Abstract
Background. Parasite clearance time after artemisinin-based combination therapy (ACT) may be increasing in Asian and African settings. The association between parasite clearance following ACT and transmissibility is currently unknown.
Methods. We determined parasite clearance dynamics by duplex quantitative polymerase chain reaction (qPCR) in samples collected in the first 3 days after treatment of uncomplicated malaria with ACT. Gametocyte carriage was determined by Pfs25 quantitative nucleic acid sequence–based amplification assays; infectiousness to mosquitoes by membrane-feeding assays on day 7 after treatment.
Results. Residual parasitemia was detected by qPCR in 31.8% (95% confidence interval [CI], 24.6–39.8) of the children on day 3 after initiation of treatment. Residual parasitemia was associated with a 2-fold longer duration of gametocyte carriage (P = .0007), a higher likelihood of infecting mosquitoes (relative risk, 1.95; 95% CI, 1.17–3.24; P = .015), and a higher parasite burden in mosquitoes (incidence rate ratio, 2.92; 95% CI, 1.61–5.31; P < .001). Children with residual parasitemia were also significantly more likely to experience microscopically detectable parasitemia during follow-up (relative risk, 11.25; 95% CI, 4.08–31.01; P < .001).
Conclusions. Residual submicroscopic parasitemia is common after ACT and is associated with a higher transmission potential. Residual parasitemia may also have consequences for individual patients because of its higher risk of recurrent parasitemia.
Keywords: artemisinin, anopheles, infectivity, transmission, resistance, submicroscopic, PCR
Reduced susceptibility of Plasmodium falciparum to artesunate monotherapy has been reported in western Cambodia [1, 2] and was recently shown to have emerged in or spread westward to the Thailand–Myanmar border [3]. The time to clearance of parasites after mefloquine-artesunate combination therapy is also becoming longer along the Thailand–Cambodia border [4], and treatment failure rates of mefloquine-artesunate or artemether-lumefantrine (AL) often exceed 10% [5–8]. Reduced susceptibility to artemisinin-combination therapy (ACT) may also affect the African continent, where the majority of countries have adopted ACT as first-line antimalarial treatment.
In the first such report from Africa, a measurable increase in parasite clearance time after AL and dihydroartemisinin-piperaquine (DP) was reported in coastal Kenya during 2005–2009 [9]. Parasite clearance time is influenced by parasite drug susceptibility, parasite density before initiation of treatment, and interindividual differences in antimalarial pharmacokinetics and immunity [10, 11]. With the decline in transmission intensity in various regions in Africa [12, 13] and consequent changes in the rate at which immunity is acquired, time trends in parasite clearance times are difficult to interpret [9]. Detailed monitoring of parasite clearance dynamics after ACT is needed to determine whether parasite responsiveness to ACT is changing. Clearance dynamics in vivo can be analyzed as the parasite half-life if blood samples are obtained more than once per day after treatment [2, 11] or as the simple presence or absence of parasites at a single time point after initiation of treatment [14]. A simple duplex quantitative polymerase chain reaction (qPCR) method has been proposed for the analysis of sequentially collected daily filter paper blood samples to sensitively detect and quantify parasites below the microscopic threshold for detection [15].
A less conventional indicator of reduced susceptibility of malaria parasites to antimalarial drugs is the malaria transmission potential after treatment [16]. Transmission potential is frequently reported as the prevalence or density of malarial transmission stages (ie, gametocytes) and can be directly quantified by determining the infectivity of naturally infected individuals in mosquito-feeding assays [17]. For chloroquine (CQ) and sulfadoxine-pyrimethamine (SP), gametocyte carriage is increased or prolonged in resistant or partially resistant parasites [18–20], and enhanced transmission to Anopheles mosquitoes has been shown for CQ-resistant parasite isolates [21]. Parasites with mutations conferring SP resistance exhibit enhanced transmissibility even when circulating asexual parasites are effectively cleared by treatment [22], suggesting that, as antimalarial resistance develops in a population, changes in transmission potential may precede changes in treatment response [16, 22, 23]. Understanding the phenotype of enhanced parasite transmissibility is important for predicting the spread of resistant parasites.
Treatment with ACT is associated with rapid reductions in asexual parasite densities, lower prevalence of gametocyte carriage, and reduced but not completely abrogated posttreatment malaria transmission [24, 25]. The association between parasite clearance following ACT and transmissibility is unknown and regarded as one of the key questions for successful containment of artemisinin resistance [26, 27]. We determined the association between parasite clearance dynamics after ACT treatment of uncomplicated malaria in Kenyan children and posttreatment gametocyte carriage, malaria transmission to mosquitoes, and recurrent asexual parasitemia during follow-up.
METHODS
Study Design, Site, and Enrollment
Between April and June 2009, a randomized, open-label trial was conducted in Mbita Point, western Kenya, and has been described in detail elsewhere [28]. Briefly, children aged 6 months–10 years with uncomplicated malaria who were attending the health facility of the International Center of Insect Physiology and Ecology were included in the study if they had a microscopically confirmed P. falciparum monoinfection with an asexual parasite density of 1000–200 000 parasites/μL and a hemoglobin level of >5 g/dL. The protocol received approval from the Kenya Medical Research Institute Ethical Review Committee and the London School of Hygiene and Tropical Medicine Ethics Committee (reference 5455). Written informed consent was obtained from a parent or guardian of each participating child.
Treatment, Follow-up, and Clinical and Laboratory Procedures
Eligible children were randomly allocated to receive supervised treatment with AL (Coartem; Novartis Pharma), administered as half a tablet (20 mg of artemether and 120 mg of lumefantrine) per 5 kg of body weight in a 6-dose regimen, or with DP (Duocotexin, Holley Pharm, 40 mg dihydroartemisinin/320 mg piperaquine tablets), administered with targeted total doses of 6.4 and 51.2 mg/kg of dihydroartemisinin and piperaquine, respectively, given in 3 equally divided daily doses to the nearest quarter tablet. New participants were enrolled into the trial on a daily basis between 10 am and 12 pm (noon). Follow-up sampling was performed on days 1, 2, 3, 7, 14, 28, and 42 between 8 am and 12 pm. Participants who did not attend the clinic on any of the follow-up days were visited at home before noon. On treatment days, sampling was done before treatment: blood smears, dried filter paper blood spots, and 50-μL whole blood samples were collected for microscopy, qPCR, and quantitative nucleic acid sequence-based amplification (QT-NASBA), respectively. QT-NASBA was done for a random selection of individuals per treatment arm, as described previously [28]. MSP-1 and MSP-2 genotyping in cases of treatment failure was performed in accordance with World Health Organization (WHO) criteria [28, 29] but with additional time points; samples collected on days 0, 1, 2, and 3 and at the visit before recurrent patent parasitemia were included in each case. The original trial report was restricted to a conventional presentation of microscopically defined treatment outcomes and transmission potential [28]. For the current study, a duplex qPCR method was used to determine parasite clearance dynamics for all patients for whom a complete set of day 0, 1, 2, and 3 filter papers were available. qPCR was used as previously described [15], using 6-mm punches from Whatman 3MM filter paper (Whatman, Maidstone, United Kingdom)]. The qPCR generates distinct fluorescent signals for human (β-tubulin) and parasite (methionine transfer RNA gene [pgmet]) DNA targets. In the course of validating the modified protocol used here, the assay displayed a sensitivity comparable with that of conventional nested PCR [30] and reliably detected parasite densities of 1 parasite/μL; lower densities were frequently detected but with a stochastic element that is also observed for nested PCR [31]. The qPCR has a coefficient of variation between 0.03% and 0.68% at densities of ≥5 parasites/μL and between 4.0% and 6.8% at lower parasite densities. The WHO international standard for P. falciparum DNA was used as positive control [15]. The following modified amplification cycling conditions were used in the current study: 95°C for 6 minutes and then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. qPCR failed initially in 3 samples (ie, no β-tubulin signal was detected); repeating the samples using the same DNA material gave interpretable results. Pfs25 QT-NASBA gametocyte carriage, and infectiousness to mosquitoes at day 7 of follow up were determined as reported elsewhere [26].
Sample Size Considerations
The current study aims to determine parasite clearance by qPCR, a newly developed assay for which no baseline data were available, and test for association of clearance time with treatment outcome, gametocyte carriage, and malaria transmission. Parasite clearance was evaluated in 154 individuals by qPCR. As an indicative estimate of study power, if it is assumed a priori that 20% of individuals might display discernibly slower parasite clearance dynamics in the qPCR analysis and if both treatment arms are analyzed together, this sample size could detect, with 86% power at the 5% significance level, a 2-fold increase in the proportion of individuals who are infectious to mosquitoes on day 7 after treatment, based on an average of 30% of individuals infecting at least one mosquito [28].
Data Analysis
The main outcome of this study was parasite clearance by qPCR. Parasite clearance dynamics were described by generating a log-linear line of best fit for parasite density plotted across the 4 time points—days 0, 1, 2, and 3. From these data, 4 parameters of interest were determined: parasite clearance time (in hours), parasite reduction rate 48 hours after treatment, time to 95% reduction in parasite density (in hours), and a binary variable denoting whether parasite DNA was detectable at day 3. The parasite reduction rate 48 hours after treatment was arbitrarily set at 105 for isolates negative by qPCR at 48 hours. Gametocyte carriage was compared between groups as microscopic and Pfs25 QT-NASBA gametocyte prevalence at enrollment and day of feeding; the mean duration of gametocyte carriage after treatment was estimated using a previously published mathematical model for repeated QT-NASBA measurements [32]. Continuous data were compared between groups, using the t test or nonparametric tests. The proportion of parasitemic or gametocytemic individuals and the proportion of infectious individuals (ie, subjects who infected at least 1 mosquito) were compared between groups by the χ2 test and logistic regression models. The proportion of infected mosquitoes and the oocyst burden in mosquitoes were compared between groups by logistic or negative binomial regression models, using generalized estimating equations to adjust for clustering between observations from the same individual.
RESULTS
Residual Parasitemia Detected by qPCR
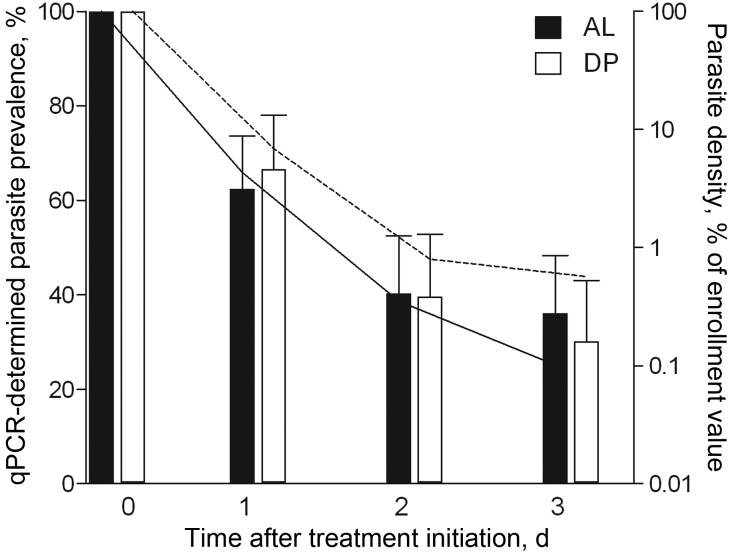
As reported elsewhere, conventional microscopic assessment of parasitological efficacy found both regimens very effective at initial parasite clearance, with only a single child in the DP arm found to harbor detectable asexual parasitemia 3 days after treatment [28]. Prevalence and relative density of parasitemia determined by qPCR over the first 72 hours following treatment are plotted in Figure 1 for 154 children with a full set of 4 daily samples available. Residual parasitemia on day 3 after initiation of treatment was observed in 33.3% (28/84) of the children treated with AL and 30.0% (21/70) of the children treated with DP (P = .66). The median estimated density of these residual parasites was 0.17% (interquartile range, 0.0001–1.54) of the enrollment parasite density and was not different between treatment arms (P = .26; Figure 1). qPCR positivity on day 3 was strongly associated with the parasite reduction rate 48 hours after treatment (P < .001) and with the time to 95% reduction in parasite density (P < .001; Table 1). We retained the binary variable residual parasitemia by qPCR on day 3 for exploring associations with other trial outcomes.
Figure 1.
Plasmodium falciparum parasite prevalence and relative density by duplex quantitative polymerase chain reaction (qPCR), by treatment arm. Left y-axis, qPCR parasite prevalence (bars) for children treated with artemether-lumefantrine (AL; n = 84) or dihydroartemisinin-piperaquine (DP; n = 70). Error bars indicate the upper limit of the 95% confidence interval. Right y-axis, median relative parasite density relative to starting infection is plotted (line) for PCR-positive individuals treated with AL (solid line) or DP (dashed line). All samples were collected between 10 am and 12 pm (day 0) or between 8 am and 12 pm (days 1, 2, and 3).
Table 1.
Parameters of Plasmodium falciparum Parasite Clearance Dynamics Over the First 3 Days Following Initiation of Treatment With Artemether-Lumefantrine (AL) or Dihydroartemisinin-Piperaquine (DP)
| Treatment, qPCR Result on Day 3 | PRR48a , Geometric Mean (95% CI) | PCT95,b h , Median (IQR) |
|---|---|---|
| AL (n = 84) | ||
| Negative (n = 56) | 54 984 (30 291–99 807) | 5 (5–13) |
| Positive (n = 28) | 186.0 (68.59–504.5) | 39 (22–45) |
| DP (n = 70) | ||
| Negative (n = 49) | 44 929 (21 625–93 347) | 9 (5–13) |
| Positive (n = 21) | 206.2 (70.43–603.6) | 44 (30–78) |
Abbreviations: CI, confidence interval; IQR, interquartile range; qPCR quantitative polymerase chain reaction.
a The parasite reduction ratio 48 hours after treatment (PRR48) was estimated from point estimates at 0 hours and 48 hours derived from qPCR data, using the DDCT formula as previously described [16].
b The time to 95% parasite clearance (PCT95) was estimated from log-linear line of best-fit of plotted point estimates of relative reductions in parasite density.
Children with residual parasitemia on day 3 were significantly younger (P < .001; Table 2), had a higher multiplicity of infection at enrollment (P = .047), but did not have a statistically significant different enrollment asexual parasite density (P = .91) or mean total drug dose (P ≥ .34). Microscopic gametocyte carriage at enrollment was significantly more common for children with residual parasitemia on day 3 (P = .006), whereas Pfs25 QT-NASBA–detected gametocyte carriage was not significantly different (P = .34). There was limited agreement between the qPCR parasite detection and the Pfs25 QT-NASBA gametocyte detection. On days 1, 2, and 3 after initiation of treatment, 45.0% (18/40), 53.4% (15/28), and 50.0% (15/30), respectively, of the qPCR-positive samples were QT-NASBA gametocyte negative. The scatter plot of qPCR signal on day 3 and QT-NASBA gametocyte prevalence is given in Supplementary Figure 1. The κ values for agreement were −0.09, 0.15, and 0.25 on days 1, 2, and 3, respectively, after initiation of treatment. The estimated parasite density determined by qPCR was not associated with the Pfs25 QT-NASBA gametocyte prevalence on the same day (P ≥ .50).
Table 2.
Characteristics of Individuals Whose Plasmodium falciparum Infections Were Cleared or Who Had Residual Parasitemia Detected by Quantitative Polymerase Chain Reaction (qPCR) on Day 3 After Initiation of Treatment With Artemether-Lumefantrine (AL) or Dihydroartemisinin-Piperaquine (DP)
| Characteristic | qPCR Result on Day 3 |
P | |
|---|---|---|---|
| Parasite Free (n = 105) | Residual Parasitemia (n = 49) | ||
| Treated with AL | 53.3 (56/105) | 57.1 (28/49) | .66 |
| Total dose of lumefantrine in AL arm, mg/5 kg body weight | 59.8 ± 10.1 | 60.1 ± 8.6 | .93 |
| Total dose of piperaquine in DP arm, mg/kg body weight | 65.6 ± 7.3 | 67.5 ± 10.5 | .34 |
| Age, y | 6 (5–8) | 4 (3–6) | <.0001 |
| Characteristics on day 0 before initiation of treatment | |||
| Temperature ≥37.5°C | 36.2 (38/105) | 49.0 (24/49) | .13 |
| Hemoglobin level, mmol/dL, mean (95% CI) | 6.8 (6.6–7.0) | 6.6 (6.3–6.9) | .28 |
| Multiplicity of infection, mean (95% CI) | 3.6 (3.2–3.9) | 4.3 (3.7–5.0) | .047 |
| Microscopy finding | |||
| Asexual parasite density, geometric mean (95% CI) | 18 560 (14 989–22 981) | 13 720 (10 411–18 081) | .91 |
| Gametocyte prevalence | 4.2 (4/95) | 18.2 (8/44) | .006 |
| Pfs25 QT-NASBA finding | |||
| Gametocyte prevalence | 71.7 (38/53) | 81.5 (22/27) | .34 |
Data are % (proportion) of participants, mean ±SD, or median (interquartile range), unless otherwise indicated.
Abbreviations: CI, confidence interval; qPCR, duplex quantitative polymerase chain reaction; QT-NASBA, quantitative nucleic acid sequence–based amplification.
Residual Parasitemia on Day 3 and Malaria Transmission Potential During Follow-up
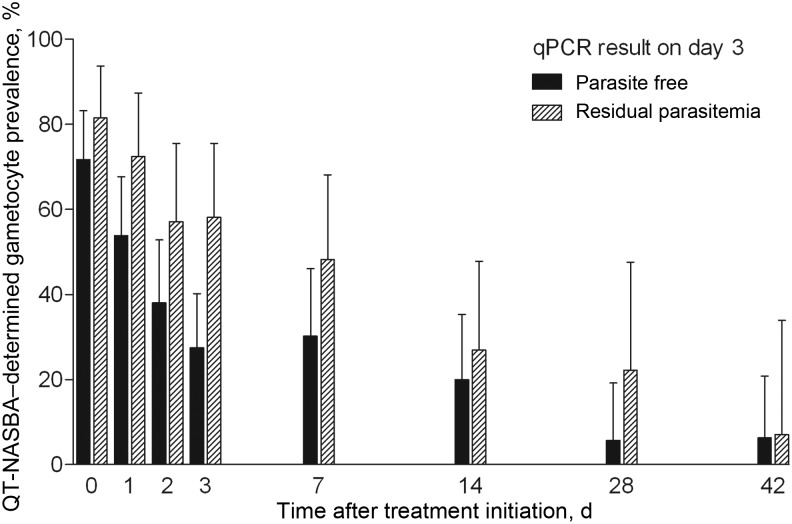
Children with residual parasitemia on day 3 had a longer duration of gametocyte carriage during the 42-day follow-up period (P = .0007; Figure 2 and Table 3). On the day of feeding experiments, children with residual parasitemia on day 3 were more likely to infect at least 1 mosquito, compared with children whose parasites were cleared by day 3 after initiation of treatment (relative risk [RR], 1.95; 95% confidence interval [CI], 1.17–3.24; P = .015; Table 3). Blood from these children infected 4.0% (34/859) of the mosquitoes with 1–6 oocysts, compared with blood donated by children who were parasite free by qPCR on day 3, which infected 1.6% (49/2998) of the mosquitoes with 1–4 oocysts. The proportion of infected mosquitoes (odds ratio [OR], 2.41; 95% CI, 1.18–4.92) and the oocyst burden in mosquitoes (incidence rate ratio [IRR], 2.92; 95% CI, 1.61–5.31; P < .001) were significantly higher in experiments in children with residual parasitemia on day 3, after adjustment for correlations between observations from the same individual. These associations were not confounded by enrollment parasite density, multiplicity of infection, treatment arm, or age.
Figure 2.
Plasmodium falciparum gametocyte prevalence by quantitative nucleic acid sequence–based amplification for children whose infection cleared or who had residual parasitemia detected by duplex quantitative polymerase chain reaction on day 3 after treatment with artemether-lumefantrine or dihydroartemisinin-piperaquine. Error bars indicate the upper limit of the 95% confidence interval.
Table 3.
Association Between Residual Parasitemia on Day 3 After Initiation of Treatment and Malaria Transmission Potential
| Characteristic | qPCR Result on Day 3 |
P | |
|---|---|---|---|
| Parasite Free | Residual Parasitemia | ||
| Duration of gametocyte carriage, d, mean (95% CI) | 6.6 (4.7–9.3) | 14.5 (9.4–22.3) | .0007 |
| Microscopy finding on feeding day | |||
| Gametocyte prevalence | 5.0 (5/100) | 11.1 (5/45) | .18 |
| Gametocyte density, gametocytes/μL, median (IQR)a | 48.0 (16.0–48.0) | 64.0 (48.0–208.0) | .24 |
| Pfs25 QT-NASBA finding on feeding day | |||
| Gametocyte prevalence | 30.2 (13/43) | 48.2 (13/27) | .13 |
| Individuals participating in membrane feedings, no. | 101 | 29 | |
| Infected ≥1 mosquito | 24.8 (25/101) | 48.3 (14/29) | .015 |
| Infected mosquitoes, no. (proportion) | 1.6 (49/2998) | 3.96 (34/859) | .016b |
| Oocysts in infected mosquitoes, no., mean (range) | 1.3 (1–4) | 1.8 (1–6) | .001b,c |
Data are % (proportion) of participants, unless otherwise indicated.
Abbreviations: CI, confidence interval; IQR, interquartile range; qPCR, duplex quantitative polymerase chain reaction; QT-NASBA, quantitative nucleic acid sequence–based amplification.
a Data are for gametocyte carriers only.
b Adjusted for correlations between observations from the same individual.
c Determined using a negative binomial regression model that incorporated both prevalence and intensity of infection among mosquitoes.
We repeated our analyses after exclusion of children who had microscopically detectable gametocytes at enrollment. Gametocyte carriage determined by microscopy on day 7, which was strongly associated with microscopic gametocyte carriage at enrollment (P < .001), was no longer elevated in children with residual parasitemia on day 3 (P = .93). Residual parasitemia was still significantly associated with a longer duration of Pfs25 NASBA gametocyte carriage (P = .021), a higher likelihood of infecting at least 1 mosquito (OR, 3.71; 95% CI, 1.01–13.66; P = .049), and a higher oocyst burden in mosquitoes (IRR, 1.85; 95% CI, 1.02–3.35; P = .040). Because 75.0% (60/80) of children with qPCR results harbored gametocytes as determined by QT-NASBA at enrollment and because de novo gametocyte production was rare, we did not restrict our analyses to children who were gametocyte free at enrollment according to QT-NASBA.
Residual Parasitemia on Day 3 and Parasite Recurrence During Follow-up
Among the 154 children for whom clearance dynamics were measured, 25 had recurrent parasitemia detected by microscopy on days 28 or 42. Of these children, 84.0% (21/25) had residual parasitemia on day 3, compared with 21.7% (28/129) among children who remained parasite free according to microscopy during follow-up (RR, 11.25; 95% CI, 4.08–31.01; P < .001). This association was not confounded by age, treatment arm, enrollment parasite density, or total administered drug dose. The multiplicity of infection at enrollment was weakly associated with recurrent parasitemia during follow-up (OR for each additional clone 1.25; 95% CI, .96–1.61; P = .093), but the association between residual parasitemia on day 3 and recurrent parasitemia during follow-up remained statistically significant after adjustment for multiplicity of infection (P < .001). For all 25 children, we performed merozoite antigen genotyping analysis on days 0, 1, 2, the scheduled follow-up day preceding failure, and the day of failure. PCR was successful for 24 of 25 samples from day 0, 23 of 24 from day 1, 18 of 25 from day 2, 17 of 24 from the day before failure, and 24 of 25 from day of failure. As a result, 96.0% (24/25) of the individuals had ≥2 observations from the parasite population within 48 hours of treatment, and 64.0% (16/25) had 2 observations from the parasite population at or shortly before microscopy-detected parasitological treatment failure. Reworking WHO criteria to suit this approach [29], recrudescent infections were defined as the presence of ≥1 allele on the day of failure or the day preceding failure that were also present on ≥1 of days 0–2. By using these criteria, 19 infections were now classified as recrudescent and 6 as new infections. Residual parasitemia on day 3 remained significantly associated with both recrudescent infections (RR, 11.43; 95% CI, 3.49–37.40; P < .001) and new infections (RR, 10.71; 95% CI, 1.29–89.27; P = .006).
DISCUSSION
In this study, we provide the first evidence from Africa that low-density residual parasitemia after treatment with ACT is common and has consequences for onward malaria transmission to mosquitoes. Children with residual parasitemia detected by qPCR on day 3 after initiation of treatment had a longer duration of gametocyte carriage, were more likely to infect mosquitoes, and infected a higher proportion of mosquitoes. In addition, they were >10-fold more likely to have recurrent microscopic asexual parasite carriage during follow-up.
Despite excellent parasite clearance rates by microscopy [28], we observed that residual parasitemia detected by PCR persisted in approximately one third of ACT-treated children until day 3 after initiation of treatment. Submicroscopic parasite carriage persisting after ACT has recently been described in Sudan and Ghana [33, 34], but because PCR is not routinely used to monitor parasite clearance dynamics, it is currently unknown how often parasites remain PCR detectable after successful ACT treatment. Our study is the first to directly relate parasite clearance dynamics after ACT to malaria transmissibility. We show that children who had residual parasitemia on day 3 after treatment initiation had a longer duration of gametocyte carriage, were more likely to be infectious to mosquitoes, and infected more mosquitoes with a higher oocyst burden. These associations remained apparent if children with microscopically detectable gametocytes at enrollment were excluded from the analyses.
The association between slow parasite clearance and gametocyte carriage is at least partly the result of ongoing gametocyte production by persisting asexual parasites [19]. Because ACT rapidly clears the pool of asexual parasites from which gametocytes are derived [8] and because the artemisinin-component of ACT is given over 3 days, during which it efficiently clears developing gametocytes [8, 35], it is striking that parasite-clearance dynamics shortly after initiation of ACT determine malaria transmission potential 1 week later. Our results confirm previous suggestions that increased gametocyte production is a valuable marker of a slow parasite-clearance phenotype after ACT [27, 36, 37] and are in line with findings from the Thailand–Myanmar border, where slow microscopic parasite clearance was associated with a longer duration of microscopic gametocyte carriage following mefloquine-artesunate treatment [4]. We confirm this trend in Africa and add 2 relevant lines of evidence: we show that this association is also apparent if residual parasitemia after treatment and gametocytes are present only at submicroscopic levels, and we provide direct evidence that this longer duration of gametocyte carriage results in higher infectivity to mosquitoes.
Similar to previous studies, we found that gametocyte carriage at enrollment may be an indicator of subsequent treatment responses [3, 4, 38]. One may hypothesize that this indicates that slow clearing infections are have an intrinsic higher commitment to gametocyte production [39] or that slow clearing infections have been present for a longer period than fast clearing infections and therefore have a higher likelihood of gametocyte development during the course of infection [40]. It was recently suggested that long-term chronic infections in equilibrium may elicit weak immune responses and thus leave drug clearance unassisted [34], which may result in slower parasite clearance and longer gametocyte production. Whatever the reason, careful monitoring of transmission potential after ACT is justified by earlier demonstrations that high gametocyte production and infectivity were linked with the development of resistance to CQ and SP [18, 19, 21].
In our setting, we observed a strong association between residual submicroscopic parasitemia and PCR-unadjusted treatment failure during follow-up. This association was independent of starting parasite density, treatment arm, multiplicity of infection, and age and was not explained by differences in the administered dose of AL or DP. Using standard methods, we previously determined that the vast majority of recurrent infections on day 28 and day 42 were new infections [28]. It was therefore surprising that residual parasitemia on day 3 was strongly associated with recurrent infections. We see 3 possible explanations for the association between early residual parasitemia and later recurrent infections that appear to be mostly “new” infections. First, higher multiplicity of infection, a characteristic of slow-clearing infections in this setting, may be associated with a higher malaria exposure [41] that may be stable over time [42] and may thereby predict a higher risk of new malaria infections during follow-up [43]. Second, immunity is an important factor in determining parasite-clearance time [10, 14], and slow parasite clearance may be indicative of a less efficient immune response in clearing parasites that may also be associated with a higher chance of new infections becoming detectable by microscopy. Third, we may have misclassified recrudescent infections as new infections.
We have provided evidence that the third explanation is plausible for a proportion of the infections by extending our parasite genotyping to multiple days. The risk of overestimating the number of recrudescent infections because some new infections may have the same genetic profile as original infections is reasonably well appreciated [44, 45]; the risk of underestimating the number of recrudescent infections as a consequence of incomplete detection of all parasite clones that are present at enrollment and failure has received less attention [46, 47]. Clones may go undetected if genotyping is done on single days as a consequence of sequestration of parasites, differences in clonal densities and a stochastic element in PCR detection [31, 48]. When we analyzed sample series from the first 3 days after enrollment and the day of failure combined with the day preceding failure, we observed that parasites were often detectable on all of the examined days. Based on MSP-1 and MSP-2 typing in these extended sample sets, 15 infections that were initially classified as new infections [28] were now classified as recrudescent infections. This is in line with a study that used a similar approach in Tanzania, where genotypes that were subsequently found to be recrudescent were undetectable at enrollment but detected as late as day 3 after initiation of treatment [49]. Our data illustrate the difficulty of distinguishing between recrudescent and new infections in areas with complex infections and suggest that the original genotyping approach may have underestimated the true number of recrudescent infections [47].
In summary, we demonstrated that 24-hour DNA sampling followed by qPCR analysis identifies submicroscopic, slow-clearing P. falciparum in approximately one third of Kenyan children treated with ACT. Children harboring such parasites were significantly more likely to be infectious to mosquitoes and are more likely to experience recurrent asexual parasitemia on day 28 or day 42.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Gibson Kibiki and Frank Mosha of the Kilimanjaro Clinical Research Institute-Kilimanjaro Christian Medical Centre, for the logistical support of this study; the parents of participants and village and district authorities, for their cooperation; the data and safety monitoring board and the local safety monitor; and Silas Otieno and Tom Guda (International Centre for Insect physiology and Ecology, Mbita Point, Kenya), for their helping during the membrane–feeding assays.
C. J. S., P. S., C. J. D., S. A. S., H. D. F. H. S., and T. B. contributed to study design. P. S., C. K. M., S. A. O., S. A. S., A. N., and J. C. collected clinical data. C. K. M. and T. B. coordinated and conducted the membrane-feeding assays. K. B. B., C. J. S., H. K., A. N., R. L. H., and T. B. did laboratory work. H. K. and H. D. F. H. S. provided reagents. K. B. B., C. J. S., L. O., R. L. H., and T. B. analyzed the data. K. B. B., C. J. S., C. J. D., R. W. S., R. L. H., and T. B. wrote the manuscript. All authors have contributed to and approved the final version of the manuscript.
Disclaimer. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Financial support. This work was supported by the European Community's Seventh Framework Programme (grant 201889 to Project MALACTRES [Multi-drug resistance in malaria under combination therapy: assessment of specific markers and development of innovative, rapid and simple diagnostics]), the Bill and Melinda Gates Foundation (grant OPP1024438 to T. B.), Public Health England. (to C. J. S.), and the EDCTP WANECAM Consortium (to. K. B. B).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phyo AP, Nkhoma S, Stepniewska K, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–6. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrara VI, Zwang J, Ashley EA, et al. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wongsrichanalai C, Meshnick SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis. 2008;14:716–9. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denis MB, Tsuyuoka R, Lim P, et al. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop Med Int Health. 2006;11:1800–7. doi: 10.1111/j.1365-3156.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 7.Denis MB, Tsuyuoka R, Poravuth Y, et al. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop Med Int Health. 2006;11:1360–6. doi: 10.1111/j.1365-3156.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 8.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–4. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 9.Borrmann S, Sasi P, Mwai L, et al. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS One. 2011;6:e26005. doi: 10.1371/journal.pone.0026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaratunga C, Sreng S, Suon S, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–8. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–55. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 13.Barnes KI, Chanda P, Ab Barnabas G. Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar J. 2009;8(Suppl 1):S8. doi: 10.1186/1475-2875-8-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepniewska K, Ashley E, Lee SJ, et al. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–9. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beshir KB, Hallett RL, Eziefula AC, et al. Measuring the efficacy of anti-malarial drugs in vivo: quantitative PCR measurement of parasite clearance. Malar J. 2010;9:312. doi: 10.1186/1475-2875-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland CJ. Comparing highly efficacious antimalarial drugs. PLoS Med. 2008;5:e228. doi: 10.1371/journal.pmed.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bousema T, Dinglasan RR, Morlais I, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One. 2012;7:e42821. doi: 10.1371/journal.pone.0042821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland CJ, Alloueche A, Curtis J, et al. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–85. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- 19.Mendez F, Munoz A, Carrasquilla G, et al. Determinants of treatment response to sulfadoxine-pyrimethamine and subsequent transmission potential in falciparum malaria. Am J Epidemiol. 2002;156:230–8. doi: 10.1093/aje/kwf030. [DOI] [PubMed] [Google Scholar]
- 20.Bousema JT, Gouagna LC, Meutstege AM, et al. Treatment failure of pyrimethamine-sulphadoxine and induction of P. falciparum gametocytaemia in children in western Kenya. Trop Med Int Health. 2003;8:427–30. doi: 10.1046/j.1365-3156.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 21.Hallett RL, Sutherland CJ, Alexander N, et al. Combination therapy counteracts the enhanced transmission of drug-resistant malaria parasites to mosquitoes. Antimicrob Agents Chemother. 2004;48:3940–3. doi: 10.1128/AAC.48.10.3940-3943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez F, Herrera S, Murrain B, et al. Selection of antifolate-resistant Plasmodium falciparum by sulfadoxine-pyrimethamine treatment and infectivity to anopheles mosquitoes. Am J Trop Med Hyg. 2007;77:438–43. [PubMed] [Google Scholar]
- 23.Hallett RL, Dunyo S, Ord R, et al. Chloroquine/sulphadoxine-pyrimethamine for gambian children with malaria: transmission to mosquitoes of multidrug-resistant Plasmodium falciparum. PLoS Clin Trials. 2006;1:e15. doi: 10.1371/journal.pctr.0010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bousema JT, Schneider P, Gouagna LC, et al. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–9. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland CJ, Ord R, Dunyo S, et al. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dondorp AM, Fairhurst RM, Slutsker L, et al. The threat of artemisinin-resistant malaria. N Engl J Med. 2011;365:1073–5. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishna S, Kremsner PG. Antidogmatic approaches to artemisinin resistance: reappraisal as treatment failure with artemisinin combination therapy. Trends Parasitol. 2013;29:313–7. doi: 10.1016/j.pt.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Sawa P, Shekalaghe SA, Drakeley CJ, et al. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infect Dis. 2013;207:1637–45. doi: 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Genotyping to identify parasite populations. Geneva: WHO; 2008. [Google Scholar]
- 30.Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 31.Baidjoe A, Stone W, Ploemen I, et al. Combined DNA extraction and antibody elution from filter papers for the assessment of malaria transmission intensity in epidemiological studies. Malar J. 2013;12:272. doi: 10.1186/1475-2875-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bousema T, Okell L, Shekalaghe S, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gadalla NB, Adam I, Elzaki SE, et al. Increased pfmdr1 copy number and sequence polymorphisms in Plasmodium falciparum isolates from Sudanese malaria patients treated with artemether-lumefantrine. Antimicrob Agents Chemother. 2011;55:5408–11. doi: 10.1128/AAC.05102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int J Parasit: Drugs and Drug Resist. 2013;3:45–50. doi: 10.1016/j.ijpddr.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adjalley SH, Johnston GL, Li T, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A. 2011;108:E1214–23. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairhurst RM, Nayyar GM, Breman JG, et al. Artemisinin-resistant malaria: research challenges, opportunities, and public health implications. Am J Trop Med Hyg. 2012;87:231–41. doi: 10.4269/ajtmh.2012.12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das D, Price RN, Bethell D, Guerin PJ, Stepniewska K. Early parasitological response following artemisinin-containing regimens: a critical review of the literature. Malar J. 2013;12:125. doi: 10.1186/1475-2875-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mockenhaupt FP, Teun BJ, Eggelte TA, et al. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop Med Int Health. 2005;10:901–8. doi: 10.1111/j.1365-3156.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 39.Schneider P, Bell AS, Sim DG, et al. Virulence, drug sensitivity and transmission success in the rodent malaria, Plasmodium chabaudi. Proc Biol Sci. 2012;279:4677–85. doi: 10.1098/rspb.2012.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price R, Nosten F, Simpson JA, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–23. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 41.Mueller I, Schoepflin S, Smith TA, et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci U S A. 2012;109:10030–5. doi: 10.1073/pnas.1200841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bousema T, Griffin JT, Sauerwein RW, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liljander A, Bejon P, Mwacharo J, et al. Clearance of asymptomatic P. falciparum Infections Interacts with the number of clones to predict the risk of subsequent malaria in Kenyan children. PLoS One. 2011;6:e16940. doi: 10.1371/journal.pone.0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwiek JJ, Alker AP, Wenink EC, Chaponda M, Kalilani LV, Meshnick SR. Estimating true antimalarial efficacy by heteroduplex tracking assay in patients with complex Plasmodium falciparum infections. Antimicrob Agents Chemother. 2007;51:521–7. doi: 10.1128/AAC.00902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenhouse B, Dokomajilar C, Hubbard A, Rosenthal PJ, Dorsey G. Impact of transmission intensity on the accuracy of genotyping to distinguish recrudescence from new infection in antimalarial clinical trials. AntimicrobAgents Chemother. 2007;51:3096–103. doi: 10.1128/AAC.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juliano JJ, Ariey F, Sem R, et al. Misclassification of drug failure in Plasmodium falciparum clinical trials in southeast Asia. J Infect Dis. 2009;200:624–8. doi: 10.1086/600892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juliano JJ, Gadalla N, Sutherland CJ, Meshnick SR. The perils of PCR: can we accurately ‘correct’ antimalarial trials? Trends Parasitol. 2010;26:119–24. doi: 10.1016/j.pt.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koepfli C, Schoepflin S, Bretscher M, et al. How much remains undetected? Probability of molecular detection of human Plasmodia in the field. PLoS One. 2011;6:e19010. doi: 10.1371/journal.pone.0019010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irion A, Felger I, Abdulla S, et al. Distinction of recrudescences from new infections by PCR-RFLP analysis in a comparative trial of CGP 56 697 and chloroquine in Tanzanian children. Trop Med Int Health. 1998;3:490–7. doi: 10.1046/j.1365-3156.1998.00253.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.