Abstract
Background. Alveolar macrophages in chronic obstructive pulmonary disease (COPD) have fundamental impairment of phagocytosis for nontypeable Haemophilus influenzae (NTHI). However, relative selectivity of dysfunctional phagocytosis among diverse respiratory pathogens: NTHI, Moraxella catarrhalis (MC), Streptococcus pneumoniae (SP), and nonbacterial particles, as well as the contribution of impaired phagocytosis to severity of COPD, has not been explored.
Methods. Alveolar macrophages, obtained from nonsmokers (n = 20), COPD ex-smokers (n = 32), and COPD active smokers (n = 64), were incubated with labeled NTHI, MC, SP, and fluorescent microspheres. Phagocytosis was measured as intracellular percentages of each.
Results. Alveolar macrophages of COPD ex-smokers and active smokers had impaired complement-independent phagocytosis of NTHI (P = .003) and MC (P = .0007) but not SP or microspheres. Nonetheless, complement-mediated phagocytosis was enhanced within each group only for SP. Defective phagocytosis was significantly greater for NTHI than for MC among COPD active smokers (P < .0001) and ex-smokers (P = .028). Moreover, severity of COPD (FEV1%predicted) correlated with impaired AM phagocytosis for NTHI (P = .0016) and MC (P = .01).
Conclusions. These studies delineate pathogen- and host-specific differences in defective alveolar macrophages phagocytosis of respiratory bacteria in COPD, further elucidating the immunologic basis for bacterial persistence in COPD and provide the first demonstration of association of impaired phagocytosis to severity of disease.
Keywords: nontypeable Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, alveolar macrophage, phagocytosis, COPD
The paradigm of bacterial colonization of the lower airways in COPD contributing to progressive lung disease is supported by the association of bacterial colonization with advanced airway inflammation, increased frequency of exacerbations, and accelerated decline in lung function [1–3]. Once initiated, chronic inflammation and failure to clear bacteria of the lower airways may promote progression of COPD [4, 5]. Moreover, bacterial colonization of the airways is associated with increased risk of exacerbation of COPD [6].
Our previous studies identified a fundamental phagocytic defect of alveolar macrophages in COPD to 3 clinical strains of NTHI that is not attributable to active smoking [7]. However, macrophages derived from peripheral blood monocytes of COPD donors had no phagocytic impairment, suggesting a compartmentalized immunologic defect. Intracellular survival of NTHI in macrophages after phagocytosis has been theorized to play a role in bacterial persistence [8, 9]. However, we found no increase in intracellular viability of bacteria in COPD alveolar macrophages.
Instead, failure to evoke a phagocytic response by alveolar macrophages in COPD may permit evasion of immune clearance by bacterial pathogens, providing a source for perpetuating inflammation. Our related findings of impaired cytokine responsiveness to outer membrane protein P6 and to lipooligosaccharide of NTHI further indicated that intrinsic alveolar macrophage impairment in COPD is not limited to a single intracellular signaling pathway [10]. Collectively, these studies support an immunologic basis for persistence of NTHI in the respiratory tract of adults with COPD. However, our previous investigation was limited to study of NTHI and did not determine if defective phagocytosis discriminated among respiratory targets [7]. Further, the impact of active smoking on alveolar macrophage dysfunction in COPD was not explored.
Each bacterial species that causes infection in adults with COPD displays unique dynamics of colonization and immune interaction with human cells. Nontypeable Haemophilus influenzae (NTHI) and Moraxella catarrhalis are the 2 most prevalent bacteria associated with exacerbations of COPD [11, 12]. Host acquisition of new strains of NTHI and host lymphocyte and antibody responses to NTHI antigens are associated with exacerbations of COPD [13–15]. Host antibody responses to outer membrane antigens of M. catarrhalis are also associated with respiratory clearance [16]. Moreover, M. catarrhalis strains have conserved genes whose proteins are expressed during infection in COPD [17]. NTHI and M. catarrhalis are predominantly mucosal pathogens [15, 18]. Although Streptococcus pneumoniae is also a mucosal pathogen, it is a leading cause of community-acquired pneumonia in COPD [19, 20]. Each of these bacterial pathogens presents a unique set of potential immunologic triggers for different host pathways. Beyond this, the link between impaired alveolar macrophage innate defense and severity of COPD has only been explored to a limited degree.
We hypothesized that the impaired immunologic responses of COPD alveolar macrophages to NTHI extend to other respiratory pathogens, and that responses to pathogen-associated molecular motifs of bacteria are different from responses to inert targets. We further theorized that dysfunctional alveolar macrophage phagocytosis in COPD, associated with failure to effectively clear respiratory pathogens, is associated with severity of disease in COPD and performed experiments to test these hypotheses.
METHODS
Recruitment of Subjects
All procedures received approval of the Institutional Review Boards, VAWNY Healthcare System and the University at Buffalo and were in strict accordance with institutional human studies guidelines. Participants were over 30 years of age and all gave informed consent. Volunteers were screened for inclusion into one of 3 groups: (1) nonsmokers (2) ex-smokers with COPD, and (3) active smokers with COPD. Groups 2 and 3 were also evaluated collectively as the COPD group. All ex-smokers had to have ceased smoking for greater than 1 year. All underwent clinical assessment, routine spirometry, and chest x-rays. Ex-smokers and nonsmokers had expired breath carbon monoxide of ≤0.02 ppm (Vitalograph Breath CO Monitor, Lenexa, KS).
For all COPD participants, exclusion criteria included a forced expiratory volume at one second (FEV1) <35%, hypercapnia and comorbid diseases that would render bronchoscopy unsafe. Inclusion criteria were (1) chronic bronchitis by history and/or emphysema by chest x-ray or CT; (2) absence of other lung disease, including asthma and bronchiectasis, based on clinical evaluation; (3) chest x-ray findings that were normal or compatible with COPD but detected no other disease; (4) FEV1/FVC ratio and FEV1 both below the lower 95% confidence limit of normal range on spirometry; (5) nonatopic by history; (6) no antibiotic or systemic steroid use for 4 weeks preceding enrollment.
Healthy nonsmokers met all inclusion criteria of the COPD group, except item 1 and item 4 (above) and that all had never smoked.
Phagocytosis
All experiments were performed with and without 8% antibody-depleted serum, as a complement source.
NTHI and M. catarrhalis
Assay for phagocytosis of 3H-bacteria was performed with adherent cells, as described elsewhere [7]. Briefly, bacteria were labeled with 3H-leucine and grown to mid-logarithmic phase; 3H-NTHI or 3H-M. catarrhalis were incubated with macrophages (200:1) with and without 8% antibody-depleted serum. No loss of macrophage viability occurred with any incubation conditions. The 3H-bacteria of extracellular supernatants and intracellular lysates were counted. Variation between wells and between experiments was accommodated by normalizing intracellular cpms against cpms of cell supernatants of the same well, and a phagocytic index was calculated. The mean cell-associated radioactivity is expressed as a percentage of mean total radioactivity of each well, calculated as follows:
Elimination of extracellular bacteria and viability of bacterial strains was confirmed with control wells of macrophage-free bacteria, treated identically to experimental wells and counted for 3H along with experimental samples. For each donor, quadruplicate values were averaged to arrive at a final phagocytic index. Further details of methodology are given in Supplementary Data.
S. pneumoniae
Owing to autolysis in long-term culture, it was not possible to consistently grow S. pneumoniae to mid-log phase. To maintain uniformity among donors, S. pneumoniae was harvested at stationary phase and labeled with fluorescein isothiocyanate (FITC) to measure phagocytosis. Labeled bacteria were incubated with adherent alveolar macrophages (200:1) with or without 8% antibody-depleted serum. No loss of macrophage viability was detected by trypan blue exclusion with any incubation conditions. Phagocytosis was calculated as the number of alveolar macrophages with intracellular bacteria, measured by fluorescence microscopy, as a percentage of the total number of cells over several fields. A minimum of 50 cells per well was counted for each experiment and results of a total of four wells per condition were averaged to arrive at a phagocytic index. Further details of methodology are given in Supplementary Data.
Latex Microspheres
Fluorescent (Nile red; 1 µm) latex microspheres (Invitrogen-Molecular Probes, Eugene, OR) were incubated with adherent macrophages (1000:1). Extracellular microspheres were removed with rinses and alveolar macrophages were evaluated for intracellular microspheres by fluorescent microscopy. Phagocytosis was measured as the number of macrophages containing intracellular microspheres as a percentage of the total and was determined over ten fields per well. At least 50 macrophages were counted per well and 4 wells per experiment were averaged for final results for each donor. Further details of methodology are given in Supplementary Data.
RESULTS
Recruitment of Participants
In total, 116 recruited volunteers met inclusion criteria and were amenable to undergoing bronchoscopy for this study, including 79 men and 37 women. Group 1 (referred to as “healthy nonsmokers”) included 20 participants; group 2 (“COPD ex-smokers”) included 32 participants; group 3 (“COPD active smokers”) included 64 participants. Demographic data for these subjects are presented in Table 1.
Table 1.
Demographic Characteristics of Participants
| Characteristics | Non-COPD |
COPD |
P Value | |
|---|---|---|---|---|
| Group 1 Nonsmokers (n = 20) | Group 2 Ex-smokers (n = 32) | Group 3 Active Smokers (n = 64) | ||
| Age, years (Mean ± SEM) | 57.3 ± 3.1 | 64.5 ± 1.3 | 56.5 ± 1.2 | P ≤ .02a,b |
| Gender, no. | ||||
| Male | 10 | 23 | 46 | P = .16 |
| Female | 10 | 9 | 18 | |
| Race, no. | ||||
| White | 16 | 30 | 41 | P = .006b |
| Black | 4 | 2 | 23 | |
| Pack-years smoking, Mean ± SEM | 0 ± 0 | 54.6 ± 4.4 | 48.4 ± 3.1 | P < .0001a,c |
| FEV1 (L) Mean ± SEM | 2.88 ± 0.18 | 1.86 ± 0.08 | 2.01 ± 0.09 | P < .0001a,c |
| FEV1 (% predicted) Mean + SEM | 99.55 ± 3.57 | 61.64 ± 2.54 | 64.90 ± 2.19 | P < .0001a,c |
| FVC (L), Mean ± SEM | 3.64 ± 0.20 | 3.27 ± 0.87 | 3.55 ± 0.12 | P > .15 |
| FVC, % predicted Mean ± SEM | 98.50 ± 3.10 | 83.56 ± 3.37 | 90.84 ± 2.13 | P ≤ .05a,b |
| FEV1/FVC, Mean ± SEM | 80.05 ± 3.0 | 56.77 ± 2.05 | 55.87 ± 1.39 | P < .0001a,c |
| GOLD classificationd | % (n) | % (n) | ||
| 1 | 12.5% (4) | 20.3% (13) | ||
| 2 | 59.4% (19) | 59.4% (38) | P = .55 | |
| 3 | 28.1% (9) | 20.3% (13) | ||
Data are expressed as mean ± SEM. Abbreviations: COPD, chronic obstructive pulmonary disease; SEM, standard error of the mean.
a P ≤ .05 - nonsmokers vs COPD ex-smokers.
b P ≤ .05 – COPD ex-smokers vs COPD active smokers.
c P ≤ .05 - nonsmokers vs COPD active smokers.
d GOLD classification is as detailed in the Global Initiative for Chronic Obstructive Lung Disease [21].
As expected, subjects of groups 2 and 3 (COPD) had significantly worse spirometric parameters (FEV1 and FEV1% predicted) than did healthy nonsmokers. Ex-smokers with COPD were also older than active smokers. Therefore, regression analyses were used to determine the independent impact of group disparities in FEV1, cumulative pack-years and age on immunologic outcomes.
The numbers of alveolar macrophages obtained by bronchoscopy (means ×105 ± SEM) were 209.8 ± 25.1 for healthy nonsmokers, 222.4 ± 60.8 for COPD ex-smokers and 541.8 ± 62.1 for COPD active smokers (P < .002). No participants were taking systemic steroids or antibiotics. In total, 57 participants (59.3%) with COPD were taking bronchoactive medications, including inhaled corticosteroids in 17 (17.7%), beta agonists in 55 (57.3%), anticholinergic medications in 36 (37.5%), and theophylline preparations in 2 (2.1%). No statistically significant differences in medication use existed between ex-smokers and active smokers with COPD.
Phagocytosis by Alveolar Macrophages
Phagocytosis of NTHI
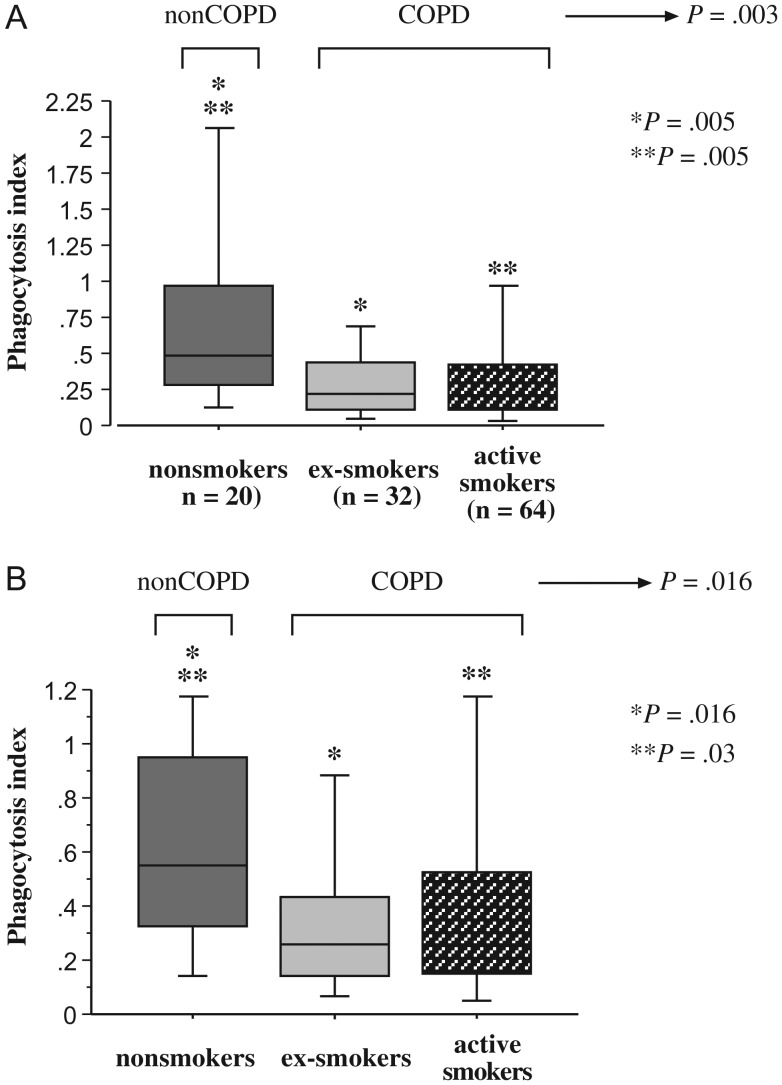
To investigate the relative phagocytic capabilities of alveolar macrophages of each group, alveolar macrophages were incubated with NTHI 11P6H1 and evaluated for intracellular radiolabel. Data, expressed as median (interquartile range), are included (see Supplementary Table 2). In all phagocytosis studies, antibody-depleted serum was used as a source of complement. In the presence of complement, phagocytosis of NTHI among all COPD participants was significantly less than that of healthy nonsmokers (P = .003; Figure 1A). Further analysis of smoking status indicated that phagocytosis by alveolar macrophages of active smokers with COPD, as well as those of COPD ex-smokers, was decreased (P = .005 for each) compared to macrophages of healthy nonsmokers (Figure 1A; Supplementary Table 2). No differences existed between ex-smokers and active smokers.
Figure 1.
Phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages. Alveolar macrophages were obtained from nonsmokers (dark gray shading), ex-smokers with COPD (light gray shading) and active smokers with COPD (stippled shading). Cells were incubated with NTHI 11P6H1 with antibody-depleted serum, as a source of complement (A) or in serum-free media (B). Phagocytosis was diminished for alveolar macrophages of COPD ex-smokers vs healthy nonsmokers (A: *P = .005; B *P = .016), and for COPD active smokers vs healthy nonsmokers (A: **P = .005; B: **P = .03). Phagocytosis among all COPD participants was significantly less than that of healthy nonsmokers (A: P = .003; B: P = .016). Phagocytosis was measured as described in Methods. Results are shown as box plots for each group. Each box encompasses the 25th to 75th interquartile range, with the horizontal line representing median values. Each vertical bar encompasses the 10th to 90th percentile ranges. Statistical comparison of all three groups was performed by Kruskal-Wallis test. Intergroup comparisons, for which P values are shown in figure, were performed by Mann–Whitney U rank test. Values correspond with data given in Supplementary Table 2.
The absence of complement did not change the pattern of impaired phagocytosis of NTHI for alveolar macrophages of COPD ex-smokers (P = .016) and COPD active smokers (P = .03) compared with healthy nonsmokers (Figure 1B; Supplementary Table 2). Paired comparisons, with or without complement, within each of the 3 participant groups, for NTHI phagocytosis, were not significantly different (P > .15 for each).
Phagocytosis of M. catarrhalis
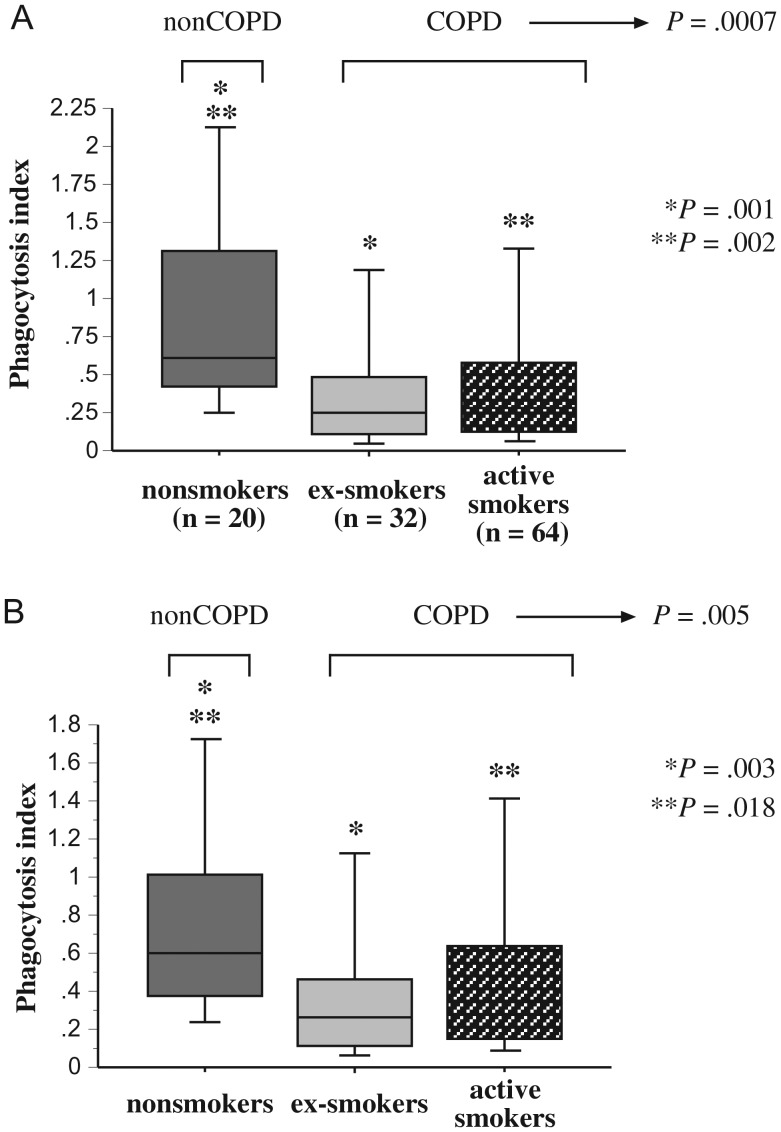
To investigate phagocytosis of M. catarrhalis, alveolar macrophages of each donor were incubated with M. catarrhalis 6P29B1 and evaluated for intracellular radiolabeled bacteria, as described for NTHI. Phagocytosis by alveolar macrophages of all COPD donors was significantly diminished compared with alveolar macrophages of healthy nonsmoker controls (P = .0007) in the presence of complement (Figure 2A: Supplementary Table 2). As with NTHI, further analysis identified phagocytic differences between alveolar macrophages of healthy nonsmokers and COPD ex-smokers (P = .001) and between healthy nonsmokers and COPD active smokers (P = .002). No differences existed between ex-smokers and active smokers.
Figure 2.
Phagocytosis of Moraxella catarrhalis by human alveolar macrophages. Alveolar macrophages were obtained from nonsmokers, ex-smokers with COPD, and active smokers with COPD. Shading denoting each individual group is as described in Figure 1. Cells were incubated with M. catarrhalis 6P29B1 with complement (A) or in serum-free media (B). Phagocytosis was diminished for alveolar macrophages of COPD ex-smokers vs healthy nonsmokers (A: *P = .001; B: *P = .003) and for COPD active smokers vs healthy nonsmokers (A: **P = .002; B: **P = .018). Phagocytosis among all COPD participants was significantly less than that of healthy nonsmokers (A: P = .0007; B: P = .005). Data are represented by box plots for each group, as detailed in Figure 1. Data correspond with values of Supplementary Table 2. Statistical comparison of all 3 groups was performed by Kruskal-Wallis test and for intergroup comparisons by Mann–Whitney U rank test.
As with NTHI, studies performed without complement did not change the pattern of impaired phagocytosis of M. catarrhalis for alveolar macrophages of ex-smokers (P = .003) and active smokers (P = .018) compared with healthy nonsmokers (Figure 2B; Supplementary Table 2). Paired comparisons within each participant group for M. catarrhalis phagocytosis were not significantly different with or without complement (P ≥ .2 for each).
Phagocytosis of S. pneumoniae
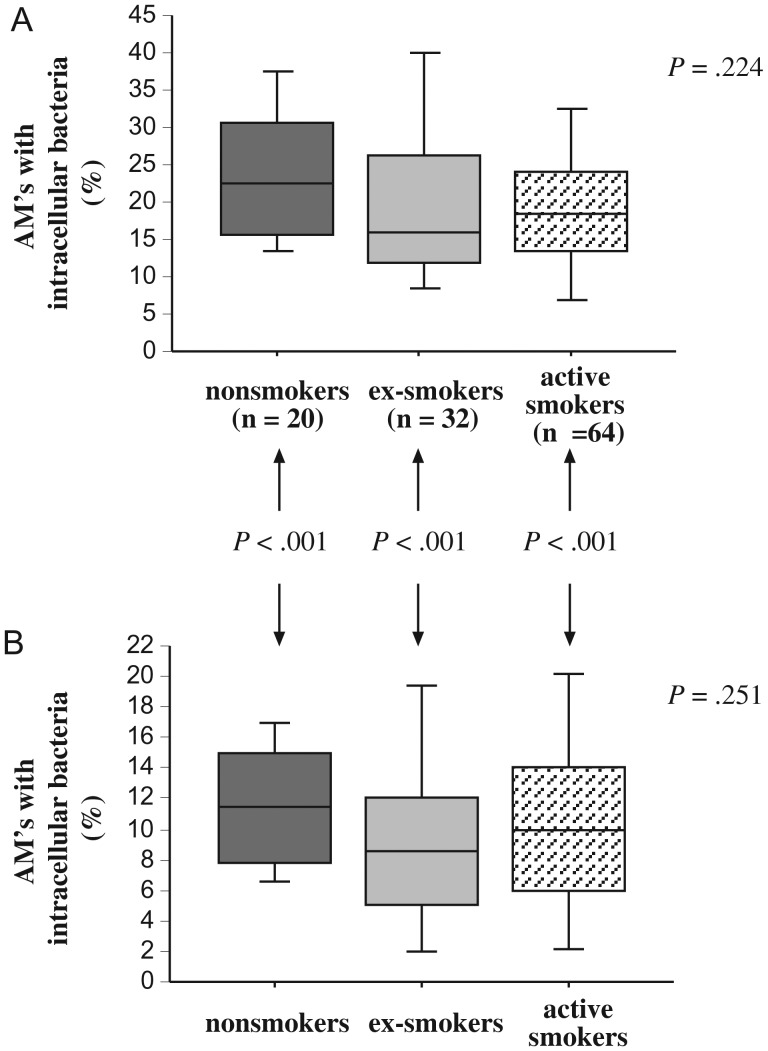
To extend our investigation to phagocytosis of S. pneumoniae, alveolar macrophages of each donor were incubated with FITC-labeled S. pneumoniae 25P55S1 and evaluated for intracellular fluorescent bacteria. Phagocytosis by alveolar macrophages of all 3 groups was not significantly different either in the presence (P = .224) or in the absence (P = .25) of a complement source (Figure 3; Supplementary Table 2). Further, there were no individual inter-group differences.
Figure 3.
Phagocytosis of Streptococcus pneumoniae by human alveolar macrophages. Alveolar macrophages were obtained from nonsmokers, ex-smokers with COPD, and active smokers with COPD. Shading denoting each individual group is as described in Figure 1. Cells were incubated with labeled S. pneumoniae 25P55S1, as detailed in Methods, with complement (A) and in serum-free media (B). Phagocytosis (y-axis) is measured as the percent of alveolar macrophages (AMs) containing intracellular bacteria. Data are represented by box plots for each group, as detailed in Figure 1, and correspond with values of Supplementary Table 2. Statistical comparison of all 3 groups was performed by Kruskal-Wallis test.
However, paired comparisons within each participant group for S. pneumoniae phagocytosis indicated significantly greater phagocytosis for alveolar macrophages of each group with complement compared to phagocytosis by alveolar macrophages of the same group without complement (P < .0001 for each group).
Phagocytosis of Latex Microspheres
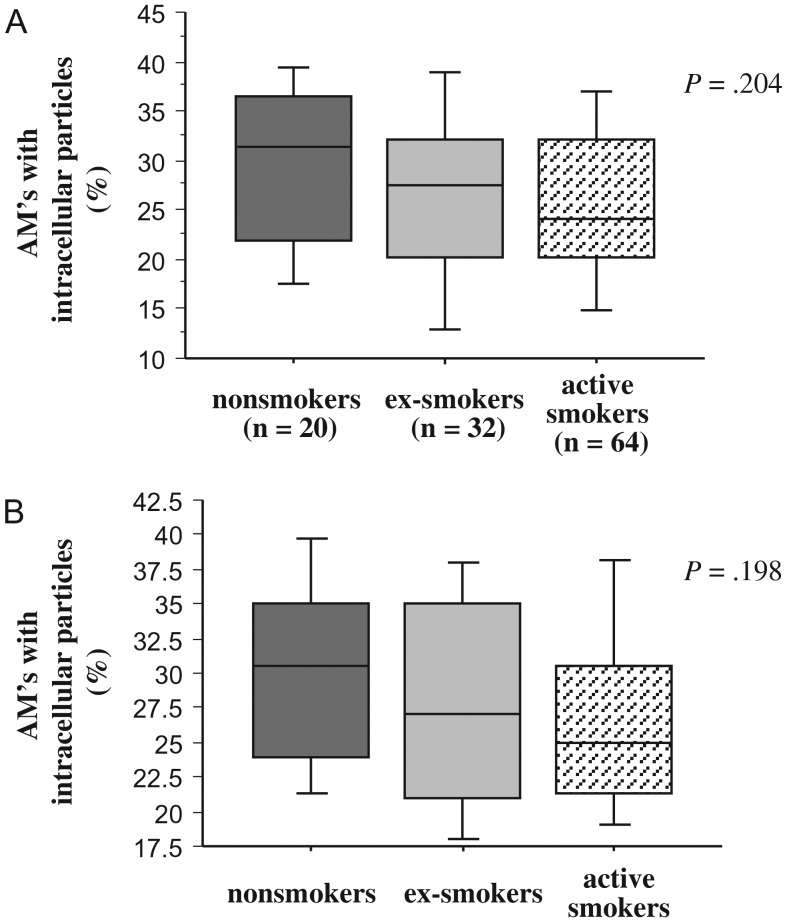
To determine if impaired phagocytosis of alveolar macrophages of any group extended to inert nonbacterial particles, alveolar macrophages of each donor were incubated with fluorescent latex microspheres (1 µm) and evaluated for intracellular spheres (Figure 4; Supplementary Table 2). Comparison of phagocytosis among groups showed no significant differences, either with (P = .204) or without (P = .198) complement. Paired comparisons within each group, for phagocytosis of microspheres with and without complement, were not significantly different for all groups (P ≥ .2 for each).
Figure 4.
Phagocytosis of latex microspheres by human alveolar macrophages. Alveolar macrophages were obtained from nonsmokers, ex-smokers with COPD, and active smokers with COPD. Shading denoting each individual group is as described in Figure 1. Cells were incubated with fluorescent latex microspheres (1 μm), as detailed in Methods, with complement (A) and in serum-free media (B). Phagocytosis (y-axis) is measured as the percent of alveolar macrophages (AMs) containing intracellular microspheres. Data are represented by box plots for each group, as detailed in Figure 1, and correspond with values of Supplementary Table 2. Statistical comparison of all 3 groups was performed by Kruskal-Wallis test.
Comparative Phagocytosis of NTHI and M. catarrhalis
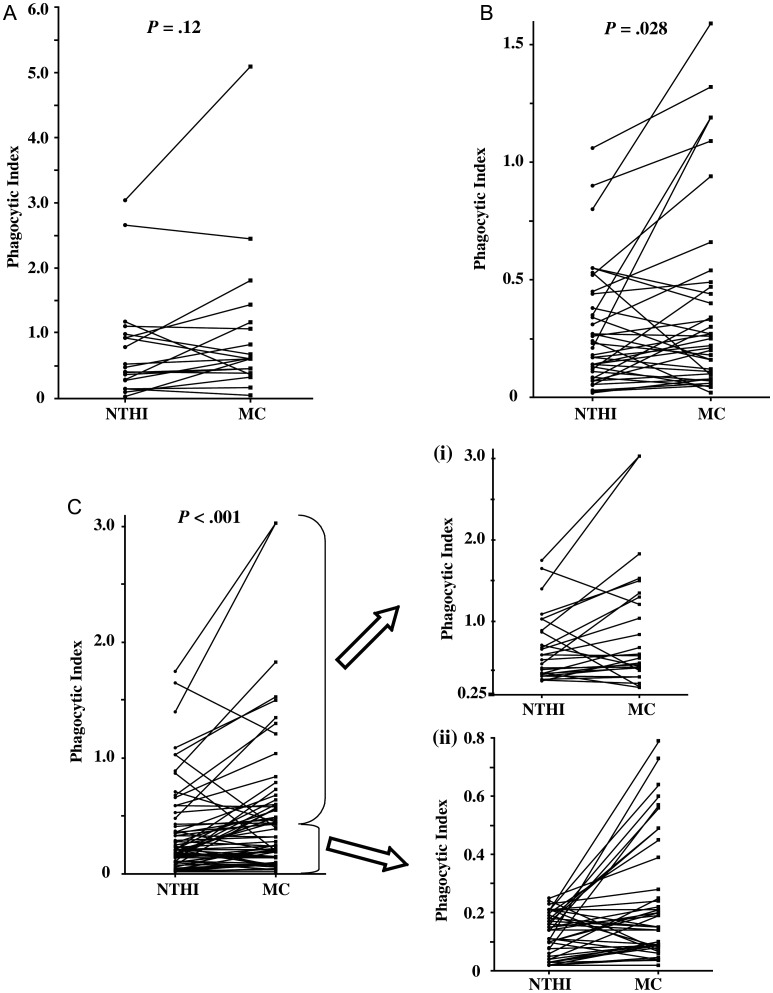
To determine if alveolar macrophages of any group exhibited preferential phagocytosis for either NTHI or M. catarrhalis, data were evaluated independently, by paired comparisons, of intracellular bacteria for each group (Figure 5). Among healthy nonsmokers (Figure 5A), differences in phagocytosis between NTHI and M. catarrhalis were not significant with (P = .12) or without (P = .37) complement.
Figure 5.
Relative phagocytosis of nontypeable Haemophilus influenza (NTHI) and Moraxella catarrhalis (MC) by human alveolar macrophages. Alveolar macrophages from nonsmokers (A), ex-smokers with COPD (B) and active smokers with COPD (C) were incubated with NTHI 11P6H1 (NTHI) or with M. catarrhalis 6P29B1 (MC) in antibody-depleted serum. Phagocytosis was evaluated by paired comparisons of phagocytosis of each bacteria from the same donor. To afford better clarity of individual data points in C, data is further separated (right side of C) into high values (i), (NTHI phagocytic index >0.25) and low values (ii) (NTHI phagocytic index ≤0.25). Data correspond with values of Supplementary Table 2. Statistical comparisons were performed by Wilcoxon signed rank test.
However, among all COPD donors (active and ex-smokers combined), alveolar macrophages had greater phagocytic impairment for NTHI than for M. catarrhalis with (P < .0001) or without (P = .002) complement. Alveolar macrophages of ex-smokers (P = .028; Figure 5B) and active smokers (P < .0001; Figure 5C) both exhibited markedly diminished phagocytosis of NTHI compared with M. catarrhalis. For better clarity of data points, Figure 5C, data are further separated into high values (i) (NTHI phagocytic index > 0.25) and low values (ii) (NTHI phagocytic index ≤ 0.25). Thus, the impairment of alveolar macrophage phagocytosis in COPD was significantly greater for NTHI than for M. catarrhalis.
Alveolar Macrophage Impairment and Severity of COPD
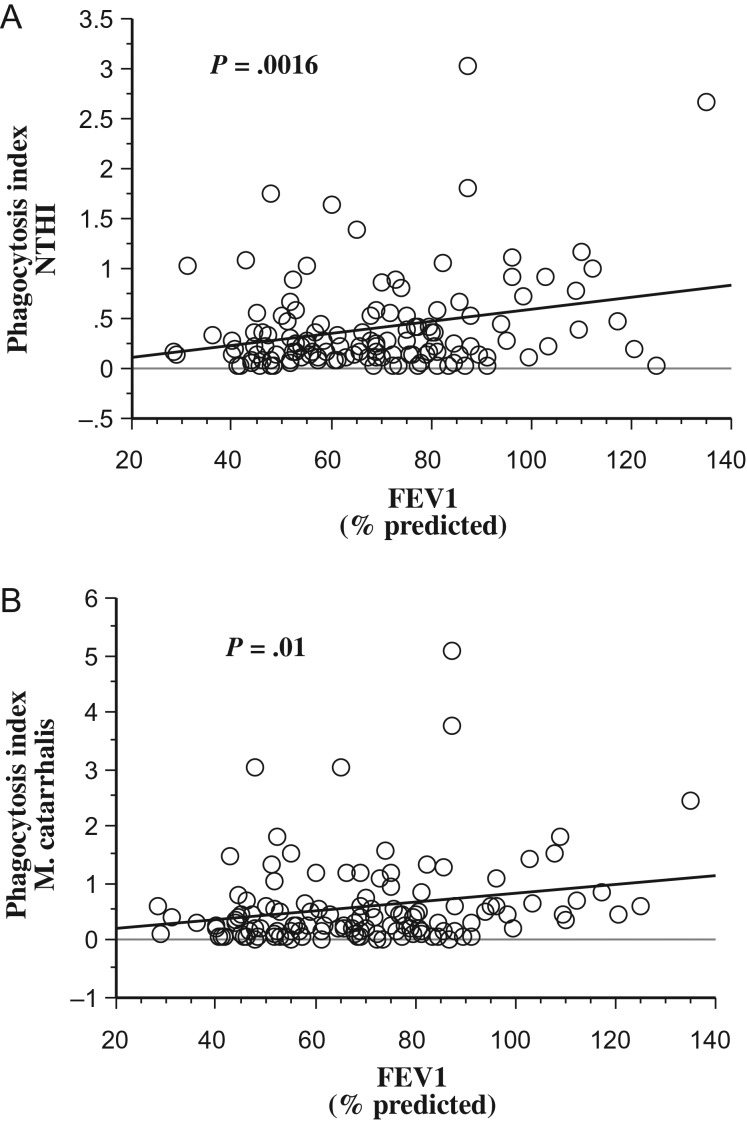
The relationship of alveolar macrophage dysfunction with severity of COPD was determined by regression analysis of macrophage functions with FEV1% predicted for all participants. Phagocytic impairment for NTHI (P = .0016; Figure 6A) and for M. catarrhalis (P = .01; Figure 6B) strongly correlated with FEV1% predicted. No statistical correlation existed between phagocytosis for S. pneumoniae or for microspheres and FEV1% predicted. Thus, strong association exists between severity of COPD and impaired phagocytosis of alveolar macrophages for NTHI and for M. catarrhalis.
Figure 6.
Alveolar macrophage phagocytosis and FEV1. Correlation of phagocytosis of nontypeable Haemophilus influenza (NTHI) (A) and of Moraxella catarrhalis (B) with FEV1% predicted is shown for all participants. Statistical correlation was determined by regression analyses.
Regression Analyses
Because demographic differences existed between groups (Table 1), regression analyses were performed to determine the relationship of age, race, and cumulative smoking on phagocytosis. In all analyses, age, race, and cumulative pack-years were not independent determinants of any alveolar macrophage phagocytic responses, nor were other demographic features independent predictors of alveolar macrophage responses.
DISCUSSION
This is the first study to our knowledge to demonstrate distinct correlation of fundamental impairment of complement-independent phagocytosis of alveolar macrophages in COPD for NTHI and M. catarrhalis, the 2 most prevalent respiratory pathogens of COPD. In related studies, GMCSF-differentiated blood monocyte-derived macrophages, rather than alveolar macrophages of COPD donors, also had impaired phagocytosis of NTHI [22]. Dysfunctional phagocytosis of alveolar macrophages of smokers with COPD for apoptotic bronchial epithelial cells is also reported, raising questions of the specificity of the phagocytic defect [23].
Our previous studies demonstrated dysfunctional phagocytosis of alveolar macrophages, but not of blood macrophages, in ex-smokers with COPD, compared to alveolar macrophages of ex-smokers without COPD [7]. Although alveolar macrophage phagocytosis was defective for different clinical strains of NTHI, our previous study excluded active smokers and did not include other respiratory pathogens or inert particles. While components of cigarette smoke are reported to have impaired in vitro phagocytosis of NTHI and S. pneumoniae, the impact of active smoking on alveolar macrophage phagocytosis in COPD has had limited investigation [24, 25]. Our current study is consistent with these previous findings but differs in design, evaluating phagocytic properties of alveolar macrophages of both active and ex-smokers with COPD, as well as healthy nonsmokers.
Our findings further delineate pathogen specificity, with greater phagocytic impairment for NTHI compared with M. catarrhalis, by alveolar macrophages of both active and ex-smokers with COPD. Although NTHI and M. catarrhalis both have integral pathologic roles in COPD, NTHI is not only more prevalent than M. catarrhalis in COPD but is more persistent and for longer periods [12]. Thus, it is noteworthy that alveolar macrophages of both active and ex-smokers with COPD had significantly poorer clearance of NTHI than M. catarrhalis, lending support to dysfunctional macrophage phagocytosis in COPD as a contributor to the greater prevalence of NTHI. Confirmation of the clinical relevance of these findings will require further study.
In contrast to results with NTHI and M. catarrhalis, phagocytosis of S. pneumoniae was no different between alveolar macrophages of COPD and non-COPD donors. A separate investigation found that differentiated blood monocyte-derived macrophages, rather than alveolar macrophages, of COPD donors had impaired phagocytosis of S. pneumoniae [22]. Our results with COPD alveolar macrophages did not corroborate these findings. Nonetheless, in the current study, phagocytosis of S. pneumoniae, but not other targets, was significantly greater for each individual group of participants in the presence of a complement source. Complement-enhanced phagocytosis is consistent with existing investigations of S. pneumoniae phagocytosis [26, 27]. In addition, intracellular trafficking of S. pneumoniae is also regulated by complement opsonization, as well as through interactions with complement receptors, pointing to regulation of phagocytosis of S. pneumoniae by different controls than phagocytosis of NTHI or M. catarrhalis. [26, 28].
The comparative defect in phagocytosis in COPD for bacterial pathogens, but not for inert microspheres, suggests diminished responsiveness of alveolar macrophages in COPD is directed at pathogen-associated molecular motifs found on bacteria. In related studies, phagocytosis of inert microspheres by COPD alveolar macrophages was not impaired and Toll-like receptor (TLR)-deficient murine macrophages also did not exhibit impaired phagocytosis of inert microspheres [22, 29]. In addition, murine macrophages treated with cigarette smoke extract also had diminished phagocytosis of NTHI, but not of latex beads, further indicating that nonbacterial phagocytosis is subject to different regulatory controls [24]. Our results do not coincide with all data from other studies. One earlier investigation indicated that alveolar macrophages in COPD may have diminished phagocytic ability for inert nonbacterial targets, a finding not corroborated by our results [30].
Phagocytosis of microbial pathogens is a remarkably complex and diverse process, governed by many cellular interactions [31]. Recent studies have begun to explore the immunomodulatory processes involved in regulation of alveolar macrophage phagocytosis. Activation of TLR signaling pathways by bacteria, as well as impaired phagocytosis of bacteria in the absence of TLR signaling indicates a role for pathogen-associated molecular pattern recognition in bacterial phagocytosis [29]. Nuclear erythroid-related factor 2 (Nrf2) signaling is supported by studies demonstrating activation of this pathway by sulforaphane, resulting in enhanced alveolar macrophage clearance of bacteria [32]. Moreover, a downstream target of Nrf2, the scavenger receptor MARCO, is also required for bacterial and particle clearance by alveolar macrophages [33]. In addition, complement receptor-mediated phagocytosis is also enhanced by surfactant protein A [34, 35]. Expression of the mannose receptor is also reported to be reduced in COPD and may be involved in alveolar macrophage phagocytosis of pathogens [36].
Aside from phagocytosis, additional features of dysregulated macrophage function in COPD and in smoking have been recognized in several investigations. Alveolar macrophages of adults with COPD had impaired inflammatory cytokine responsiveness to outer membrane protein P6 and to lipooligosaccharide of nontypeable Haemophilus influenzae (NTHI), suggesting impaired TLR regulation in COPD [10]. Histone deacetylase, a nuclear enzyme that regulates chromatin structure, also has diminished expression in alveolar macrophages of COPD donors [37]. Human sirtuin (SIRT1), an antiinflammatory protein, is also downregulated in lung tissue and in macrophages of smokers and COPD donors, possibly modulating pulmonary inflammation [38].
Our results reveal a conspicuous association between impaired phagocytosis of both NTHI and M. catarrhalis and severity of COPD. Impaired phagocytic responses by alveolar macrophages in COPD may contribute to pathologic features of COPD, as evasion of alveolar macrophage phagocytosis permits persistence of bacterial pathogens, providing a source for ongoing inflammation and progression of COPD. It is intriguing that progression of COPD is also associated with occlusion of the lumen of small airways in surgically resected lung tissue by inflammatory exudates and with development of lymphoid follicles, despite increased percentages of tissue macrophages [39]. Our findings support the possibility that impaired alveolar macrophage phagocytic function may play a key role in these changes that contribute to airway remodeling in COPD.
Although our results are definitive, there are inherent limitations to our study. Our investigation was limited to mild and moderate COPD because of the need for bronchoscopy. Thus the possibility that alveolar macrophages of adults with severe COPD might have greater immunologic impairment is not addressed in this study. In addition, differences in methodology were used to measure phagocytosis of different targets. Therefore, cross-methodologic comparisons are not used to denote pathogen-specificity in the current study. However, our analysis of comparisons among macrophage donor groups, and not between phagocytosis of targets measured by alternate methods, should allay concerns with this limitation. Finally, heterogeneity among strains of NTHI and M. catarrhalis raises questions of the phagocytic capabilities of COPD alveolar macrophages toward multiple strains of both bacteria.
The present study advances our understanding of innate immune dysfunction in COPD through several key observations: (1) Alveolar macrophages, specifically of COPD donors, had diminished complement-independent phagocytosis of both NTHI and M. catarrhalis. (2) Although phagocytosis of S. pneumoniae was not impaired for COPD alveolar macrophages, it was significantly enhanced by complement in all groups. (3) Despite the immunologic impact of active smoking, alveolar macrophage phagocytosis was also diminished among active smokers with COPD. (4) Alveolar macrophage phagocytosis in COPD was significantly worse for NTHI than for M. catarrhalis. (5) Most notably, significant correlation existed between severity of COPD and impaired complement-independent phagocytosis of NTHI and M. catarrhalis.
Although it is tempting to speculate that impaired alveolar macrophage phagocytosis for respiratory pathogens contributes to chronic persistence of bacteria in the airways in COPD and thus to the decline of lung function, further studies will be required to establish a causal relationship. Our findings provide further insight into the immunologic basis for persistence of bacteria in COPD.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors are grateful for scientific advice offered by Timothy F. Murphy, MD, and for assistance with graphics provided by Alan J. Lesse, MD. This article is dedicated to the memory to our colleague, Jane Maloney, without whom this work would not have been possible.
Financial support. This work was supported by research grant R01HL082561-01 (C. S. B., S. S.) from the National Institutes of Health and with support from the Department of Veterans Affairs.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection: comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:947–52. doi: 10.1164/ajrccm.162.3.9908103. [DOI] [PubMed] [Google Scholar]
- 2.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–95. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 3.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–64. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev. 2001;14:336–63. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–5. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:491–7. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- 7.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–84. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 8.Craig JE, Cliffe A, Garnett K, High NJ. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol Lett. 2001;203:55–61. doi: 10.1111/j.1574-6968.2001.tb10820.x. [DOI] [PubMed] [Google Scholar]
- 9.Forsgren J, Samuelson A, Ahli A, Jonasson J, Rynnel-Dagoo B, Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoidal tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun. 1994;62:673–9. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berenson CS, Wrona CT, Grove LJ, et al. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Resp Crit Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–9. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:266–72. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 13.Abe Y, Murphy TF, Sethi S, et al. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:967–71. doi: 10.1164/ajrccm.165.7.2109009. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–71. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S, Wrona C, Grant BJ, Murphy TF. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169:448–53. doi: 10.1164/rccm.200308-1181OC. [DOI] [PubMed] [Google Scholar]
- 16.Murphy TF, Brauer AL, Aebi C, Sethi S. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect Immun. 2005;73:3471–8. doi: 10.1128/IAI.73.6.3471-3478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruckdeschel EA, Kirkham C, Lesse AJ, Hu Z, Murphy TF. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76:1599–1607. doi: 10.1128/IAI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy TF, Brauer AL, Aebi C, Sethi S. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect Immun. 2005;73:8161–6. doi: 10.1128/IAI.73.12.8161-8166.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calbo E, Valdés E, Ochoa de Echagüen A, et al. Bacteraemic pneumococcal pneumonia in COPD patients: better outcomes than expected. Eur J Clin Microbiol Infect Dis. 2009;28:971–6. doi: 10.1007/s10096-009-0737-1. [DOI] [PubMed] [Google Scholar]
- 20.Malley R, Lipsitch M, Bogaert D, et al. Serum antipneumococcal antibodies and pneumococcal colonization in adults with chronic obstructive pulmonary disease. J Infect Dis. 2007;196:928–35. doi: 10.1086/520937. [DOI] [PubMed] [Google Scholar]
- 21.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039–47. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 23.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81:289–96. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 24.Martí-Lliteras P, Regueiro V, Morey P, et al. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect Immun. 2009;77:4232–42. doi: 10.1128/IAI.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun. 2010;78:1214–20. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68:2286–93. doi: 10.1128/iai.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hof DG, Repine JE, Peterson PK, Hoidal JR. Phagocytosis by human alveolar macrophages and neutrophils: qualitative differences in the opsonic requirements for uptake of Staphylococcus aureus and Streptococcus pneumoniae in vitro. Am Rev Respir Dis. 1980;121:65–71. doi: 10.1164/arrd.1980.121.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Wang JP, Ghiran I, et al. Complement receptor 1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence. Infect Immun. 2010;78:3129–35. doi: 10.1128/IAI.01263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304:1014–18. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara F, D'adda D, Falchi M, Dall'asta L. The macrophagic activity of patients affected by pneumonia or chronic obstructive pulmonary disease. Int J Tiss Reac. 1996;25:109–14. [PubMed] [Google Scholar]
- 31.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 32.Harvey CJ, Thimmulappa RK, Sethi S, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002042. 78ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arredouani M, Yang Z, Ning Y, et al. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–72. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil M, McCormack FX, Levine AM. Surfactant protein A modulates cell surface expression of CR3 on alveolar macrophages and enhances CR3-mediated phagocytosis. J Biol Chem. 2009;284:7495–504. doi: 10.1074/jbc.M808643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuronuma K, Sano H, Kato K, et al. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem. 2004;279:21421–30. doi: 10.1074/jbc.M312490200. [DOI] [PubMed] [Google Scholar]
- 36.Hodge S, Hodge G, Jersmann H, et al. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:139–48. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–76. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 38.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–70. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.