Birmingham. Brown et al have undertaken a prospective quality of life evaluation in 130 adult patients, including 34 who had previously been “intolerant” of TIS.111 As observed in clinical trials, the most commonly reported side effect of dry cough was observed in 24% of individuals. Patient discontinuations before 1 month of use were observed in 18% (23/130), with the most frequently cited cause for treatment discontinuation that being increased dry cough. However, overall subjective treatment satisfaction, ease of administration, and likelihood to continue with therapy measured on a 10-point scale (with 1 = very satisfied/very likely) associated with TIP use was 1 (interquartile range [IQR] 1–2) measured after 1 cycle. Furthermore, after 3 months of use, these values were maintained. While those individuals previously known to be intolerant of TIS were more likely to experience dry cough and discontinue therapy with TIP owing to adverse events, 48% of these individuals were tolerant and continued its use. These data would suggest that clinicians should consider a TIP treatment trial for all those patients previously not prescribed TIS for chronic P. aeruginosa lower airways infection due to concerns of prior intolerance.
Nash et al sought to determine if the ease and convenience of TIP was associated with an increased rate of prescription fill, as a surrogate measure for compliance.112
They followed 23 patients previously on TIS and compared pharmacy refill rates for TIP relative to the prior 12 months of TIS use. Owing to the short duration of time with which TIP was available for clinical use, the duration of follow-up after switching to TIP was a mere 7.6 months. Individuals who remained on TIP had more compliant pharmacy prescription refill rates after changing to TIP (0.53 refills/month [IQR 0.43–0.63] vs. 0.24 refills/month [IQR 0.04–0.42], P < 0.001). Given that TIS data linking increased compliance with improved patient outcomes, TIP may offer a therapeutic advantage.
Conclusions
Despite technical issues in trial data collection owing to patient recruitment in areas without established CF expertise, multiple replicate placebo-controlled trials continue to validate TIP as being superior than placebo with respect to improvements in pulmonary function, reduction of pulmonary exacerbations and subsequent hospitalizations, and decrease in sputum P. aeruginosa density. A subsequent randomized, open-label trial comparing TIP with the gold standard of TIS revealed TIP to be noninferior with respect to the primary end point of pulmonary function improvement and secondary end points including pulmonary exacerbations. TIP was demonstrated to be capable of being administered in almost a quarter of the time of TIS and resulting in increased time-saving. TIP offers the theoretical potential for further improved outcomes, as its lower burden of treatment appears to enable improved treatment compliance, which has been associated with improved clinical outcomes.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: JL, MDP. Contributed to the writing of the manuscript: JL, SV. Agree with manuscript results and conclusions: JL, SV, MDP. Jointly developed the structure and arguments for the paper: JL, MDP. Made critical revisions and approved final version: JL, SV, MDP. All authors reviewed and approved of the final manuscript.
Competing Interests
MDP has served on advisory boards for Novartis, Gilead and Roche. JL and SV have no conflicts to report.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- 1.Ratjen F. Update in cystic fibrosis 2008. Am J Respir Crit Care Med. 2009;179:445–8. doi: 10.1164/rccm.200812-1927UP. [DOI] [PubMed] [Google Scholar]
- 2.Dequeker E, Stuhrmann M, Morris MA, et al. Best practice guidelines for molecular genetic diagnosis of cystic fibrosis and CFTR-related disorders—updated European recommendations. Eur J Hum Genet. 2009;17:51–65. doi: 10.1038/ejhg.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 4.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 5.Ratjen F. Recent advances in cystic fibrosis. Paediatr Respir Rev. 2008;9:144–8. doi: 10.1016/j.prrv.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995–2005. Chest. 2009;136(6):1554–60. doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- 7.Lambiase A, Raia V, Del Pezzo M, Sepe A, Carnovale V, Rossano F. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis. 2006;6:4. doi: 10.1186/1471-2334-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 9.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–8. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 11.Nixon GM, Armstrong DS, Carzino R, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. 2001;138:699–704. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 12.Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkins MD, Elborn JS. Newer antibacterial agents and their potential role in cystic fibrosis pulmonary exacerbation management. J Antimicrob Chemother. 2010;65:1853–61. doi: 10.1093/jac/dkq245. [DOI] [PubMed] [Google Scholar]
- 14.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 15.Szaff M, Hoiby N, Flensborg EW. Frequent antibiotic therapy improves survival of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection. Acta Paediatr Scand. 1983;72:651–7. doi: 10.1111/j.1651-2227.1983.tb09789.x. [DOI] [PubMed] [Google Scholar]
- 16.Etherington C, Bosomworth M, Clifton I, Peckham DG, Conway SP. Measurement of urinary N-acetyl-b-D-glucosaminidase in adult patients with cystic fibrosis: before, during and after treatment with intravenous antibiotics. J Cyst Fibros. 2007;6:67–73. doi: 10.1016/j.jcf.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Smyth A, Lewis S, Bertenshaw C, Choonara I, McGaw J, Watson A. Case-control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2008;63:532–5. doi: 10.1136/thx.2007.088757. [DOI] [PubMed] [Google Scholar]
- 18.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39:15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 19.Elborn JS, Prescott RJ, Stack BH, et al. Elective versus symptomatic antibiotic treatment in cystic fibrosis patients with chronic Pseudomonas infection of the lungs. Thorax. 2000;55:355–8. doi: 10.1136/thorax.55.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cystic Fibrosis Trust. Antibiotic Treatment for Cystic Fibrosis. Bromley, Kent, UK: Cystic Fibrosis Trust; 2009. pp. 1–102. [Google Scholar]
- 21.di Sant’Agnese PaDA. Chemotherapy in infections of the respiratory tract associated with cystic fibrosis of the pancreas; observations with penicillin and drugs of the sulfonamide group, with special reference to penicllin aerosol. Am J Dis Child. 1946;72:17–61. [PubMed] [Google Scholar]
- 22.LiPuma JJ. Microbiological and immunologic considerations with aerosolized drug delivery. Chest. 2001;120:118S–23. doi: 10.1378/chest.120.3_suppl.118s. [DOI] [PubMed] [Google Scholar]
- 23.Smith AL. Inhaled antibiotic therapy: What drug? What dose? What regimen? What formulation? J Cyst Fibros. 2002;1:189–93. doi: 10.1016/s1569-1993(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn RJ. Formulation of aerosolized therapeutics. Chest. 2001;120:94S–8. doi: 10.1378/chest.120.3_suppl.94s. [DOI] [PubMed] [Google Scholar]
- 25.Foweraker JE, Laughton CR, Brown DF, Bilton D. Phenotypic variability of Pseudomonas aeruginosa in sputa from patients with acute infective exacerbation of cystic fibrosis and its impact on the validity of antimicrobial susceptibility testing. J Antimicrob Chemother. 2005;55:921–7. doi: 10.1093/jac/dki146. [DOI] [PubMed] [Google Scholar]
- 26.Foweraker JE, Laughton CR, Brown DF, Bilton D. Comparison of methods to test antibiotic combinations against heterogeneous populations of multiresistant Pseudomonas aeruginosa from patients with acute infective exacerbations in cystic fibrosis. Antimicrob Agents Chemother. 2009;53:4809–15. doi: 10.1128/AAC.00269-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Workentine ML, Sibley CD, Glezerson B, et al. Phenotypic heterogeneity of Pseudomonas aeruginosa populations in a cystic fibrosis patient. PLoS One. 2013;8:e60225. doi: 10.1371/journal.pone.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morosini MI, García-Castillo M, Loza E, Pérez-Vázquez M, Baquero F, Cantón R. Breakpoints for predicting Pseudomonas aeruginosa susceptibility to inhaled tobramycin in cystic fibrosis patients: use of high-range Etest strips. J Clin Microbiol. 2005;43:4480–5. doi: 10.1128/JCM.43.9.4480-4485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skindersoe ME, Alhede M, Phipps R, et al. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:3648–63. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hybenova D, Majtan V. The influence of postantibiotic effects and postantibiotic effects of sub-inhibitory concentrations of quinolones and aminoglycosides on phospholipase C of Pseudomonas aeruginosa. Pharmazie. 1997;52:157–9. [PubMed] [Google Scholar]
- 31.Jensen T, Pedersen SS, Garne S, et al. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother. 1987;19:831–8. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 32.Schuster A, Haliburn C, Döring G, Goldman MH Freedom Study Group. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax. 2013;68:344–50. doi: 10.1136/thoraxjnl-2012-202059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–8. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Retsch-Bogart GZ, Burns JL, Otto KL, et al. AZLI Phase II Study Group. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol. 2008;43:47–58. doi: 10.1002/ppul.20736. [DOI] [PubMed] [Google Scholar]
- 35.Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135:1223–32. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oermann CM, Retsch-Bogart GZ, Quittner AL, et al. An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr Pulmonol. 2010 doi: 10.1002/ppul.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assael BM, Pressler T, Bilton D, et al. For the AZLI Active Comparator Study Group. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: A comparative efficacy trial. J Cyst Fibros. doi: 10.1016/j.jcf.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Parkins MD, Elborn JS. Aztreonam lysine: a novel inhalational antibiotic for cystic fibrosis. Expert Rev Respir Med. 2010;4:435–44. doi: 10.1586/ers.10.48. [DOI] [PubMed] [Google Scholar]
- 39.Moskowitz SM, Silva SJ, Mayer-Hamblett N, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis (ESCF) Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–81. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 41.Konstan MW, Geller DE, Minić P, Brockhaus F, Zhang J, Angyalosi G. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: The EVOLVE trial. Pediatr Pulmonol. 2010 doi: 10.1002/ppul.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawicki GS, Signorovitch JE, Zhang J, et al. Reduced mortality in cystic fibrosis patients treated with tobramycin inhalation solution. Pediatr Pulmonol. 2012;47:44–52. doi: 10.1002/ppul.21521. [DOI] [PubMed] [Google Scholar]
- 43.Stephens D, Garey N, Isles A, Levison H, Gold R. Efficacy of inhaled tobramycin in the treatment of pulmonary exacerbations in children with cystic fibrosis. Pediatr Infect Dis. 1983;2:209–11. doi: 10.1097/00006454-198305000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Pechere JC, Roy B, Dugal R. Distribution and elimination kinetics of intravenously and intramusculary administered tobramycin in man. Int J Clin Pharmacol Biopharm. 1976;14:313–8. [PubMed] [Google Scholar]
- 45.Pechere JC, Dugal R. Pharmacokinetics of intravenously administered tobramycin in normal volunteers and in renal-impaired and hemodialyzed patients. J Infect Dis. 1976;134(Suppl):S118–24. doi: 10.1093/infdis/134.supplement_1.s118. [DOI] [PubMed] [Google Scholar]
- 46.Thompson RQ, Presti EA. Nebramycin, a new broad-spectrum antibiotic complex. III. Isolation and chemical-physical properties. Antimicrob Agents Chemother (Bethesda) 1967;7:332–40. [PubMed] [Google Scholar]
- 47.Stark WM, Hoehn MM, Knox NG. Nebramycin, a new broad-spectrum antibiotic complex. I. Detection and biosynthesis. Antimicrob Agents Chemother (Bethesda) 1967;7:314–23. [PubMed] [Google Scholar]
- 48.Lynch SR, Puglisi JD. Structural origins of aminoglycoside specificity for prokaryotic ribosomes. J Mol Biol. 2001;306:1037–58. doi: 10.1006/jmbi.2000.4420. [DOI] [PubMed] [Google Scholar]
- 49.Peterson AA, Hancock RE, McGroarty EJ. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol. 1985;164:1256–61. doi: 10.1128/jb.164.3.1256-1261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taber HW, Mueller JP, Miller PF, et al. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987;51:439–57. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig WA, Redington J, Ebert SC. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother. 1991;27(Suppl C):29–40. doi: 10.1093/jac/27.suppl_c.29. [DOI] [PubMed] [Google Scholar]
- 52.Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis. 1999;179:1190–6. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 53.Islam S, Oh H, Jalal S, et al. Chromosomal mechanisms of aminoglycoside resistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Clin Microbiol Infect. 2009;15:60–66. doi: 10.1111/j.1469-0691.2008.02097.x. [DOI] [PubMed] [Google Scholar]
- 54.Westbrock-Wadman S, Sherman DR, Hickey MJ, et al. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother. 1999;43:2975–83. doi: 10.1128/aac.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraud S, Poole K. Oxidative stress induction of the MexXY multidrug efflux genes and promotion of aminoglycoside resistance development in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2011;55:1068–74. doi: 10.1128/AAC.01495-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:479–87. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konstan M, Parkins M, Angyalosi G, Higgins M. Tobramycin inhalation powder is as effective as tobramycin inhalation solution in patients with cystic fibrosis: a subgroup analysis of the EAGER trial. Am J Respir Crit Care Med. 2013;187(meeting abstracts) Abstract 39401. [Google Scholar]
- 58.Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, Davis RL. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis. 1985;132:761–5. doi: 10.1164/arrd.1985.132.4.761. [DOI] [PubMed] [Google Scholar]
- 59.Hunt BE, Weber A, Berger A, Ramsey B, Smith AL. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother. 1995;39:34–9. doi: 10.1128/aac.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaudaux P, Waldvogel FA. Gentamicin inactivation in purulent exudates: role of cell lysis. J Infect Dis. 1980;142:586–93. doi: 10.1093/infdis/142.4.586. [DOI] [PubMed] [Google Scholar]
- 61.Smith AL, Ramsey BW, Hedges DL, et al. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr Pulmonol. 1989;7:265–71. doi: 10.1002/ppul.1950070413. [DOI] [PubMed] [Google Scholar]
- 62.Ramsey BW, Dorkin HL, Eisenberg JD, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328:1740–6. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 63.Le Brun PP, de Boer AH, Gjaltema D, et al. Inhalation of tobramycin in cystic fibrosis. Part 2: optimization of the tobramycin solution for a jet and an ultrasonic nebulizer. Int J Pharm. 1999;189:215–25. doi: 10.1016/s0378-5173(99)00252-5. [DOI] [PubMed] [Google Scholar]
- 64.Le Brun PP, de Boer AH, Gjaltema D, et al. Inhalation of tobramycin in cystic fibrosis. Part 1: the choice of a nebulizer. Int J Pharm. 1999;189:205–14. doi: 10.1016/s0378-5173(99)00251-3. [DOI] [PubMed] [Google Scholar]
- 65.Mogayzel PJ, Naureckas ET, Robinson KA, et al. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–9. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 66.Murphy TD, Anbar RD, Lester LA, et al. Treatment with tobramycin solution for inhalation reduces hospitalizations in young CF subjects with mild lung disease. Pediatr Pulmonol. 2004;38:314–20. doi: 10.1002/ppul.20097. [DOI] [PubMed] [Google Scholar]
- 67.Iles R, Legh-Smith J, Drummond M, Prevost A, Vowler S. Economic evaluation of Tobramycin nebuliser solution in cystic fibrosis. J Cyst Fibros. 2003;2:120–8. doi: 10.1016/S1569-1993(03)00064-X. [DOI] [PubMed] [Google Scholar]
- 68.LeLorier J, Perreault S, Birnbaum H, Greenberg P, Sheehy O. Savings in direct medical costs from the use of tobramycin solution for inhalation in patients with cystic fibrosis. Clin Ther. 2000;22:140–51. doi: 10.1016/s0149-2918(00)87985-0. [DOI] [PubMed] [Google Scholar]
- 69.Foundation CF. Cystic Fibrosis Foundation Patient Registry: Annual report to the center directors. Bethesda, MD: 2010. [Google Scholar]
- 70.Foundation CCF. Canadian Cystic Fibrosis Foundation Patient Data Registry Report. Toronto, CANADA: 2011. [Google Scholar]
- 71.Ashlock MA, Beall RJ, Hamblett NM, et al. A pipeline of therapies for cystic fibrosis. Semin Respir Crit Care Med. 2009;30:611–26. doi: 10.1055/s-0029-1238919. [DOI] [PubMed] [Google Scholar]
- 72.Marshall BC, Penland CM, Hazle L, et al. Cystic fibrosis foundation: achieving the mission. Respir Care. 2009;54:788–795. doi: 10.4187/002013209790983223. discussion 795. [DOI] [PubMed] [Google Scholar]
- 73.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8:91–6. doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foundation CF. Cystic Fibrosis Foundation Patient Registry: Annual report to the centre directors. Bethesda, Maryland: Cystic Fibrosis Foundation; 2008. [Google Scholar]
- 75.Sawicki GS, Ren CL, Konstan MW, et al. Treatment complexity in cystic fibrosis: Trends over time and associations with site-specific outcomes. J Cyst Fibros. 2013 doi: 10.1016/j.jcf.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5:177–85. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Modi AC, Quittner AL. Barriers to treatment adherence for children with cystic fibrosis and asthma: what gets in the way? J Pediatr Psychol. 2006;31:846–58. doi: 10.1093/jpepsy/jsj096. [DOI] [PubMed] [Google Scholar]
- 78.Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med. 2011;11:5. doi: 10.1186/1471-2466-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Briesacher BA, Quittner A, Saiman L, Fouayzi H, Sacco P, Quiettel L. Adherence to tobramycin inhaled solution and health care utilization. Am J Respir Crit Care Med. 2009;179(meeting abstracts) Absract A1184. [Google Scholar]
- 80.Arias Llorente, Bousono RP, Garcia C, Diaz Martin JJ. Treatment compliance in children and adults with cystic fibrosis. J Cyst Fibros. 2008;7:359–67. doi: 10.1016/j.jcf.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Dziuban EJ, Saab-Abazeed L, Chaudhry SR, Streetman DS, Nasr SZ. Identifying barriers to treatment adherence and related attitudinal patterns in adolescents with cystic fibrosis. Pediatr Pulmonol. 2010;45:450–8. doi: 10.1002/ppul.21195. [DOI] [PubMed] [Google Scholar]
- 82.Hubert D, Leroy S, Nove-Josserand R, et al. Pharmacokinetics and safety of tobramycin administered by the PARI eFlow rapid nebulizer in cystic fibrosis. J Cyst Fibros. 2009;8:332–7. doi: 10.1016/j.jcf.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Govoni M, Poli G, Acerbi D, et al. Pharmacokinetic and tolerability profiles of tobramycin nebuliser solution 300 mg/4 ml administered by PARI eFlow((R)) rapid and PARI LC Plus((R)) nebulisers in cystic fibrosis patients. Pulm Pharmacol Ther. 2013;26:249–55. doi: 10.1016/j.pupt.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Li Z, Zhang Y, Wurtz W, et al. Characterization of nebulized liposomal amikacin (Arikace) as a function of droplet size. J Aerosol Med Pulm Drug Deliv. 2008;21:245–54. doi: 10.1089/jamp.2008.0686. [DOI] [PubMed] [Google Scholar]
- 85.Sweeney LG, Wang Z, Loebenberg R, Wong JP, Lange CF, Finlay WH. Spray-freeze-dried liposomal ciprofloxacin powder for inhaled aerosol drug delivery. Int J Pharm. 2005;305:180–5. doi: 10.1016/j.ijpharm.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Geller DE, Konstan MW, Smith J, Noonberg SB, Conrad C. Novel tobramycin inhalation powder in cystic fibrosis subjects: pharmacokinetics and safety. Pediatr Pulmonol. 2007;42:307–13. doi: 10.1002/ppul.20594. [DOI] [PubMed] [Google Scholar]
- 87.Duddu SP, Sisk SA, Walter YH, et al. Improved lung delivery from a passive dry powder inhaler using an Engineered PulmoSphere powder. Pharm Res. 2002;19:689–95. doi: 10.1023/a:1015322616613. [DOI] [PubMed] [Google Scholar]
- 88.Shah SP, Misra A. Liposomal amikacin dry powder inhaler: effect of fines on in vitro performance. AAPS Pharm Sci Tech. 2004;5:e65. doi: 10.1208/pt050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Tsifansky MD, Wu CJ, Yang HI, Schmidt G, Yeo Y. Inhalable antibiotic delivery using a dry powder co-delivering recombinant deoxyribonuclease and ciprofloxacin for treatment of cystic fibrosis. Pharm Res. 2010;27:151–60. doi: 10.1007/s11095-009-9991-2. [DOI] [PubMed] [Google Scholar]
- 90.Burdock GA. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem Toxicol. 2007;45:2341–51. doi: 10.1016/j.fct.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Geller DE, Weers J, Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using pulmosphere technology. J Aerosol Med Pulm Drug Deliv. 2011 doi: 10.1089/jamp.2010.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavkov RG, Geller DE, Ament B, Heuerding S, Gupta A. Dynamic inspiratory flow characteristics through the T-326 dry powder inhaler in cystic fibrosis patients. RDD Europe. 2013;2:325–8. [Google Scholar]
- 93.Geller DE, Ament B, Heuerding S, Maykut R, Pavkov R. Inspiratory flow characteristics of the T-326 dry powder inhaler (DPI) in CF patients. J Cyst Fibros. 2013;12(Suppl 1):S66. [Google Scholar]
- 94.Tiddens HA, Geller DE, Challoner P, et al. Effect of dry powder inhaler resistance on the inspiratory flow rates and volumes of cystic fibrosis patients of six years and older. J Aerosol Med. 2006;19:456–65. doi: 10.1089/jam.2006.19.456. [DOI] [PubMed] [Google Scholar]
- 95.Standeardt T, Speirs RJ, Raon N, et al. Young cystic fibrosis patients can effectively use a novel high-payload capsule based dry powder inhaler with tobramycin powder for inhalation (TPI) Pediatr Pulmonol. 2004;38 Abstract 279. [Google Scholar]
- 96.Novartis Pharmaceuticals Canada Inc. TOBI Podhaler Product Mongraph. Dorval, Quebec: Novartis Pharmaceuticals Canada Inc; 2011. [Google Scholar]
- 97.Galeva I, Konstan MW, Higgins M, et al. Tobramycin inhalation powder manufactured by improved process in cystic fibrosis: the randomized EDIT trial. Curr Med Res Opin. 2013 doi: 10.1185/03007995.2013.805122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konstan MW, Flume PA, Kappler M, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J Cyst Fibros. 2011;10:54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Regnault A, Balp MM, Kulich K, Viala-Danten M. Validation of the Treatment Satisfaction Questionnaire for Medication in patients with cystic fibrosis. J Cyst Fibros. 2012;11:494–501. doi: 10.1016/j.jcf.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 101.Bertino JS, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis. 1993;167:173–9. doi: 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 102.Kahlmeter G, Dahlager JI. Aminoglycoside toxicity—a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984;13(Suppl A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 103.Brummett RE, Fox KE. Aminoglycoside-induced hearing loss in humans. Antimicrob Agents Chemother. 1989;33:797–800. doi: 10.1128/aac.33.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brummett RE, Morrison RB. The incidence of aminoglycoside antibiotic-induced hearing loss. Arch Otolaryngol Head Neck Surg. 1990;116:406–10. doi: 10.1001/archotol.1990.01870040028008. [DOI] [PubMed] [Google Scholar]
- 105.Pittinger CB. Convergence of interests in antibiotic-induced neuromuscular blockade. Acta Physiol Pharmacol Latinoam. 1989;39:393–6. [PubMed] [Google Scholar]
- 106.Hoffmann IM, Rubin BK, Iskandar SS, Schechter MS, Nagaraj SK, Bitzan MM. Acute renal failure in cystic fibrosis: association with inhaled tobramycin therapy. Pediatr Pulmonol. 2002;34:375–7. doi: 10.1002/ppul.10185. [DOI] [PubMed] [Google Scholar]
- 107.Mulrennan SA, Helm J, Thomas RB, Dodd M, Jones A, Webb K. Aminoglycoside ototoxicity susceptibility in cystic fibrosis. Thorax. 2009;64:271–2. doi: 10.1136/thx.2008.103010. [DOI] [PubMed] [Google Scholar]
- 108.Patatanian L. Inhaled tobramycin-associated hearing loss in an adolescent with renal failure. Pediatr Infect Dis J. 2006;25:276–8. doi: 10.1097/01.inf.0000202126.44544.41. [DOI] [PubMed] [Google Scholar]
- 109.Nikolaizik WH, Vietzke D, Ratjen F. A pilot study to compare tobramycin 80 mg injectable preparation with 300 mg solution for inhalation in cystic fibrosis patients. Can Respir J. 2008;15:259–62. doi: 10.1155/2008/202464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ratjen F, Rietschel E, Kasel D, et al. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother. 2006;57:306–11. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 111.Brown C, Nash EF, Camerson S, Rashid R, Whitehouse JL. Clinical outcomes and patient satisfaction following initation of the TOBI podhaler in CF adults. Journal of cystic fibrosis. 2013;12(Suppl 1):s65. [Google Scholar]
- 112.Nash E, Ahitan B, Brown C, Rashid R, Whitehouse JL. Comparison of pharamacy presecription refill frequency in CF adults before and after switching from tobramycin inhalation solution (TIS) to TOBI podhaler – ‘real world’ evidence of improved adherence. Journal of cystic fibrosis. 2013 [Google Scholar]